Abstract
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family of growth factors. It is expressed throughout the nervous system. A unique feature of the BDNF gene is the existence of multiple mRNA transcripts, all of which are translated into BDNF protein, suggesting a multilevel regulation of expression. In particular, the BDNF exon IV promoter region is a preferential target for epigenetic alterations, as it contains binding sites for CREB and MeCP2, two transcriptional regulators known to mediate epigenetic changes. Exposure to drugs of abuse is known to modulate epigenetic regulation of BDNF gene expression. This review will discuss how exposure to cocaine, one of the most addictive drugs known to mankind, can produce alterations in BDNF gene expression, especially in the mesolimbic dopaminergic system, which lead to alterations in the reward-mediated behaviors involved in addiction.
Keywords: BDNF, cocaine, dopamine, animal models, addiction, epigenetics, synaptic plasticity
Introduction
Brain-derived neurotrophic factor (BDNF) is a member of a family of secreted proteins known as neurotrophins that regulate the survival, development, and function of neurons [1,2]. BDNF was identified and purified from pig brain by Yves Alain Barde and colleagues in 1982 [3] and is now recognized as the most widely and abundantly expressed neurotrophin in the nervous system [4]. BDNF is synthetized as proBDNF (32kDa), a precursor protein, and secreted as a mixture of proBDNF and mature BDNF (14kDa). Both forms have been shown to play vital roles in learning, memory, and other higher cognitive functions [5-8]. BDNF also plays a role in the regulation of normal development of the nervous system. BDNF knockout mice display developmental defects in the brain and sensory nervous system and usually die soon after birth [9]. Thus, BDNF plays critical roles in the regulation of the structure and function of neuronal circuits throughout life [7,8].
BDNF and other neurotrophins are endogenous ligands for one or more members of the tropomyosin-receptor kinase (Trk) family of receptors. BDNF has the highest affinity for the TrkB receptor, and BDNF-TrkB binding at the cell surface initiates autophosphorylation of the TrkB receptor through one of at least three signaling cascades: 1) The MAP kinase pathway, which has been implicated in neuronal differentiation and neurite outgrowth; 2) The PI3-Kinase pathway, which enables cell survival; and 3) The PLCγ pathway, which is involved in synaptic plasticity and transmission [10].
The rodent BDNF gene consists of at least eight untranslated 5’ exons that are spliced onto a single 3’ coding exon and driven by eight unique promoters [11,12]. Of particular significance for this review is the observation that BDNF exon IV expression is induced by drugs of abuse within brain regions associated with the reward circuitry [13]. The promoter region of exon IV contains specific binding sites for the transcriptional regulators cAMP response element-binding (CREB) and methyl CpG binding protein 2 (MeCP2), both of which have established associations with regulation of gene expression at the epigenetic level.
Epigenetics literally translates as “above the genome,” and as such refers to the chemical modifications of the DNA and associated histone proteins promoting changes in transcription without altering the DNA sequence. Methylated DNA is typically condensed and transcriptionally repressed. DNA methylation is tightly linked to a variety of chemical modifications on the tails of histone proteins [14,15], which can also influence genomic condensation/silencing by changing the charge of the DNA-associated histone proteins. For the purpose of this review, epigenetic regulation of gene expression may be simplified by the following two concepts: Acetylation of histones H3/H4 in the promoter region of a gene is correlated with “open” or transcriptionally active DNA, whereas methylation of histone H3K9 is correlated with repression. The chemical changes associated with epigenetic regulation can be transient or persistent, are regulated throughout development, can confer cell-specific or response-specific expression patterns and, when transmitted to the germ line, can be heritable [16].
Impaired BDNF signaling is associated with a variety of neurological and psychiatric conditions, including depression, schizophrenia, obsessive-compulsive disorder, Alzheimer’s disease, Huntington’s disease, Rett syndrome, dementia, anorexia, depression, and epilepsy [2,17-19]. Recent studies demonstrate that many neurological and behavioral changes associated with these disorders and following drug exposure are regulated at the epigenetic level [20]. In this review, we focus on cocaine-induced epigenetic changes in BDNF gene expression and how these changes can play a role in mediating addiction. In particular, we will focus on synaptic plasticity and reward-mediated behaviors in rodent models.
