Abstract
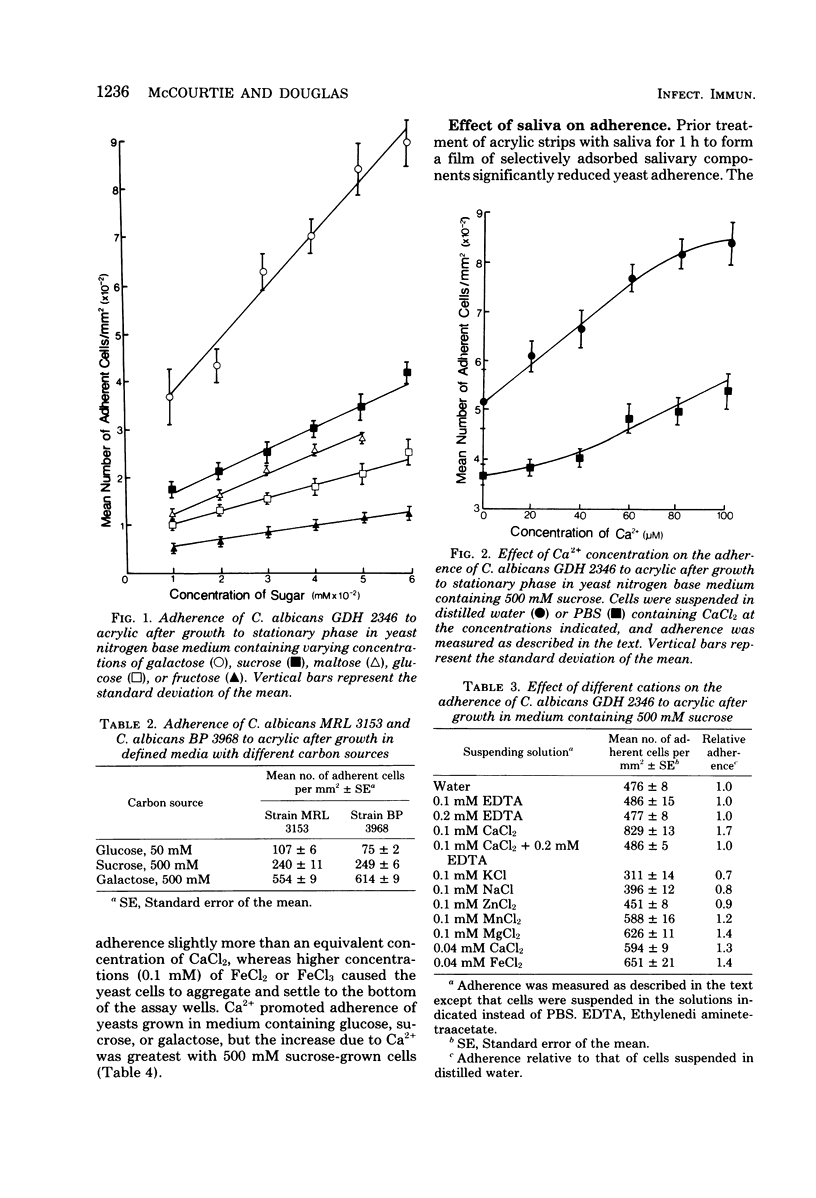
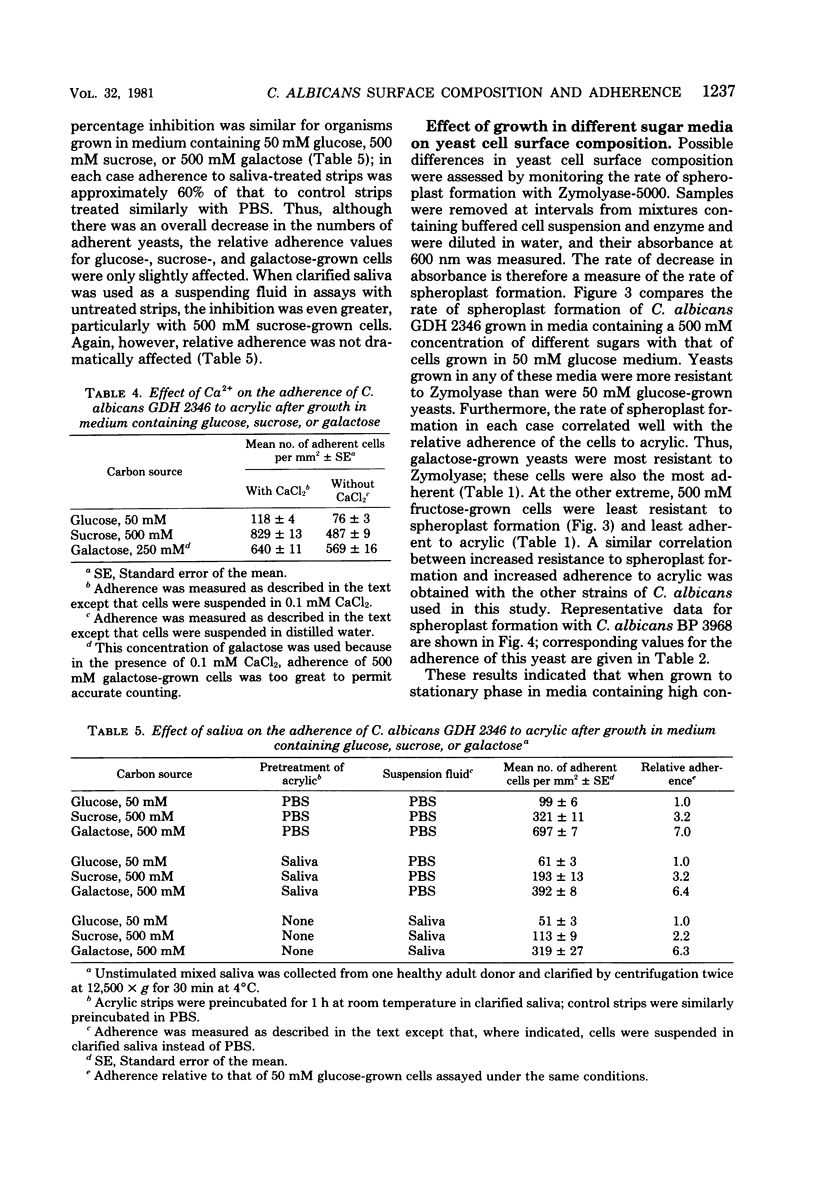
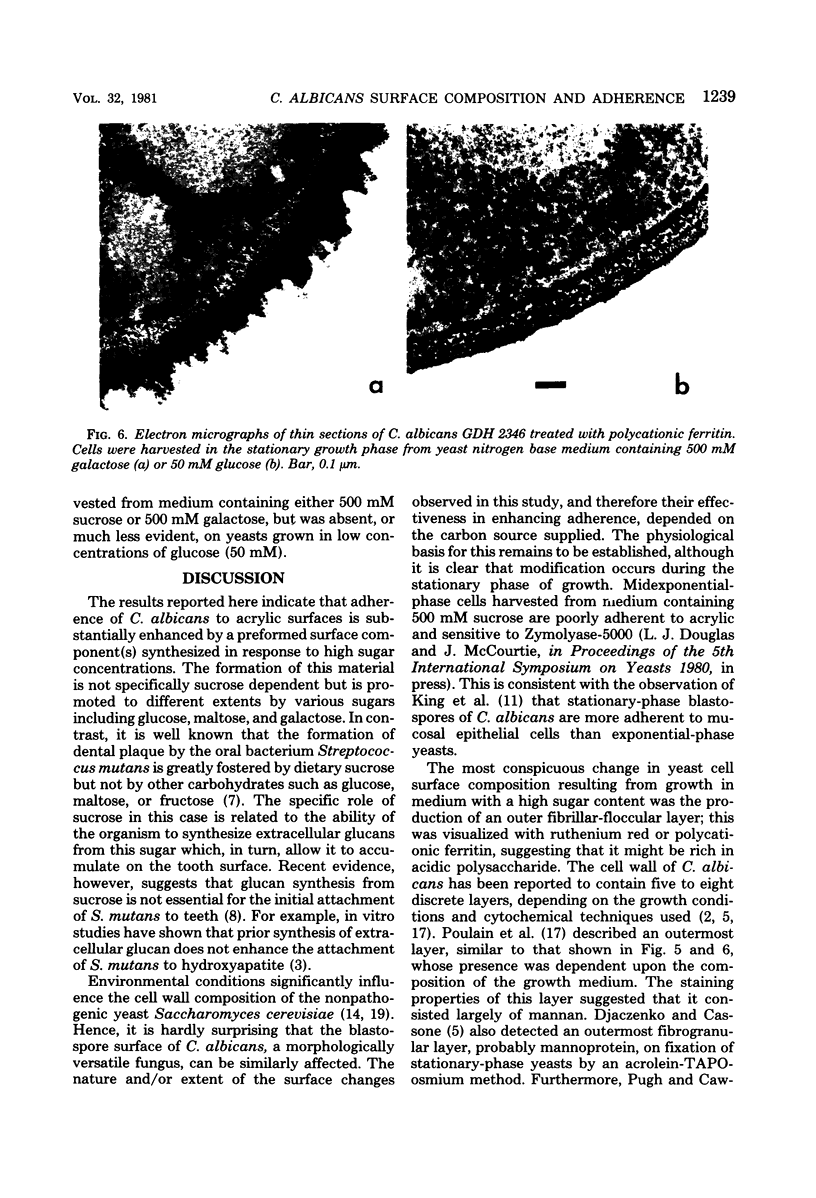
The adherence of Candida albicans to acrylic was measured in vitro after growth of the yeast to stationary phase in defined medium containing glucose, sucrose, galactose, fructose, or maltose as the carbon source. In each case, yeast adherence was proportional to the concentration of sugar in the growth medium, but equimolar concentrations of different sugars promoted adherence to different extents. In vitro adherence was further increased by the addition of divalent cations to assay mixtures but was inhibited when saliva-treated acrylic strips were used or when yeasts were suspended in mixed saliva during the assay. The rate of spheroplast formation of yeasts grown in media containing a 500 mM concentration of the different sugars correlated well with the relative adherence of the cells to acrylic. Galactose-grown yeasts were most resistant to spheroplast formation with Zymolyase-5000 and most adherent to acrylic, whereas fructose-grown organisms were least resistant to spheroplast formation and least adherent to acrylic. These results indicate that when grown to stationary phase in media containing high concentrations of certain sugars, C. albicans undergoes a change in cell surface composition which facilitates its adherence to acrylic surfaces. Electron microscopy of yeasts harvested from such media revealed the presence of an additional surface layer which may be responsible for this enhanced adherence.