Summary
Behavioral analyses of the deletion mutants of the four known myosin II heavy chain (Mhc) kinases of Dictyostelium discoideum revealed that all play a minor role in the efficiency of basic cell motility, but none play a role in chemotaxis in a spatial gradient of cAMP generated in vitro. However, the two kinases MhckA and MhckC were essential for chemotaxis in a spatial gradient of Ca2+, shear-induced directed movement, and reorientation in the front of waves of cAMP during natural aggregation. The phenotypes of the mutants mhckA− and mhckC− were highly similar to that of the Ca2+ channel/receptor mutant iplA− and the myosin II phosphorylation mutant 3XALA, which produces constitutively unphosphorylated myosin II. These results demonstrate that IplA, MhckA and MhckC play a selective role in chemotaxis in a spatial gradient of Ca2+, but not cAMP, and suggest that Ca2+ chemotaxis plays a role in the orientation of cells in the front of cAMP waves during natural aggregation.
Key words: Calcium chemotaxis, Microfluidic chamber, Calcium receptor, Myosin II
Introduction
Phosphorylation and dephosphorylation of the heavy chain plays a major role in the polymerization, localization and function of nonmuscle myosin II in both lower and higher eukaryotes (Middelbeek et al., 2010; Bosgraaf and van Haastert, 2006; Clark et al., 2008). In the lower eukaryote Dictyostelium discoideum, phosphorylation of the myosin heavy chain plays a role in a variety of basic cell behaviors, including cell polarity, the extension of the anterior pseudopod, repression of lateral pseudopods, uropod formation and the dynamics of cell migration (Stites et al., 1998; De La Roche et al., 2002; Heid et al., 2004). Phosphorylation, therefore, impacts the directed translocation of a D. discoideum amoeba up a spatial gradient of the chemoattractant cAMP during chemotaxis (Yumura and Uyeda, 1997; Heid et al., 2004). In human cells there are several myosin II isoforms (Vicente-Manzanares et al., 2009; Conti et al., 2008; Berg et al., 2001). Phosphorylation and dephosphorylation of the myosin IIA, which regulates the polymerized state of this isoform, has been shown to play a role in migration, lamellipod formation and the stabilization of stress fibers (Dulyaninova et al., 2007; Redowicz, 2001). Therefore, the myosin II heavy chain kinases and phosphatases must play roles in basic cell motility and the efficiency of chemotaxis. Here, we have explored the roles of the four identified myosin II heavy chain (Mhc) kinases of D. discoideum by analyzing the behavior of null mutants during translocation in the absence of chemoattractant, chemotaxis in a spatial gradient of either of the two chemoattractants, cAMP or Ca2+, chemokinesis in temporal gradients of cAMP, and chemotaxis in naturally aggregating cell populations.
During natural aggregation, D. discoideum amoebae respond chemotactically and chemokinetically to the spatial and temporal dynamics, respectively, of waves of the chemoattractant cAMP. These waves are relayed outwardly through an aggregating cell population (Tomchik and Devreotes 1981; Soll et al., 2002). During the developmental program leading to aggregation, cells not only acquire the receptor and signal transduction pathways for cAMP chemotaxis, but they also acquire the capacity to undergo chemotaxis in a spatial gradient of Ca2+ (Scherer et al., 2010). The discovery of Ca2+ chemotaxis (Scherer et al., 2010; Soll et al., 2011) and its selective loss in the Ca2+ channel/receptor mutant iplA− (Lusche et al., 2012), has led to the hypothesis that transient Ca2+ gradients formed between cells at the onset of each natural cAMP wave may augment orientation in the direction of the aggregation center.
When D. discoideum amoebae polarize and translocate on a substratum, myosin II polymerizes in the cortex of the posterior cell body and uropod (Yumura et al., 1984; Yumura and Fukui, 1985; Soll et al., 2009). The role of the phosphorylation–dephosphorylation cycle of the myosin heavy chain, MhcA, was revealed in the behavioral analyses of two mutants. In the mutant 3XASP, the three threonine phosphorylation sites were substituted with aspartic acid to mimic the constitutively phosphorylated state, and in the mutant 3XALA, the three sites were substituted with alanine to mimic the constitutively unphosphorylated state (Egelhoff et al., 1993; Lück-Vielmetter et al., 1990). In 3XASP cells, myosin II was primarily disassembled, and in 3XALA cells, it was overassembled (Egelhoff et al., 1993). 3XASP cells exhibited decreases in velocity, polarity, the repression of lateral pseudopod formation and directional persistence (Heid et al., 2004). They did not possess a tapered uropod and formed lateral pseudopods on average at twice the rate of wild-type cells (Heid et al., 2005). 3XASP cells, however, oriented normally in a spatial gradient of cAMP, although they still exhibited defects in basic cell motility (Heid et al., 2004). 3XALA cells, however, translocated relatively normally, except for frequent bifurcations of the anterior pseudopod (Yumura and Uyeda, 1997; Stites et al., 1998), suggesting an increase in cortical tension (Egelhoff et al., 1993; Stites et al., 1998; Laevsky and Knecht, 2003). In contrast to 3XASP cells, 3XALA cells exhibited a 50% reduction in the efficiency of chemotactic orientation. The defects in the 3XASP and 3XALA mutants indicate that the phosphorylation–dephosphorylation cycle plays a role both in basic cell motility and chemotaxis. This cycle is regulated by myosin heavy chain kinases and phosphatases (Murphy and Egelhoff, 1999; Rai and Egelhoff, 2011), which one would assume are the targets of signal transduction pathways that coordinate remodeling of the cortical cytoskeleton during basic cell motility and chemotaxis.
To investigate the role of the myosin heavy chain kinases, the behavior of the individual Mhc null mutants mhckA− (Côte and Bukiejko, 1987), mhckB− (Clancy et al., 1997; Rico and Egelhoff, 2003), mhckC− (Nagasaki et al., 2002) and mhckD− (Yumura et al., 2005), and the triple mutant, mhckA− mhckB− mhckC− (Yumura et al., 2005) (hereafter referred to as mhckA−B−C−), has been analyzed by computer-assisted live-cell reconstruction and motion analysis systems. We fully expected to find that one or more of the mutants would exhibit behavioral defects similar to those of the mutant 3XALA (Heid et al., 2004). The behavior of each mutant and its parent strain were analyzed during rapid perfusion with buffer (in the absence of attractants), in a spatial gradient of cAMP, in temporal gradients of cAMP, in spatial gradients of Ca2+ and in wild-type aggregation territories. We found that three of the mutants, mhckA−, mhckC−, mhckD− and the triple mutant, mhckA−B−C−, exhibited defects in basic motile behavior in the absence of attractant as well as in a spatial gradient of cAMP. We found, however, that all five mutants oriented normally in spatial gradients of cAMP generated in vitro and exhibited normal chemokinetic responses to temporal increases and decreases in cAMP in temporal waves generated in vitro to mimic the dynamics of natural waves. However, while mhckB− and mhckD− cells oriented normally in a spatial gradient of Ca2+, mhckA−, mhckC− and mhckA−B−C− had completely lost the capacity to orient in a spatial gradient of Ca2+. They also lost flow-directed orientation. More importantly, the three mutants lost the capacity to reorient accurately in the front of each natural cAMP wave relayed through a wild-type aggregation territory. These results indicate that none of the Mhcks are each essential for orientation in a cAMP gradient, but the two Mhcks, MhckA and MhckC, are essential for Ca2+ chemotaxis and flow-directed orientation, the same phenotype exhibited by the null mutant of the putative Ca2+ channel/receptor, IplA (Lusche et al., 2012).
