Abstract
Rats repeatedly exposed to variable prenatal stress (PNS) exhibit schizophrenia-like behavioral signs such as social withdrawal, elevations in amphetamine-induced locomotor activity, deficits in sensory-motor gating, as well as impairments in memory-related task performance. However, to date there have been no studies designed to test the hypothesis that variable PNS would lead to disruptions in sustained attention and inhibitory response control (i.e., symptoms also commonly observed in schizophrenia and other neuropsychiatric disorders such as Attention Deficit Hyperactivity Disorder). In the current study, the effects of variable PNS in rats were evaluated in fixed and variable stimulus duration (VSD) as well as variable intertrial interval (VITI) versions of a five choice serial reaction time task (5C-SRTT). In a separate series of experiments, the glutamate (NMDA) antagonist, MK-801 (0.025–0.05 mg/kg), and the norepinephrine reuptake inhibitor, atomoxetine (0.30–3.0 mg/kg), were administered acutely to assess the sensitivity of PNS subjects to glutamatergic and noradrenergic manipulations. The results indicated that exposure to variable PNS significantly impaired accuracy in the VSD version of the 5C-SRTT and increased premature and timeout responses in the VITI version. In addition, both doses of MK-801 impaired accuracy and increased premature and timeout responses in PNS, but not control subjects. In contrast, atomoxetine decreased premature and timeout responses in both PNS and control subjects in the VITI version of the task and improved accuracy in the PNS subjects. The results suggest that exposure to variable PNS in rats results in impairments of sustained attention and inhibitory response control and that these deficits can be exacerbated by NMDA antagonism and improved by a norepinephrine uptake inhibitor. Collectivity, these data further support the premise that variable PNS in rats is a valid model system for the study of neuropsychiatric disorders and their treatment.
Keywords: schizophrenia, attention deficit, impulsivity compulsivity, glutamate, atomoxetine
INTRODUCTION
During the prenatal period, the rapid growth of the central nervous system which includes the development and migration of neurons and the establishment of proper synaptic connections makes the fetus particularly vulnerable to insults (reviewed, Markham and Koenig, 2011). Accordingly, environmental stressors, including adverse life events experienced by the pregnant mother, may exert a significant impact on brain development resulting in harmful effects on the mental health of the child throughout life (Weinstock, 2005). Several epidemiological studies have established associations between prenatal stress and childhood neurodevelopmental disorders including attention-deficit/hyperactivity disorder (ADHD) and autism (Linnet et al., 2003; Grizenko et al., 2008; Kinney et al., 2008). Additional epidemiologic studies demonstrate correlations between prenatal stress and neuropsychiatric illnesses that are manifested later in life including depression, anxiety, and schizophrenia (van Os and Selten, 1998; Lewis and Levitt, 2002; Spauwen et al., 2004; Rice et al., 2007; Davis and Sandman, 2012). This has led to a growing interest in the development of prenatal stress models in animals for the purpose of elucidating neurobiological mechanisms of neuropsychiatric disorders as well as for evaluating novel therapeutic approaches (Wilson and Terry, 2010; Marco et al., 2011).
It has been suggested that repeated variable prenatal stress in rodents (henceforth referred to as PNS) might be an etiologically appropriate neurodevelopmental model for some components of schizophrenia (Koenig et al., 2005). Following acute restraint stress, rats exposed to variable PNS were found to have significantly higher levels of plasma corticosterone than control animals suggesting an altered response of the hypothalamic–pituitary–adrenal axis to acute stress (Kinnunen et al., 2003; Kapoor et al., 2006). Additionally, exposure to variable PNS also resulted in social withdrawal, elevated amphetamine-induced locomotor activity, and deficits in sensory-motor gating; behavioral characteristics commonly associated with a schizophrenia-related phenotype (Koenig et al., 2005; Lee et al., 2007). PNS subjects were also found to exhibit impairments in the performance of several behavioral tasks that have been designed to assess object recognition memory, spatial reference memory, and conditioned fear memory (Markham et al., 2010). Each of the domains of cognition modeled in these tasks (recognition memory, spatial memory, emotional processing) has have been reported to be negatively affected in schizophrenia (reviewed, Hagan and Jones, 2005, see also Phillips et al., 2003)
While several domains of cognition have been evaluated in the variable PNS model, sustained attention and inhibitory response control have not been evaluated to date. Impairments in these important behavioral functions are often characteristic of schizophrenia as well as several other neuropsychiatric disorders mentioned above (e.g., ADHD and Autism, Birkett et al., 2007; Grizenko et al., 2008; Park et al., 2012; Han et al., 2012). The 5-choice serial reaction time task (5C-SRTT), first described by Carli and colleagues in 1983, is considered a rodent analog of the continuous performance task (CPT) in humans and is useful for detecting impairments of sustained attention and inhibitory response control in several neuropsychiatric disorders (e.g. ADHD, schizophrenia) (Carli et al., 1983; Riccio et al., 2001; Lee and Park, 2006). The 5C-SRTT has increasingly gained popularity in drug discovery and development studies to identify novel compounds for the treatment of disorders associated with deficits of attention (reviewed, Higgins and Breysse, 2008). Like the CPT, the rodent 5C-SRTT is useful for evaluating sustained attention and inhibitory response control (e.g., impulsive and compulsive-like behaviors). An additional strength of the 5C-SRTT is the ability to increase the demands of the task by altering specific components of the procedure such as duration of the stimulus or the intertrial interval, factors that can distinguish subtle phenotypic differences in study groups (Higgins and Breysse, 2008; Bari et al., 2008).
There were, therefore, two major objectives of the current study: 1) to determine (using fixed and variable stimulus duration (VSD) as well as variable intertrial interval (VITI) versions of 5C-SRTT) if PNS in rats is associated with impairments in sustained attention and inhibitory response control and 2) to determine the sensitivity of PNS subjects to glutamatergic and noradrenergic manipulations using the glutamate (NMDA) antagonist, MK-801 and the norepinephrine reuptake inhibitor, atomoxetine, respectively. MK-801, has been found to disrupt areas of performance of the 5C-SRTT thought to be relevant to schizophrenia (e.g., increase premature responses) in other animals studies (Paine and Carlezon, 2009; Amitai and Markou, 2010). In contrast, atomoxetine is a clinically efficacious ADHD medication that has been found to enhance attention and attenuate impulsivity in preclinical studies utilizing the 5C-SRTT (Caballero and Nahata, 2003; Navarra et al., 2008; Paterson et al., 2011).
EXPERIMENTAL PROCEDURES
Animals
Timed pregnant Sprague-Dawley female rats (Harlan Sprague-Dawley, Inc., Indianapolis, IN, USA) arriving on day five of gestation were housed individually in a temperature-controlled (25°C) and light-controlled (12-h light/dark cycle) facility. Pregnant animals had free access to food (Teklad Rodent Diet 8604 pellets, Harlan, Madison, WI, USA) and water following their arrival. All procedures employed during this study were reviewed and approved by the Georgia Health Sciences University Institutional Animal Care and Use Committee and are consistent with the AAALAC guidelines. Measures were taken to minimize pain or discomfort in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Stress paradigm
The repeated variable PNS paradigm used in this study was adapted from Koenig and colleagues (Kinnunen et al., 2003; Koenig et al., 2005). Pregnant rats were exposed to the paradigm beginning on day 14 of gestation until delivery of pups on gestational day 22 or 23. The stress paradigm consisted of: (1) restraint in Broome style rodent restrainers (PLAS Labs, Inc) (1 h); (2) exposure to a cold environment (4 ± 1°C, 6 h); (3) overnight food deprivation; (4) forced swim in room temperature water (15 min); (5) reversal of the light–dark cycle; and (6) social stress induced by overcrowded housing during the dark phase of the cycle. Stressors were applied in a randomized manner to prevent accommodation and one to three stress sessions were administered per day. Pregnant control rats were exposed to normal animal care and maintenance procedures during this period. Following birth, all dams and pups were left undisturbed until weaning on postnatal day 22. Offspring were double housed with same sex littermate (6 females and 6 males per group), food and water was allowed ad libitum. Exposure to the prenatal stress procedures did not result in changes to the number of live born pups or the latency to parturition. Further, there were no differences in the number of pups per litter or the ratio of male to female pups between the control groups or those exposed to the paradigm (data not shown)
5-Choice Serial Reaction Time Task
Offspring of stressed and control dams were tested using an automated 5-Choice Serial Reaction Time Task (5C-SRTT) previously described by Terry and colleagues (Middlemore-Risher et al., 2010; Terry et al., 2012). Training and testing in the 5C-SRTT was conducted using six ventilated, sound attenuated operant chambers (Med Associates, St. Albans, VT, USA). Each operant chamber consisted of nine nose pokes/apertures (2.5 cm wide, 4 cm deep), four of which were closed off with metal inserts thus every other nose poke was available. The apertures, arranged on a curved panel 2 cm above the floor of the chamber, were equipped with a photocell beam to detect nose pokes. Each aperture was equipped with a lamp (2.8 W) on the rear wall that could be illuminated randomly and for varying durations. Food pellets (45 mg chow pellet, BioServ, Frenchtown, NJ, USA) were delivered automatically to a magazine, located on the opposite wall to the nose pokes, that was also equipped with a light that turned on to indicate that a pellet had been dispensed. The food magazine was equidistant from all nose poke apertures. The house light remained on for the entire session unless an error or omission occurred. The apparatus was controlled using MedPC software (Med Associates, St. Albans, VT, USA).