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alterthum F., Rose A. H. Osmotic lysis of sphaeroplasts from Saccharomyces cerevisiae grown anaerobically in media containing different unsaturated fatty acids. J Gen Microbiol. 1973 Aug;77(2):371–382. doi: 10.1099/00221287-77-2-371. [DOI] [PubMed] [Google Scholar]
- Cassone A., Simonetti N., Strippoli V. Ultrastructural changes in the wall during germ-tube formation from blastospores of Candida albicans. J Gen Microbiol. 1973 Aug;77(2):417–426. doi: 10.1099/00221287-77-2-417. [DOI] [PubMed] [Google Scholar]
- Clark W. B., Gibbons R. J. Influence of salivary components and extracellular polysaccharide synthesis from sucrose on the attachment of Streptococcus mutans 6715 to hydroxyapatite surfaces. Infect Immun. 1977 Nov;18(2):514–523. doi: 10.1128/iai.18.2.514-523.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport J. C. The oral distribution of candida in denture stomatitis. Br Dent J. 1970 Aug 18;129(4):151–156. doi: 10.1038/sj.bdj.4802540. [DOI] [PubMed] [Google Scholar]
- Djaczenko W., Cassone A. Visulization of new ultrastructural components in the cell wall of Candida albicans with fixatives containing TAPO. J Cell Biol. 1972 Jan;52(1):186–190. doi: 10.1083/jcb.52.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Kimura L. H., Pearsall N. N. Adherence of Candida albicans to human buccal epithelial cells. Infect Immun. 1978 Jul;21(1):64–68. doi: 10.1128/iai.21.1.64-68.