Results
Basic motile behavior in the absence of attractrant
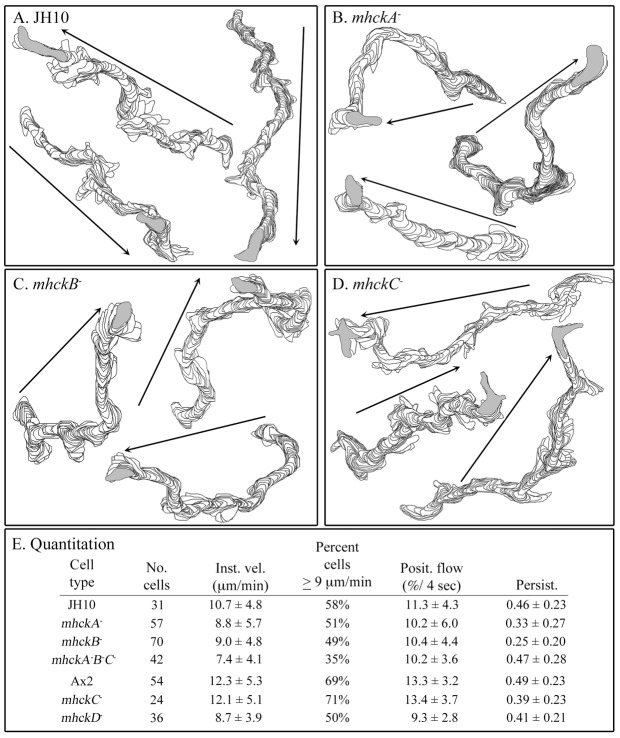
Cells of the mutants mhckA−, mhckB−, mhckC−, mhckD−, mhckA−B−C− and their parent strains JH10 and Ax2, were allowed to attach at low density to the glass wall of a Sykes–Moore chamber and were then perfused with Tricine buffer (TB) containing 10 mM Ca2+ (10 mM Ca2+ solution), which has been demonstrated to facilitate maximum polarity, directional persistence and velocity in the absence of attractant (Lusche et al., 2009; Lusche et al., 2011). Buffer solution was perfused at a rate that precluded cell-cell signaling (Varnum et al., 1985). Tracks of the cell perimeters were reconstructed by the computer-assisted program 2D-DIAS (Soll, 1995; Soll and Voss, 1998). The tracks of the mutants revealed no obvious qualitative differences with those of the parental strains (Fig. 1A–D). However, quantification revealed that the parameters reflecting the speed of cellular translocation (i.e. instantaneous velocity, percentage of cells ≧9 µm per second, positive flow) for the individual mutants mhckA−, mhckB− and mhckD−, were consistently below those of their parental strains (Fig. 1E). The decreases in instantaneous velocity were significant (P<0.05). The persistence measurements of mhckA−, mhckB− cells and mhckD− cells were also below those of their respective parental strains (Fig. 1E). The results for the triple mutant mhckA−B−C− were consistent with those for individual mutants mhckA− and mhckB− and, if anything, revealed some additivity (Fig. 1E). In marked contrast, the same motility parameters for the mutant mhckC− were indistinguishable from those of parental cells (Fig. 1E; P>0.1). These results demonstrate that none of the four Mhcks is essential for cellular translocation, but three of them (MhckA, MhckB, MhckD) play roles in fine tuning basic motile behavior and in attaining maximum velocity.
Fig. 1.
Deleting the individual MHCK genes can have minor effects on the basic motile behavior of cells in the absence of chemoattractant. Cell behavior was assessed in a chamber perfused with buffer lacking attractant. (A–D) Representative perimeter tracks of cells of parental strain JH10 and mutants mhckA−, mhckB− and mhckC−, respectively. (E) Quantification of the motility paremeters instantaneous velocity (Inst. vel.), percentage of cells with velocities ≧9 µm per min (Percent cells ≧9 µm/min), positive flow (Posit. Flow) and persistence (Persist.). Arrows in A–D indicate net direction of a cell.
All mutants undergo cAMP chemotaxis
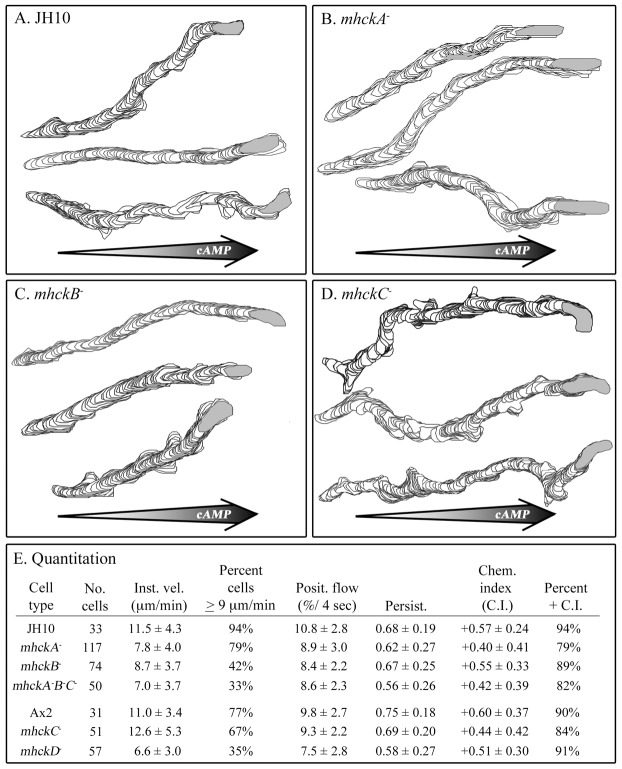
Cells of the mutant and parental strains were analyzed in spatial gradients of cAMP generated in a chamber consisting of a bridge, which supports test cells, and two bordering troughs, one filled with 10 mM Ca2+ solution and the other filled with 10 mM Ca2+ solution containing 10−6 M cAMP. In this chamber, a gradient forms across the bridge after five minutes and remains steep enough to induce chemotaxis for 15 additional minutes (Varnum and Soll, 1981; Varnum and Soll, 1984). Cell behavior was video recorded between 5 and 15 minutes for computer-assisted analysis. Cells of all five mutants moved in a directed fashion up a cAMP gradient like parental cells (Fig. 2A–D). The chemotactic index for the five mutants varied between +0.40 and +0.55, while those of parental strains JH10 and Ax2 were +0.57 and +0.60, respectively (Fig. 2E). The percentage of cells of the five mutant strains with positive chemotactic indices varied between 79 and 91%, while those of the parental strains JH10 and Ax2 were 94 and 90%, respectively (Fig. 2E). These results demonstrate that none of the Mhcks are individually essential for cAMP chemotaxis. The triple mutant underwent cAMP chemotaxis, demonstrating that at least in the case of MhckA, MhckB and MhckC, the results do not reflect a case of redundancy.
Fig. 2.
Deleting the individual MHCK genes does not affect the efficiency of chemotaxis in a spatial gradient of cAMP. Chemotaxis was assessed in a gradient chamber designed after that of Zigmond (Zigmond, 1977; Varnum and Soll, 1984). (A–D) Representative perimeter tracks of cells of parental strain JH10 and mutants mhckA−, mhckB− and mhckC−, respectively. (E) Quantification of motility and chemotaxis parameters. The motility parameters are defined in the legend in Fig. 1. The chemotaxis parameters were chemotactic index (Chem. Index, C.I.) and percentage of cells with a positive chemotactic index (Percent + C.I.). Large arrows in A–D represent direction of cAMP gradient.
As was the case in the absence of attractant (Fig. 1), the three motility parameters instantaneous velocity, percentage of cells with velocities ≧9 µm per minute and positive flow of the mutants mhckA−, mhckB−, mhckD− and mhckA−B−C− cells were lower than that of parental cells (Fig. 2E). The differences were statistically significant (P<0.05). As was the case in the absence of attractant (Fig. 1), the motility parameters of mutant mhckC− were similar to those of the parental strain Ax2 (P>0.05; Fig. 2E). Therefore, the velocity defects of mhckA−, mhckB−, mhckD− and mhckA−B−C− observed in the absence of chemoattractant (Fig. 1E) were also observed when cells were undergoing chemotaxis in a spatial gradient of cAMP (Fig. 2E).
All mutants respond normally to temporal waves of cAMP
During natural aggregation, cells undergo changes in motility in response to the temporal dynamics of each relayed wave of cAMP. Instantaneous velocity increases in response to the increasing temporal gradient in the front of each wave, and decreases in response to the peak and decreasing temporal gradient in the back of each wave (Varnum et al., 1985; Soll et al., 2002). These chemokinetic responses can be assessed in vitro by measuring instantaneous velocity in a series of temporal waves of cAMP generated with pumps attached to a round perfusion chamber (Geiger et al., 2003; Wessels et al., 2009). Under these conditions, spatial gradients of cAMP were not established. The wave periodicity was seven minutes, the average periodicity of natural waves in an aggregation territory (Tomchik and Devreotes, 1981). The response of a representative parental wild-type cell is shown in Fig. 3A. In each temporal wave, instantaneous velocity increased in the increasing phase, and decreased at the peak and in the decreasing phase (Fig. 3A). All five MhckA mutants (mhckA−, mhckB−, mhckC−, mhckD−, mhckA−B−C−) responded normally to a series of four temporal waves (Fig. 3B–F, respectively). In the front of each of the last three in a series of four temporal waves there was an increase in instantaneous velocity. Although the triple mutant exhibited an increase in the front of each wave, it consistently exhibited aberrant surges in the backs of waves (Fig. 3F). Ten cells of each parental and mutant strain were analyzed, with similar results.