Beginning at postnatal day 60, animals were separated into single housed conditions in preparation for food restriction and handled daily for one week to reduce stress and anxiety in preparation for training. From week 2 until the end of the study animals were food restricted to approximately 85% of their age-dependent, free-feeding weights based upon Harlan Laboratories growth rate curves. At week 3, animals began habituation to the 5C-SRTT apparatus in preparation for training consisting of a non-spatial habituation program. Study subjects were transferred (in their home cages) to the behavioral testing rooms each morning approximately 30 min before testing.
5C-SRTT Training
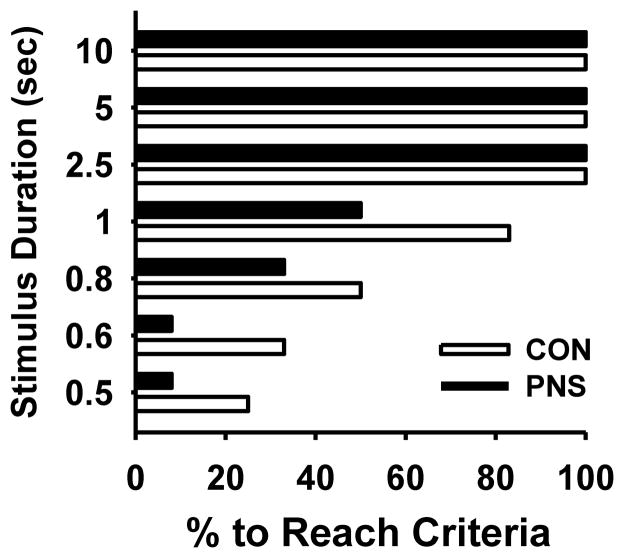
At postnatal day 81, animals began 5C-SRTT training with the stimulus duration (SD) of 10 seconds, each session being 100 trials or 30 minutes in duration with intertrial intervals (ITI) of 5 seconds. Animals were trained 5 days per week until they reached stable performance levels (defined as 2 consecutive days at >80% accuracy, <20% omissions and completion of all 100 trials) at the 10 seconds stimulus duration Once criterion was achieved at a given stimulus duration, the animals were moved to the next more challenging stimulus durations (5, 2.5, 1.0, 0.8, 0.6, 0.5 seconds) accordingly. Due to the small number of animals, especially in the prenatal stress group, that were able to meet the performance criteria described above after 45 days of training at the more challenging stimulus durations (0.8, 0.6, 0.5 seconds) (see Fig 1), subjects were trained at the 1 seconds stimulus duration until both group performed stably at approximately 75–85% accuracy. At the end of training, one animal from each group was removed from the study due to an average completion of less than 30 % of trials per session.
Fig. 1.
Percentage of control (CON) and prenatally stressed (PNS) rats able to achieve the predetermined performance criteria in the 5C-SRTT (defined as 2 consecutive days at >80% accuracy, <20% omissions and completion of all 100 trials) at stimulus durations of 10, 5, 2.5, 1.0, 0.8, 0.6, 0.5 seconds with a 5 seconds intertrial interval. N = 12.
5C-SRTT Performance Assessment
To determine the effects of PNS on sustained attention, animals were tested at i) a standard 1 second stimulus duration with a 5 second intertrial interval, ii) a randomized, varying stimulus duration (VSD) of 0.25, 0.50 and 1 second with a 5 second intertrial interval iii) and a randomized varying intertrial interval (VITI) of 1, 5, and 10 seconds with a 1 second stimulus duration. To assess performance the following parameters were measured: % correct ((# correct /(# correct + # incorrect))x100), % of omissions, premature responses (total # of responses performed after the trial began but before onset of the light stimulus), timeout responses (total # of nose pokes made in any aperture during a timeout period, perseverative responses (total # of nose pokes performed after the correct response had been made but before collecting the reward), trials completed, latency to correct response (time taken from onset of nose poke light stimulus to making the correct nose poke response), latency to incorrect response (time taken from onset of nose poke light stimulus to making the incorrect nose poke response), and latency to reward (i.e., the magazine latency, time taken from making a correct nose poke response to retrieving the reward from the magazine).
Drug administration
Following baseline (vehicle) assessments in the Fixed, VSD, and VITI versions of the 5C-SRTT, both PNS and control animals were treated acutely with the glutamate (NMDA) antagonist, MK-801 and the norepinephrine reuptake inhibitor, atomoxetine to assess sensitivity to glutamatergic and noradrenergic manipulations. A drug-free washout period of at least three days was imposed between individual drug doses and at least two weeks of washout was imposed between exposures to the different drugs (i.e., MK-801 and atomoxetine). Subjects were injected subcutaneously (s.c) with MK-801 (0.025 and 0.05 mg/kg; Sigma, Cat. No. M-107) or vehicle (0.9% NaCl) 30 minutes prior to testing in the 5C-SRTT at the standard 1 second stimulus duration and 5 second intertrial interval. The MK-801 doses chosen for this study are slightly lower than those typically found to produce robust deficits in normal (control) rats in previous studies of the 5C-SRTT (see Paine and Carlezon, 2009; Smith et al., 2011; Terry et al., 2012). This approach was taken so that potential differences in sensitivity to NMDA antagonism between the PNS and control subjects could be identified as well as to reduce the possibility of confounding motor impairments or sedation produced by MK-801 (Paine and Carlezon, 2009; Smith et al., 2011; Terry et al., 2012). MK-801 and vehicle were administered in a pseudorandom manner to obviate any effects associated with the order of drug administration.
Atomoxetine hydrochloride ((3R)-N-methyl-3-(2-methylphenoxy)-3-phenylpropan-1-amine) obtained from Pfizer (Ann Arbor, MI) 0.3, 1, 3 mg/kg or vehicle (0.01 mol/l PBS) was subsequently administered by intraperitoneal (i.p.) injection 45 minutes prior to testing in the 5C-SRTT with randomized, VITIs of 1, 5, and 10 seconds and a 1 second stimulus duration. Doses were chosen based on efficacy in previously published 5C-SRTT studies (Navarra et al., 2008, Robinson et al., 2008). Atomoxetine and vehicle were administered in a pseudorandom manner to obviate any effects associated with the order of drug administration.
Statistical, Analyses
To determine whether differences in the proportion of subjects (PNS versus control) reaching the training criterion across the different stimulus durations existed, a generalized estimating equation (GEE) model was used assuming a binomial distribution of the outcome and an identity link (SAS 9.3). Animal nested within group was considered a random effect. Included in the model were the main effects of group and stimulus duration and the two-factor interaction between group and stimulus. A Bonferroni adjustment to the overall alpha level was used to examine post hoc pair-wise differences between groups within stimulus duration. Due to all animals meeting the criterion for stimuli 10, 5 and 2.5 the analysis was restricted to stimuli 1, 0.8, 0.6 and 0.5. In trained subjects (for the remainder of the study), single factor comparisons between the study groups were made via unpaired two-tailed t-tests. Comparisons of groups with two-factors were conducted using a two way repeated measures ANOVA followed by post hoc analysis using the Student-Newman–Keuls multiple comparison method (SigmaStat 2.03, SPSS Inc., Chicago, IL, USA). Statistical significance was assessed using an alpha level of 0.05.