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura L. H., Pearsall N. N. Relationship between germination of Candida albicans and increased adherence to human buccal epithelial cells. Infect Immun. 1980 May;28(2):464–468. doi: 10.1128/iai.28.2.464-468.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. D., Lee J. C., Morris A. L. Adherence of Candida albicans and other Candida species to mucosal epithelial cells. Infect Immun. 1980 Feb;27(2):667–674. doi: 10.1128/iai.27.2.667-674.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight L., Fletcher J. Growth of Candida albicans in saliva: stimulation by glucose associated with antibiotics, corticosteroids, and diabetes mellitus. J Infect Dis. 1971 Apr;123(4):371–377. doi: 10.1093/infdis/123.4.371. [DOI] [PubMed] [Google Scholar]
- Masler L., Sikl D., Bauer S., Sandula J. Extracellular polysaccharide-protein complexes produced by selected strains of Candida albicans (Robin) Berkhout. Folia Microbiol (Praha) 1966;11(5):373–378. doi: 10.1007/BF02887694. [DOI] [PubMed] [Google Scholar]
- McMurrough I., Rose A. H. Effect of growth rate and substrate limitation on the composition and structure of the cell wall of Saccharomyces cerevisiae. Biochem J. 1967 Oct;105(1):189–203. doi: 10.1042/bj1050189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen I., Birkeland J. M. Initiation and aggravation of denture stomatitis by sucrose rinses. Scand J Dent Res. 1976 Mar;84(2):94–97. doi: 10.1111/j.1600-0722.1976.tb00466.x. [DOI] [PubMed] [Google Scholar]
