Abstract
The brain is the most intricate, energetically active, and plastic organ in the body. These features extend to its cellular elements, the neurons and glia. Understanding neurons, or nerve cells, at the cellular and molecular levels is the cornerstone of modern neuroscience. The complexities of neuron structure and function require unusual methods of culture to determine how aberrations in or between cells give rise to brain dysfunction and disease. Here we review the methods that have emerged over the past century for culturing neurons in vitro, from the landmark finding by Harrison (1910) — that neurons can be cultured outside the body — to studies utilizing culture vessels, micro-islands, Campenot and brain slice chambers, and microfluidic technologies. We conclude with future prospects for neuronal culture and considerations for advancement. We anticipate that continued innovation in culture methods will enhance design capabilities for temporal control of media and reagents (chemotemporal control) within sub-cellular environments of three-dimensional fluidic spaces (microfluidic devices) and materials (e.g., hydrogels). They will enable new insights into the complexities of neuronal development and pathology.
Keywords: brain slice culture, cell culture, co-culture, culture chamber, culture flask, culture substrate, hanging drop, organotypic culture, microfluidics, neuron, polydimethylsiloxane, PDMS, substrate patterning
Introduction
Early in embryogenesis, neuron precursor cells, the neuroblasts, divide and differentiate into the cellular components of the brain and peripheral nervous system. They develop specific cellular fates while migrating to pre-determined destinations. These cells are destined to become either neurons or glial cells. Nascent neurons extend multiple protrusions that can become highly elaborate with many tree-like branches, or arborizations. They become highly polarized structurally and are specialized for electrical and chemical communication. The most highly branched protrusions are the dendrites, which act as antennae that receive incoming signals and transmit them to the cell body. A single axon, a neuronal extension specialized for signal transmission downstream to other cells, communicates with neurons, organs, muscles, and skin. As the brain forms, neurons participate in increasingly extensive networks. These multiple extensions exist in many complex extracellular microenvironments. Communication between neurons and their downstream targets is typically achieved via synaptic specializations, where chemical or electrical signals are transmitted between neuronal circuit elements.
Unlike other types of cells, neurons are elongate. The soma extends processes that become highly branched and whose length is often many times the soma’s diameter. Terminal points of these branches reside in different spatial domains from the soma and other processes with distinct chemical, physical, and fluidic features. Consequently, studying neuronal cytoarchitecture is challenging and complex [1,2]. Nevertheless, understanding how the structure of the neuron influences its function is critical [3]. Discovering how local signals direct neuronal development has been impeded by the inability to access and control local microenvironments of the complex neuronal cytoarchitecture. Since in vitro studies in many cases require simulating the in vivo microenvironment of cells, convenient and viable in vitro techniques are critical prerequisites for studying neuron cells. The challenge has been to make them accessible for in vitro laboratory manipulation. New ways of interrogating sub-regions and their microenvironments are being developed that enable differential control of sub-regions of individual dendrites and axons [4].
The emergence and implementation of traditional, in vitro-based cell culture methods have advanced understanding of neuronal development in highly significant ways. The first notable thrust occurred more than a century ago. While conducting embryological research at Yale University, Ross Granville Harrison published findings on the developing neuron that quickly transformed biological sciences by seeding new directions for developmental investigations, substantially changing neuroscience [5]. By applying the bacteriologists’ tools to vertebrate embryology, Harrison developed a method that demonstrated — for the first time — that living tissues from vertebrates could be cultivated and studied outside the body. His studies also answered important questions relating to the growth and development of nerve cells into nerve fibers.
Borrowed or invented, techniques for culturing neurons in vitro have evolved to answer uniquely neurobiological questions. These techniques have been applied in studies on the development and function of cells, tissues, and organs. As newly developed methods are refined, further insights into cell function can be obtained; new ideas and questions emerge to further propel the field of study toward answering more weighty and complex conundrums. In this way, methodological developments in basic scientific research advance our understanding of the nervous system, improving access to specific cellular relationships and elucidating both the mechanistic and integrative bases of diseases and disorders of the nervous system.
By reviewing the origins and progress of neuron culture methods, beginning with Harrison’s pivotal publication, we can appreciate how acquiring, improving, and interjecting new methodologies into conventional practices enhances our ability to advance understanding of neuronal structure and function. Harrison’s contributions changed the way we view and investigate cells of the body and brain. Here, we address fundamental technological shifts in the ability to culture neurons over the past century since Harrison proved the neuron doctrine, that the nervous system is composed of discrete cells. We first address methods to culture nervous tissue and neurons and then focus on approaches to manipulate neuron growth and differentiation in vitro using micro-islands, Campenot chambers, and microfluidic devices. These approaches have enhanced understanding of how neuronal development and function are shaped by the local chemical, physical, and fluidic environments of its intricate morphology. They are enabling new insights into how signaling mechanisms change in neuronal pathologies. Finally, we discuss future directions, looking toward prospects for neuron culture over the coming years.
The Development of Cell Culture Methods
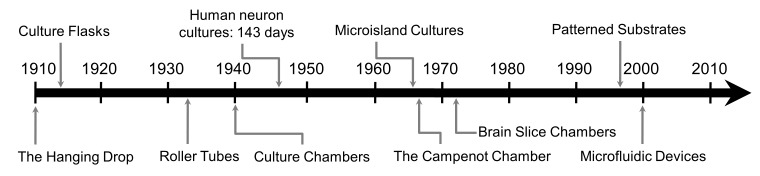
Over the past century, diverse methods to enable the culture, observation, and development of neurons in vitro have emerged. Figure 1 provides a timeline overview of the development of culture techniques, each reviewed here in greater detail. Table 1 presents the benefits and limitations of tissue and neuron culture methodologies.
Figure 1.
Timeline of emergence of methods for culturing neurons. After the successful culture of vertebrate cells and tissues by Ross Granville Harrison at Yale in 1910, successive refinements for culturing neurons emerged. The timeline shows the approximate date of publications introducing the use of each method.
Table 1. Benefits and limitations of tissue and neuron culture methodologies.
| Hanging Drop [5-7] |
| Benefits |
| Simple design: Easy to assemble and requires no special equipment. |
| Culture integrity: Contamination isolated to individual cultures. |
| Convenience: Convenient closed-system assembly for handling tissue cultures. |
| Histology: Compatible with histological preparations. |
| Visualization: Acceptable for microscopic imaging. |
| Limitations |
| Device handling: Hermetically sealed device is cumbersome for extending culture duration. |
| Fluidic control: Uncontrollable fluidic environment. |
| Longevity: Low media-to-cell ratio causes nutrient depletion and waste accumulation |
| Culture uniformity: Ranging from individual cells to tissue fragments. |
| Necrosis: Tissue chunks in culture are prone to necrosis. |
| Visualization: Challenging for cell and morphological identification. |
|
|
| Carrel Flask [13,21] |
| Benefits |
| Longevity: Extends viable cultures months beyond the hanging drop method. |
| Fluidic control: Increases culture media to cell ratio. |
| Gas exchange: Improves gas exchange for pH control by introducing known gas mixtures. |
| Large capacity: Increased capacity for cell and tissue cultures. |
| Biochemistry: Useful for biochemical studies, e.g., cell/tissue extraction. |
| Limitations |
| Fluid control: Difficult to control lateral spread and size of the plasma coagulum. |
| Variability: Plasma coagulum variability influences cell spread, growth rates. |
| Sample control: Cultures prone to plasma clot dissolution, reducing culture longevity. |
| Media stagnation: Impacts culture uniformity. |
| Histology: Cumbersome for histological preparations and sample preservation. |
| Contamination: Contamination can affect numerous cultures in a single flask. |
|
|
| Roller Tube [22,32] |
| Benefits |
| Utility: Broad, for suspended tissue cultures, organotypic slice cultures, or combined for coverslip cultures ("flying coverslips"). |
| Gas exchange: Improves media and gas circulation for improved uniformity. |
| Longevity: Reduces tissue necrosis and enables culture durations for months. |
| Convenience: A low-maintenance method. |
| Limitations |
| Visualization: Challenging and cumbersome for cell imaging, especially microscopic cinematography. |
|
|
| Slide Chamber [22,43] |
| Benefits |
| Utility: Different chamber designs confer a range of benefits. |
| Longevity: Continuously supplies new media to cultured tissues. |
| Biochemistry: Amenable for testing effects of pharmacological agents on cultures. |
| Visualization: Improves imaging of cultured cells and tissue. |
| Limitations |
| Low throughput: Costly chambers prohibit many parallel cultures. |
|
|
| Campenot Chamber [63,68,69] |
| Benefits |
| Utility: Permits compartmentalization of peripheral neurons in vitro for sympathetic neuron growth and signaling studies in an easily accessible cell culture Petri dish. |
| Visualization: Neurons easily visualized using conventional microscopic methods. |
| Fluid control: Media exchange and fluid control not complicated or cumbersome. |
| Low cost: Simple, cost-effective constructs for cell culture. |
| High throughput: Many samples can be analyzed in parallel within the same chamber device. |
| Limitations |
| Assembly: Requires silicone grease and prone to leakage. |
| Versatility: Due to the size, the Campenot chamber is limited to large peripheral neurons that grow very long processes; is not compatible with many neurons of the brain and spine. |
|
|
| Brain Slice Chamber [38,39] |
| Benefits |
| Utility: Permits slices of brain tissue to be maintained in vitro with neural circuitry and integrative functions intact. Highly controlled access to subcellular regions, single cells, and neuron networks. Design pattern produces nearly identical replicates with chemotemporal, spatial control. High reproducibility. |
| Experimental feasibility: Brain slice chambers can be made or purchased with many designs that permit controlled fluid exchange, optical transparency for imaging studies, exposure to moist gas, low water evaporation; thermally stabile; physically confine neurons to tissue. |
| Visualization: Cells, tissues, and nerves easily visualized using conventional microscopic methods. |
| Electrophysiology: Direct access to the neurons, axonal tracts and nerves by extracellular, sharp and patch electrodes, as well as multi-electrode arrays (MEAs). |
| Fluidic control: Temporal and spatial fluidic control permits numerous possibilities for stimulation, inhibition, and perturbations. |
| Low cost: Simple, cost-effective constructs for tissue culture. |
| Versatility: Highly versatile in materials and design; can be tailored to experimental design needs. |
| Shear stress: Laminar flow fields can be kept low for experiments with sensitive tissues. |
| Limitations |
| Assembly: Requires purchase or machine-shop fabrication of designer environments. |
| Throughput: Low, although several brain slices can be co-cultured within the same chamber device. |
| Evaporation: Inevitable if the access port is left open more than briefly; drying can cause a surface scab to form, which is a barrier to oxygen and leads to tissue death; changes in media osmolarity can confound results, killing cells if not controlled. |
|
|
| Microfluidic Device [4,91,118,139,140] |
| Benefits |
| Utility: Compartmentalization of single or co-cultured neurons, glia in PDMS device with flexible design. Highly controlled access to subcellular regions, single cells, and neuron networks. Template produces many, nearly identical replicates, with chemotemporal, spatial control. High reproducibility. |
| Experimental feasibility: PDMS-based devices permit controlled fluid exchange, optical transparency for imaging studies, gas permeation; low in water evaporation; thermally stabile; physically confine neurons to control connectivity. |
| Visualization: Cells easily visualized using conventional microscopic methods. |
| Fluidic control: Temporal and spatial fluidic control permits numerous possibilities for fluidic isolation, stimulation, and perturbations; valves can be built-in. |
| Low cost: Simple, cost-effective constructs for cell culture. |
| Throughput: Many samples can be analyzed in parallel within the same chamber/device. |
| Versatility: Highly versatile in materials and design; can be fabricated for specific cell and process sizes and geometries. Designs highly flexible, tailored to experimental design needs. |
| Limitations |
| Assembly: Requires purchase or training to fabricate designer environments. |
| Behavior of gas fluxes not optizimized vs. conventional cultures: Gas solubility, permeability, and diffusion may be material- and/or dimension-dependent. |
| Evaporation: Inevitable at micro- and nano-scale volumes; changes in media osmolarity can confound results, kill cells if not controlled. |
| Shear stress: Laminar flow fields can potentially confound experiments with sensitive neurons. |
| Substrate uptake of media components/release of materials: Adsorption, absorption, and desorption have the potential to bias cell growth or response in microfluidics. |
The Hanging Drop
In the early 20th century, the development of the capability to culture living cells enabled observations that were a marked change from conventional fixed-tissue histology of the 19th century. To answer the contentious questions surrounding the unusual morphology of neurons, Ross Harrison sought “… to obtain a method by which the end of a growing nerve could be brought under direct observation while alive” [6]. While working at Yale in 1910, Harrison conducted one of the most significant studies in the field of neuroscience, using the hanging drop technique to establish culture conditions that permitted direct observations of living cells in vitro. He determined for the first time that the nervous system is cellular.