BDNF and Addiction
Although roles for BDNF signaling in a variety of physiological processes are well established, its involvement in drug addiction is a more recent revelation. To understand the link between BDNF and drug addiction, one must systematically examine the links between BDNF signaling and learning and between learning and drug addiction. Addiction, or substance dependence, is characterized by at least three phases: 1) the continued use of a substance despite adverse consequences; 2) the diminishing effectiveness of the substance over time or the need for larger doses to achieve the same desired effect (tolerance); and 3) physical and psychological symptoms of distress, discomfort, or impairment upon reduction or cessation of substance use (withdrawal) [21]. These three phases of addiction are associated with structural and functional changes in the brain that can be explained collectively as neuronal plasticity. Lasting changes in synaptic strength underlie neuronal plasticity, and these changes are necessary for successful information storage and memory formation. In fact, these synaptic changes are critical for an organism’s adaptive responses underlying various modifications in behavior, including drug addiction, and are facilitated by increased BDNF synthesis and release [22,23]. Thus, a link between BDNF and drug addiction emerges via a well-established link between BDNF and neuronal plasticity.
The earliest studies of BDNF’s effects on synaptic transmission in Xenopus [24] demonstrated that acute exposure to BDNF increased the frequency of miniature excitatory postsynaptic currents, a phenomenon associated with development of long-term potentiation (LTP), the core mechanism of learning and memory. Induction of LTP is dependent on NMDA-facilitated influx of Ca2+ into a neuron, subsequent phosphorylation of AMPA receptors, and increased sensitivity of the cell to depolarization. Additionally, groundbreaking evidence for BDNF’s role in LTP came from knockout mouse models. In the mid-1990s, two groups [25,26] independently reported impairment of early LTP in homozygous or heterozygous BDNF knockout mice, which was restored by treatment with recombinant BDNF. Thus, BDNF appears to play important roles in mediating synaptic changes involved in learning and memory, which in turn underlies behavioral and structural adaptations associated with drug addiction.
Transcriptional Regulation of the BDNF Gene Expression
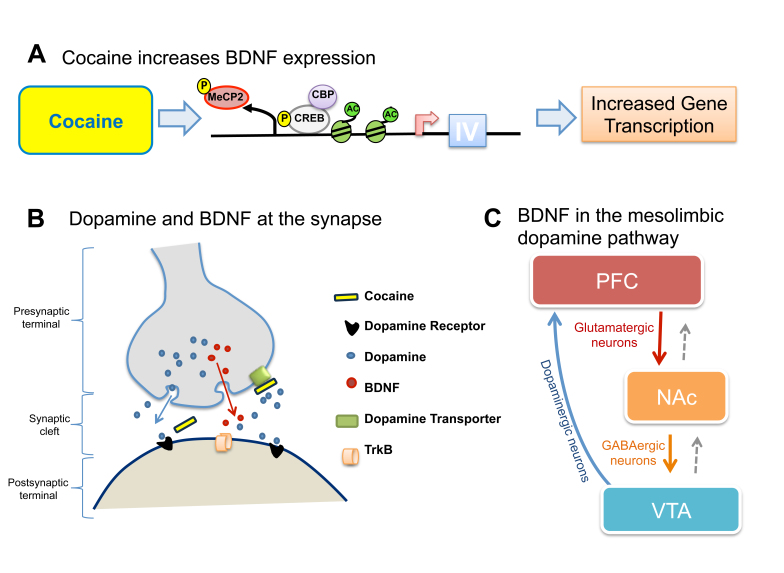
The promoter region of BDNF exon IV contains specific binding sites for the transcriptional regulators CREB and MeCP2 (Figure 1A). Both of these transcription factors are known to play important roles in the regulation of synaptic plasticity. CREB is involved in the regulation of a vast array of biological processes including development of the nervous system, learning, and memory and can regulate transcription of genes containing Ca2+-response elements (CRE). Evidence from different model organisms, such as the mollusk Aplysia, the fruitfly Drosophilia, and the mouse, shows that disruption of CREB function in neurons is associated with defects in LTP, thus memory [27]. MeCP2 is another transcription factor that regulates expression of a large number of genes, including BDNF, through diverse mechanisms. Predominantly recognized as a transcriptional repressor and for its role in the pathology of Rett syndrome [28], it has recently emerged as a key modulator of neural-activity related gene expression, as well as learning and memory [28-31]. Additionally, it has been shown to have a role in neuroplasticity and drug addiction [32].
Figure 1.
Cocaine-induced alterations in BDNF expression within the mesolimbic dopamine pathway. A. Cocaine-induced activation of gene expression at the promoter region of BDNF exon IV is facilitated by phosphorylation of CREB and association of phosphorylated CREB with CREB-binding protein. Subsequently, phosphorylated MeCP2 is dissociated from methylated DNA and histones become acetylated, resulting in transcriptionally active chromatin (modified from [79]). B. Simplified example of cocaine-induced changes in BDNF and dopamine at the synapse. Cocaine binds to the dopamine transporter of the presynaptic terminal of dopaminergic neurons, blocking reuptake of dopamine and an increased concentration of the neurotransmitter in the synaptic cleft. Increased BDNF release from dopaminergic neurons of the VTA has been associated with enhanced drug seeking. C. Repeated exposure to cocaine leads to increased BDNF expression in the mesolimbic pathway. BDNF is released from the dopaminergic neurons of the VTA (blue), glutamatergic neurons of the PFC (red) and GABAergic neurons of the NAc (orange). Colored lines represent anterograde transport from a specific cell type and dotted grey lines represent retrograde transport of BDNF (modified from [46]).