RESULTS
Effects of PNS on Training in the 5C-SRTT
While there was a trend toward differences in the proportion of subjects (PNS versus control) reaching the training criterion across the different stimulus durations (Fig 1), the effect did not reach the required level of statistical significance, group, χ2= 2.82, p=0.09; stimulus duration, χ2= 24.73, p<0.05; group x stimulus duration interaction, χ2= 2.42, p=0.49. Post hoc pairwise comparisons within stimulus duration also indicated that there was no difference in the proportion meeting criterion between the PNS and control groups.
Effects of PNS on Standard 5C-SRTT Performance
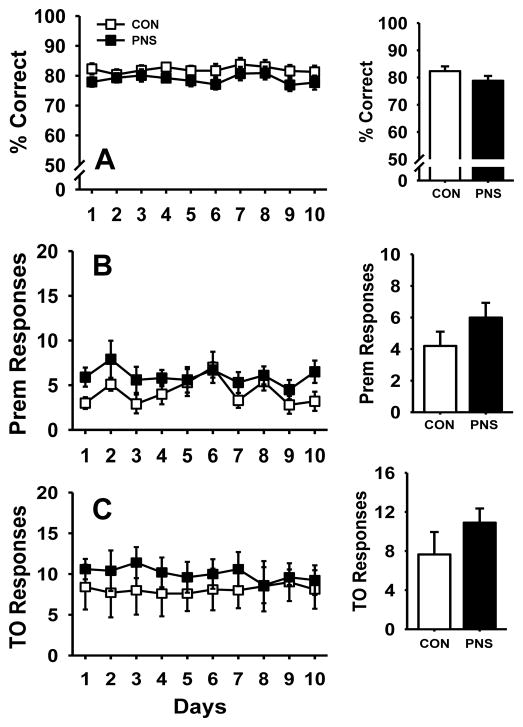
Following training to the performance criteria described in the methods, PNS and control subjects were evaluated for baseline stability and potential differences in daily performance at a fixed 1 second stimulus duration (SD) and 5 second intertrial interval (ITI) over a ten day period (Fig. 2 and Table 1). Exposure to PNS resulted in no significant differences compared to controls in accuracy (Fig. 2A), number of premature responses (Fig. 2B), or the number of timeout responses (Fig. 2C). Likewise, there were also no significant differences in the number of perseverative responses, latencies, or the percentage of omissions (Table 1). Analysis of sex-specific differences in the performance of all parameters measured using the various versions of 5C-SRTT determined no significant difference in either the prenatally stressed or control group (data not shown), thus data of females and males were combined to determine overall effect of prenatal stress on performance.
Fig. 2.
Effects of prenatal stress on performance of the 5C-SRTT at a fixed 1 second stimulus duration (SD) and 5 second intertrial interval (ITI) over a ten day observation period. (A) percent correct, (B) premature responses, or (C) timeout responses. Insets illustrate the effects averaged over the 10 day observation period. Each symbol and bar represents the mean ± SEM for each test group. PNS = prenatally stressed; CON = non-stressed controls. N = 10.
Table 1.
Effects of prenatal stress (PNS) on perseverative responses, latencies, % omissions, and trials completed in the standard (1 second stimulus duration, 5 second intertrial interval), variable stimulus durations (VSD) and variable intertrial intervals (VITI) versions of the 5C-SRTT.
| Task | Group | Persev Responses | Latency Correct (s) | Latency Incorrect (s) | Magazine Latency (s) | % Omissions | Trials Completed |
|---|---|---|---|---|---|---|---|
| Standard | Control | 2.6 ± 0.59 | 0.90 ± 0.05 | 1.97 ± 0.14 | 1.95 ± 0.18 | 4.46 ± 0.8 | 98.9 ± 0.9 |
| PNS | 3.5 ± 0.41 | 1.02 ± 0.12 | 1.87 ± 0.16 | 1.72 ± 0.11 | 4.61 ± 1.4 | 99.7 ± 0.2 | |
| VSD (0.25, 0.5, 1s) | Control | 2.0 ± 0.62 | 0.83 ± 0.05 | 1.77 ± 0.12 | 1.89 ± 0.15 | 6.50 ± 1.8 | 100.0 ± 0.0 |
| PNS | 2.3 ± 1.03 | 0.98 ± 0.10 | 1.76 ± 0.13 | 1.78 ± 0.13 | 7.50 ± 1.9 | 100.0 ± 0.0 | |
| VITI (1, 5, 10s) | Control | 0.9 ± 0.32 | 0.99 ± 0.04 | 2.21 ± 0.26 | 1.66 ± 0.12 | 13.3 ± 2.0 * | 61.0 ± 7.0 * |
| PNS | 1.0 ± 0.27 | 1.11 ± 0.08 | 2.13 ± 0.09 | 1.62 ± 0.09 | 14.7 ± 4.5 * | 63.8 ± 7.0 * |
Data are presented as the mean ± SEM.
represents a significant difference between performance associated with the standard (fixed SD and ITI) task and the VITI task (p < 0.05).
Effects of PNS on Performance of the 5C-SRTT with Variable Stimulus Durations (VSDs)
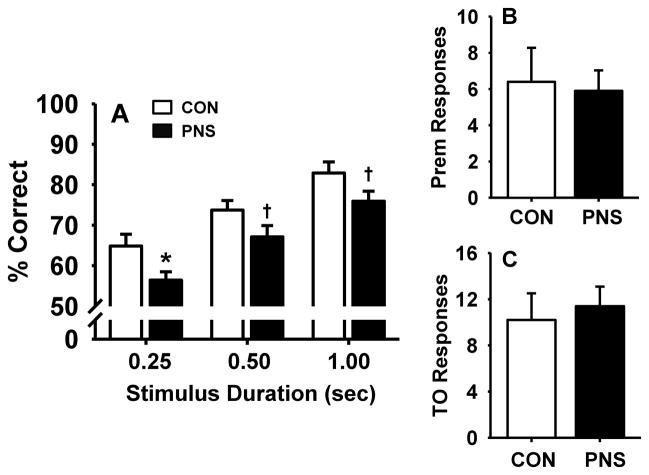
To determine the effects of PNS on sustained attention when demands of the task are increased, PNS and control animals were evaluated for performance of the 5C-SRTT with pseudorandom presentation of VSDs of 1.0, 0.5, and 0.25 second and a fixed 5 second intertrial interval (Fig. 3 and Table 1). Statistical analysis revealed the following results, main effect of group F(1,18) = 8.2, p<0.05; stimulus duration, F(2,36) = 31.6, p<0.05; group by stimulus duration interaction F(2,36) = 0.08, p>0.1). As expected, accuracy in all subjects increased with the increase of stimulus duration (see Fig 3A). However, post hoc analysis further indicated that accuracy in prenatally stressed rats was impaired (relative to controls) at the 0.25 second stimulus duration (p<0.05), with a trend toward significant impairment at the 0.5 and 1 second stimulus duration (p = 0.08, 0.07 respectively). The number of premature responses (Fig. 3B), number of timeout responses (Fig. 3C), and other parameters tested (Table 1) were not significantly affected in either group by the presentation of VSDs.
Fig. 3.
Effects of prenatal stress on performance of the 5C-SRTT with pseudorandom presentation of variable stimulus durations (VSD, 0.25, 0.50, and 1.00 second). (A) percent correct, (B) premature responses, or (C) timeout responses. Each bar represents the mean ± SEM for each test group.* represents a significant difference (p < 0.05) in performance between PNS and control rats, † represents a trend towards a significant difference (p < 0.10). PNS =prenatally stressed; CON = non-stressed controls. N = 10.