The hanging drop is a culture technique whereby cells or tissue fragments are attached to a coverslip and bathed in a drop of partially coagulated serum or lymph (Figure 2). The coverslip with the tissue-containing coagulum is inverted so that the clot is hanging, then the coverslip is attached and sealed to a microscope slide with a glass impression in which the drop hangs. Through this elegant approach, Harrison demonstrated that nerve fibers form from cells. His results provided incontrovertible proof that the nervous system is composed of individual, discrete cells: the neuron doctrine hypothesis. The neuron doctrine was in direct opposition to the reticular theory, which posited that the nervous system was an acellular, reticular network of fibrils. In settling this debate, Harrison also reported the biological significance of in vitro cultures, including the normal cellular differentiation of various cell types, the contraction of muscle cells, and ciliary movement. For individual nerve cells, he demonstrated differentiation, migration, neurite elongation rate, interactions, and anastomoses [5].
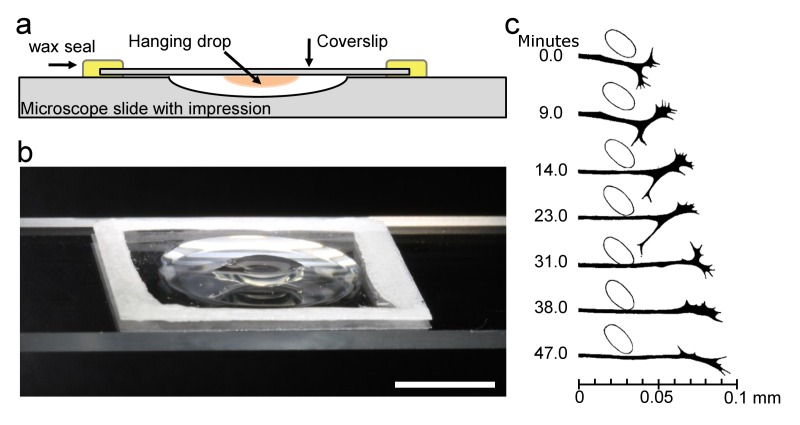
Figure 2.
The hanging drop. (a) Schematic diagram of the hanging drop technique. (b) Photograph of a hanging drop replica assembled as previously described [5]. Scale bar = 7.5 mm. (c) Ross Harrison’s 1909 camera lucida drawings of a growing nerve fiber in the hanging drop culture. The fiber shown was cultured from the brachial region of a Rana sylvatica embryo; prior to tracing, the fiber extended 800 µm over 4 days. The stationary silhouetted red blood corpuscle gives perspective to the dynamic growth of the nerve fiber over 47 minutes. Modified from [5] and used with permission.
The simplicity of the experiment belies the cumbersome logistics of developing successful cultures. Neural tube fragments from the embryonic frog were placed in a drop of fresh frog lymph, on flame- or heat-sterilized coverslips, and bathed in moist environments. Pioneering cell cultures was a difficult task without bulk quantities of sterile supplies, defined reagents, and antibiotics. Nevertheless, when addressing his successes, Harrison credited the common utility of the “hanging drop culture” to bacteriological studies and acknowledged the amalgamation of ideas rather than taking credit to himself [7,8].
By 1913, another significant application of the hanging drop technique was achieved when Levaditi used the hanging drop to investigate interactions of polio and rabies viruses with cells and obtained the first culture of the poliomyelitis virus using neurons from monkey spinal ganglia [9-12]. He concluded that viruses are intracellular parasites incapable of reproduction in nutrient media alone. These studies substantiated his claim against the prevailing dogma that viruses were the product of degenerated lethal genes. Furthermore, Levaditi’s cultures in the hanging drop were early contributions to the armamentarium used in understanding the nature of the virus, producing a vaccine, and eradicating disease.
Harrison’s methods of cell culture were rapidly adopted and adapted by others and applied to mammalian tissues. Also in 1910, Burrows and Carrel used conditioned and unconditioned plasma in hollow glass slides for cultivating differentiated mammalian tissues, including vascular tissues, cartilage, endothelium, bone, epidermis, thyroid, spleen, and kidney. This method enabled the first culturing of adult mammalian tissues and organs outside the body [13]. In the following year, Lewis and Lewis, researchers at Johns Hopkins, reported improvements on tissue cultures of Carrel and Burrows, who used body fluids as the culture media. Lewis and Lewis used an artificial media consisting of a simple salt solution and chicken bouillon [14]. Artificial media rapidly replaced lymphatic fluids as the medium of choice for cultivating cells and defined culture media formulations, enabling more control of the environment, improving reliability and reproducibility [15-17]. Cell culture methods expanded, enabling the observation of spontaneously contracting cardiac myocytes in isolation [18], connective tissues in culture [19], and mitochondria in cultured cells [20]. New methodologies for live cell investigations were exciting, for they added a new level of observation and analysis well beyond the technical limitations of histopathological techniques.
These early experiments led to the emergence during the 1920s of identifiable scientific foci. The first was to resolve problems of viability, longevity associated with cell growth, nutrition, and multiplication. Experimental criteria for defining and improving culture requirements continued to follow the assessments Harrison had applied, assaying cell migration and culture duration. These criteria proved valuable in determining the effectiveness of various formulations of salts, osmolites, pH, temperature, glucose, and other nutrients. Research for decades thereafter would be concentrated primarily on developing precise conditions for culturing a broad range of animal tissues, from insects to man. A second focus was on defining the processes of cellular differentiation and tissue organization. Common to these developments was the invention of culture-assisting devices devised to answer specific questions, solve immediate problems, or overcome technical limitations or constraints of existing methods. The result has been a developmental progression of optimizing culture conditions with new devices for culturing neurons and brain tissue.
Despite the effectiveness of the hanging drop, its limitations proved obvious when dealing with long-term cultures or its compatibility with different types of cells and cultures. This drove the development of a range of devices designed to meet the many requirements for aseptic cultivation of cells and tissues in vitro.
Carrel Flasks
A range of culture flasks were developed in 1923 by Alexis Carrel to “maintain tissues in a condition of uninterrupted growth in a medium which does not deteriorate spontaneously” [21]. The flat surfaces and angled necks of these culture flasks enabled long-term cultivation by providing easier access to the culture for exchanging media and improving cell observation. Features of the five different models include one or two angular necks, flat bottoms that facilitate visualization, and removable mica or glass plates for easy access to the inner flask (Figure 3). These advances improved culture techniques and prolonged viability through accessibility for culture maintenance. This enabled the biologist to approach research questions with a control beyond that which was achievable with the hanging drop. The flask permitted greater media volumes, supported more cultures in the same device, enabled the introduction of select gas mixtures, and extended culture durations. However, the new apparatus did not circumvent variabilities associated with the plasma coagulum, particularly clot spread and proteolytic clot dissolution. Because clot size influences cell spread and growth rates, uniform cultures could not be achieved [22]. At one point, perforated cellophane was substituted as a culture substrate for tissue attachment and cell spread, including an effort to reduce confounding effects of tissue fragments in clots [23-26]. Notwithstanding these disadvantages, the improvements of the Carrel flask proved useful for studies on mitosis and cell migration, media/tissue extracts, proteases, amino acids, and toxins [22]. The efficacy and influence of the culture flask extended far beyond Carrel’s initial studies, and the essence of the culture vessel persists today in studies throughout the biological sciences, including neuroscience.
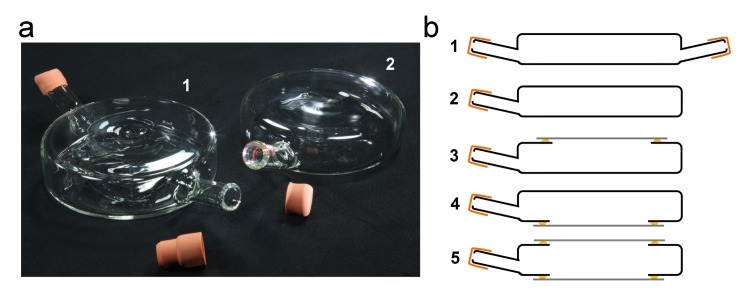
Figure 3.
Culture flasks. Replicas of two glass culture flasks introduced by Alexis Carrel for long-term tissue culture. a) Culture flasks labeled 1 and 2 correspond to the cross-sectional schematics of flasks 1 and 2 shown in b. b) Five types of culture flasks were developed by Carrel for cell and tissue culture [21]. The range of fabrication approaches were modifications to permit easy access to the sample for placement and maintenance. Thin mica sheets 3 to 5 (top and/or bottom) were used in place of glass for imaging cultures with higher power microscope objectives [8,9]. Gaskets and ports were incorporated in the flask designs to permit access to the culture and for media exchange.
Roller Tubes
Cultures using the test tube, the predecessor to the roller tube, pre-date the hanging drop technique by at least a decade, in reports as early as 1897 [27]. Throughout the history of their usage, roller tubes offered some advantages to the static culture systems by providing a gently kinetic environment. Early tube cultures were particularly useful in determining the effects of hydrogen and oxygen on cultures from various organ tissues [27]. Then, in 1933, the test tube was modified into the roller tube and introduced as “an improved technique” for large-scale tissue culture [28]. Aside from the Carrel flask, the roller tube approach was used in early studies to improve culture uniformity and media circulation for free-floating and adherent tissues in a coagulum. Mary Hogue's detailed report in 1947 describes both the ability to extend cultures for 3 to 4 months (up to 143 days) and the benefits and challenges of using hanging drop and roller tube cultures [29].