The link between cocaine and BDNF gene expression is underscored by the finding that CREB plays critical roles in establishing and/or maintaining drug-induced behavioral alterations, including addiction to opiates, cocaine, amphetamines, nicotine, and alcohol [33]. CREB also associates with CREB-binding protein (CBP), an enzyme with histone acetyltransferase (HAT) activity [34]. Acetylation of histone H3 in CRE-containing promoter regions by CBP facilitates transcription and may be the source of CREB’s identity as a “transcription factor.” Regulation of CREB and its function in addiction are variable and dependent on the substance, nature of exposure, and region of the brain involved [35]. Regardless, the association between CREB, BDNF, and the behavioral effects of exposure to addictive drugs highlights the potential role for BDNF in mediating drug addiction.
Further support for a link between BDNF gene expression and neuronal activity emerges from the finding that both CREB and MeCP2 are phosphorylated in response to Ca2+-mediated membrane depolarization following neuronal activity [29,36]. Upon phosphorylation, phosphorylated CREB (pCREB) becomes associated with CRE elements in the exon IV promoter of BDNF, while the MeCP2/HDAC/Sin3A complex dissociates from it, thus inducing transcription of the BDNF exon IV transcript (Figure 1A) [29,30,37]. Kinetics of these interactions indicate that, although CREB phosphorylation occurs within minutes of depolarization and MeCP2 up to 30 minutes later, transcription of BDNF does not occur until MeCP2 is phosphorylated, removed from the promoter, and the DNA is demethylated [29]. Changes in the DNA methylation status at the BDNF exon IV promoter correspond with the reported changes in BDNF transcript abundance throughout development [12,38].
Recent studies have shown that cocaine induces the transcription of several microRNAs, which, through interactions with CREB and MeCP2, can either promote or repress cocaine-induced BDNF expression and reward [39,40]. Thus, regulation of chromatin status and transcriptional activity by CREB and MeCP2 have recently become an area of considerable interest with respect to BDNF gene expression and development of potential treatment approaches for drug addiction and psychiatric disorders [13].
Cocaine-BDNF Interactions in the Mesolimbic Dopamine Pathway
Psychostimulant compounds such as cocaine interfere with dopaminergic signaling by elevating dopamine levels at the synapse (Figure 1B) [39-41]. Repeated administration of stimulant drugs can cause permanent changes in dopamine levels as well as both transient and permanent alterations in BDNF and tyrosine hydroxylase expression, implicating both BDNF and dopamine in drug-induced long-term neuroadaptations [42-46]. A major CNS dopaminergic pathway is the mesolimbic pathway (Figure 1C). In this pathway, dopamine synthetized in the ventral tegmental area (VTA) of the mid brain is transported to the nucleus accumbens (NAc), amygdala, hippocampus, and prefrontal cortex (PFC) in the forebrain. The mesolimbic dopaminergic pathway is also referred to as the “reward pathway” because of its critical involvement in mediating rewarding effects of drugs such as cocaine. BDNF is expressed robustly in the mesolimbic pathway. Therefore, the mesolimbic pathway is the anatomical substrate for intimate interactions between cocaine, dopamine, and BDNF.
Pyramidal neurons of the PFC are the main source of BDNF in the caudate-putamen and NAc [47]. BDNF potentiates dopamine release in the NAc via activation of TrkB receptors on VTA dopaminergic neurons [48]. Synaptic plasticity in the VTA plays an essential role in early behavioral responses as well as long-term adaptations to drug exposure [49]. Although the underlying mechanisms of drug addiction are complex, there is a strong correlation between exposure to drugs of abuse, such as cocaine, amphetamine, morphine, and alcohol, and neuroadaptations in the mesolimbic dopamine system. Animal models, specifically rodent models, have provided exquisite insights into the behavioral and pharmacological dimensions of drug abuse. A common paradigm employed in such studies is exposure of the mice or rats to an addictive substance followed by analyses of the animal’s behavioral, molecular, and neurological changes. These studies show that BDNF mRNA and protein are differentially regulated in the different phases of addiction. For example, acute exposure to cocaine results in increased BDNF expression in the PFC, VTA, striatum, and NAc as a result of a transient increase in pCREB [45,50-52]. These changes are accompanied by corresponding, region-specific epigenetic changes in the BDNF gene. The increased expression of BDNF following acute exposure to cocaine is associated with cocaine-induced changes in MeCP2/pCREB-association with the BDNF exon IV promoter and transient histone modifications permitting transcription of the BDNF gene [20]. In particular, H4 acetylation and H3 phosphoacetylation are increased 30 minutes after an acute exposure and induce the expression of around 100 genes, but expression and acetylation patterns return to normal by 3 hours [53]. In particular, H4 acetylation and H3 phosphoacetylation are generally considered transient marks of gene expression, and the subset of genes with these marks has very little overlap with the genes induced by repeated cocaine exposure. These acute changes in BDNF expression may represent the initial stages of plasticity, or formation of a “drug memory,” which lay the foundation for subsequent drug-induced alterations in behavior [54].