Effects of PNS on Performance of the 5C-SRTT with Variable Intertrial Interval (VITIs)
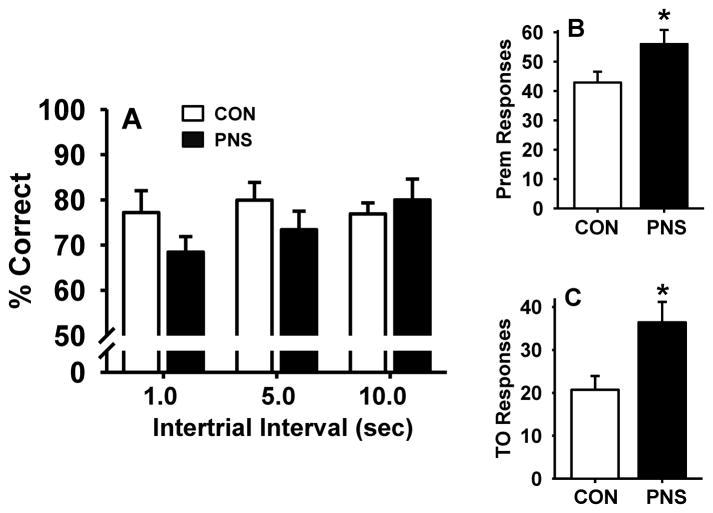
To further assess the effects of PNS on performance of the 5C-SRTT when demands of the task are increased, PNS and control animals were evaluated for performance of the 5C-SRTT with a pseudorandom presentation of VITIs of 1.0, 5.0, and 10.0 seconds and a fixed 1 second stimulus duration (Fig. 4 and Table 1). No significant differences in accuracy were observed in PNS subjects compared to controls (Fig. 4A) in this portion of the study. However, when presented with varying ITIs, PNS animals had a significant increase in the total number of premature responses (t = −2.18, df = 18.0, p<0.05) and in the total number of timeout responses (t = −2.48, df = 18.0, p<0.05) compared to control (Fig. 4B and Fig. 4C). There were no differences between PNS subjects and controls in the number of perseverative responses, latencies, or the percentage of omissions when performing the VITI version of the 5C-SRTT (Table 1).
Fig. 4.
Effects of prenatal stress on performance of the 5C-SRTT with pseudorandom presentation of variable intertrial intervals (VITI, 1.0, 5.0, and 10.0 seconds). (A) percent correct, (B) premature responses, or (C) timeout responses. Each bar represents the mean ± SEM for each test group. * represents a significant difference (p < 0.05) in performance between PNS and control rats. PNS = prenatally stressed; CON = non-stressed controls. N = 10.
Comparisons in Performance between the Various 5C-SRTT Tasks
Additional (within group and between group) statistical analyses were performed to make an assessment of whether there were differences in performance associated with the standard (fixed SD and ITI) task versus the VSD and VITI task. The only significant (p<0.05) differences detected were an increase in the percentage of omissions and a decrease in the number of trials completed in both the PNS and control groups when the VITI task was used compared the standard task (see Table 1).
Effects of MK-801 on PNS and Control Performance
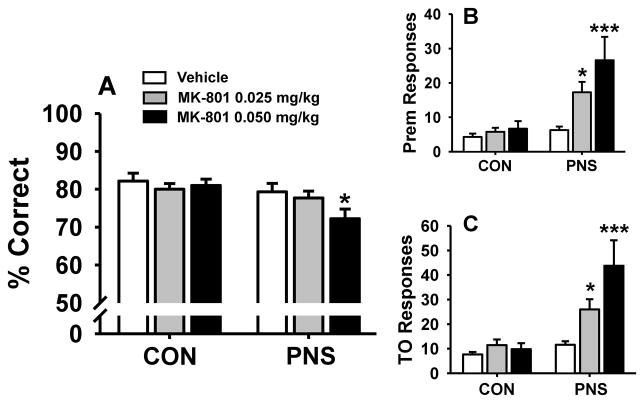
MK-801 was evaluated for dose-related effects on performance of PNS and control animals at the standard (fixed) 1 second stimulus duration and 5 second intertrial interval (Fig. 5 and Table 2). For the accuracy assessment, the following statistical results were obtained, main effect of group, F(1,18) = 5.03, p<0.05; treatment F(2,36) = 3.02, p=0.06; group by treatment interaction F(2,36) = 2.30, p=0.12. Post hoc analysis indicated that the highest dose of MK-801 (0.05 mg/kg) significantly impaired accuracy (Fig. 5A) in PNS animals (compared to vehicle-associated performance, p<0.05), but not in controls. For the premature response assessment, the following statistical results were obtained, main effect of group F(1,18) = 15.87, p<0.05; treatment F(2,36) = 6.50, p<0.05; group by treatment interaction F(2,36) = 4.04, p<0.05. Post hoc analysis indicated that PNS rats (but not controls) had a significant increase in premature responses associated with both of the doses of MK-801 that were evaluated (p<0.05). For the timeout response assessment, the following statistical results were obtained, main effect of group F(1,18) = 11.47, p<0.05; treatment F(2,36) = 6.56, p<0.05; group by treatment interaction F(2,36) = 5.16, p<0.05). Post hoc analysis indicated that PNS rats (but not controls) had a significant increase in timeout responses associated with both of the doses of MK-801 that were evaluated (p<0.05).
Fig. 5.
Dose-related effects of the glutamate (NMDA) antagonist MK-801 on performance of the standard 5C-SRTT (1 second SD, 5 second ITI) by prenatally stressed (PNS) and control (CON) rats. (A) percent correct, (B) premature responses, or (C) timeout responses. Each bar represents the mean ± SEM for each test group. *(p<0.05); ***(p<0.001) = significantly different compared to vehicle-associated performance level. N = 10.
Table 2.
Dose-related effects of acute administration of MK-801 in control and prenatally stressed (PNS) rats on perseverative responses, latencies, % omissions, and trials completed in the standard (1 second stimulus duration, 5 second intertrial interval) version of the 5C-SRTT.
| Treatment | Group | Persev Responses | Latency Correct (s) | Latency Incorrect (s) | Magazine Latency (s) | % Omissions | Trials Completed |
|---|---|---|---|---|---|---|---|
| Vehicle | Control | 3.1 ± 0.86 | 0.90 ± 0.06 | 1.82 ± 0.18 | 1.94 ± 0.16 | 3.45 ± 0.7 | 99.9 ± 0.1 |
| PNS | 3.7 ± 0.67 | 1.03 ± 0.14 | 1.88 ± 0.20 | 1.72 ± 0.10 | 4.75 ± 1.8 | 99.8 ± 0.2 | |
| MK-801 0.025 mg/kg | Control | 2.2 ± 0.61 | 0.80 ± 0.06 | 1.74 ± 0.31 | 1.77 ± 0.29 | 3.00 ± 0.5 | 98.5 ± 0.6 |
| PNS | 3.6 ± 0.48 | 0.90 ± 0.12 | 1.47 ± 0.26 | 1.57 ± 0.13 | 2.7 ± 0.45 | 100 ± 0.0 | |
| MK-801 0.05 mg/kg | Control | 3.8 ± 0.81 | 0.86 ± 0.03 | 2.01 ± 0.20 | 1.91 ± 0.33 | 5.10 ± 0.5 | 90.9 ± 5.9 |
| PNS | 3.1 ± 0.92 | 0.88 ± 0.14 | 1.61 ± 0.27 | 1.56 ± 0.17 | 4.70 ± 1.5 | 83.7 ± 5.0 * |
Data are presented as the mean ± SEM.
represents a significant difference between vehicle-associated performance and drug treatment-associated performance (p < 0.05).
MK-801 was also associated with a decrease in the number of trials completed, main effect of treatment F(2,36) = 9.69, p<0.05). Post analysis indicated that PNS animals completed significantly fewer trials (p<0.05) at the 0.05 mg/kg dose of MK-801 compared to when vehicle was administered. (Table 2). There were no MK-related differences in the number of perseverative responses, latencies, or the percentage of omissions in either PNS or control animals.