To improve visualization of flask-cultured tissues, flattened portions were made similar to the Carrel flask [22]. Additional modifications included hexagonal tubes [22], sponges [30], and the “flying coverslip,” where cultures of adult human cerebrum and cerebellum were established on a coverslip and maintained in a roller tube through continuous rotation [31,32]. Over the last decade, new methodological reports employ the roller tube for sustaining organotypic brain slice cultures (slices of living brain tissue usually thinner than 500 µm thickness and sustained in culture) [33-37]. Notwithstanding the benefits conferred through the roller tube, the most significant factors limiting the utility of this device were low compatibility with imaging and poor access to the tissue.
Brain Slice Chambers
Studying individual neurons in dissociated cultures has been the method of choice for decades owing to the ease with which neurons can be isolated, maintained, and observed. However, brain tissue can survive up to 6 min of hypoxia, enabling it to be removed from the skull and brain, sliced thinly enough to permit oxygenation by diffusion, and then transferred to a life-support environment [38]. Conditions must approximate those in vivo: a warm, moist, biocompatible environment with high O2 tension and bathed in minimal salts and glucose or enriched media. Individual neurons and their processes within the 100-500 µm-thick brain slice are relatively undisturbed, compared with single neurons isolated for dispersed culture. The complex neural circuitry and spatial relationships established during development are maintained, and tissue level function is largely preserved [39]. Furthermore, more critical for brain slices than dissociated neurons dispersed in culture, gas and nutrient exchange are vital for maintaining endogenous tissue function ex vivo [40].
In addition to the roller tube method for maintaining tissue slices, perfusion systems, membrane inserts, and culture chambers have been developed and refined to improve tissue health and vitality. This enables significantly greater access and control of the neurons and circuits of selected brain regions than in vivo. From dissociated cells to neurons in brain slices, improvements in imaging methods are increasing the resolution and ability to interrogate neuron structure and function non-invasively and dynamically [41]. For example, through multi-photon confocal microscopy of live organotypic brain slices, individual neurons were simultaneously studied metabolically and electrophysiologically, enabling discovery of novel mechanisms by which cellular metabolism regulates neuronal excitability of the mammalian circadian pacemaker, the suprachiasmatic nucleus [42].
Slide Culture Chambers
In an effort to improve visualization of cells and exchange of media, a variety of chambers were developed on microscope slides. After working under the direction of Ross Harrison, Montrose Burrows adapted the tissue culture technique to continuously supply fresh media. This method improves viability of cells and tissues, extending the life and health of the culture [18]. These attributes would be realized only decades later through methodological progression of designs. From the 1940s through the 1960s, more than 20 different designs for perfusion systems appeared (e.g., Mackaness-type and Pulvertaft-type chambers). They increased culture longevity and permitted time-lapse imaging (capturing images of dynamic events at predefined intervals of time) and testing of pharmacological agents [22]. Among the various iterations are a two-gasket chamber design for improving fluidic circulation and exchange, a one-coverslip design modeled on the hanging drop apparatus, and the dual coverslip device (the Rose Chamber, Figure 4) [43]. During chamber assembly and sealing, small bubbles can be intentionally trapped inside to stir the media with rotational planes. Today, commercially available chambers hold close to the original design of the Rose Chamber, while other models have been developed that incorporate and make use of new technologies, including indium tin oxide glass coatings for thermal stability and the glass-bottomed Petri dish for fluorescence microscopy.
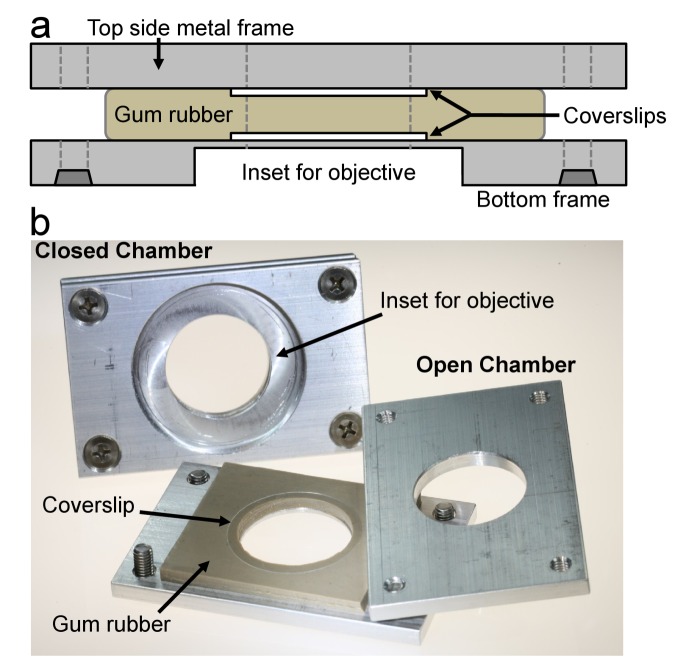
Figure 4.
Slide culture chambers. A replica of the Rose chamber developed according to the prescribed specifications for sustaining cells in culture [43]. Numerous chamber types and modifications have been developed and are used for culturing and imaging neurons in vitro. a) Schematic representation of the Rose chamber in cross-section. Two microscope coverslips spaced by a gasket to seal the culture chamber are sandwiched between two metal frames. The bottom frame has a recessed surface to permit access of the microscope objectives to the coverslip and four counter-holes for the bolts to hold the chamber assembly. b) Photograph of two separate Rose chamber replicas. The top chamber is completely assembled; the bottom chamber is partly disassembled to show the coverslip on the gum rubber gasket.
Dispersed neurons are most often cultivated in static baths on hard planar substrates of glass or polystyrene. Vessels range from macroscopic culture flasks to microscopic chambers. When removed from the supporting structure and perfusing vascularization of the brain and maintained in vitro, organotypic slices become very soft and fragile. More advanced culture methods that integrate neurons or specialized substrates for controlling neuronal development and function are being developed.
Controlling Neuron Development
In the remainder of this review, we address these advanced methodologies (e.g., microchips of silicon-based substrates, morphologically and chemically defined material substrates and/or biochemically relevant agents, and microfluidic devices). With the ability to investigate neurons in culture, numerous discoveries relevant to the development of neuronal morphology and function were achieved in the 60 years following the inception of in vitro culture methods. Scientific advances often parallel the technical refinement of the investigation. To access the biology with greater resolution, scientists began moving beyond merely culturing neurons to controlling neurons, driving interactions, or challenging cells with greater precision than could be accomplished through conventional methods. The micro-island culture and Campenot chamber provided the means to accomplish such investigations and pioneered the era in which neurons could be probed, controlled, and cultured under greater constraints. Through lithography, microtechnology, engineering, and chemistry, exquisite microenvironments can be produced that present microenvironmental cues that reproduce, in fundamental form, those experienced in vivo (e.g., diffusible chemical gradients, pulses of stimulants, micro- and nano-scale topography, and compartmentalized microdomains). The evolution of these methods for controlling neuron subregions locally provides insights on the developmental, functional, and regenerative properties of neurons [4].
The Micro-Island Culture
In 1976, Furshpan and colleges devised a micro-culture method (cultures confined to smaller spatial dimensions) to better understand the cell-to-cell interactions of sympathetic neurons with cardiac myocytes in co-culture [44]. In comparison to the methods addressed above, micro-island cultures consist of small islands (300-500 µm diameter) of cell-adhesion permissive substrates situated against a background substrate that precludes cell attachment and growth. On these micro-island substrates, cells can be cultured in physical isolation through confinement of a few (≤ 5) cells in co-cultures that embody cells of the same or different tissue origins. On a macro-scale, the Petri dish can have numerous micro-islands and support cell populations housed within the same dish to provide beneficial trophic support and reduce media evaporation. By restricting cell contact between solitary rat sympathetic neurons to a micro-island of cultured cardiac myocytes, the first access to neuromuscular connections was achieved. In contrast, co-cultures in the Petri dish without substrate patterns or guidance cues developed neuron-myocyte connections at random, so they were challenging to find and study.
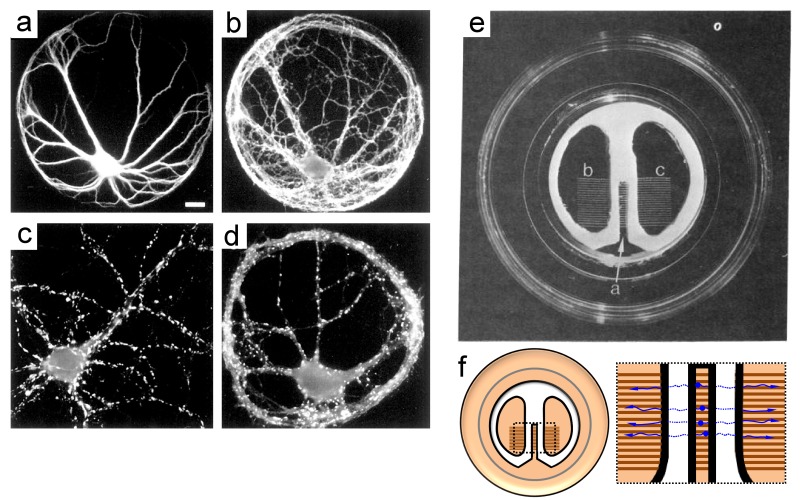
The logical and practical advantages of micro-island cultures are evident when comparing the ability to locate and identify the high-density neuromuscular connections with the conventional approach in the Petri dish. With this self-innervating (autaptic) micro-island preparation (Figure 5a-d), significant changes in neurite arborizations and membrane properties are evident compared with neurons in mass cultures [45,46].
Figure 5.
Micro-islands and the Campenot chamber. (a-d) Effects of isolation on neuronal development using micro-island cultures. Modified from [50], reproduced with permission from The Journal of Neuroscience. a-b) A pyramidal cell in a micro-island and stained with antibodies to MAP2 (a) and de-phospho-tau (b), labeling dendrites and axons, respectively. The axon grows profusely over the soma and dendrites, resulting in many contact sites where synapses could form. c) Pyramidal cell in contiguous culture labeled with an antibody to synaptophysin to show the density of synaptic vesicle clusters. d) Pyramidal island labeled with an antibody to synaptophysin showing a large number of puncta, presumptive autaptic sites. Scale bar, 10 µm [50]. e) The Campenot chamber was introduced as a Teflon divider that compartmentalizes the neuronal soma from the growing processes. In this setup, the Teflon divider is attached to a glass-bottomed, collagen-coated dish with silicon grease. Parallel scratches in the substrate guide the elongating neurites into adjacent chambers for investigating how the selective exposure of neurotrophic compounds influences local neuron development [63]. Reproduced with permission from Robert B. Campenot. f) Schematic of a Campenot chamber (L) and enlargement (R) of the inset (L) depicting how neurons (blue) grow and become compartmentalized within the device.