A time-dependent increase in BDNF protein expression was observed in the mesolimbic structures (VTA, NAc, and amygdala) up to 90 days after withdrawal from repeated self-administration of cocaine [55]. During the same period, extracellular glutamate, pMeCP2, pCREB, and H3 acetylation are increased, extracellular signal-regulated kinase (ERK) signaling and DNA methylation are decreased, and expression/activity of the enzymes mediating these changes are also altered [53,56,57]. These observations raise the possibility that BDNF is regulated differentially in regions of the mesolimbic pathway during the withdrawal or abstinence period following repeated cocaine administration.
Following repeated cocaine exposure, there is an increased sensitivity to drug-associated cues that trigger cocaine cravings. Since BDNF modulates synaptic plasticity, increased BDNF following cocaine exposure may cause synaptic changes that contribute to enhanced drug seeking [58]. In the PFC, there is a transient decrease in BDNF mRNA 21 hours after the final exposure of a series of repeated cocaine administrations. However, by 21 days post-cocaine, BDNF mRNA levels returned to baseline [46]. During the 21-day withdrawal period, DNA methylation and MeCP2 association are decreased,while H3 acetylation and pCREB are increased at the BDNF exon IV promoter in the PFC, thus promoting a permissive transcriptional state. It is not yet entirely clear whether the elevated BDNF expression in the PFC following the 21-day withdrawal period is solely due to increased local synthesis by PFC neurons and/or an additional increase in transport from other mesolimbic brain regions. The variable expression of BDNF within these interconnected brain regions adds additional weight to a potential role for BDNF as a critical component of cocaine-induced plasticity [46].
In order to elucidate the role of cocaine-induced changes in BDNF expression in specific regions of the mesolimbic pathway, exogenous BDNF was infused into the different regions in drug-naïve rats or rats experiencing a period of cocaine abstinence. It is important to note that the timing of BDNF infusion (during or immediately following the final administration or during the abstinence period) and the dose of BDNF (single or repeated) are key to the behavioral outcome and must be considered in the analysis of BDNF’s region-specific effects. Primarily, infusion of BDNF into the VTA promoted transition from a drug-naïve to a drug-dependent motivational state by inducing an inhibitory to excitatory switch in the GABAergic neurons [59]. In cocaine-experienced rats, infusion of BDNF into the VTA or NAc reinstated cocaine seeking following a period of abstinence [51,60-63] and led to long-lasting changes in the mesolimbic dopamine system [62] via the TrkB-MAPK signaling cascade [64]. Alternatively, infusion of BDNF into the PFC, which is a critical region for goal-directed behaviors and impulse control, attenuated cocaine-seeking behavior in rodents following a period of abstinence. Infusion of BDNF into the PFC following cocaine self-administration restored pERK expression, basal glutamate levels, and cocaine-induced increases in extracellular glutamate release in the NAc [64,65], possibly associated with the observed reduction of MeCP2 phosphorylation in the PFC [66]. Although endogenous BDNF expression is increased in all three brain regions following acute and chronic cocaine exposure and during the abstinence period, the differing effects of BDNF infusion into subcortical regions, such as the VTA and NAc, or cortical regions, such as the PFC, highlight region-specific roles for BDNF signaling.
Collectively, these data indicate that BDNF can have local effects at the site of infusion/expression in addition to distant effects in target areas that are critical to cocaine-induced neuroadaptations. Moreover, these effects appear to mediate different changes in neurotransmitter homeostasis, intracellular signaling mechanisms, and associated behaviors. While BDNF infusion into the VTA and NAc may enhance the rewarding effects of cocaine, infusion of BDNF into the PFC appears to normalize cocaine-mediated changes in intracellular signaling and drug-seeking behavior. More detailed analyses of BDNF expression, epigenetic regulation, uptake, secretion, and properties of anterograde/retrograde transport in a time-dependent, region-specific manner are needed in order to fully understand the roles of BDNF in response to cocaine exposure and addiction.