Effects of Atomoxetine on PNS and Control Performance of the VITI Task
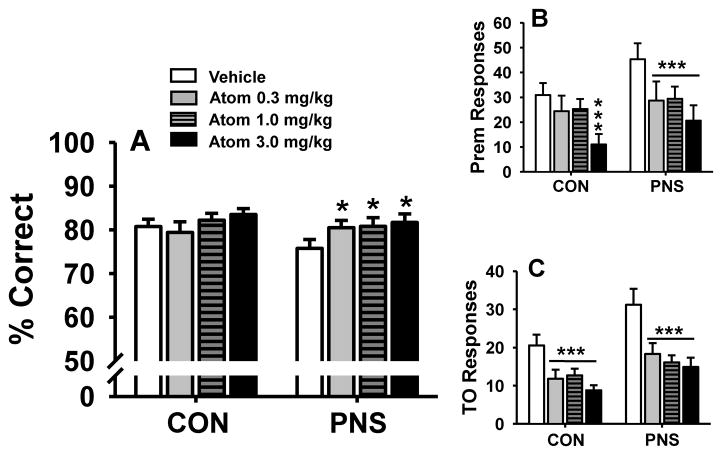
Atomoxetine was evaluated for dose-related effects on performance of PNS and control animals in the VITI version of the 5C-SRTT task (Fig. 6). Doses of atomoxetine (0.3–3.0 mg/kg) were acutely administered i.p. 45 minutes prior to testing. For the accuracy assessment (Fig 6A), the following statistical results were obtained main effect of group F(1,18) = 0.46, p=0.51; treatment F(2,36) = 3.62, p<0.05; group by treatment interaction F(2,36) = 1.58, p=0.20. Post hoc analysis indicated that all three doses of atomoxetine (0.3, 1.0, 3.0 mg/kg, compared to vehicle) were associated with modest (but statistically significant) improvements in accuracy in prenatally stressed animals, but not controls.
Fig. 6.
Dose-related effects of the norepinephrine reuptake inhibitor atomoxetine on performance of the 5C-SRTT with pseudorandom presentation of variable intertrial intervals (VITI, 1.0, 5.0, and 10.0 seconds) by prenatally stressed (PNS) and control (CON) rats. (A) percent correct, (B) premature responses, or (C) timeout responses. Each bar represents the mean ± SEM for each test group. *(p<0.05); ***(p<0.001) = significantly different compared to vehicle-associated performance level. N = 10.
For the premature response assessment (Fig 6B), the following statistical results were obtained, main effect of group F(1,18) = 1.29, p = 0.27; treatment F(2,36) = 18.23, p<0.05; group by treatment interaction F(2,36) = 1.33, p = 0.28). Post hoc analysis indicated that all 3 doses of atomoxetine reduced the number of premature responses in PNS subjects (p<0.05 for all three doses) and that the higher dose (3.0 mg/kg) reduced the number of premature responses in control subjects (p<0.05).
For the timeout response assessment (Fig 6C), the following statistical results were obtained, main effect of group F(1,18) = 4.73, p<0.05; treatment F(2,36) = 17.37, p<0.05; group by treatment interaction F(2,36) = 1.01, p = 0.40). Post hoc analysis indicated that all three doses of atomoxetine reduced the number of timeout responses in both PNS subjects and controls (p<0.05 for all doses evaluated). In addition, in the between group comparisons (i.e., PNS subjects versus controls) for both premature and timeout responses, the vehicle-associated response was significantly different (p<0.05) but, the response associated with each of the doses of atomoxetine in the PNS rats was not statistically different from the control animals administered vehicle. This would suggest that atomoxetine may reverse some of the behavioral alterations observed following PNS. The 1.0 mg/kg dose of atomoxetine also significantly (p<0.05 versus vehicle-related performance) improved the number of trials completed in the PNS subjects (see Table 3).
Table 3.
Dose-related effects of acute administration of Atomoxetine in control and prenatally stressed (PNS) rats on perseverative responses, latencies, % omissions, and trials completed during the 5C-SRTT with pseudorandom presentation of variable intertrial interval (1, 5, and 10 seconds).
| Treatment | Group | Persev Responses | Latency Correct (s) | Latency Incorrect (s) | Magazine Latency (s) | % Omissions | Trials Completed |
|---|---|---|---|---|---|---|---|
| Vehicle | Control | 1.0 ± 0.31 | 0.92 ± 0.03 | 2.06 ± 0.15 | 1.78 ± 0.16 | 14.9 ± 1.9 | 69.6 ± 4.8 |
| PNS | 1.0 ± 0.25 | 1.12 ± 0.10 | 2.12 ± 0.16 | 1.66 ± 0.11 | 13.3 ± 1.9 | 67.1 ± 5.0 | |
| Atomoxetine 0.3 mg/kg | Control | 1.4 ± 0.48 | 1.03 ± 0.03 | 2.15 ± 0.10 | 1.77 ± 0.13 | 16.3 ± 2.0 | 74.2 ± 4.7 |
| PNS | 1.5 ± 0.39 | 1.13 ± 0.10 | 2.09 ± 0.24 | 1.87 ± 0.14 | 17.0 ± 2.5 | 78.9 ± 5.4 | |
| Atomoxetine 1.0 mg/kg | Control | 0.8 ± 0.47 | 0.96 ± 0.03 | 2.03 ± 0.10 | 1.76 ± 0.22 | 14.4 ± 1.4 | 81.0 ± 4.0 |
| PNS | 2.4 ± 0.71 | 1.05 ± 0.10 | 2.41 ± 0.16 | 1.62 ± 0.08 | 14.0 ± 2.1 | 83.7 ± 5.7 * | |
| Atomoxetine 3.0 mg/kg | Control | 0.9 ± 0.37 | 1.03 ± 0.06 | 2.26 ± 0.17 | 1.86 ± 0.10 | 16.1 ± 2.4 | 64.4 ± 6.0 |
| PNS | 1.5 ± 0.66 | 1.14 ± 0.11 | 2.17 ± 0.17 | 1.89 ± 0.21 | 16.8 ± 1.9 | 68.5 ± 3.7 |
Data are presented as the mean ± SEM.
represents a significant difference between vehicle-associated performance and drug treatment-associated performance (p < 0.05).
Finally, there were no atomoxetine-related differences in the number of perseverative responses, latencies, or the percentage of omissions when performing the VITI version of the 5C-SRTT.
DISCUSSION
The results of this study can be summarized as follows: 1) under standard 5C-SRTT conditions (i.e., fixed ITI and SDs) there were few notable differences in performance between PNS rats and controls, 2) when the demands of the task were increased by varying the stimulus durations or intertrial intervals, accuracy and inhibitory response control (premature and timeout responses) was impaired, respectively, in the PNS rats relative to controls, 3) relatively low doses of the NMDA antagonist MK-801 impaired accuracy and inhibitory response control in PNS rats, but not control subjects (under standard 5C-SRTT conditions), and 4) the norepinephrine reuptake inhibitor, atomoxetine improved inhibitory response control (i.e., it decreased premature and timeout responses) in both PNS and control subjects in the VITI version of the task and improved accuracy in the PNS subjects. Collectively, these experimental results suggest that exposure to variable PNS results in impairments of sustained attention and inhibitory response control (especially when the demands of the task are increased) and that these deficits can be exacerbated by NMDA antagonism and improved by a norepinephrine uptake inhibitor.
The lack of performance differences between PNS and control subjects under standard 5C-SRTT conditions was not altogether surprising. The 5C-SRTT requires long training times to attain stable performance by the animals, which can lead to task performance being mediated by “automatic processing” (i.e., involvement of processes exogenously triggered by stimulus sequence association from one trial to the next, rather than by internal volitional expectations) (see Amitai and Markou, 2011; Capizzi et al., 2012). However, by altering various task parameters from the standard procedure on which the animals were trained, the animals can no longer rely on automatic processes, but have to continuously sustain response readiness using high-resource “control processing” to perform the task. Thus, subtle cognitive deficits that may be undetectable in the standard 5C-SRTT task may become manifested when demands of the task are increased (Amitai and Markou, 2011).
The VSD version of the 5C-SRTT is used to increase attentional load, thus it often results in decreased accuracy (% correct), the main measure of attentional performance of the task (Higgins et al., 2008; Bari et al., 2008). It has become widely accepted that attention does not refer to a single cognitive process, but is divided into three sub-domains of selective (i.e., process by which environmental stimuli are chosen for attention), sustained (i.e., the continuous allocation of processing resources on a particular stimuli for prolong periods of time), and divided attention (i.e., focus on stimuli despite distractors and/or multiple tasks) (Young et al., 2009). While it has been suggested that the 5C-SRTT can be used to assess deficits and/or improvements in all three sub-domains of attention, changes in accuracy are often thought to be representative of alterations in sustained attention, also referred to as vigilance (Young et al., 2009; Bari et al., 2008). In the 5C-SRTT, accuracy is defined as the total number correct responses divided by the sum of correct and incorrect responses, and thus it is a conservative measure of attention, due to its independence of omissions. As expected, when tested using VSDs, all experimental subjects had a significant (stimulus-dependent) decrease in accuracy. However, the magnitude of the decrease in accuracy was greater in rats exposed to variable PNS indicating a vulnerability to deficits of sustained attention when attentional load is increased (see Higgins and Breysse, 2008). The deficits do not appear to reflect a general nonselective suppression of responding as no (group-related) differences in the number of trials completed or the percentage of omissions were observed. The lack of changes in magazine latency (i.e., the latency to collect food rewards) further supports the argument that impairments in attention in the PNS subjects are not a result in general deficits in motivation, locomotor activity, or malaise. Other measures such as inappropriate responses (i.e., premature, perseverative, and timeout responses) where not different in the VSD version of the task compared to that associated with the standard 5C-SRTT.