Micro-island cultures have revealed numerous molecular and cellular mechanisms of neuron development and synaptic transmission [47-49], mechanisms that govern synapse formation and mismatch [50], and synaptic modulation [51-53], depression [54], and potentiation [55]. Further, insights have been discovered on the cellular basis of epilepsy [56-58], neurotransmitter production and release [59,60], and activity-dependent autapse maturation [61].
Due to the broad impact of the micro-island culture approach in elucidating substrates of synaptic activity, contemporary detailed protocols are available that enable the researcher to implement this simple, yet elegant approach [62]. Within a year of the first micro-island culture publication, the Campenot chamber emerged for subcellular fluidic control in vitro.
The Campenot Chamber
Whereas the hanging drop afforded a limited-volume culture environment and the micro-island confined cell development on micro-substrates, the Campenot chamber, introduced in 1977, offered the ability to compartmentalize sympathetic neurons of the rat superior cervical ganglia for selective chemical manipulation of neurotrophic factors locally, at neurites of the developing neurons (Figure 5e-f) [63]. Prior to the development of the Campenot chamber, investigations into the responses of cultured neurons to differential chemical environments were achieved through explant co-cultures [64-66]. Robert Campenot’s three-chamber design enabled subcellular control of manipulations, across ranges from hundreds of microns to millimeters, and led to the discovery that nerve growth factor (NGF) enhances local neurite growth. This simple and effective technique has been improved and utilized beyond the initial study [67-69], facilitating local fluidic control, spatial access to distinct regions of the compartmentalized neuron, and microelectrode-based assessments of local signal transduction events [70-77]. Modifications to the device have included glass cloning cylinders sealed onto a scratched collagen substrate [77], microfluidic devices [4,74,75], and even aluminum foil [78]. In these two-chambered variants of the initial design, neurons are introduced into one compartment and axons grow into and become compartmentalized within the second. This design extended study of cultured neurons beyond the spinal ganglionic neurons to central and peripheral neurons of any type. The two-compartment chamber has the advantages: 1) as a simplified, user-friendly version of the original three-compartment device [77]; 2) in permitting alternative fabrication methods that refined the micro-environment for axonal isolation [78]; and 3) for enabling recording of electrical signals from fluidically isolated axons via embedded micro-electrode arrays [74].
While the practice of compartmentalizing neurons remains advantageous, further spatiotemporal control has been integrated and advanced through microfluidic devices. By merging microtechnologies of the physical sciences and engineering with Campenot’s original concept, designs are rapidly evolving to produce complex, integrated, and highly controllable devices for investigating cell and tissue development.
Microelectrode Arrays
Microelectrode arrays (also termed multi-electrode arrays, MEA) have been utilized in cellular neuroscience and engineering for over nearly 30 years. MEAs have been developed as an on-chip approach to probe and record neuronal communication [79]. These devices consist of multiple microfabricated electrodes, usually with only conductive nodes exposed, often arranged in patterns designed by the user. MEAs range in size from ~50 to >80,000 electrodes on planar surfaces. This flexibility of design offers a range of electrophysiological capabilities, from sub-cellular resolution of single cells to field potentials [80-83]. Primary neurons seeded at a range of densities, stem cell-derived neuronal networks, and slices of brain tissue have been integrated with MEAs [80,84,85]. The drive to understand the emergence of neuronal networks, the organization within them, and information flow and integration in signaling between neurons and neuron clusters is being advanced through present-day applications of MEA with devices of a range of designs.
Microfluidic Devices
While techniques of molecular biology enable dynamic molecular imaging and manipulations for dissecting intracellular signaling mechanisms, the goal of achieving high-resolution spatiotemporal control of the extracellular environment is presently advancing through applications of microfluidic devices. Development of new microfluidic devices permits the control of fluidic microdomains surrounding discrete neurites of an individual neuron and portions of neuronal microcircuits [86]. New ways of assessing the microenvironment and subregions are critical for neuroscience, because the neuron extends its fine processes into spatially dispersed regions distant (millimeters in vitro) from the cell body.
Photolithography and replicate molding enable the fabrication of inexpensive, disposable microfluidic devices made of moldable gels and polymers. Polydimethylsiloxane (PDMS) is the most widely used material in the fabrication of microfluidic devices, yet other materials can be used, including poly(silazane) and gelatin [87,88]. Microfluidic devices further refine the ability to culture neurons by offering a number of advantages (Table 1). They retain many of the attributes of the aforementioned culture devices, namely fluid exchange (used in flasks, dishes, and chambers), density control and cellular confinement (used in micro-islands), and compatibility with optical imaging (dishes, chambers, and the hanging drop). After generating a “master” mold, microfluidic devices can be easily and reproducibly manufactured with inexpensive reagents in a hood or on a bench top in almost any laboratory setting. Microfluidic devices can be reversibly, or irreversibly, sealed to glass, polystyrene, silicon wafers, or other microfabricated microdevices to render a 3-D microenvironment (fluid-filled domain with growth surfaces in x, y, and z planes) capable of chemotemporal control. PDMS microfluidic devices have intrinsic properties amenable for cell culture [89], including optical transparency with high gas permeation [90,91], low water permeability, practical scalability, thermal stability, and biocompatibility [92].
By understanding these advantages, predetermined designs for the microfluidic environment can be developed to address specific applications and research questions. Analogous to the hanging drop technique of the bacteriologist, microfluidic devices enable a direct approach to study neurons in highly controllable ways, from subcellular regions of an individual cell to neuron pairs and networks of neurons [93]. Unlike the Carrel flask or Petri dish, these fluidically controlled microdevices enable sub-cellular environments and manipulations beyond traditional cultures; moreover, they offer defined microdomains that can be used to mimic, at a fundamental level, aspects of brain microenvironments, such as focal dendritic stimulation [93], glial mylenation of axons [94], and the development of cortico-striatal synaptic connections [95]. In this way, spatiotemporal fluidic control can be imposed on neurons in nanoliter cultures with 3-D topography for precise manipulations.
The tremendous flexibility of design control enables the neuroscientist to address new questions with tailored culture methods offering exquisite precision. Here, we briefly summarize progress on the fundamental uses of microfluidic devices in neurobiology. Controlled substrates are addressed first, as they are products of microfluidic device applications for neurobiology. This is followed by 3D microdomain cultures and co-cultures in microchannels, and biocompatibility. Finally, we address progress on chemotemporal fluidic control using microfluidic devices. Due to the multifunctional nature of microfluidic devices, these topics are not mutually exclusive; rather, they are individual applications of a device that provides a range of possibilities.
Controlled substrates
Dissociated neurons in culture polarize and undergo a characteristic developmental sequence, as in vivo. Initial neurites undergo specification wherein one primary neurite becomes an axon and the remaining neurites develop into dendrites [96]. Axon guidance during development is regulated in part by external signals, both long-range diffusive cues and short-range contact cues. Both attractive and repulsive cues fine-tune axonal pathfinding and cellular associations.
Defining and regulating extrinsic cues can be accomplished by improved environmental control. The ability to control the pattern and chemical composition of substrates has been used for a broad spectrum of biological applications to control surface contacts and signals. While microfluidics can be used for patterning substrates to guide neuronal populations [97], other non-microfluidic-based methods exist [98-102] and are used alternatively due to the different fabrication advantages they provide. Here we consider the emerging area of studying neurons in micro-engineered environments with nano-scale volumes and complex substrate patterns.
Patterning techniques produce defined geometrical substrate in monolayers of adhesive, attractive, and/or repulsive compounds. Substrate molecules can be laid down singly, adjacently, or combined with or within other substrates and are compatible for culturing dissociated neurons [3,102-111]. To investigate how extrinsic signals influence axo-dendritic polarity, Banker and colleagues presented embryonic hippocampal neurons with adjacent stripes of molecules known to influence cell adhesion to determine whether these ligands could preferentially induce one of the several primary neurites to become an axon [112,113]. Through further extensions of this approach, intersecting lines of different recombinant or purified cell-adhesion molecules further refined the analysis of neurite navigation in vitro [104,114,115]. These patterned substrates demonstrated that various immobilized substrates predictably guide neuron polarization, axon formation, and growth cone navigation.
In addition to adhesion molecules, neuronal differentiation is influenced by trophic factors, diffusible extrinsic molecules that provide critical signals during nervous system development. Trophic factors influence the outgrowth and trajectory axons and dendrites and confer neuroprotection from environmental insults. Due to their beneficial role in development, trophic factors have been used as substrates to expose neurons to activated surfaces and gradients for regulating development [116].
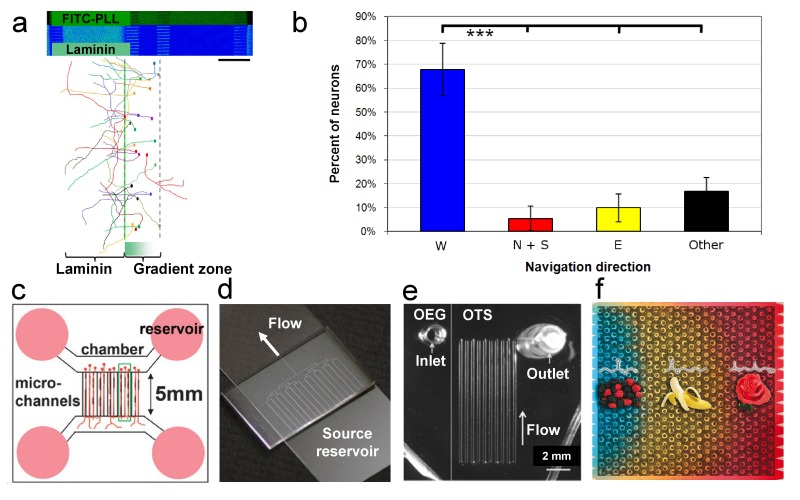
Establishing neuronal circuits in vivo relies upon cues present during the developmental period that may not be sustained. The ability to understand how developmentally instructive gradients encourage neurite extension is facilitated by flow- and diffusion-controlled devices [117]. Fluidically stable and tunable biomolecular gradients have been well characterized, and numerous means exist for producing the desired results [118]. However, some require special equipment, are cumbersome, and are not compatible with neuronal cultures due to shear stresses and/or device designs. Through microfluidic devices, laminar flow can be used to generate defined diffusive or substrate-bound gradients to investigate the context-dependent response of a neuron and its sub-cellular extensions to the local environment (Figure 6a-b) [97].
Figure 6.