Prenatal Cocaine Exposure
Cocaine abuse by pregnant women continues to be a major public health concern throughout the world. Although the effects of prenatal cocaine exposure are difficult to interpret in individuals due to the possibility that pregnant women may also abuse other stimulants and/or alcohol, it has been reported that prenatally cocaine-exposed individuals show lasting changes in cognitive functions, including learning and language skills. Rodent models of prenatal cocaine exposure also report cognitive abnormalities in the offspring [67]. Additionally, BDNF signaling is emerging as a mediator of the effects of prenatal cocaine exposure on the brain and behavior [68-71]. Recent studies report changes in BDNF expression, synaptic plasticity, and behavioral responses to administration of cocaine or dopamine agonist in adult rodents exposed to cocaine prenatally. Our recent work demonstrated a transient decrease in BDNF expression in the striatum of the cocaine-exposed embryos [69]. Cocaine-induced perturbations in the level of BDNF during development may have a long-term impact on multiple signaling mechanisms including dopamine, BDNF, and GABA receptor signaling mechanisms in the striatum, NAc [70], and the PFC [72]. Such persistent changes may be responsible for changes in LTP, reduced GABAA receptor subunit expression, decreased cocaine locomotor sensitization, and cocaine place preference [73-75].
Correlating the electrophysiological and behavioral changes in the prenatally cocaine-exposed adults with changes in BDNF expression in the mesolimbic pathway has been difficult. For example, there is no change in the levels of BDNF at postnatal day 60 in the PFC, NAc, VTA, and striatum of the prenatally cocaine-exposed mice [70]. One explanation for the apparent lack of correlation may be that the studies thus far have not dissociated the effects of prenatal cocaine exposure from the effects of postnatal cocaine withdrawal. Time series studies are necessary to reveal transient versus persistent alterations in these parameters in specific brain regions, in response to prenatal cocaine exposure and the long period of withdrawal during postnatal development. A recent study of hippocampal neurons revealed a reduction in global DNA methylation in prenatally cocaine exposed 3-day old mice and an increase in 30-day old mice via increased expression of DNMT1 and DNMT3a, suggesting an epigenetic mechanism for developmental regulation of gene expression in response to prenatal cocaine exposure [76]. Additionally, two recent studies in prenatal cocaine-exposed mice reveal interactions among BDNF signaling, synaptic plasticity, and cognitive function using a mouse model harboring a single nucleotide polymorphism (SNP) in the BDNF gene (Val66Met) commonly associated with vulnerability to drug-abuse and addiction [71,77].
Concluding Remarks
This review proposes the idea that cocaine exerts its effects upon the mesolimbic dopamine pathway to stimulate reward and addiction, in part by increasing synaptic plasticity via epigenetic regulation of the BDNF gene. A single exposure to cocaine has transient effects, which induce a small subset of genes, including BDNF, through the actions of several histone and DNA modifying enzymes. This increase in gene expression drives the rewarding effects of cocaine, presumably by interfering with dopamine homeostasis following repeated cocaine exposure. However, the histone modifications (H4 acetylation and phospho-acetylation) following acute exposure to cocaine are short-lived. After repeated administration of cocaine, a larger subset of genes carry more permanent chemical modifications (H3 acetylation and H3K9 dimethylation), which shift the chromatin to a more permanent permissive state and thus become sensitized to cocaine-induced neuronal activation. Once the chromatin is maintained in this permissive state, pCREB is poised to induce transcription of BDNF and other plasticity-related genes. This is believed to create a “genomic scar,” locking the chromatin in the permissive or inducible state, even after long periods of abstinence. Prenatal exposure to cocaine also changes the epigenetic status of a number of genes throughout development. Evidence from human studies indicates that children of drug-using parents are more likely to seek drugs, even when said children are adopted and raised in a drug-free home [78]. While the reward component of drug abuse and addiction is becoming clear, the identity of genes that may influence an individual to initially experiment with these harmful substances is still a mystery. However, the changes in chromatin structure caused by drug exposure, which render the genome vulnerable to further attacks by harmful stimuli, may help explain the rise in developmental neurological disorders such as depression, ADHD, autism, and substance abuse seen in recent decades. Understanding the mechanisms of genetic vulnerability to drug-seeking and addictive behavior will aid in the discovery of pharmacological treatments and may help to minimize the heritable transmission of such susceptibilities in future generations.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- Trk