The VITI version of the 5C-SRTT is often used to increase the demand on inhibition of inappropriate responding by making the appearance of the stimuli unpredictable, thus it often results in increased premature responses, generally interpreted as a form of impulsive behavior (Bari et al., 2008; Amitai and Markou, 2011). Premature responses occur inappropriately, during the intertrial interval before the target stimulus has been presented, a period in which the rodents are anticipating the occurrence of the stimuli. Predictably, when experimental animals were exposed to a pseudorandom presentation of different intertrial intervals, there was a significant increase in premature responses (compared to when fixed intertrial intervals were presented). However, prenatally stressed animals had significantly more premature responses compared to controls, suggesting a loss of impulse control and disinhibition of inappropriate responding (see review, Robbins 2002). While there was a significant decrease in accuracy in all subjects exposed to this procedure, there were no significant differences between control and PNS animals, most likely due to the fact that this version of the task does not increase attentional load as much as the VSD version of the task (where group-related differences were observed). While all experimental subjects exposed to the VITI version of the task had a significant increase in the percent of omissions as well as a significant decrease in the number of trials completed, no significant difference was found between the PNS and control groups. The overall decrease in trials completed may be reflective of the fact that trials involving errors such as premature responses last longer because of timeout periods.
An increase was also observed in the number of timeout responses (i.e., nose pokes made during the timeout interval) in all experimental subjects in the VITI task compared to the standard 5C-SRTT, although the increase was more pronounced in PNS animals relative to controls. Timeout responses, another measure of response inhibition, have been suggested to represent a measure of compulsivity and/or cognitive inflexibility (or disorganized responses) that are not tied to the stimulus presentation. Notably, cognitive inflexibility (i.e., the inability to alter behavior in reaction to changing situational demands) is a characteristic deficit in schizophrenia and other mental health disorders (see Amitai and Markou, 2010)..
Interestingly, perseverative responses (i.e., a repeated nose poke into the same aperture after a correct response), also generally interpreted as a form of compulsive-like behavior, were unaffected during the VITI version of the task. This may be due to the fact that when perseverative responses are punished by a timeout period, additional perseverative responses would be recorded as timeout responses. Another line of reasoning may be that the increase in timeout responses is due to the increase of premature responses which are punished with timeout periods and consequently provide more opportunity for this type of response to be recorded. Collectively, the results described above are suggestive of a loss of inhibitory response control in rats exposed to PNS. It is important to note that several of the neuropsychiatric illnesses associated with PNS (e.g., schizophrenia, ADHD) exhibit impulsivity characterized by response disinhibition (Wykes et al., 2000; Sagvolden et al., 2005).
The next series of experiments were designed to determine the sensitivity of PNS rats to alterations in the glutamatergic system. Disturbance of the glutamatergic system has been implicated in the pathophysiology of several neuropsychiatric disorders including schizophrenia, autism, and depression (Javitt et al., 2011). Studies have shown that NMDA receptor antagonism exacerbates symptoms in schizophrenia patients, including the cognitive dysfunction (Lahti et al., 1995; Malhotra et al., 1997). Further, NMDA receptor antagonists are commonly used to produce schizophrenia-like symptoms in animals (Rung et al., 2005). Administration of NMDA antagonists has been shown to disrupt multiple performance measures of the 5C-SRTT (Amitai and Markou, 2010). We found that acute exposure to relatively low doses of MK-801 in PNS rats (but not controls) significantly altered several performance measures in the 5C-SRTT that are often associated with NMDA antagonism including a decrease in accuracy and an increase in premature and timeout responses. While we have not (to date) evaluated the glutamatergic system (directly) in PNS rats, our findings suggest that these subjects may be useful for modeling the cognitive dysfunction (e.g., impairments in sustained attention, increased impulsivity, and cognitive inflexibility) often observed with several neuropsychiatric disorders where the glutamatergic system is altered We did detect a slight decrease in the number of trials completed in the PNS subjects administered the 0.05 mg/kg dose of MK-801. This observation could suggest that the NMDA antagonist (specifically in the PNS rats) could have altered motivation (i.e., effects that would confound interpretations related to MK-801 effects on attention, impulsivity, etc). To argue against this possible conclusion, or that the MK-801 effects might be related to alterations in locomotor activity, neither the percentage of omissions nor the response or magazine latencies were altered in the current study by MK-801. Moreover, other investigators have demonstrated that changes in motor activity and motivation are dissociated from changes in accuracy and response control in the 5C-SRTT (Carli and Samanin, 1992 Robbins 2002; Mirjana et al., 2004).
The final series of experiments were designed to determine the sensitivity of PNS rats to the noradrenaline-specific reuptake inhibitor atomoxetine. Atomoxetine has been shown clinically to enhance attention and attenuate impulsivity in both children and adults with ADHD (Michelson et al., 2003; Chamberlain et al., 2007). The exact mechanism of action of atomoxetine on impulsive behaviors is unclear but may be due to either enhanced cortical noradrenergic neurotransmission or by stimulation of dopamine overflow via regulation by the norepinephrine transporter (Carboni et al., 1990; Bymaster et al., 2002; Swanson et al., 2006). In previous rodent studies using the 5C-SRTT, the most notable effects of atomoxetine has been the decrease in premature responses (Navarra et al., 2008; Robinson et al., 2008; Koffarnus and Katz, 2011). Accordingly, in the present study, we employed the VITI version of the 5C-SRTT (known to increase premature responses) to determine the effects of atomoxetine in PNS subjects. Interestingly, atomoxetine reduced premature responses both in control animals (similar to previously published studies) as well as in PNS subjects and it also reduced the number of timeout responses. In addition, all three of the doses of atomoxetine were associated with modest improvements in accuracy (% correct) in the PNS animals. Navarra and colleagues also found a modest (atomoxetine-related) improvement in accuracy using a VITI version of the task (Navarra et al., 2008), although in most of the previous studies where atomoxetine was evaluated in the 5C-SRTT no improvements in accuracy were noted (Robinson et al., 2008; Koffarnus and Katz, 2011, Paterson et al., 2011). These results may suggest that attentional processes of PNS animals are more sensitive to atomoxetine possibly due to alterations in noradrenergic or dopamine neurotransmission. Alternatively, the results may reflect differential effects of atomoxetine in various versions of the 5C-SRTT, since only our study and the Navarra study utilized the VITI version of the task. The effects of atomoxetine do not appear to reflect changes in motivation or hyperactivity produced by the compound, as there were no significant changes in latencies (i.e., correct, incorrect and magazine latencies). Further, atomoxetine administration did not result in fewer trials completed; in fact, one dose significantly increased the number of trials completed by PNS animals.
Collectively, the results described here (i.e., demonstrating sensitivity to a clinically useful ADHD therapeutic agent) indicate that PNS rats may be useful as a model system for neuropsychiatric disorders where impairments of attention and impulse control are present. Early clinical studies appear to support this notion and suggest that atomoxetine may have efficacy in autism, bipolar disorders, and depression (Bangs et al., 2007; Chang et al., 2009; Zeiner et al., 2011). There have also been several studies of the use of atomoxetine as an adjunctive treatment in schizophrenia, although no significant cognitive improvement has yet to be detected, possibly due small sample sizes (Friedman et al., 2008; Kelly et al., 2009; Sacco et al., 2009).