Substrate patterns and microfluidic devices for neuronal cultures. a) Laminin substrate gradients guide axonal navigation on glass (step 1, blue field of image) followed by a rinse with FITC-PLL (step 2, fluorescent green field of image). The microfluidic channels for patterning were removed and discarded. Neurons were cultured on the surface cues to determine the direction of axonal growth, as shown by individual neuron traces. b) Graphical summary of axonal navigation direction (W, N/S, E, Other) shows a statistically significant preference for laminin (mean ± standard deviation, p ≤ 0.0001, unpaired t-test). (a-b) Modified from [97], reproduced with permission from The Royal Society of Chemistry (RSC). c) Neurons (pink in central connecting microchannels) can be cultured in compartmentalized microfluidic channels to segregate subregions of neurons in different microdomains. The dual-chamber microfluidic device is the most widely used for neuronal analysis [131-132,137]. Modified from [137] and reproduced with permission from RSC. d) Converging parallel channels enable the optimization of culture conditions and investigation of neuronal cultures at low-densities in PDMS. The single-outlet channel can be cut to customized channel lengths to control media-flow velocity to sustain perfusion for days. Modified from [92] and reproduced with permission from RSC. e) Microfluidic devices can be used to collect neuropeptides for post-hoc detection via mass spectrometry. The microfluidic channel is positioned across glass surfaces that have been functionalized to prevent peptide adherence (OEG-functionalization), then collect (OTS-functionalization) neuropeptides from Aplysia neurons. Peptides are retained on the OTS-coated glass of the serpentine microfluidic channel, then subjected to analysis. Modified from [129] and reproduced with permission from RSC. f) Multi-well microfluidic devices enable the simultaneous screening of over 20,000 single olfactory sensory neuron cells. A portion of the microfluidic well array (273 wells) is shown that contains individual cells retained within the array. A pictographic representation of a few odorants used to test sensitivity of the neurons, using Ca2+ imaging, are shown superimposed on the cell array. As a result, individual cells can be categorized into populations based on odorant responsiveness. Modified from [138] and reproduced with permission from RSC.
3-D microenvironments
Planar two-dimensional substrate surfaces provide the means for culturing dispersed neurons on a uniform substrate, which offers valuable insights into nerve cell growth behavior and function in an easily viewable plane. However, the brain is a 3-D structure. Microfluidic techniques enable the fabrication of defined topographical structures and facilitate chemotemporal manipulation (temporal control of media and reagents in a given fluidic space) that better approximates the microenvironmental dynamics of the brain. In the context of controlling substrate-bound cues and topography, there is a need to understand how structural changes of select regions of the neuron occur in response to precise input from the ambient biological, chemical, and physical environment. With the combination of substrate cues and material devices, Gomez and colleagues investigated neuronal responses to competitive chemical stimuli and contact cues. As a result, they found that topographical cues are stronger inducers of neuronal polarity than immobilized chemical cues [119,120].
Co-cultures
Co-culturing cells of different types within microfluidic devices has the power to interrogate cell-to-cell contacts and interactions while achieving differential fluidic exposure, sample manipulation, and collection. These co-cultures can be of different types of neurons or of neurons and glial cells. Cells with distinct functionalities and from different brain regions can be maintained in adjacent culture domains while being permitted to form cell-to-cell connections. Beyond enhancing neuronal survival [94], co-culturing glia with neurons improves the transfection efficiency of co-cultured neurons. Interconnecting large populations of neurons from tissue preparations of different brain regions can be achieved easily through microfluidic devices, and multiple samples can be set up on a single microfluidic device to produce multiple replicates through one process [121]. Further, isolated cells and organotypic slices from different brain regions cross-innervate via micro-channels fabricated within the device. This architecture enables functional studies, while manipulating the chemical composition of local compartments [122]. Tissue-level studies can be accomplished through the ability to build and manipulate neural pathways. These approaches can advance understanding brain tissue development, plasticity, pathology of epileptiform disorders, and circadian clock entrainment, to name a few.
Extrinsic signals are altered during disease states. Whether they are contributors to, or products of, the disease is a matter of debate. For example, β-amyloid is proposed to affect axonal retrograde transport and the downstream propagation of TrkB signaling [123]. To test this hypothesis, embryonic cortical neurons from wild-type mice and an Alzheimer’s disease (AD) transgenic model (Tg2576, over-expressing amyloid precursor protein) were co-cultured in compartmentalized microfluidic devices. Cleavage products of amyloid precursor protein impair TrkB processing and retrograde transport of brain-derived neurotrophic factor in diseased neurons. ϒ-secretase inhibitors reverse a retrograde signaling deficit in AD mouse neurons. This elegant use of microfluidic devices reveals that β-amyloid attenuates signaling via brain-derived neurotrophic factor by impairing axonal transport. This suggests a possible explanation of synaptic dysfunction in AD. AD-like pathological states can be generated in only a portion of neuronal networks within microfluidic devices [124]. Experiments manipulating co-cultures with microfluidic devices to study interactions of neurons from different sources are just appearing; this approach has tremendous potential.
Biocompatibility
Neurons are highly sensitive to the surrounding environment. Establishing cell cultures of dispersed neurons and glia can require reagents of high purity and refined protocols [125]. Building microfluidic devices with biocompatible materials improves culture reliability and the likelihood of success for integrating neurons within the device. Gas permeability is a material property of PDMS, enabling development of 3-D closed systems [90,91]. Notwithstanding the demonstrated biocompatibility of PDMS in numerous microfluidic culture applications, we recently demonstrated that by introducing a solvent-extraction step during fabrication that PDMS biocompatibility can be enhanced for scaling down channel systems to sustain low densities of primary postnatal hippocampal neurons within nanoliter-scale microchannels [92]. Therefore, using solvent-extracted PDMS with channels of high surface-area-to-volume ratios improves culture viability for cultivating neurons in these engineered microdomains.
Chemotemporal control
Extrinsic signals establish and enrich neuron-neuron interactions during development. Micro-neural circuits may become altered by damage or disease over the lifespan and, thus, underlie mental health and neurological dysfunctions. Understanding the signals that shaped nervous system wiring during development may enable us to identify and implement key effectors of repair and restoration of function. Media and substrates can be selectively directed through fabricated microchannels by laminar flow for controlling the chemical environment and treatment application (Figure 6c-d) [93,112,126,127]. Media processing and peptide extraction for mass spectrometry can be applied directly, with the knowledge that the signals originated from a defined neuron population (Figure 6e) [128,129]. With these advantages, an increasing number of reports are utilizing microfluidic devices as tools for direct physiological or biochemical applications in neurobiology (Figure 6f) [130-133].
Precise temporal delivery of stimulatory or inhibitory molecules to neurons in culture could be greatly improved through integrating microfluidic channels into defined, fluidic networks, but this has yet to be tested extensively. Recent reports demonstrate the ability to perform focused chemotemporal manipulations of neuronal subregions in microfluidics [93]. Furthermore, competitive signals or confounding variables of surrounding tissues can be removed to characterize secreted signaling molecules in finite volumes for neurochemical signaling studies [128]. Neurochemicals released from living neurons can be preserved and manipulated by harnessing the spatiotemporal fluidic control enabled by this technology.
Future Directions
No single device or method can yet fully re-create the natural, unaltered environment of the brain for studying neurons under the range of contexts and dynamics in which they function. However, the knowledge that has been acquired from reduced neuronal preparations is invaluable. From Ross Harrison’s first culture in the hanging drop to the present, advancements in culture methodologies have emerged and advanced neuroscience. As new techniques are developed, they should be embraced for their ability to address unanswered questions, strategic research priorities, and provide insights into the contextual cellular development and operation of the brain. Examples include 1) novel drug discovery for treatment of nervous system disorders; 2) resolving mechanisms governing dendrite development and guidance; 3) finding and treating brain reservoirs of HIV (human immunodeficiency virus); and 4) discovering elements of the synaptome (i.e., genes, proteins, biochemicals, etc.).
Methodological approaches are established for culturing brain tissues and controlling neurons in vitro, including the hanging drop for stem cell, cell signaling, and embryological studies [134-136] and the roller tube for organotypic cultures [33,35]. The degree to which these techniques will be used in the future will depend on many factors, including ease of use, affordability, commercial availability, and the range of scientific productivity afforded by each. Flasks, dishes, micro-chambers, and Campenot chambers are readily available, and micro-islands can be easily generated. Considering the newness of applications of microfluidic devices in neurobiology, their full potential has yet to be realized. In part, this will depend on the availability of, or access to, facilities for the fabrication of master devices to mold the microfluidic channels. It will also depend on the how well investigators can become adept at exploiting the potential of the devices and how microfluidics can be effectively incorporated with standard biological methods and toolsets. Notwithstanding these considerations, microfluidic devices are empowering chemotemporal control at the sub-cellular level and offer the opportunity to create regulated environments in three dimensions. Applications and insights in the second century of neuron culture are likely only limited by our imaginations.
Acknowledgments
The development of this paper was supported by the National Institutes of Health: National Institute of Child Health and Human Development Developmental Psychobiology and Neurobiology Training Grant HD007333 (LJM); National Institute of Mental Health R21MH 085220, National Heart, Lung, and Blood Institute R01HL092571Z ARRA and RO1HL086870 (MUG); and the National Science Foundation Integrative Organismal Systems 08-18555 and Science & Technology Center: Emergent Behaviors of Integrated Cellular Systems CBET 0939511 (MUG).