tropomyosin-receptor kinase
- MAP
mitogen-activation protein
- NMDA
N-methyl-D-aspartate
- LTP
long-term potentiation
- CREB
cAMP response element-binding
- MeCP2
methyl CpG binding protein 2
- CRE
Ca2+ response elements
- CBP
CREB-binding protein
- HDAC1
histone deacetylase 1
- HAT
histone acetyltransferase
- HMTs
histone methyltransferases
- DNMTs
DNA methyltransferase
- pCREB
phosphorylated CREB
- VTA
ventral tegmental area
- NAc
nucleus accumbens
- PFC
prefrontal cortex
- GABA
γ-aminobutyric acid
References
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22(3):123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1(5):549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270(5236):593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Izquierdo I, Medina JH. BDNF and memory formation and storage. Neuroscientist. 2008;12(2):147–156. doi: 10.1177/1073858407305850. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76(2):99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn Mem. 2002;9(5):224–237. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2(1):24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Kucera J, Lee KF, Loring J, Jaenisch R. Studies on the physiological role of brain-derived neurotrophic factor and neurotrophin-3 in knockout mice. Int J Dev Biol. 1995;39(5):799–807. [PubMed] [Google Scholar]
- Atwal JK, Massie B, Miller FD, Kaplan DR. The TrkB-Shc site signals neuronal survival and local axon growth via MEK and P13-kinase. Neuron. 2000;27(2):265–277. doi: 10.1016/s0896-6273(00)00035-0. [DOI] [PubMed] [Google Scholar]
- Liu QR, Lu L, Zhu XG, Gong JP, Shaham Y, Uhl GR. Rodent BDNF genes, novel promoters, novel splice variants, and regulation by cocaine. Brain Res. 2006;1067(1):1–12. doi: 10.1016/j.brainres.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85(3):525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulle F, van den Hove DL, Jakob SB, Rutten BP, Hamon M, van Os J. et al. Epigenetic regulation of the BDNF gene: implications for psychiatric disorders. Mol Psychiatry. 2012;17(6):584–596. doi: 10.1038/mp.2011.107. [DOI] [PubMed] [Google Scholar]
- Jones PL, Veenstra GJ, Wade PA, Vermaak D, Kass SU, Landsberger N. et al. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet. 1998;19(2):187–191. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN. et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393(6683):386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128(4):707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Castren E. Neurotrophic effects of antidepressant drugs. Curr Opin Pharmacol. 2004;4(1):58–64. doi: 10.1016/j.coph.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Cattaneo E, Zuccato C, Tartari M. Normal huntingtin function: an alternative approach to Huntington's disease. Nat Rev Neurosci. 2005;6(12):919–930. doi: 10.1038/nrn1806. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt AA, Chen MJ. Brain-derived neurotrophic factor and antidepressant activity. Curr Pharm Des. 2005;11(12):1495–1510. doi: 10.2174/1381612053764788. [DOI] [PubMed] [Google Scholar]
- LaPlant Q, Nestler EJ. CRACKing the histone code: cocaine's effects on chromatin structure and function. Horm Behav. 2011;59(3):321–330. doi: 10.1016/j.yhbeh.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DSM-IV. Diagnostic and Statistical Manual of Mental Disorders. 4th edition. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Morris RG. Long-term potentiation and memory. Philos Trans R Soc Lond B Biol Sci. 2003;358(1432):643–647. doi: 10.1098/rstb.2002.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Lohof AM, Ip NY, Poo MM. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993;363(6427):350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci USA. 1995;92(19):8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16(6):1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56(3):422–437. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC. et al. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302(5646):885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y. et al. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302(5646):890–893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Kavalali ET. Rett syndrome and the impact of MeCP2 associated transcriptional mechanisms on neurotransmission. Biol Psychiatry. 2009;65(3):204–210. doi: 10.1016/j.biopsych.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Nestler EJ. MeCP2 and drug addiction. Nat Neurosci. 2010;13(9):1039–1041. doi: 10.1038/nn0910-1039. [DOI] [PubMed] [Google Scholar]
- Blendy JA, Maldonado R. Genetic analysis of drug addiction: the role of cAMP response element binding protein. J Mol Med (Berl) 1998;76(2):104–110. doi: 10.1007/s001090050197. [DOI] [PubMed] [Google Scholar]
- Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87(5):953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- Briand LA, Blendy JA. Molecular and genetic substrates linking stress and addiction. Brain Res. 2010;1314:219–234. doi: 10.1016/j.brainres.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg ME, Thompson MA, Sheng M. Calcium regulation of immediate early gene transcription. J Physiol Paris. 1992;86(1-3):99–108. doi: 10.1016/s0928-4257(05)80013-0. [DOI] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20(4):709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Dennis KE, Levitt P. Regional expression of brain derived neurotrophic factor (BDNF) is correlated with dynamic patterns of promoter methylation in the developing mouse forebrain. Brain Res Mol Brain Res. 2005;140(1-2):1–9. doi: 10.1016/j.molbrainres.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Jones SR, Garris PA, Wightman RM. Different effects of cocaine and nomifensine on dopamine uptake in the caudate-putamen and nucleus accumbens. J Pharmacol Exp Ther. 1995;274(1):396–403. [PubMed] [Google Scholar]
- Venton BJ, Seipel AT, Phillips PE, Wetsel WC, Gitler D, Greengard P. et al. Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool. J Neurosci. 2006;26(12):3206–3209. doi: 10.1523/JNEUROSCI.4901-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger D. Cocaine, metamfetamine, and MDMA abuse: the role and clinical importance of neuroadaptation. Clin Toxicol (Phila) 2010;48(7):695–708. doi: 10.3109/15563650.2010.516263. [DOI] [PubMed] [Google Scholar]
- Schroeder FA, Penta KL, Matevossian A, Jones SR, Konradi C, Tapper AR. et al. Drug-induced activation of dopamine D(1) receptor signaling and inhibition of class I/II histone deacetylase induce chromatin remodeling in reward circuitry and modulate cocaine-related behaviors. Neuropsychopharmacology. 2008;33(12):2981–2992. doi: 10.1038/npp.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit HO, Pan HT, Parsons LH, Justice JB Jr.. Extracellular concentrations of cocaine and dopamine are enhanced during chronic cocaine administration. J Neurochem. 1990;55(3):798–804. doi: 10.1111/j.1471-4159.1990.tb04562.x. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Fillmore J, Kreek MJ. Chronic repeated cocaine administration increases dopamine D1 receptor-mediated signal transduction. Eur J Pharmacol. 1996;318(1):31–35. doi: 10.1016/s0014-2999(96)00841-2. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Di Pasquale L, Caffino L, Racagni G, Riva MA. Repeated exposure to cocaine differently modulates BDNF mRNA and protein levels in rat striatum and prefrontal cortex. Eur J Neurosci. 2007;26(10):2756–2763. doi: 10.1111/j.1460-9568.2007.05918.x. [DOI] [PubMed] [Google Scholar]
- McGinty JF, Whitfield TW Jr., Berglind WK. Brain-derived neurotrophic factor and cocaine addiction. Brain Res. 2010;1314:183–193. doi: 10.1016/j.brainres.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL. et al. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389(6653):856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- Goggi J, Pullar IA, Carney SL, Bradford HF. Signalling pathways involved in the short-term potentiation of dopamine release by BDNF. Brain Res. 2003;968(1):156–161. doi: 10.1016/s0006-8993(03)02234-0. [DOI] [PubMed] [Google Scholar]
- Kauer JA. Learning mechanisms in addiction: synaptic plasticity in the ventral tegmental area as a result of exposure to drugs of abuse. Annu Rev Physiol. 2004;66:447–475. doi: 10.1146/annurev.physiol.66.032102.112534. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Diaz J, Sokoloff P. A single cocaine exposure increases BDNF and D3 receptor expression: implications for drug-conditioning. Neuroreport. 2005;16(2):175–178. doi: 10.1097/00001756-200502080-00022. [DOI] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10(8):1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW Jr., Miller SW. et al. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci. 2007;26(3):757–766. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- Maze I, Covington HE 3rd, Dietz DM, LaPlant Q, Renthal W, Russo SJ. et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science. 2010;327(5962):213–216. doi: 10.1126/science.1179438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maze I, Nestler EJ. The epigenetic landscape of addiction. Ann NY Acad Sci. 2011;1216:99–113. doi: 10.1111/j.1749-6632.2010.05893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23(3):742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT. et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48(2):303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Sadri-Vakili G, Kumaresan V, Schmidt HD, Famous KR, Chawla P, Vassoler FM. et al. Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. J Neurosci. 2010;30(35):11735–11744. doi: 10.1523/JNEUROSCI.2328-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156(1):11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Perez H, Ting AKR, Walton CH, Hansen DM, Razavi R, Clarke L. et al. Ventral tegmental area BDNF induces an opiate-dependent-like reward state in naive rats. Science. 2009;324(5935):1732–1734. doi: 10.1126/science.1168501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Iverson MT, Todd KG, Altar CA. Brain-derived neurotrophic factor and neurotrophin-3 activate striatal dopamine and serotonin metabolism and related behaviors: interactions with amphetamine. J Neurosci. 1994;14(3 Pt 1):1262–1270. doi: 10.1523/JNEUROSCI.14-03-01262.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Iverson MT, Altar CA. Spontaneous behaviours of rats are differentially affected by substantia nigra infusions of brain-derived neurotrophic factor and neurotrophin-3. Eur J Neurosci. 1996;8(8):1696–1706. doi: 10.1111/j.1460-9568.1996.tb01313.x. [DOI] [PubMed] [Google Scholar]