CONCLUSION
The results of this study suggest that exposure to variable PNS results in impairments of sustained attention and inhibitory response control and that these deficits can be exacerbated by NMDA antagonism and improved by a norepinephrine uptake inhibitor. These observations along with previous reports of impairments in object recognition memory, spatial reference memory and conditioned fear memory (Koenig et al., 2005; Markham et al., 2010) suggest a phenomenological similarity between prenatally stressed rodents and humans afflicted with one of several neuropsychiatric disorders. Such observations support the face validity of this model system for studying some aspects of these conditions. The ability of atomoxetine to attenuate the deficits associated with PNS during the 5C-SRTT also provides some evidence of predictive validity of the model. It should be noted, however, that predictive validity of animal models of neuropsychiatric disorders is difficult to achieve as drugs that reliably improve cognitive symptoms in human patients are not currently available.
HIGHLIGHTS.
Prenatal stress in rats leads to impairments of sustained attention.
Prenatal stress in rats results in a loss of inhibitory response control.
The deficits are exacerbated by NMDA antagonism
The deficits can be improved by a norepinephrine uptake inhibitor.
Rat prenatal stress is a valid model system for studying neuropsychiatric disorders.
Acknowledgments
The experiments described in this manuscript were supported in part by grants from the National Institute on Drug Abuse (DA029127), the National Institute of Environmental Health Sciences (ES012241), and the National Institute on Aging (AG029617).
Abbreviations
- PNS
Prenatal Stress
- ADHD
Attention Deficit/Hyperactivity Disorder
- 5C-SRTT
5 Choice Serial Reaction Time Task
- VSD
Variable Stimulus Duration
- VITI
Variable Intertrial Interval
- NMDA
N-methyl-D-aspartate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amitai N, Markou A. Disruption of performance in the five-choice serial reaction time task induced by administration of N-methyl-D-aspartate receptor antagonists: relevance to cognitive dysfunction in schizophrenia. Biol Psychiatry. 2010;68(1):5–16. doi: 10.1016/j.biopsych.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amitai N, Markou A. Comparative effects of different test day challenges on performance in the 5-choice serial reaction time task. Behav Neurosci. 2011;125(5):764–774. doi: 10.1037/a0024722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bangs ME, Emslie GJ, Spencer TJ, Ramsey JL, Carlson C, Bartky EJ, Busner J, Duesenberg DA, Harshawat P, Kaplan SL, Quintana H, Allen AJ, Sumner CR. Efficacy and safety of atomoxetine in adolescents with attention-deficit/hyperactivity disorder and major depression. J Child Adolesc Psychopharmacol. 2007;17(4):407–420. doi: 10.1089/cap.2007.0066. [DOI] [PubMed] [Google Scholar]
- 4.Bari A, Dalley JW, Robbins TW. The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nature Protocols. 2008;3(5):759–767. doi: 10.1038/nprot.2008.41. [DOI] [PubMed] [Google Scholar]
- 5.Birkett P, Sigmundsson T, Sharma T, Toulopoulou T, Griffiths TD, Reveley A, Murray R. Reaction time and sustained attention in schizophrenia and its genetic predisposition. Schizophr Res. 2007;95(1–3):76–85. doi: 10.1016/j.schres.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 6.Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- 7.Caballero J, Nahata MC. Atomoxetine hydrochloride for the treatment of attention-deficit/hyperactivity disorder. Clin Ther. 2003;25(12):3065–83. doi: 10.1016/s0149-2918(03)90092-0. [DOI] [PubMed] [Google Scholar]
- 8.Capizzi M, Sanabria D, Correa A. Dissociating controlled from automatic processing in temporal preparation. Cognition. 2012;123(2):293–302. doi: 10.1016/j.cognition.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Carboni E, Tanda GL, Frau R, Di Chiara Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals. J Neurochem. 1990;55:1067–1070. doi: 10.1111/j.1471-4159.1990.tb04599.x. [DOI] [PubMed] [Google Scholar]
- 10.Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurons on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- 11.Carli M, Samanin R. Serotonin2 receptor agonists and serotonergic anorectic drugs affect rats’ performance differently in a five-choice serial reaction time task. Psychopharmacology (Berl) 1992;106:228–234. doi: 10.1007/BF02801977. [DOI] [PubMed] [Google Scholar]
- 12.Chamberlain SR, Del Campo N, Dowson J, Müller U, Clark L, Robbins TW, Sahakian BJ. Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biol Psychiatry. 2007;62:977–984. doi: 10.1016/j.biopsych.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Chang K, Nayar D, Howe M, Rana M. Atomoxetine as an adjunct therapy in the treatment of co-morbid attention-deficit/hyperactivity disorder in children and adolescents with bipolar I or II disorder. J Child Adolesc Psychopharmacol. 2009;19(5):547–551. doi: 10.1089/cap.2009.0030. [DOI] [PubMed] [Google Scholar]
- 14.Davis EP, Sandman CA. Prenatal psychobiological predictors of anxiety risk in preadolescent children. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2011. 12.016. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman JI, Carpenter D, Lu J, Fan J, Tang CY, White L, Parrella M, Bowler S, Elbaz Z, Flanagan L, Harvey PD. A pilot study of adjunctive atomoxetine treatment to seconds-generation antipsychotics for cognitive impairment in schizophrenia. J Clin Psychopharmacol. 2008;28(1):59–63. doi: 10.1097/jcp.0b013e318161318f. [DOI] [PubMed] [Google Scholar]
- 16.Grizenko N, Shayan YR, Polotskaia A, Ter-Stepanian M, Joober R. Relation of maternal stress during pregnancy to symptom severity and response to treatment in children with ADHD. Rev Psychiatr Neurosci. 2008;33(1):10–16. [PMC free article] [PubMed] [Google Scholar]
- 17.Hagan JJ, Jones DN. Predicting drug efficacy for cognitive deficits in schizophrenia. Schizophr Bull. 2005;31:830–853. doi: 10.1093/schbul/sbi058. [DOI] [PubMed] [Google Scholar]
- 18.Han G, Klimes-Dougan B, Jepsen S, Ballard K, Nelson M, Houri A, Kumra S, Cullen K. Selective neurocognitive impairments in adolescents with major depressive disorder. J Adolesc. 2012;35(1):11–20. doi: 10.1016/j.adolescence.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins GA, Breysse N. Rodent model of attention: the 5-Choice serial reaction time task. Current Protocols in Pharmacology. 2008:5.49.1–5.49.20. doi: 10.1002/0471141755.ph0549s41. [DOI] [PubMed] [Google Scholar]
- 20.Javitt DC, Schoepp D, Kalivas PW, Volkow ND, Zarate C, Merchant K, Bear MF, Umbricht D, Hajos M, Potter WZ, Lee CM. Translating glutamate: from pathophysiology to treatment. Sci Transl Med. 2011;3(102):102mr2. doi: 10.1126/scitranslmed.3002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J Physiol. 2006;572(1):31–44. doi: 10.1113/jphysiol.2006.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly DL, Buchanan RW, Boggs DL, McMahon RP, Dickinson D, Nelson M, Gold JM, Ball MP, Feldman S, Liu F, Conley RR. A randomized double-blind trial of atomoxetine for cognitive impairments in 32 people with schizophrenia. J Clin Psychiatry. 2009;70(4):518–25. doi: 10.4088/jcp.08m04358. [DOI] [PubMed] [Google Scholar]
- 23.Kinney DK, Munir KM, Crowley DJ, Miller AM. Prenatal stress and risk for autism. Neurosci Biobehav Rev. 2008;32(8):1519–1532. doi: 10.1016/j.neubiorev.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinnunen AK, Koenig JI, Bilbe G. Repeated variable prenatal stress alters pre- and postsynaptic gene expression in the rat frontal pole. J Neurochem. 2003;86:736–748. doi: 10.1046/j.1471-4159.2003.01873.x. [DOI] [PubMed] [Google Scholar]