Fig. 3.
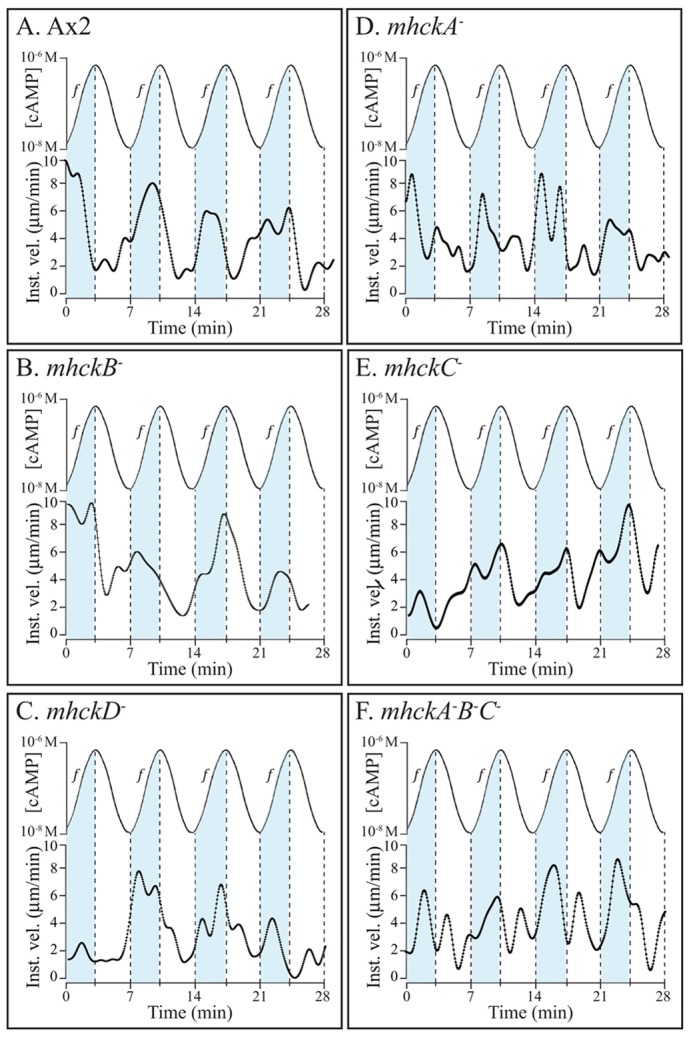
Deleting the individual MHCK genes does not affect the chemokinetic response to temporal waves of cAMP generated in vitro that mimic the temporal dynamics of naturally relayed cAMP waves. Instantaneous velocity is plotted as a function of time. The cAMP waves are plotted above the velocity plots. “f” and blue shading denotes the front (increasing temporal gradient) of each wave.
mhckA−, mhckC− and mhckA−B−C− cells do not undergo Ca2+ chemotaxis
To test whether the five mutants underwent Ca2+ chemotaxis, TB plus 10 mM Ca2+ was pumped along one side of a microfluidic chamber, and TB lacking Ca2+ along the other (Scherer et al., 2010). A series of chevron micromixers between the two flow channels of the chamber generated a stable Ca2+ gradient that was perpendicular to the direction of flow (Scherer et al., 2010). We also demonstrated that wild-type cells were mechanoresponsive to the shear force caused by the high level of flow (Scherer et al., 2010), as others have shown (Décavé et al., 2003; Fache et al., 2005; Lombardi et al., 2008). Because wild-type cells respond to both a chemotactic signal and mechanosignal, wild-type cells move up a Ca2+ gradient generated in the microfluidic chamber with a bias in the direction of flow (i.e. at an angle between the direction of the Ca2+ gradient and the angle of flow) (Scherer et al., 2010). Parental JH10 and Ax2 cells, in the experiments reported here, moved up Ca2+ gradients with chemotactic indices of +0.20±0.23 and +0.23±0.22, respectively, and with percentage positive chemotaxis measures of 75 and 80%, respectively (Fig. 4A). These values were very close to those previously reported for wild-type cells (Scherer et al., 2010). Cells of both parental strains exhibited rightward bias, which was evident in the rightward direction parameter, calculated as the degrees directed away from the rightward vector (i.e. the direction of flow) (Scherer et al., 2010) (Fig. 4A), and in representative cell tracks (Fig. 4B).
Fig. 4.
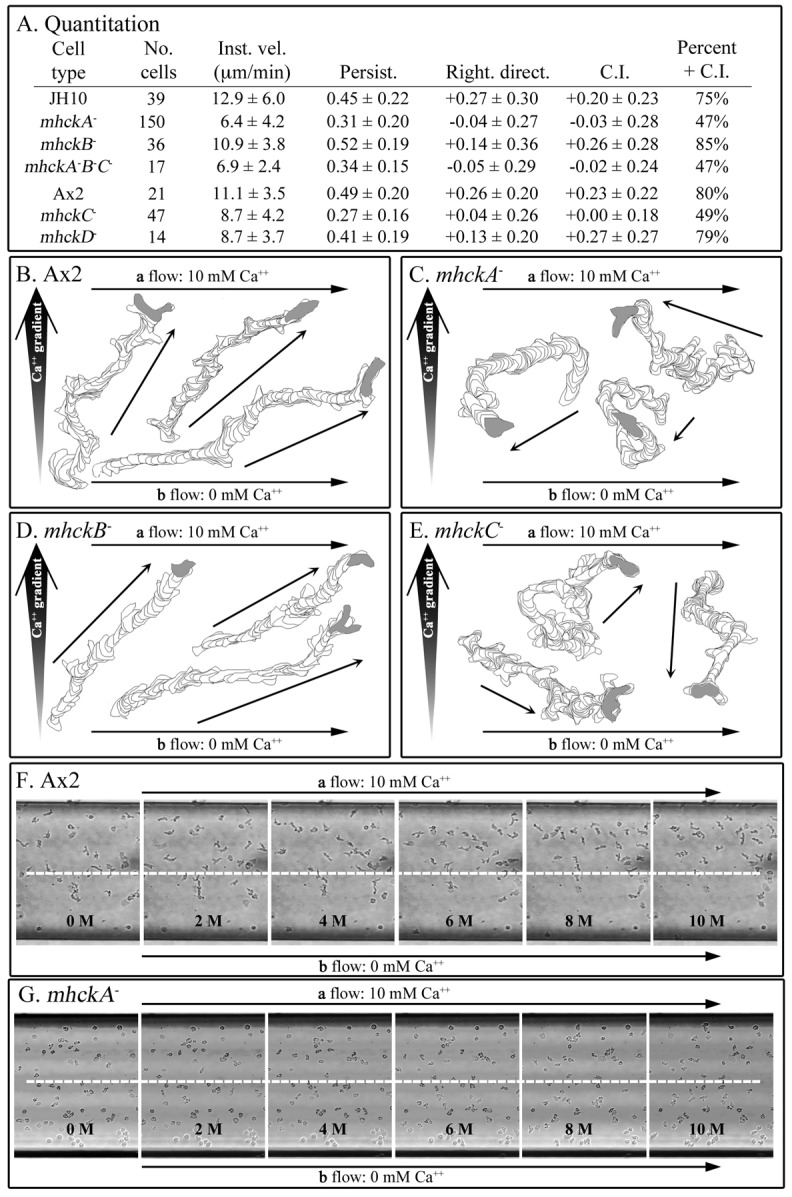
The mutants mhckA−, mhckB− and mhckA−B−C− have lost the capacity to undergo chemotaxis in a spatial gradient of Ca2+. They also lose flow-induced directed motility. Chemotaxis and flow-induced directed motility were assessed in a microfluidic chamber (Scherer et al., 2010). (A) Quantification of motility parameters, flow-induced movement and chemotaxis. Motility and chemotaxis parameters are defined in the legends to Figs 1 and 2, respectively. Right. direct., rightward directionality. (B–E) Representative perimeter tracks of cells of parental strain Ax2 and mutants mhckA−, mhckB− and mhckC−, respectively. (F,G) Consecutive video frames at 2 minute intervals of Ax2 and mhckA− cells, respectively. In B–E, thick and thin arrows represent direction of Ca2+ gradient and cell movement, respectively. In F and G, the white dashed line denotes the middle of the chamber, and time is presented in minutes (M).