Abbreviations
- PDMS
polydimethylsiloxane
- NGF
nerve growth factor
- MEA
microelectrode arrays
- AD
Alzheimer’s disease
- HIV
human immunodeficiency virus
- RSC
Royal Society of Chemistry
References
- Pilati N, Barker M, Panteleimonitis S, Donga R, Hamann M. A rapid method combining Golgi and Nissl staining to study neuronal morphology and cytoarchitecture. J Histochem Cytochem. 2008;56(6):539–550. doi: 10.1369/jhc.2008.950246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankle WR, Romney AK, Landing BH, Ara H. Developmental patterns in the cytoarchitecture of the human cerebral cortex from birth to 6 years examined by correspondence analysis. Proc Natl Acad Sci USA. 1998;95(7):4023–4028. doi: 10.1073/pnas.95.7.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanova EV, Fosser KA, Rubakhin SS, Nuzzo RG, Sweedler JV. Engineering the morphology and electrophysiological parameters of cultured neurons by microfluidic surface patterning. Faseb J. 2004;18(11):1267–1269. doi: 10.1096/fj.03-1368fje. [DOI] [PubMed] [Google Scholar]
- Millet LJ, Gillette MU. New perspectives on neuronal development via microfluidic environments. Trends Neurosci. 2012 doi: 10.1016/j.tins.2012.09.001. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RG. The outgrowth of the nerve fiber as a mode of protoplasmic movement. J Exp Zool. 1910;9(4):787–846. doi: 10.1002/jez.1401420103. [DOI] [PubMed] [Google Scholar]
- Harrison RG. Observations on the living developing nerve fibre. Proc Soc Exp Biol. 1907;4:140. [Google Scholar]
- Harrison RG. The life of tissues outside the organism from the embryological standpoint. Trans Congr Am Phys Surg. 1913;9:63–75. [Google Scholar]
- Landecker H. New times for biology: Nerve cultures and the advent of cellular life in vitro. Stud Hist Philos Biol Biomed Sci C. 2002;33(4):667–694. [Google Scholar]
- Iftimovici R. Constantin Levaditi, a forerunner of the modern research and interpretation of virus multiplication. Virologie. 1979;30(3):237–242. [PubMed] [Google Scholar]
- Kalantzis G, Skiadas P, Lascaratos J. Constantin Levaditi (1874-1953): a pioneer in Immunology and Virology. J Med Biogr. 2006;14(3):178–182. doi: 10.1258/j.jmb.2006.05-30. [DOI] [PubMed] [Google Scholar]
- Levaditi C. Virus de la poliomyelite et culture des cellules in vitro. Soc Biol Compt Rend. 1913;75:202–205. [Google Scholar]
- Levaditi C. Symbiose entre le virus de la poliomyelite et les cullules des ganglions spinaux, a’ l'etat de vie prolongee in vitro. Soc Biol Compt Rend. 1913;74:1179. [Google Scholar]
- Carrel A, Burrows M. Cultivation of adult tissues and organs outside of the body. J Am Med Assoc. 1910;55:1379–1381. [Google Scholar]
- Lewis MR, Lewis WH. The growth of embryonic chick tissues in artificial media, agar and bouillon. Johns Hopkins Hosp Bull. 1911;22:126. [Google Scholar]
- Chen Y, Stevens B, Chang J, Milbrandt J, Barres BA, Hell JW. NS21: re-defined and modified supplement B27 for neuronal cultures. J Neurosci Methods. 2008;171(2):239–247. doi: 10.1016/j.jneumeth.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S, Zhang S, Chiu J, Wirth EK, Schweizer U. Development of a serum-free supplement for primary neuron culture reveals the interplay of selenium and vitamin E in neuronal survival. J Trace Elem Med Biol. 2010;24(2):130–137. doi: 10.1016/j.jtemb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- van der Valk J, Brunner D, De Smet K, Fex Svenningsen A, Honegger P, Knudsen LE. et al. Optimization of chemically defined cell culture media--replacing fetal bovine serum in mammalian in vitro methods. Toxicol In Vitro. 2010;24(4):1053–1063. doi: 10.1016/j.tiv.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Burrows MT. Rhythmical activity of isolated heart muscle cells in vitro. Science. 1912;36(916):90–92. doi: 10.1126/science.36.916.90. [DOI] [PubMed] [Google Scholar]
- Ebeling AH. The permanent life of connective tissue outside of the organism. J Exp Med. 1913;17:273. doi: 10.1084/jem.17.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MR, Lewis WH. Mitochondria in tissue culture. Science. 1914;39:330–333. doi: 10.1126/science.39.1000.330. [DOI] [PubMed] [Google Scholar]
- Carrel A. A method for the physiological study of tissues in vitro. J Exp Med. 1923;38:407–418. doi: 10.1084/jem.38.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmer EN. Cells and tissues in culture: methods, biology, and physiology. London, New York: Academic Press; 1965. [Google Scholar]
- Earle WR, Evans VJ, Edwards MF, Duchesne EJ. Influence of perforation design on growth of tissue cells under perforated cellophane sheets in vitro. J Natl Cancer Inst. 1949;10(2):291–295. [PubMed] [Google Scholar]
- Earle WR, Evans VJ, Schilling EL. Use of perforated cellophane substrate in slide preparation tissue cultures. J Natl Cancer Inst. 1950;10(4):883–891. [PubMed] [Google Scholar]
- Earle WR, Evans VJ, Sandford KK, Shannon JE Jr., Waltz HK. Influence of glass and cellophane substrates on proliferation of strain L cells in tissue culture. J Natl Cancer Inst. 1951;12(3):563–567. [PubMed] [Google Scholar]
- Evans VJ, Earle WR. The use of perforated cellophane for the growth of cells in tissue culture. J Natl Cancer Inst. 1947;8(3):103–119. [PubMed] [Google Scholar]
- Loeb L, Fleisher MS. The growth of tissues in the test tube under experimentally varied conditions with special reference to mitotic cell proliferation. J Exp Med. 1919;40(3):509–550. [PMC free article] [PubMed] [Google Scholar]
- Grey GO. An improved technique for massive tissue culture. Amer J Cancer. 1933;17:752. [Google Scholar]
- Hogue MJ. Human fetal ependymal cells in tissue cultures. Anat Rec. 1947;99(4):523–529. [PubMed] [Google Scholar]
- Leighton J. A sponge matrix method for tissue culture; formation of organized aggregates of cells in vitro. J Natl Cancer Inst. 1951;12(3):545–561. [PubMed] [Google Scholar]
- Costero I, Pomerat CM. Cultivation of neurons from the adult human cerebral and cerebellar cortex. Am J Anat. 1951;89(3):405–467. doi: 10.1002/aja.1000890304. [DOI] [PubMed] [Google Scholar]
- De Boni U, Chong AA, Hawthorn LA. Organotypic development of neonate rabbit hippocampus in roller tube culture. Acta Neuropathol. 1984;65(1):53–61. doi: 10.1007/BF00689828. [DOI] [PubMed] [Google Scholar]
- Andres RH, Ducray AD, Perez-Bouza A, Schlattner U, Huber AW, Krebs SH. et al. Creatine supplementation improves dopaminergic cell survival and protects against MPP+ toxicity in an organotypic tissue culture system. Cell Transplant. 2005;14(8):537–550. doi: 10.3727/000000005783982756. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH, Thompson SM, Muller D. Preparation and maintenance of organotypic slice cultures of CNS tissue. Curr Protoc Neurosci. 2001 doi: 10.1002/0471142301.ns0611s09. Chapter 6: Unit 6.11. [DOI] [PubMed] [Google Scholar]
- Josephson EM, Yilma S, Vodyanoy V, Morrison EE. Structure and function of long-lived olfactory organotypic cultures from postnatal mice. J Neurosci Res. 2004;75(5):642–653. doi: 10.1002/jnr.20007. [DOI] [PubMed] [Google Scholar]
- Morales P, Huaiquin P, Bustamante D, Fiedler J, Herrera-Marschitz M. Perinatal asphyxia induces neurogenesis in hippocampus: an organotypic culture study. Neurotox Res. 2007;12(1):81–84. doi: 10.1007/BF03033903. [DOI] [PubMed] [Google Scholar]
- Studer L. Culture of substantia nigra neurons. Curr Protoc Neurosci. 2001 doi: 10.1002/0471142301.ns0303s00. Chapter 3: Unit 3.3. [DOI] [PubMed] [Google Scholar]
- Yamamoto C. Activation of hippocampal neurons by mossy fiber stimulation in thin brain sections in vitro. Exp Brain Res. 1972;14(4):423–435. doi: 10.1007/BF00235037. [DOI] [PubMed] [Google Scholar]
- Gillette MU. In: Suprachiasmatic Nucleus: The Mind’s Clock. Klein DC, Moore RY, Reppert SM, editors. New York, NY: Oxford University Press; 1991. SCN electrophysiology in vitro: Rhythmic activity and endogenous clock properties; pp. 125–143. [Google Scholar]
- Gähwiler BH, Capogna M, Debanne D, McKinney RA, Thompson SM. Organotypic slice cultures: a technique has come of age. Trends Neurosci. 1997;20(10):471–477. doi: 10.1016/s0166-2236(97)01122-3. [DOI] [PubMed] [Google Scholar]
- Wang Z, Millet L, Mir M, Ding H, Unarunotai S, Rogers J. et al. Spatial light interference microscopy (SLIM) Opt Express. 2011;19(2):1016–1026. doi: 10.1364/OE.19.001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TA, Yu YV, Govindaiah G, Ye X, Artinian L, Coleman TP. et al. Circadian rhythm of redox state regulates excitability in suprachiasmatic nucleus neurons. Science. 2012;337(6096):839–842. doi: 10.1126/science.1222826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose G. A separable and multipurpose tissue culture chamber. Tex Rep Biol Med. 1954;12(4):1074–1083. [PubMed] [Google Scholar]
- Furshpan EJ, MacLeish PR, O’Lague PH, Potter DD. Chemical transmission between rat sympathetic neurons and cardiac myocytes developing in microcultures: evidence for cholinergic, adrenergic, and dual-function neurons. Proc Natl Acad Sci USA. 1976;73(11):4225–4229. doi: 10.1073/pnas.73.11.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennerick S, Que J, Benz A, Zorumski CF. Passive and synaptic properties of hippocampal neurons grown in microcultures and in mass cultures. J Neurophysiol. 1995;73(1):320–332. doi: 10.1152/jn.1995.73.1.320. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Zorumski CF. Paired-pulse modulation of fast excitatory synaptic currents in microcultures of rat hippocampal neurons. J Physiol. 1995;488(Pt 1):85–101. doi: 10.1113/jphysiol.1995.sp020948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque MJ, Trudeau LE. GDNF enhances the synaptic efficacy of dopaminergic neurons in culture. Eur J Neurosci. 2000;12(9):3172–3180. doi: 10.1046/j.1460-9568.2000.00219.x. [DOI] [PubMed] [Google Scholar]
- Schluter OM, Basu J, Sudhof TC, Rosenmund C. Rab3 superprimes synaptic vesicles for release: implications for short-term synaptic plasticity. J Neurosci. 2006;26(4):1239–1246. doi: 10.1523/JNEUROSCI.3553-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarsa L, Goda Y. Synaptophysin regulates activity-dependent synapse formation in cultured hippocampal neurons. Proc Natl Acad Sci USA. 2002;99(2):1012–1016. doi: 10.1073/pnas.022575999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Cha EM, Craig AM. Mismatched appositions of presynaptic and postsynaptic components in isolated hippocampal neurons. J Neurosci. 2000;20(22):8344–8353. doi: 10.1523/JNEUROSCI.20-22-08344.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm S. Presynaptic alpha2-adrenoceptors control excitatory, but not inhibitory, transmission at rat hippocampal synapses. J Physiol. 1999;519(Pt 2):439–449. doi: 10.1111/j.1469-7793.1999.0439m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm S, Betz H. Somatostatin inhibits excitatory transmission at rat hippocampal synapses via presynaptic receptors. J Neurosci. 1997;17(11):4066–4075. doi: 10.1523/JNEUROSCI.17-11-04066.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Tong G, Jahr CE. β-Adrenergic regulation of synaptic NMDA receptors by cAMP-dependent protein kinase. Neuron. 1996;16(2):415–421. doi: 10.1016/s0896-6273(00)80059-8. [DOI] [PubMed] [Google Scholar]