- Poulain D., Tronchin G., Dubremetz J. F., Biguet J. Ultrastructure of the cell wall of Candida albicans blastospores: study of its constitutive layers by the use of a cytochemical technique revealing polysaccharides. Ann Microbiol (Paris) 1978 Feb-Mar;129(2):141–153. [PubMed] [Google Scholar]
- Pugh D., Cawson R. A. The surface layer of Candida albicans. Microbios. 1978;23(91):19–23. [PubMed] [Google Scholar]
- Ramsay A. M., Douglas L. J. Effects of phosphate limitation of growth on the cell-wall and lipid composition of Saccharomyces cerevisiae. J Gen Microbiol. 1979 Jan;110(1):185–191. doi: 10.1099/00221287-110-1-185. [DOI] [PubMed] [Google Scholar]
- Samaranayake L. P., MacFarlane T. W. An in-vitro study of the adherence of Candida albicans to acrylic surfaces. Arch Oral Biol. 1980;25(8-9):603–609. doi: 10.1016/0003-9969(80)90075-8. [DOI] [PubMed] [Google Scholar]
- Samaranayake L. P., McCourtie J., MacFarlane T. W. Factors affecting th in-vitro adherence of Candida albicans to acrylic surfaces. Arch Oral Biol. 1980;25(8-9):611–615. doi: 10.1016/0003-9969(80)90076-x. [DOI] [PubMed] [Google Scholar]
- Springer E. L., Roth I. L. The ultrastructure of the capsules of Diplococcus pneumoniae and Klebsiella pneumoniae stained with ruthenium red. J Gen Microbiol. 1973 Jan;74(1):21–31. doi: 10.1099/00221287-74-1-21. [DOI] [PubMed] [Google Scholar]
- Sugarman B. Effect of heavy metals on bacterial adherence. J Med Microbiol. 1980 May;13(2):351–354. doi: 10.1099/00222615-13-2-351. [DOI] [PubMed] [Google Scholar]