Cells of the mutants mhckB− and mhckD− exhibited chemotactic indices and percentage positive chemotaxis measures similar to those of cells of the parental strains (Fig. 4A). mhckB− and mhckD− cells also exhibited positive rightward directionality values of +0.14 and +0.13 (Fig. 4A), which were half that of parental strains, but still far above 0.00, the measure of unresponsiveness (Scherer et al., 2010). The capacity of mhckB− cells (Fig. 4D) and mhckD− cells (data not shown) to undergo chemotaxis and mechanoreception albeit reduced, in a Ca2+ gradient was evident in representative perimeter tracks. In marked contrast, the chemotactic indices of mhckA−, mhckC− and mhckA−B−C− cells were −0.03±0.28, +0.00±0.18 and −0.02±0.24, respectively, and the percentage positive chemotaxis measure in all cases was close to 50% (Fig. 4A). Rightward directionality measurements were −0.04±0.27, +0.04±0.26 and −0.05±0.29, respectively (Fig. 4A). The differences between the mutants (mhckA−, mhckC−and mhckA−B−C−) and their parental strains for the two chemotactic parameters and rightward directionality were significant (P<0.05). Representative tracks of mhckA− (Fig. 4C), mhckC− (Fig. 4E) and mhckA−B−C− (data not shown) cells reflected directional randomness in a Ca2+ gradient. A comparison of fields of cells in Ca2+ gradients at low magnification revealed that although mhckA− cells were mobile, they remained randomly dispersed throughout the field of analysis (Fig. 4G) whereas parental cells moved towards the upper right hand corner of the field (Fig. 4F). These results demonstrate that each of the two Mhcks, MhckA and MhckC, but not MhckB or MhckD, is essential for Ca2+ chemotaxis and flow-induced directed movement.
Defects in chemotactic orientation in the front of natural waves
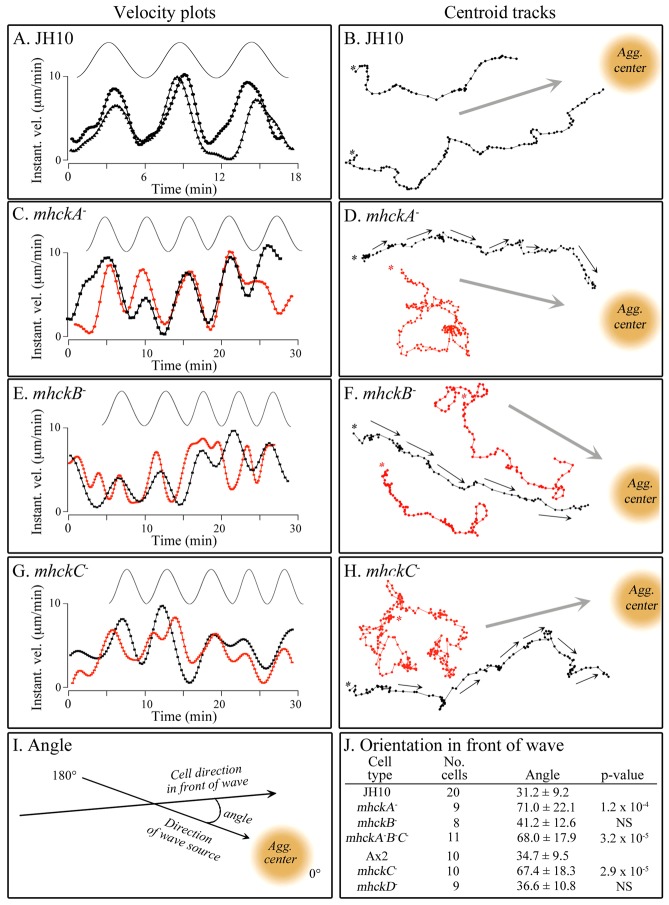
Since all four individual Mhck mutants underwent normal chemotaxis in a spatial gradient of cAMP and normal chemokinetic responses to increasing and decreasing temporal gradients of cAMP in vitro, one might expect all four to respond normally to the spatial and temporal dynamics of cAMP waves relayed in a natural aggregation territory of majority wild-type cells. This expectation was based on the premise that every aspect of the complex behavior of cells in a natural wave of cAMP could be explained by their responses to the increasing and decreasing spatial gradients of cAMP, and the increasing and decreasing temporal gradients of cAMP that accompany the front and back, respectively, of each relayed wave (Soll et al., 2002). To test this, vitally stained mutant cells and unstained JH10 cells were mixed at a ratio of 1:9, respectively, and allowed to aggregate on the surface of a 35 mm plastic Petri dish (Wessels et al., 2000; Wessels et al., 2007; Lusche et al., 2012). The outwardly moving, nondissipating waves of cAMP in these mixed populations are dictated by the majority wild-type cells. The form and frequency of each natural wave passing over an individual minority mutant cell was deduced by the transient increase in instantaneous velocity of neighboring majority wild-type cells. Majority wild-type cells responded chemotactically and chemokinetically to the front of each wave (Fig. 5A,B). In the front of each deduced wave, wild-type cells moved with increased velocity in the direction of the aggregation center, and at the peak and in the back of each deduced wave, the velocity of cells decreased dramatically, with little net movement towards the aggregation center. These behaviors were evident in velocity plots (Fig. 5A) and tracks of the cell centroid (Fig. 5B). Minority mhckA−, mhckB− and mhckC−, mhckD− and mhckA−B−C− cells all exhibited normal chemokinetic increases and decreases in instantaneous velocity in the fronts and backs of consecutive relayed wild-type waves (red dot plots), behaviors similar to neighboring wild-type cells (black dot plots; Fig. 5C, E and G, respectively; data for mhckD− and mhckA−B−C− not shown). The majority of cell centroid tracks (90%) of the mutants mhckB− and mhckD− (n = 8 and 10, respectively) also appeared to be similar to those of neighboring wild-type cells, although they did display a slightly higher tendency to make sharp turns (Fig. 5F; data for mhckD− not shown). However, only a minority (<20%) of the cell centroid tracks of the mutants mhckA− (Fig. 5D), mhckC− (Fig. 5H) and mhckA−B−C− (data not shown) exhibited normal trajectories (n = 9 and 11, respectively). In the majority of cases, the cells did not appear to reorient at the onset of each wave, resulting in a high frequency of sharp turns (Fig. 5D,H). To quantify this abnormality in reorientation, the angle was measured between the direction of movement in the front of each of four successive waves and the direction of the aggregation center, the source of the wave, as diagrammed in Fig. 5I. In a previous study using this technique (Lusche et al., 2012), we found the average angle of orientation for wild-type cells to be 30°. Here, parental JH10 and Ax2 cells oriented with average angles of 31.2±9.2° and 34.7±9.5°, respectively (Fig. 5J). Mutant mhckB− and mhckD− cells oriented at angles (± standard deviation) of 41.2±12.6° and 36.6±10.8°, respectively (Fig. 5J). These values were found to be statistically indistinguishable from those of the respective controls (Fig. 5J). Cells of the mutants mhckA−, mhckC− and mhckA−B−C−, however, exhibited average angles of 71.0±22.1°, 67.4±18.3° and 68.0±17.9°, respectively (Fig. 5J). The difference in angle between the three latter mutants and their respective parental strain was highly significant (Fig. 5J). Together, these results indicate that MhckA and MhckC play major roles in reorientation in the front of each natural, relayed wave, but MhckB and MhckD do not.
Fig. 5.
The mutants mhckA−,mhckC−, and mhckA−B−C− have lost the capacity to reorient in the front of each natural wave, in the direction of the aggregation center, the source of the waves. Parental and mutant cells were mixed at a ratio of 9∶1 and allowed to aggregate. (A,C,E,G) Representative velocity plots of cells responding to the temporal dynamics of successive natural waves (drawn above each velocity plot) for parent strain JH10 and mutants mhckA−, mhckB− and mhckC−, respectively (mhckD− and mhckA−B−C− not shown). In A, the data are for two representative JH10 cells in a homogeneous JH10 population (both black plots), and in C, E and G, the data are for a JH10 (black) and mutant (red) cell. (B,D,F,H) Representative centroid tracks at 20 sec. intervals in successive natural waves. In B, the data are for two representative JH10 cells (black). In D, F and H, data are for a representative control cell (black) and representative mutant (red) cells in 9∶1 mixtures. (I) Method for assessing the angle in the front of a wave. (J) Measured average angle in the front of successive waves.