- Goda Y, Stevens CF. Readily releasable pool size changes associated with long term depression. Proc Natl Acad Sci USA. 1998;95(3):1283–1288. doi: 10.1073/pnas.95.3.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong G, Malenka RC, Nicoll RA. Long-term potentiation in cultures of single hippocampal granule cells: a presynaptic form of plasticity. Neuron. 1996;16(6):1147–1157. doi: 10.1016/s0896-6273(00)80141-5. [DOI] [PubMed] [Google Scholar]
- Segal MM. Epileptiform activity in microcultures containing one excitatory hippocampal neuron. J Neurophysiol. 1991;65(4):761–770. doi: 10.1152/jn.1991.65.4.761. [DOI] [PubMed] [Google Scholar]
- Segal MM, Furshpan EJ. Epileptiform activity in microcultures containing small numbers of hippocampal neurons. J Neurophysiol. 1990;64(5):1390–1399. doi: 10.1152/jn.1990.64.5.1390. [DOI] [PubMed] [Google Scholar]
- Segal MM. Endogenous bursts underlie seizurelike activity in solitary excitatory hippocampal neurons in microcultures. J Neurophysiol. 1994;72(4):1874–1884. doi: 10.1152/jn.1994.72.4.1874. [DOI] [PubMed] [Google Scholar]
- Johnson MD. Synaptic glutamate release by postnatal rat serotonergic neurons in microculture. Neuron. 1994;12(2):433–442. doi: 10.1016/0896-6273(94)90283-6. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Joyce MP, Lin L, Geldwert D, Haber SN, Hattori T. et al. Dopamine neurons make glutamatergic synapses in vitro. J Neurosci. 1998;18(12):4588–4602. doi: 10.1523/JNEUROSCI.18-12-04588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomperts SN, Carroll R, Malenka RC, Nicoll RA. Distinct roles for ionotropic and metabotropic glutamate receptors in the maturation of excitatory synapses. J Neurosci. 2000;20(6):2229–2237. doi: 10.1523/JNEUROSCI.20-06-02229.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TGJ. Preparation and maintenance of single-cell micro-island cultures of basal forebrain neurons. Nature Protocols. 2006;1(6):2543–2550. doi: 10.1038/nprot.2006.394. [DOI] [PubMed] [Google Scholar]
- Campenot RB. Local control of neurite development by nerve growth factor. Proc Natl Acad Sci USA. 1977;74(10):4516–4519. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamley JH, Campbell GR, Burnstock G. An analysis of the interactions between sympathetic nerve fibers and smooth muscle cells in tissue cultur. Dev Biol. 1973;33(2):344–361. doi: 10.1016/0012-1606(73)90142-5. [DOI] [PubMed] [Google Scholar]
- Chamley JH, Dowel JJ. Specificity of nerve fibre “attraction” to autonomic effector organs in tissue culture. Exp Cell Res. 1975;90(1):1–7. doi: 10.1016/0014-4827(75)90349-3. [DOI] [PubMed] [Google Scholar]
- Ebendal T, Jacobson CO. Tests of possible role of NGF in neurite outgrowth stimulation exerted by glial cells and heart explants in culture. Brain Res. 1977;131(2):373–378. doi: 10.1016/0006-8993(77)90531-5. [DOI] [PubMed] [Google Scholar]
- Campenot RB. Independent control of the local environment of somas and neurites. Methods Enzymol. 1979;58:302–307. doi: 10.1016/s0076-6879(79)58146-4. [DOI] [PubMed] [Google Scholar]
- Campenot RB. Development of sympathetic neurons in compartmentalized cultures. l Local control of neurite growth by nerve growth factor. Dev Biol. 1982;93(1):1–12. doi: 10.1016/0012-1606(82)90232-9. [DOI] [PubMed] [Google Scholar]
- Campenot RB. Development of sympathetic neurons in compartmentalized cultures. II. Local control of neurite survival by nerve growth factor. Dev Biol. 1982;93(1):13–21. doi: 10.1016/0012-1606(82)90233-0. [DOI] [PubMed] [Google Scholar]
- Bertrand J, Winton MJ, Rodriguez-Hernandez N, Campenot RB, McKerracher L. Application of Rho antagonist to neuronal cell bodies promotes neurite growth in compartmented cultures and regeneration of retinal ganglion cell axons in the optic nerve of adult rats. J Neurosci. 2005;25(5):1113–1121. doi: 10.1523/JNEUROSCI.3931-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ch’ng TH, Enquist LW. Neuron-to-cell spread of pseudorabies virus in a compartmented neuronal culture system. J Virol. 2005;79(17):10875–10889. doi: 10.1128/JVI.79.17.10875-10889.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerssen HM, Segal RA. Location, location, location: a spatial view of neurotrophin signal transduction. Trends Neurosci. 2002;25(3):160–165. doi: 10.1016/s0166-2236(02)02144-6. [DOI] [PubMed] [Google Scholar]
- Kimpinski K, Campenot RB, Mearow K. Effects of the neurotrophins nerve growth factor, neurotrophin-3, and brain-derived neurotrophic factor (BDNF) on neurite growth from adult sensory neurons in compartmented cultures. J Neurobiol. 1997;33(4):395–410. doi: 10.1002/(sici)1097-4695(199710)33:4<395::aid-neu5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Ravula SK, McClain MA, Wang MS, Glass JD, Frazier AB. A multielectrode microcompartment culture platform for studying signal transduction in the nervous system. Lab Chip. 2006;6(12):1530–1536. doi: 10.1039/b612684g. [DOI] [PubMed] [Google Scholar]
- Ravula SK, Wang MS, Asress SA, Glass JD, Frazier AB. A compartmented neuronal culture system in microdevice format. J Neurosci Methods. 2007;159(1):78–85. doi: 10.1016/j.jneumeth.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Senger DL, Campenot RB. Rapid retrograde tyrosine phosphorylation of trkA and other proteins in rat sympathetic neurons in compartmented cultures. J Cell Biol. 1997;138(2):411–421. doi: 10.1083/jcb.138.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler RJ, Herman RE. Peripheral infection in culture of rat sensory neurons by herpes simplex virus. Infect Immun. 1980;28(2):620–623. doi: 10.1128/iai.28.2.620-623.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda K, Jimbo K, Suzuki K, Yoda K. Separation of a process from the neuronal cell body using a thin aluminum foil separator. Brain Res Brain Res Protoc. 1998;2(4):352–356. doi: 10.1016/s1385-299x(98)00014-2. [DOI] [PubMed] [Google Scholar]
- Nam Y, Wheeler BC. In vitro microelectrode array technology and neural recordings. Crit Rev Biomed Eng. 2011;39(1):45–61. doi: 10.1615/critrevbiomedeng.v39.i1.40. [DOI] [PubMed] [Google Scholar]
- Massobrio P, Tedesco M, Giachello C, Ghirardi M, Fiumara F, Martinoia S. Helix neuronal ensembles with controlled cell type composition and placement develop functional polysynaptic circuits on Micro-Electrode Arrays. Neurosci Lett. 2009;467(2):121–126. doi: 10.1016/j.neulet.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Frey U, Egert U, Heer F, Hafizovic S, Hierlemann A. Microelectronic system for high-resolution mapping of extracellular electric fields applied to brain slices. Biosens Bioelectron. 2009;24(7):2191–2198. doi: 10.1016/j.bios.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Berdondini L, Imfeld K, Maccione A, Tedesco M, Neukom S, Koudelka-Hep M. et al. Active pixel sensor array for high spatio-temporal resolution electrophysiological recordings from single cell to large scale neuronal networks. Lab Chip. 2009;9(19):2644–2651. doi: 10.1039/b907394a. [DOI] [PubMed] [Google Scholar]
- Johnson LJ, Cohen E, Ilg D, Klein R, Skeath P, Scribner DA. et al. A novel high electrode count spike recording array using an 81,920 pixel transimpedance amplifier-based imaging chip. J Neurosci Meth. 2012;205(2):223–232. doi: 10.1016/j.jneumeth.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Baruchi I, Volman V, Raichman N, Shein M, Ben-Jacob E. The emergence and properties of mutual synchronization in in vitro coupled cortical networks. Eur J Neurosci. 2008;28(9):1825–1835. doi: 10.1111/j.1460-9568.2008.06487.x. [DOI] [PubMed] [Google Scholar]
- Illes S, Theiss S, Hartung H-P, Siebler M, Dihné M. Niche-dependent development of functional neuronal networks from embryonic stem cell-derived neural populations. BMC Neuroscience. 2009;10:93. doi: 10.1186/1471-2202-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CJ, Levchenko A. Microfluidics technology for systems biology research. Methods Mol Biol. 2009;500:203–219. doi: 10.1007/978-1-59745-525-1_7. [DOI] [PubMed] [Google Scholar]
- Asthana A, Asthana Y, Sung I-K, Kim D-P. Novel transparent poly(silazane) derived solvent-resistant, bio-compatible microchannels and substrates: application in microsystem technology. Lab Chip. 2006;6(9):1200–1204. doi: 10.1039/b603763c. [DOI] [PubMed] [Google Scholar]
- Paguirigan A, Beebe DJ. Gelatin based microfluidic devices for cell culture. Lab Chip. 2006;6(3):407–413. doi: 10.1039/b517524k. [DOI] [PubMed] [Google Scholar]
- Kartalov EP, Anderson WF, Scherer A. The analytical approach to polydimethylsiloxane microfluidic technology and its biological applications. J Nanosci Nanotechnol. 2006;6(8):2265–2277. doi: 10.1166/jnn.2006.504. [DOI] [PubMed] [Google Scholar]
- Mehta G, Mehta K, Sud D, Song JW, Bersano-Begey T, Futai N. et al. Quantitative measurement and control of oxygen levels in microfluidic poly(dimethylsiloxane) bioreactors during cell culture. Biomed Microdevices. 2007;9(2):123–134. doi: 10.1007/s10544-006-9005-7. [DOI] [PubMed] [Google Scholar]
- Merkel TC, Bondar VI, Nagai K, Freeman BD, Pinnau I. Gas sorption, diffusion, and permeation in poly(dimethylsiloxane) J Polym Sci Pol Phys. 2000;38(3):415–434. [Google Scholar]
- Millet LJ, Stewart ME, Sweedler JV, Nuzzo RG, Gillette MU. Microfluidic devices for culturing primary mammalian neurons at low densities. Lab Chip. 2007;7(8):987–994. doi: 10.1039/b705266a. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Dieterich DC, Ito HT, Kim SA, Schuman EM. Microfluidic local perfusion chambers for the visualization and manipulation of synapses. Neuron. 2010;66(1):57–68. doi: 10.1016/j.neuron.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar D, Gao Y, Li D, Webb DJ. Co-culture of neurons and glia in a novel microfluidic platform. J Neurosci Methods. 2011;196(1):38–44. doi: 10.1016/j.jneumeth.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrin JM, Deleglise B, Saias L, Vignes M, Gougis P, Magnifico S. et al. Axon diodes for the reconstruction of oriented neuronal networks in microfluidic chambers. Lab Chip. 2011;11(21):3663–3673. doi: 10.1039/c1lc20014c. [DOI] [PubMed] [Google Scholar]