- 25.Koenig JI, Elmer GI, Shepard PD, Lee PR, Mayo C, Joy B, Hercher E, Brady DL. Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: potential relevance to schizophrenia. Behav Brain Res. 2005;156:251–261. doi: 10.1016/j.bbr.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 26.Koffarnus MN, Katz JL. Response requirement and increases in accuracy produced by stimulant drugs in a 5-choice serial reaction-time task in rats. Psychopharmacology (Berl) 2011;213(4):723–733. doi: 10.1007/s00213-010-2027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13(1):9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- 28.Lee J, Park S. The role of stimulus salience in CPT-AX performance of schizophrenia patients. Schizophr Res. 2006;81:191–197. doi: 10.1016/j.schres.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. Prenatal stress generates deficits in rat social behavior: reversal by oxytocin. Brain Res. 2007;1156:152–167. doi: 10.1016/j.brainres.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- 31.Linnet KM, Dalsgaard S, Obel C, Wisborg K, Henriksen TB, Rodriguez A, Kotimaa A, Moilanen I, Thomsen PH, Olsen J, Jarvelin MR. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. Am J Psychiatry. 2003;160:1028–1040. doi: 10.1176/appi.ajp.160.6.1028. [DOI] [PubMed] [Google Scholar]
- 32.Malhotra AK, Pinals DA, Adler CM, Elman I, Clifton A, Pickar D. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. 1997;17:141–150. doi: 10.1016/S0893-133X(97)00036-5. [DOI] [PubMed] [Google Scholar]
- 33.Marco EM, Macrì S, Laviola G. Critical age windows for neurodevelopmental psychiatric disorders: evidence from animal models. Neurotox Res. 2011;19(2):286–307. doi: 10.1007/s12640-010-9205-z. [DOI] [PubMed] [Google Scholar]
- 34.Markham JA, Taylor AR, Taylor SB, Bell DB, Koenig JI. Characterization of the cognitive impairments induced by prenatal exposure to stress in the rat. Front Behav Neurosci. 2010;4:173. doi: 10.3389/fnbeh.2010.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markham JA, Koenig JI. Prenatal stress: Role in psychotic and depressive diseases. Psychopharmacology. 2011;214(1):89–106. doi: 10.1007/s00213-010-2035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michelson D, Adler L, Spencer T, Reimherr FW, West SA, Allen AJ, Kelsey D, Wernicke J, Dietrich A, Milton D. Atomoxetine in adults with ADHD: two randomized, placebo-controlled studies. Biol Psychiatry. 2003;53:112–120. doi: 10.1016/s0006-3223(02)01671-2. [DOI] [PubMed] [Google Scholar]
- 37.Middlemore-Risher ML, Buccafusco JJ, Terry AV., Jr Repeated exposures to low-level chlorpyrifos results in impairments in sustained attention and increased impulsivity in rats. Neurotoxicol Teratol. 2010;32(4):415–24. doi: 10.1016/j.ntt.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mirjana C, Baviera M, Invernizzi RW, Balducci C. The serotonin 5-HT2A receptors antagonist M100907 prevents impairment in attentional performance by NMDA receptor blockade in the rat prefrontal cortex. Neuropsychopharmacology. 2004;29:1637–1647. doi: 10.1038/sj.npp.1300479. [DOI] [PubMed] [Google Scholar]
- 39.Navarra R, Graf R, Huang Y, Logue S, Comery T, Hughes Z, Day M. Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(1):34–41. doi: 10.1016/j.pnpbp.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 40.Paine TA, Carlezon WA., Jr Effects of antipsychotic drugs on MK-801-induced attentional and motivational deficits in rats. Neuropharmacology. 2009;56(4):788–97. doi: 10.1016/j.neuropharm.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park SH, Noh JC, Kim JH, Lee J, Park JY, Lee YR, Kim CH, Lee KH. Interactive effects of background facial emotion stimulus and target salience on sustained attention performance in schizophrenia. Schizophr Res. 2012;135(1–3):90–94. doi: 10.1016/j.schres.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 42.Paterson NE, Ricciardi J, Wetzler C, Hanania T. Sub-optimal performance in the 5-choice serial reaction time task in rats was sensitive to methylphenidate, atomoxetine and d-amphetamine, but unaffected by the COMT inhibitor tolcapone. Neurosci Res. 2011;69(1):41–50. doi: 10.1016/j.neures.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 44.Riccio CA, Waldrop JJ, Reynolds CR, Lowe P. Effects of stimulants on the continuous performance test (CPT): implications for CPT use and interpretation. J Neuropsychiatry Clin Neurosci. 2001;13:326–335. doi: 10.1176/jnp.13.3.326. [DOI] [PubMed] [Google Scholar]
- 45.Rice F, Jones I, Thapar A. The impact of gestational stress and prenatal growth on emotional problems in offspring: a review. Acta Psychiatr Scand. 2007;115:171–183. doi: 10.1111/j.1600-0447.2006.00895.x. [DOI] [PubMed] [Google Scholar]
- 46.Robbins TW. The 5-choice serial reaction time task: behavioral pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- 47.Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, Dalley JW, Robbins TW. Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology. 2008;33:1028–1037. doi: 10.1038/sj.npp.1301487. [DOI] [PubMed] [Google Scholar]
- 48.Rung JP, Carlsson A, Rydén Markinhuhta K, Carlsson ML. (+)-MK-801 induced social withdrawal in rats; a model for negative symptoms of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(5):827–832. doi: 10.1016/j.pnpbp.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Sacco KA, Creeden C, Reutenauer EL, Vessicchio JC, Weinberger AH, George TP. Effects of atomoxetine on cognitive function and cigarette smoking in schizophrenia. Schizophr Res. 2009;107(2–3):332–333. doi: 10.1016/j.schres.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 50.Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav Brain Sci. 2005;28(3):397–419. doi: 10.1017/S0140525X05000075. [DOI] [PubMed] [Google Scholar]
- 51.Smith JW, Gastambide F, Gilmour G, Dix S, Foss J, Lloyd K, Malik N, Tricklebank M. A comparison of the effects of ketamine and phencyclidine with other antagonists of the NMDA receptor in rodent assays of attention and working memory. Psychopharmacology (Berl) 2011;217(2):255–269. doi: 10.1007/s00213-011-2277-5. [DOI] [PubMed] [Google Scholar]
- 52.Spauwen J, Krabbendam L, Lieb R, Wittchen HU, van Os J. Early maternal stress and health behaviours and offspring expression of psychosis in adolescence. Acta Psychiatr Scand. 2004;110:356–364. doi: 10.1111/j.1600-0447.2004.00429.x. [DOI] [PubMed] [Google Scholar]
- 53.Swanson CJ, Perry KW, Koch-Krueger S, Katner J, Svensson KA, Bymaster FP. Effect of the attention deficit/hyperactivity disorder drug atomoxetine on extracellular concentrations of norepinephrine and dopamine in several brain regions of the rat. Neuropharmacology. 2006;50:755–760. doi: 10.1016/j.neuropharm.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 54.Terry AV, Jr, Buccafusco JJ, Schade RF, Vandenhuerk L, Callahan PM, Beck WD, Hutchings EJ, Chapman JM, Li P, Bartlett MG. The nicotine metabolite, cotinine, attenuates glutamate (NMDA) antagonist-related effects on the performance of the five choice serial reaction time task (5C-SRTT) in rats. Biochem Pharmacol. 2012;83(7):941–951. doi: 10.1016/j.bcp.2011.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Os J, Selten J. Prenatal exposure to maternal stress and subsequent schizophrenia. The May 1940 invasion of The Netherlands. Br J Psychiatry. 1998;172:324–326. doi: 10.1192/bjp.172.4.324. [DOI] [PubMed] [Google Scholar]
- 56.Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain, Behavior, and Immunity. 2005;19:296–308. doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 57.Wilson C, Terry AV., Jr Neurodevelopmental Animal Models of Schizophrenia: Role in Novel Drug Discovery and Development. Clinical Schizophrenia & Related Psychosis. 2010;4(2):124–137. doi: 10.3371/CSRP.4.2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wykes T, Reeder C, Corner J. The prevalence and stability of an executive processing deficit, response inhibition, in people with chronic schizophrenia. Schizophr Res. 2000;46(2–3):241–253. doi: 10.1016/s0920-9964(99)00233-9. [DOI] [PubMed] [Google Scholar]
- 59.Young JW, Powell SB, Risbrough V, Marston HM, Geyer MA. Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacol Ther. 2009;122(2):150–202. doi: 10.1016/j.pharmthera.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zeiner P, Gjevik E, Weidle B. Response to atomoxetine in boys with high-functioning autism spectrum disorders and attention deficit/hyperactivity disorder. Acta Paediatr. 2011;100(9):1258–1261. doi: 10.1111/j.1651-2227.2011.02263.x. [DOI] [PubMed] [Google Scholar]