Myosin II localization in mutants
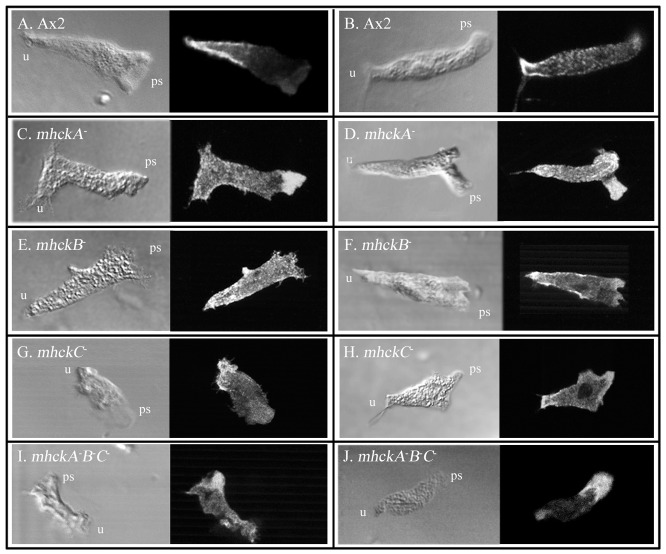
In parental Ax2 (Fig. 6A,B) and JH10 (data not shown) cells translocating in buffer, anti-myosin II antibody stained the cytoplasm diffusely and the posterior cell cortex and uropod intensely (n = 25 for both strains), as previously reported (Yumura et al., 1984; Yumura and Fukui, 1985). A similar pattern was observed for JH10 cells (data not shown). In a minority (<10%) of control cells, there was distinct myosin II staining in pseudopods (data not shown), presumably reflecting pseudopod retraction, as suggested by Spudich and co-workers, who monitored GFP-tagged myosin II in live translocating cells (Moores et al., 1996). Cells of all five mutants exhibited diffuse myosin II staining in the cytoplasm, and more intense staining in the uropod and posterior cell cortex (Fig. 6C–J). However, the pseudopods of a majority (>60%, n = 20) of mhckA− cells (Fig. 6C,D) and a majority (>60%, n = 20) of mhckA−B−C− cells (Fig. 6I,J) also stained intensely for myosin II. These latter results suggest that in the absence of MhckA, which localizes in pseudopods (Steimle et al., 2001a; Steimle et al., 2001b; Steimle et al., 2002; Liang et al., 2002), myosin II abnormally accumulates in that structure, presumably in an unphosphorylated and thus polymerized state.
Fig. 6.
Myosin II distribution in wild-type and mutant strains during cellular translocation. Cells were mixed and stained with anti-myosin II antibody. ps, pseudopod; u, uropod.
Ca2+ and in vitro MhckA activity
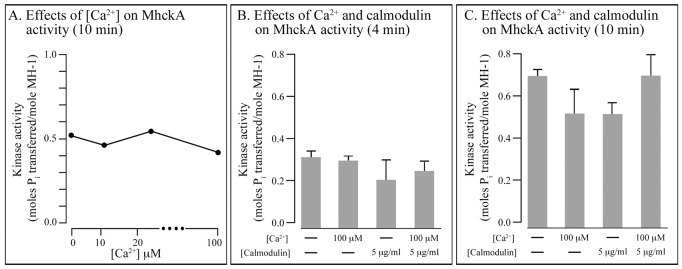
Assessing the direction of a spatial gradient of Ca2+ (Scherer et al., 2010) most likely functions through surface receptors that activate signal transduction pathways, as is the case for cAMP chemotaxis in D. discoideum (Swaney et al., 2010; Wang et al., 2011; Cai and Devreotes, 2011) and chemotaxis to a number of attractants in animal cells (von Philipsborn and Bastmeyer, 2007), including Ca2+ chemotaxis (Aguirre et al., 2010). In addition, assessing a Ca2+ gradient may also be mediated by the gradient-dependent release of Ca2+ if the receptor is a Ca2+ channel (Treves et al., 2010; Prevarskaya et al., 2010). In the latter case, orientation may be effected by generating a cytosolic Ca2+ gradient that activates MhckA and MhckC in a gradient-dependent fashion (Yumura et al., 1996). To explore this latter possibility, we tested whether changes in the concentration of Ca2+ affected MhckA and MhckC activity in vitro. The substrate in this assay was the MH-1 peptide described elsewhere (Steimle et al., 2001a). The cytosolic concentration of Ca2+ has been estimated to be 50 nM in the absence of cAMP and 200 nM upon global stimulation with very high concentrations of cAMP (Schlatterer et al., 1994; Yumura et al., 1996; Nebl and Fisher, 1997). Increasing the in vitro Ca2+ concentration from 0 to 100 µM in the reaction mixture had only a minor effect on MhckA activity (Fig. 7A). Increasing it to 500 µM, a nonphysiological concentration, caused a 30–40% decrease in activity. Addition of calmodulin, which binds Ca2+ and facilitates its interaction with target proteins, had little effect on MhckA activity (Fig. 7B,C). Similar results were obtained in measurements of MhckC activity (data not shown). These results suggest that the activity of MhckA and MhckC are not under the regulation of receptor- or channel-dependent changes in the concentration of cytosolic Ca2+. They do not, however, contradict the suggestion that Ca2+ chemotaxis is receptor mediated (Scherer et al., 2010).
Fig. 7.
Varying Ca2+ concentration or adding calmodulin has little effect on MhckA activity in vitro. (A–C) The phosphorylation reaction was performed with the MH-1 peptide described elsewhere (Steimle et al., 2001a). Similar results were obtained with MhckC.
Revisiting 3XALA
If MhckA and MhckC regulate Ca2+, but not cAMP, chemotaxis, through the phosphorylation of Mhc, then the mutant 3XALA, which produces a constitutively unphosphorylated Mhc, should share the characteristics of the mutants mhckA−, mhckC− and mhckA−B−C−. A previous computer-assisted study (Heid et al., 2004) revealed that cells of the 3XALA mutant underwent chemotaxis in a spatial gradient of cAMP, but with reduced efficiency. 3XALA also underwent chemokinesis in the front of temporal waves of cAMP generated in vitro and in the front of deduced natural waves of cAMP generated in mixtures of wild-type and mutant cells (9:1), as described here. We therefore retested whether 3XALA cells underwent chemotaxis in a spatial gradient of cAMP in 10 mM Ca2+ solution and tested for the first time whether they underwent chemotaxis in a spatial gradient of Ca2+. Cells of the mutant strain 3XALA, which was derived from strain JH10 as previously reported, underwent chemotaxis in a cAMP gradient with an average chemotactic index of +0.28±0.36, and a percentage positive chemotaxis value of 75% (n = 48), results very similar to those previously reported (Heid et al., 2004). However, 3XALA cells did not undergo chemotaxis in a spatial gradient of Ca2+. The chemotactic index in the latter case was +0.03±0.32 (n = 44). As was the case for cells of the mutants mhckA−, mhckC− and mhckA−B−C−, 3XALA cells did not exhibit rightward directionality in response to flow (rightward directionality = 0.04±0.30). And as previously reported for 3XALA cells in a spatial gradient (Stites et al., 1998), myosin II stained strongly in the cortex and uropod of 3XALA cells in buffer (data not shown), reflecting overpolymerization (Egelhoff et al., 1993).
Discussion
The role of myosin II phosphorylation
Behavioral analyses of the phosphorylation mutants of the myosin II heavy chain (Mhc), 3XASP and 3XALA (Egelhoff et al., 1993; Egelhoff et al., 1996), indicated that the phosphorylation–dephosphorylation cycle played a role not only in the basic motile behavior of a cell, but also in chemotaxis in a spatial gradient of cAMP and in response to temporal gradients of cAMP (Heid et al., 2004; Soll et al., 2009; Stites et al., 1998). The aberrant phenotypes of these mutants suggested that the dynamic phosphorylation–dephosphorylation cycle of myosin II was not absolutely essential for these behaviors, but rather played a role in fine tuning chemotaxis in a spatial gradient of cAMP. Because the Mhcks had been shown to phosphorylate myosin II in vitro, we fully expected that one or more of the Mhck null mutants would partially or fully exhibit the behavioral defects of the mutant 3XALA.