- Craig AM, Banker G. Neuronal polarity. Annu Rev Neurosci. 1994;17:267–310. doi: 10.1146/annurev.ne.17.030194.001411. [DOI] [PubMed] [Google Scholar]
- Millet LJ, Stewart ME, Nuzzo RG, Gillette MU. Guiding neuron development with planar surface gradients of substrate cues deposited using microfluidic devices. Lab Chip. 2010;10(12):1525–1535. doi: 10.1039/c001552k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Philipsborn AC, Lang S, Bernard A, Loeschinger J, David C, Lehnert D. et al. Microcontact printing of axon guidance molecules for generation of graded patterns. Nat Protoc. 2006;1(3):1322–1328. doi: 10.1038/nprot.2006.251. [DOI] [PubMed] [Google Scholar]
- von Philipsborn AC, Lang S, Loeschinger J, Bernard A, David C, Lehnert D. et al. Growth cone navigation in substrate-bound ephrin gradients. Development. 2006;133(13):2487–2495. doi: 10.1242/dev.02412. [DOI] [PubMed] [Google Scholar]
- von Philipsborn AC, Lang S, Jiang Z, Bonhoeffer F, Bastmeyer M. Substrate-bound protein gradients for cell culture fabricated by microfluidic networks and microcontact printing. Sci STKE. 2007;2007(414):16. doi: 10.1126/stke.4142007pl6. [DOI] [PubMed] [Google Scholar]
- Wheeler BC, Corey JM, Brewer GJ, Branch DW. Microcontact printing for precise control of nerve cell growth in culture. J Biomech Eng. 1999;121(1):73–78. doi: 10.1115/1.2798045. [DOI] [PubMed] [Google Scholar]
- Oliva AA Jr., James CD, Kingman CE, Craighead HG, Banker GA. Patterning axonal guidance molecules using a novel strategy for microcontact printing. Neurochem Res. 2003;28(11):1639–1648. doi: 10.1023/a:1026052820129. [DOI] [PubMed] [Google Scholar]
- Chang JC, Brewer GJ, Wheeler BC. A modified microstamping technique enhances polylysine transfer and neuronal cell patterning. Biomaterials. 2003;24(17):2863–2870. doi: 10.1016/s0142-9612(03)00116-9. [DOI] [PubMed] [Google Scholar]
- Corey JM, Feldman EL. Substrate patterning: an emerging technology for the study of neuronal behavior. Exp Neurol. 2003;184(Suppl):S89–S96. doi: 10.1016/s0014-4886(03)00392-3. [DOI] [PubMed] [Google Scholar]
- Corey JM, Wheeler BC, Brewer GJ. Compliance of hippocampal neurons to patterned substrate networks. J Neurosci Res. 1991;30(2):300–307. doi: 10.1002/jnr.490300204. [DOI] [PubMed] [Google Scholar]
- Corey JM, Wheeler BC, Brewer GJ. Micrometer resolution silane-based patterning of hippocampal neurons: critical variables in photoresist and laser ablation processes for substrate fabrication. IEEE Trans Biomed Eng. 1996;43(9):944–955. doi: 10.1109/10.532129. [DOI] [PubMed] [Google Scholar]
- Vogt AK, Lauer L, Knoll W, Offenhausser A. Micropatterned substrates for the growth of functional neuronal networks of defined geometry. Biotechnol Prog. 2003;19(5):1562–1568. doi: 10.1021/bp034016f. [DOI] [PubMed] [Google Scholar]
- Vogt AK, Stefani FD, Best A, Nelles G, Yasuda A, Knoll W. et al. Impact of micropatterned surfaces on neuronal polarity. J Neurosci Methods. 2004;134(2):191–198. doi: 10.1016/j.jneumeth.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Vogt AK, Wrobel G, Meyer W, Knoll W, Offenhausser A. Synaptic plasticity in micropatterned neuronal networks. Biomaterials. 2005;26(15):2549–2557. doi: 10.1016/j.biomaterials.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Vogt AK, Brewer GJ, Offenhausser A. Connectivity patterns in neuronal networks of experimentally defined geometry. Tissue Eng. 2005;11(11-12):1757–1767. doi: 10.1089/ten.2005.11.1757. [DOI] [PubMed] [Google Scholar]
- Weinl C, Drescher U, Lang S, Bonhoeffer F, Loschinger J. On the turning of Xenopus retinal axons induced by ephrin-A5. Development. 2003;130(8):1635–1643. doi: 10.1242/dev.00386. [DOI] [PubMed] [Google Scholar]
- Esch T, Lemmon V, Banker G. Local presentation of substrate molecules directs axon specification by cultured hippocampal neurons. J Neurosci. 1999;19(15):6417–6426. doi: 10.1523/JNEUROSCI.19-15-06417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers GS, James CD, Kingman CE, Craighead HG, Banker GA. Effects of substrate geometry on growth cone behavior and axon branching. J Neurobiol. 2006;66(11):1183–1194. doi: 10.1002/neu.20298. [DOI] [PubMed] [Google Scholar]
- Clark P, Britland S, Connolly P. Growth cone guidance and neuron morphology on micropatterned laminin surfaces. J Cell Sci. 1993;105(Pt 1):203–212. doi: 10.1242/jcs.105.1.203. [DOI] [PubMed] [Google Scholar]
- Shi P, Shen K, Kam LC. Local presentation of L1 and N-cadherin in multicomponent, microscale patterns differentially direct neuron function in vitro. Dev Neurobiol. 2007;67(13):1765–1776. doi: 10.1002/dneu.20553. [DOI] [PubMed] [Google Scholar]
- Gomez N, Schmidt CE. Nerve growth factor-immobilized polypyrrole: bioactive electrically conducting polymer for enhanced neurite extension. J Biomed Mater Res A. 2007;81(1):135–149. doi: 10.1002/jbm.a.31047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dertinger SK, Jiang X, Li Z, Murthy VN, Whitesides GM. Gradients of substrate-bound laminin orient axonal specification of neurons. Proc Natl Acad Sci USA. 2002;99(20):12542–12547. doi: 10.1073/pnas.192457199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan TM, Folch A. Biomolecular gradients in cell culture systems. Lab Chip. 2008;8(1):34–57. doi: 10.1039/b711887b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez N, Chen S, Schmidt CE. Polarization of hippocampal neurons with competitive surface stimuli: contact guidance cues are preferred over chemical ligands. J R Soc Interface. 2007;4(13):223–233. doi: 10.1098/rsif.2006.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez N, Lu Y, Chen S, Schmidt CE. Immobilized nerve growth factor and microtopography have distinct effects on polarization versus axon elongation in hippocampal cells in culture. Biomaterials. 2007;28(2):271–284. doi: 10.1016/j.biomaterials.2006.07.043. [DOI] [PubMed] [Google Scholar]
- Hosmane S, Yang IH, Ruffin A, Thakor N, Venkatesan A. Circular compartmentalized microfluidic platform: Study of axon-glia interactions. Lab Chip. 2010;10(6):741–747. doi: 10.1039/b918640a. [DOI] [PubMed] [Google Scholar]
- Berdichevsky Y, Staley KJ, Yarmush ML. Building and manipulating neural pathways with microfluidics. Lab Chip. 2010;10(8):999–1004. doi: 10.1039/b922365g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon WW, Blurton-Jones M, Tu CH, Feinberg LM, Chabrier MA, Harris JW. et al. beta-Amyloid impairs axonal BDNF retrograde trafficking. Neurobiol Aging. 2011;32(5):821–833. doi: 10.1016/j.neurobiolaging.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze A, Meissner R, Brando S, Renaud P. Co-pathological connected primary neurons in a microfluidic device for alzheimer studies. Biotechnol Bioeng. 2011;108(9):2241–2245. doi: 10.1002/bit.23128. [DOI] [PubMed] [Google Scholar]
- Foo LC, Allen NJ, Bushong EA, Ventura PB, Chung WS, Zhou L. et al. Development of a method for the purification and culture of rodent astrocytes. Neuron. 2011;71(5):799–811. doi: 10.1016/j.neuron.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch T, Lemmon V, Banker G. Differential effects of NgCAM and N-cadherin on the development of axons and dendrites by cultured hippocampal neurons. J Neurocytol. 2000;29(3):215–223. doi: 10.1023/a:1026515426303. [DOI] [PubMed] [Google Scholar]
- Fosser KA, Nuzzo RG. Fabrication of patterned multicomponent protein gradients and gradient arrays using microfluidic depletion. Anal Chem. 2003;75(21):5775–5782. doi: 10.1021/ac034634a. [DOI] [PubMed] [Google Scholar]
- Jo K, Heien ML, Thompson LB, Zhong M, Nuzzo RG, Sweedler JV. Mass spectrometric imaging of peptide release from neuronal cells within microfluidic devices. Lab Chip. 2007;7(11):1454–1460. doi: 10.1039/b706940e. [DOI] [PubMed] [Google Scholar]
- Zhong M, Lee CY, Croushore CA, Sweedler JV. Label-free quantitation of peptide release from neurons in a microfluidic device with mass spectrometry imaging. Lab Chip. 2012;12(11):2037–2045. doi: 10.1039/c2lc21085a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin F, Nishimura N, Griscom L, Lepioufle B, Fujita H, Takamura Y. et al. Constraining the connectivity of neuronal networks cultured on microelectrode arrays with microfluidic techniques: a step towards neuron-based functional chips. Biosens Bioelectron. 2006;21(7):1093–1100. doi: 10.1016/j.bios.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Blurton-Jones M, Rhee SW, Cribbs DH, Cotman CW, Jeon NL. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat Methods. 2005;2(8):599–605. doi: 10.1038/nmeth777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Berchtold NC, Perreau VM, Tu CH, Li Jeon N, Cotman CW. Axonal mRNA in uninjured and regenerating cortical mammalian axon. J Neurosci. 2009;29(15):4697–4707. doi: 10.1523/JNEUROSCI.6130-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Koito H, Li J, Han A. Microfluidic compartmentalized co-culture platform for CNS axon myelination research. Biomed Microdevices. 2009;11(6):1145–1153. doi: 10.1007/s10544-009-9331-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed BH, McMillan SC, Chaudhary R. The preparation of Drosophila embryos for live-imaging using the hanging drop protocol. J Vis Exp. 2009;(25):pii 1206. doi: 10.3791/1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepny A, Hogarth CA, Young J, Loveland KL. Identification of Hedgehog signaling outcomes in mouse testis development using a hanging drop-culture system. Biol Reprod. 2009;80(2):258–263. doi: 10.1095/biolreprod.108.067926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Yang P. n vitro differentiation of mouse embryonic stem (mES) cells using the hanging drop method. J Vis Exp. 2008;(17):pii 825. doi: 10.3791/825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Karthikeyan K, Chirvi S, Davé DP. Neuro-optical microfluidic platform to study injury and regeneration of single axons. Lab Chip. 2009;9(17):2576–2581. doi: 10.1039/b903720a. [DOI] [PubMed] [Google Scholar]
- Figueroa XA, Cooksey GA, Votaw SV, Horowitz LF, Folch A. Large-scale investigation of the olfactory receptor space using a microfluidic microwell array. Lab Chip. 2010;10(9):1120–1127. doi: 10.1039/b920585c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ren L, Li L, Liu W, Zhou J, Yu W. et al. Microfluidics: a new cosset for neurobiology. Lab Chip. 2009;9(5):644–652. doi: 10.1039/b813495b. [DOI] [PubMed] [Google Scholar]
- Lee JN, Jiang X, Ryan D, Whitesides GM. Compatibility of mammalian cells on surfaces of poly(dimethylsiloxane) Langmuir. 2004;20(26):11684–11691. doi: 10.1021/la048562+. [DOI] [PubMed] [Google Scholar]