- Horger BA, Iyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J Neurosci. 1999;19(10):4110–4122. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci. 2004;24(7):1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield TW Jr., Shi X, Sun WL, McGinty JF. The suppressive effect of an intra-prefrontal cortical infusion of BDNF on cocaine-seeking is Trk receptor and extracellular signal-regulated protein kinase mitogen-activated protein kinase dependent. J Neurosci. 2011;31(3):834–842. doi: 10.1523/JNEUROSCI.4986-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglind WJ, Whitfield TW Jr., LaLumiere RT, Kalivas PW, McGinty JF. A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. J Neurosci. 2009;29(12):3715–3719. doi: 10.1523/JNEUROSCI.5457-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao LM, Horton E, Guo ML, Xue B, Jin DZ, Fibuch EE. et al. Cocaine increases phosphorylation of MeCP2 in the rat striatum in vivo: a differential role of NMDA receptors. Neurochem Int. 2011;59(5):610–617. doi: 10.1016/j.neuint.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SJ, Mazzio E, Kolta MG, Soliman KF. Prenatal cocaine exposure affects postnatal dopaminergic systems in various regions of the rat brain. Ann NY Acad Sci. 1998;844:293–302. [PubMed] [Google Scholar]
- Yan QS, Zheng SZ, Yan SE. Prenatal cocaine exposure decreases brain-derived neurotrophic factor proteins in the rat brain. Brain Res. 2004;1009(1-2):228–233. doi: 10.1016/j.brainres.2004.02.052. [DOI] [PubMed] [Google Scholar]
- McCarthy DM, Zhang X, Darnell SB, Sangrey GR, Yanagawa Y, Sadri-Vakili G. et al. Cocaine alters BDNF expression and neuronal migration in the embryonic mouse forebrain. J Neurosci. 2011;31(38):13400–13411. doi: 10.1523/JNEUROSCI.2944-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropea TF, Kabir ZD, Kaur G, Rajadhyaksha AM, Kosofsky BE. Enhanced dopamine D1 and BDNF signaling in the adult dorsal striatum but not nucleus accumbens of prenatal cocaine treated mice. Front Psychiatry. 2011;2:67. doi: 10.3389/fpsyt.2011.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir ZD, Lourenco F, Byrne ME, Katzman A, Lee F, Rajadhyaksha AM. et al. Brain-Derived Neurotrophic Factor Genotype Impacts the Prenatal Cocaine-Induced Mouse Phenotype. Dev Neurosci. 2012;34(2-3):184–197. doi: 10.1159/000337712. [DOI] [PubMed] [Google Scholar]
- McCarthy DM, Bhide PG. Prenatal Cocaine Exposure Decreases Parvalbumin-Immunoreactive Neurons and GABA-to-Projection Neuron Ratio in the Medial Prefrontal Cortex. Dev Neurosci. 2012;34(2-3):174–183. doi: 10.1159/000337172. [DOI] [PubMed] [Google Scholar]
- Lu H, Cheng PL, Lim BK, Khoshnevisrad N, Poo MM. Elevated BDNF after cocaine withdrawal facilitates LTP in medial prefrontal cortex by suppressing GABA inhibition. Neuron. 2010;67(5):821–833. doi: 10.1016/j.neuron.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyser CJ, Spear NE, Spear LP. Effects of prenatal exposure to cocaine on conditional discrimination learning in adult rats. Behav Neurosci. 1992;106(5):837–845. doi: 10.1037//0735-7044.106.5.837. [DOI] [PubMed] [Google Scholar]
- Malanga CJ, Pejchal M, Kosofsky BE. Prenatal exposure to cocaine alters the development of conditioned place-preference to cocaine in adult mice. Pharmacol Biochem Behav. 2007;87(4):462–471. doi: 10.1016/j.pbb.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikova SI, He F, Bai J, Cutrufello NJ, Lidow MS, Undieh AS. Maternal cocaine administration in mice alters DNA methylation and gene expression in hippocampal neurons of neonatal and prepubertal offspring. PLoS One. 2008;3(4):e1919. doi: 10.1371/journal.pone.0001919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Bath KG, Perez-Castro R, Lee FS, Chao MV, Ninan I. The BDNF Val66Met polymorphism impairs synaptic transmission and plasticity in the infralimbic medial prefrontal cortex. J Neurosci. 2012;32(7):2410–2421. doi: 10.1523/JNEUROSCI.5205-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Sundquist K, Ohlsson H, Palmer K, Maes H, Winkleby MA. et al. Genetic and Familial Environmental Influences on the Risk for Drug Abuse: A National Swedish Adoption Study. Arch Gen Psychiatry. 2012;69(7):690–697. doi: 10.1001/archgenpsychiatry.2011.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, McGinty JF, West AE, Sadri-Vakili G. In: Addiction. Pierce RC, Kenny PJ, Reppert SM, editors. New York: Cold Spring Harbor Laboratory Press; 2012. Epigenetics and Psychostimulant addiction. [Google Scholar]