The role of the Mhck proteins in basic motile behavior
All of the Mhck null mutants except mhckC− exhibited reductions in velocity. MhckA had been shown to localize to the pseudopod (Steimle et al., 2001a; Steimle et al., 2001b; Steimle et al., 2002; Liang et al., 2002), which, when extending, is relatively devoid of myosin II (Moores et al., 1996). The accumulation of myosin II in the pseudopods of mhckA− cells, as we have shown here, supports the suggestion that MhckA phosphorylates Mhc in the pseudopod, thus blocking myosin II polymerization in the dynamic actin gel that drives pseudopodial expansion (Steimle et al., 2001a; Steimle et al., 2001b; Steimle et al., 2002). In marked contrast, MhckB localizes to the cytoplasm (Liang et al., 2002; Underwood et al., 2010), where it may play a role in maintaining the monomeric myosin II pool necessary for remodeling the cortical cytoskeleton during cellular translocation and lateral pseudopod formation. MhckC localizes to the myosin II-rich posterior cortex and uropod (Nagasaki et al., 2002; Yumura et al., 2005), where it may play a role in dismantling the actin–myosin cytoskeleton during remodeling. Remodeling has been indicated in morphometric studies to occur at the interface between the cell body and uropod (Soll et al., 2009). However, this suggested role is not reflected in the mhckC− mutant, which does not exhibit any measurable defect in velocity, polarity or uropod maintenance. MhckD localization has not been reported. These observations suggest that in a wild-type cell, at least three of the four Mhcks exhibit differences in localization and, therefore, are unlikely to be functionally redundant.
Mhcks and cAMP chemotaxis
All four of the individual Mhck mutants and the triple mutant mhckA−B−C−, underwent robust chemotaxis in spatial gradients of cAMP generated in vitro. All five mutants also exhibited relatively normal chemokinetic responses to increasing and decreasing temporal gradients of cAMP generated in vitro in temporal waves that mimicked the temporal dynamics of natural waves. These results indicate that no single Mhck, nor a combination of the three Mhcks, MhckA, MhckB and MhckC, is essential for chemotaxis in a spatial gradient of cAMP or for the chemokinetic responses to temporal gradients of cAMP generated in vitro. This leads to the tentative conclusion that none of these kinases may represent an exclusive, or essential, target for the signal transduction pathways regulating cAMP chemotaxis (Yumura et al., 2005). One might suggest either that other kinases may be responsible for Mhc phosphorylation and hence serve as targets for these pathways, or that another as yet unidentified pathway not involving phosphorylation may regulate myosin assembly (Levi et al., 2002). However, even this explanation seems unlikely given that 3XALA, which contains myosin II heavy chain in a constitutively unphosphorylated state, undergoes chemotaxis in a spatial gradient of cAMP and chemokinesis in temporal gradients of cAMP generated in vitro (Heid et al., 2004).
Mhcks and Ca2+ chemotaxis
Although none of the Mhcks were essential for chemotaxis in a spatial gradient of cAMP, two of them were essential for chemotaxis in a spatial gradient of Ca2+. The two mutants mhckA− and mhckC−, and the triple mutant mhckA−B−C−, were incapable of undergoing chemotaxis in a spatial gradient of Ca2+ (Scherer et al., 2010). These mutants also lost shear-induced motility in the direction of fluid flow (Décavé et al., 2002; Décavé et al., 2003; Fache et al., 2005; Lombardi et al., 2008). The two mutants mhckB− and mhckD−, however, underwent Ca2+ chemotaxis and shear-induced directional movement, although the latter at a reduced level. These results indicate that MhckA and MhckC are each essential in a nonredundant fashion for chemotaxis in a spatial gradient of Ca2+, but not cAMP. This result is surprising for several reasons. First, because the behavioral response appears so similar during cAMP and Ca2+ chemotaxis (Scherer et al., 2010; Lusche et al., 2012), we hypothesized that while the receptors and upstream components of the signal transduction machinery may differ, the downstream pathways and targets regulating the response, such as the Mhcks, would be common. This expectation was reinforced by the observation that the capacity to undergo chemotaxis in both a cAMP and a Ca2+ gradient were attained at approximately the same time in the developmental program initiated by starvation (Scherer et al., 2010). Demonstration here that MhckA and MhckC are each essential for Ca2+, but not cAMP, chemotaxis suggests that either the downstream targets of the signal transduction pathways differ in the two chemotactic processes, or that Mhc phosphorylation plays a role upstream in the regulation of Ca2+, but not cAMP, chemotaxis.
Mhck proteins and natural aggregation
Given that all four of the individual Mhck null mutants underwent chemotaxis in a spatial gradient of cAMP and chemokinesis in an increasing temporal gradient of cAMP, we expected all of them to behave normally in natural wild-type aggregation territories. This expectation was predicated on the assumption that the behavior of cells during natural aggregation is dictated solely by the spatial and temporal dynamics of cAMP in the natural wave (Soll et al., 2002). We found instead in mixing experiments (Lusche et al., 2012) that neither minority mhckA− cells nor minority mhckC− cells undergo normal chemotaxis in natural waves generated by majority wild-type cells. They were unable to reorient in the front of each successive wave. In contrast, the mutants mhckB− and mhckD− reoriented in the front of natural wild-type waves. Loss of the capacity to reorient by mhckA− and mhckC− cells correlated with loss of the capacity to undergo chemotaxis in a spatial gradient of Ca2+, not cAMP. These observations provide additional support for the hypothesis that Ca2+ chemotaxis plays a fundamental role in natural chemotaxis in an aggregation territory (Scherer et al., 2010; Lusche et al., 2012).
mhckA− and mhckC− are phenocopies of the mutant iplA−
Like cells of the Mhck null mutants mhckA− and mhckC−, cells of iplA−, the null mutant of IplA, a putative Ca2+ channel/receptor (Traynor et al., 2000), also undergo chemotaxis in a spatial gradient of cAMP, but not Ca2+ (Lusche et al., 2012). Like mhckA− and mhckC− cells, iplA− cells have also lost shear-induced movement in the direction of fluid flow (Lusche et al., 2012). Finally, like mhckA− and mhckC− cells, iplA− cells have lost the capacity to reorient in the front of cAMP waves, during natural aggregation (Lusche et al., 2012). These similarities suggest that IplA, MhckA and MhckC are components of a signal transduction pathway for Ca2+, but not cAMP, chemotaxis.
mhckA−, mhckC− and iplA− are phenocopies of 3XALA
Perhaps our most telltale observation relates to the phenotype of the mutant 3XALA. If the phenotypic consequences of deleting either MHCKA or MHCKC is an overabundance of unphosphorylated Mhc, and, therefore, higher levels of more stable polymerized myosin II, then the mutant 3XALA should be phenotypically similar to mhckA− and mhckC−, and that was indeed the case. Therefore, it is reasonable to conclude that the consequence of deleting either MHCKA or MHCKC is the overpolymerization of myosin II, in the cortex and uropod in the former case and in the pseudopod in the latter case. And based on the assumption that cAMP chemotaxis employs the same downstream motility machinery as Ca2+ chemotaxis and shear-induced directed motility, but is intact in the mutants mhckA−, mhckC−, iplA− and 3XALA, we suggest that myosin phosphorylation plays a role upstream in the pathway regulating Ca2+ chemotaxis and shear-induced directed motility. If IplA proves to be the common receptor for these two responses, as has been suggested (Lombardi et al., 2008; Lusche et al., 2012), then the possibility can be entertained that the phosphorylation of Mhc by MhckA and MhckC plays a role in receptor (IplA) function, rather than downstream in the motile response. The in vitro experiments reported here reveal that since neither MhckA nor MhckC appears to be affected in vitro by the changes in Ca2+ concentration in the physiological range, the possibility must be entertained that IplA may function as a classical chemotactic receptor at the cell surface, where it interacts directly or indirectly with MhckA and MhckC, rather than as a Ca2+ channel that regulates these kinases by affecting the intracellular concentration of Ca2+. However, the disparate locations of the two Mhcks and the disparate effects deleting either has on myosin localization are difficult to reconcile with the common phenotype exhibited by either deletion mutant. Nonetheless we have identified components in a novel and selective pathway for Ca2+ chemotaxis and shear-induced motility. Because the components are just emerging, it seems premature to attempt to design a model for this pathway at the present time.
Materials and Methods
Strain maintenance, growth and development
The mhckA−, mhckB−, mhckC−, and mhckA−B−C− strains were provided by Thomas Egelhoff (Cleveland Clinic Foundation, Cleveland, OH) and are also available in the Dictyostelium stock center (http://dictybase.org/StockCenter/StockCenter.html. The 3XALA and mhckD− strains were deposited by Thomas Egelhoff in the Dictyostelium stock center and obtained from there.
To generate the mhckA− strain (Kolman et al., 1996), the pD15 gene replacement vector containing 5′ flanking and 3′ regions of the mhckA gene was electroporated into JH10, a thymidine auxotrophic cell line. The JH10 control strain was grown in HL-5 medium (http://dictybase.org/techniques/index.html) supplemented with 100 µg/ml thymidine (Sigma Aldrich, St. Louis, MO) while the mhckA− cells were maintained in HL-5 alone.
The mhckB− strain was also obtained by insertion of a gene targeting construct into JH10 (Rico and Egelhoff, 2003). In this case, the construct contained 5′ and 3′ sequences of the mhckB gene while the central portion of the gene had been replaced with the blasticidin resistance cassette. In studies presented here, mhckB− cells were grown in HL-5 medium supplemented with 10 µg/ml Blasticidin S (Sigma Aldrich, St. Louis, MO).
The mhckC− and mhckD− strains were generated by insertion of the appropriate blasticidin resistance disruption cassette into the mhckC or mhckD genes of the Ax2 control strain (Nagasaki et al., 2002). These cells were grown in HL-5 supplemented with 10 µg/ml Blasticidin S.
The mhckA−B−C− strain was obtained using the nonselected co-transformation protocol described in detail by Betapudi et al. (Betapudi et al., 2004). This approach was designed to generate a multiple gene knockout strain while circumventing problems inherent to G418 and hygromycin selection (Betapudi et al., 2004). Briefly, targeting vectors for the mhckB and mhckC genes containing blasticidin and hygromycin resistance cassettes, respectively, within the coding regions were mixed in a 1∶6 molar ratio, electroporated into the mhckA− cell line, and selected for blasticidin resistance. Transformants were screened by PCR to confirm disruption of all genes (Yumura et al., 2005).
Finally, the 3XALA strain (Egelhoff et al., 1993) in which the three threonines in the tail region of the myosin II heavy chain were replaced with nonphosphorylatable alanines, was obtained from Dr Egelhoff (Egelhoff et al., 1993). These cells were grown in HL-5 supplemented with 10 µg/ml G418 (Sigma Aldrich, St. Louis, MO).
Frozen stocks of the strains were reconstituted every two weeks for experimental use (Wessels et al., 2007; Lusche et al., 2009). Methods for obtaining aggregation-competent amoebae for motility assays are described elsewhere in detail (Scherer et al., 2010). For analyses of basic motility and chemotaxis, cells were harvested from developmental filters at the onset of aggregation, when velocity and chemotactic responsiveness were maximal (Varnum et al., 1986).
Analysis of basic motile behavior and behavior in temporal cAMP waves
The method and conditions for analyzing basic motile behavior in the Sykes–Moore perfusion chamber have been described previously in detail (Soll et al., 2009; Varnum et al., 1986; Lusche et al., 2009; Wessels et al., 2009). Here, basic motile behavior was analyzed during perfusion with Tricine buffer (TB) containing 10 mM Ca2+. Sequential temporal waves of cAMP were generated in 40 mM K+ buffer (Varnum et al., 1985; Lusche et al., 2011) in the Sykes–Moore chamber by pressure-driven pumps as previously described (Geiger et al., 2003; Wessels et al., 2009). The wave periodicity was 7 min. Digital images were acquired at an interval of 4 secs for 10 mins as described elsewhere (Lusche et al., 2011).
Analysis of chemotaxis
Cells were distributed across the bridge of the plexiglass gradient chamber (Zigmond, 1977) according to methods previously described (Varnum and Soll, 1984). Gradients were generated by filling one trough bordering the bridge with 10 mM Ca2+ solution in TB lacking cAMP and the other trough with 10 mM Ca2+ solution in TB plus 10−6 M cAMP. Cells were video recorded for a 10 minute period following 5–7 minutes of incubation after adding solutions to the troughs.
Cells were inoculated into the custom built microfluidic chamber and a Ca2+ gradient generated using a method identical to the one described elsewhere in considerable detail (Scherer et al., 2010). The concentration of Ca2+ used in the microfluidic chamber in this study was 10 mM.
Myosin II immunofluorescent staining
Myosin II was stained with Dictyostelium anti-myosin II polyclonal antibody (Burns et al., 1995), a generous gift from Arturo de Lozanne (University of Texas, Austin). The methods were described elsewhere in detail (Wessels et al., 2000).
DIAS analysis of behavior
Cell behavior under all conditions was analyzed by 2-D DIAS software and a description of the parameters is described elsewhere in detail (Soll, 1995; Soll and Voss, 1998; Wessels et al., 2009). Briefly, instantaneous velocity, percentage of cells with a velocity of ≧9 µm per minute (% cells ≧9 µm/min), directional persistence, chemotactic index and percentage positive chemotaxis were computed from centroid positions (Soll, 1995; Soll and Voss 1998; Wessels et al., 2009; Lusche et al., 2012). ‘Instantaneous velocity’ was computed at 4 second intervals between each consecutive pair of centroids over a ten minute period (Soll, 1995; Soll and Voss, 1998). The parameter ‘% cells ≧9 µm/min’ was computed as the proportion of cells in a population with an average instantaneous velocity greater than or equal to 9 µm per minute. ‘Positive flow’ was determined from overlapping perimeter outlines of two consecutive cell images. The area in the second of the two images that did not overlap the first was calculated and expressed as a percentage of the area of the first image. This was performed at 4 second intervals over a 10 min period for each cell. ‘Directional persistence’ was computed as the net distance between the first and last centroid of a centroid track divided by the summed distances between consecutive centroid positions of the track. The ‘chemotactic index’ (C.I.) was computed as the net distance traveled in the direction of the source of chemoattractant divided by the total distance traveled. Similarly, rightward directionality (RD) was computed as the net distance traveled towards the right (direction of flow in the microfluidic chamber) divided by the total distance traveled. The ‘percentage positive chemotaxis’ parameter was measured as the proportion of cells in a population with a chemotactic index greater than 0.00. Values given are the mean (± standard deviation) computed for the population.
Mixing experiments for analysis of natural aggregation
To analyze the behavior of mhck mutants in wild-type aggregation territories, mutant cells were labeled with the vital dye DiI (Invitrogen, Carlsbad, CA), mixed with a majority of unlabeled control cells, and motion analyzed during aggregation according to methods described in detail elsewhere (Lusche et al., 2012). Briefly, mhck mutants were labeled by incubation in HL-5 containing 0.05 mM DiI (Invitrogen) for 24 hrs in the dark. HL-5 was then removed from labeled mutant cells and excess dye removed by washing in 40 mM K+ solution (Lusche et al., 2011). The appropriate control strain (JH10 or Ax2) and labeled mhck cell populations were then mixed at a 9∶1 ratio, respectively, to a final density of 5×106 cells per 2 ml. This suspension was inoculated into a 35 mm Petri dish and the dish placed on the stage of a Nikon Eclipse TE-2000 microscope connected to a Bio-Rad Radiance 2100MP laser scanning confocal microscope (Bio-Rad Microscience Ltd, Hemel Hempstead, UK). After 6 hours, image acquisition was begun using a green HeNe laser at 543 nm and a 10× objective. Transmitted light images were continuously collected through a transmitted light detector at 543 nm. Transmitted and fluorescent images were collected with LaserSharp 2000 software at 20 sec intervals and converted to QuickTimeTM format. Labeled mhck− cells and unlabeled control cells were outlined from the QuickTimeTM movie and motion analyzed using 2D-DIAS as described above (Soll, 1995; Soll and Voss 1998; Wessels et al., 2009).
Footnotes
Funding
This research was supported by the Developmental Studies Hybridoma bank at the University of Iowa, a National Resource initiated by the National Institutes of Health; and by National Institutes of Health [grant numbers GM50009 to T.T.E., 2R15GM066789 to P.A.S.]. Deposited in PMC for release after 12 months.
References
- Aguirre A., González A., Planell J. A., Engel E. (2010). Extracellular calcium modulates in vitro bone marrow-derived Flk-1+ CD34+ progenitor cell chemotaxis and differentiation through a calcium-sensing receptor. Biochem. Biophys. Res. Commun. 393, 156–161 10.1016/j.bbrc.2010.01.109 [DOI] [PubMed] [Google Scholar]
- Berg J. S., Powell B. C., Cheney R. E. (2001). A millennial myosin census. Mol. Biol. Cell 12, 780–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betapudi V., Shoebotham K., Egelhoff T. T. (2004). Genertion of double gene disruptons in Dictyostelium discoideum using a single antibiotic marker selection. Biotechniques 36, 106–112 [DOI] [PubMed] [Google Scholar]
- Bosgraaf L., van Haastert P. J. (2006). The regulation of myosin II in Dictyostelium. Eur. J. Cell Biol. 85, 969–979 10.1016/j.ejcb.2006.04.004 [DOI] [PubMed] [Google Scholar]
- Burns C. G., Larochelle D. A., Erickson H., Reedy M., De Lozanne A. (1995). Single-headed myosin II acts as a dominant negative mutation in Dictyostelium. Proc. Natl. Acad. Sci. USA 92, 8244–8248 10.1073/pnas.92.18.8244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H., Devreotes P. N. (2011). Moving in the right direction: how eukaryotic cells migrate along chemical gradients. Semin. Cell Dev. Biol. 22, 834–841 10.1016/j.semcdb.2011.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy C. E., Mendoza M. G., Naismith T. V., Kolman M. F., Egelhoff T. T. (1997). Identification of a protein kinase from Dictyostelium with homology to the novel catalytic domain of myosin heavy chain kinase A. J. Biol. Chem. 272, 11812–11815 10.1074/jbc.272.18.11812 [DOI] [PubMed] [Google Scholar]
- Clark K., Middelbeek J., Lasonder E., Dulyaninova N. G., Morrice N. A., Ryazanov A. G., Bresnick A. R., Figdor C. G., van Leeuwen F. N. (2008). TRPM7 regulates myosin IIA filament stability and protein localization by heavy chain phosphorylation. J. Mol. Biol. 378, 790–803 10.1016/j.jmb.2008.02.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti M., Kawamoto S., Adelsatein R. (2008). Nonmuscle myosin II. Myosins: A Superfamily of Molecular Motors, Vol. 7, Proteins and Cell Regulation (ed. Coluccio L M.), pp. 223–264, Dordrecht, The Netherlands: Springer [Google Scholar]
- Côte G. P., Bukiejko U. (1987). Purification and characterization of a myosin heavy chain kinase from Dictyostelium discoideum. J. Biol. Chem. 262, 1065–1072 [PubMed] [Google Scholar]
- De La Roche M. A., Smith J. L., Betapudi V., Egelhoff T. T., Côté G. P. (2002). Signaling pathways regulating Dictyostelium myosin II. J. Muscle Res. Cell Motil. 23, 703–718 10.1023/A:1024467426244 [DOI] [PubMed] [Google Scholar]
- Décavé E., Garrivier D., Bréchet Y., Fourcade B., Bruckert F. (2002). Shear flow-induced detachment kinetics of Dictyostelium discoideum cells from solid substrate. Biophys. J. 82, 2383–2395 10.1016/S0006-3495(02)75583-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Décave E., Rieu D., Dalous J., Fache S., Brechet Y., Fourcade B., Satre M., Bruckert F. (2003). Shear flow-induced motility of Dictyostelium discoideum cells on solid substrate. J. Cell Sci. 116, 4331–4343 10.1242/jcs.00726 [DOI] [PubMed] [Google Scholar]
- Dulyaninova N. G., House R. P., Betapudi V., Bresnick A. R. (2007). Myosin-IIA heavy-chain phosphorylation regulates the motility of MDA-MB-231 carcinoma cells. Mol. Biol. Cell 18, 3144–3155 10.1091/mbc.E06-11-1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egelhoff T. T., Lee R. J., Spudich J. A. (1993). Dictyostelium myosin heavy chain phosphorylation sites regulate myosin filament assembly and localization in vivo. Cell 75, 363–371 10.1016/0092-8674(93)80077-R [DOI] [PubMed] [Google Scholar]
- Egelhoff T. T., Naismith T. V., Brozovich F. V. (1996). Myosin-based cortical tension in Dictyostelium resolved into heavy and light chain-regulated components. J. Muscle Res. Cell Motil. 17, 269–274 10.1007/BF00124248 [DOI] [PubMed] [Google Scholar]
- Fache S., Dalous J., Engelund M., Hansen C., Chamaraux F., Fourcade B., Satre M., Devreotes P., Bruckert F. (2005). Calcium mobilization stimulates Dictyostelium discoideum shear-flow-induced cell motility. J. Cell Sci. 118, 3445–3458 10.1242/jcs.02461 [DOI] [PubMed] [Google Scholar]
- Geiger J., Wessels D., Soll D. R. (2003). Human polymorphonuclear leukocytes respond to waves of chemoattractant, like Dictyostelium. Cell Motil. Cytoskeleton 56, 27–44 10.1002/cm.10133 [DOI] [PubMed] [Google Scholar]
- Heid P. J., Wessels D., Daniels K. J., Gibson D. P., Zhang H., Voss E., Soll D. R. (2004). The role of myosin heavy chain phosphorylation in Dictyostelium motility, chemotaxis and F-actin localization. J. Cell Sci. 117, 4819–4835 10.1242/jcs.01358 [DOI] [PubMed] [Google Scholar]
- Heid P. J., Geiger J., Wessels D., Voss E., Soll D. R. (2005). Computer-assisted analysis of filopod formation and the role of myosin II heavy chain phosphorylation in Dictyostelium. J. Cell Sci. 118, 2225–2237 10.1242/jcs.02342 [DOI] [PubMed] [Google Scholar]
- Kolman M. F., Futey L. M., Egelhoff T. T. (1996). Dictyostelium myosin heavy chain kinase A regulates myosin localization during growth and development. J. Cell Biol. 132, 101–109 10.1083/jcb.132.1.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laevsky G., Knecht D. A. (2003). Cross-linking of actin filaments by myosin II is a major contributor to cortical integrity and cell motility in restrictive environments. J. Cell Sci. 116, 3761–3770 10.1242/jcs.00684 [DOI] [PubMed] [Google Scholar]
- Levi S., Polyakov M. V., Egelhoff T. T. (2002). Myosin II dynamics in Dictyostelium: determinants for filament assembly and translocation to the cell cortex during chemoattractant responses. Cell Motil. Cytoskeleton 53, 177–188 10.1002/cm.10068 [DOI] [PubMed] [Google Scholar]
- Liang W., Licate L., Warrick H., Spudich J., Egelhoff T. (2002). Differential localization in cells of myosin II heavy chain kinases during cytokinesis and polarized migration. BMC Cell Biol. 3, 19 10.1186/1471-2121-3-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi M. L., Knecht D. A., Lee J. (2008). Mechano-chemical signaling maintains the rapid movement of Dictyostelium cells. Exp. Cell Res. 314, 1850–1859 10.1016/j.yexcr.2008.02.001 [DOI] [PubMed] [Google Scholar]
- Lück–Vielmetter D., Schleicher M., Grabatin B., Wippler J., Gerisch G. (1990). Replacement of threonine residues by serine and alanine in a phosphorylatable heavy chain fragment of Dictyostelium myosin II. FEBS Lett. 269, 239–243 10.1016/0014-5793(90)81163-I [DOI] [PubMed] [Google Scholar]
- Lusche D. F., Wessels D., Soll D. R. (2009). The effects of extracellular calcium on motility, pseudopod and uropod formation, chemotaxis, and the cortical localization of myosin II in Dictyostelium discoideum. Cell Motil. Cytoskeleton 66, 567–587 10.1002/cm.20367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusche D. F., Wessels D., Ryerson D. E., Soll D. R. (2011). Nhe1 is essential for potassium but not calcium facilitation of cell motility and the monovalent cation requirement for chemotactic orientation in Dictyostelium discoideum. Eukaryot. Cell 10, 320–331 10.1128/EC.00255-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusche D., Wessels D., Scherer A., Daniels K., Kuhl S., Soll D. R. (2012). The IplA Ca++ channel of Dictyostelium discoideum is necessary for chemotaxis mediated through Ca++, but not through cAMP chemotaxis, and plays a fundamental role in natural aggregation. J. Cell Sci. 125, 1770–1783 10.1242/jcs.098301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middelbeek J., Clark K., Venselaar H., Huynen M. A., van Leeuwen F. N. (2010). The alpha-kinase family: an exceptional branch on the protein kinase tree. Cell. Mol. Life Sci. 67, 875–890 10.1007/s00018-009-0215-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moores S. L., Sabry J. H., Spudich J. A. (1996). Myosin dynamics in live Dictyostelium cells. Proc. Natl. Acad. Sci. USA 93, 443–446 10.1073/pnas.93.1.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. B., Egelhoff T. T. (1999). Biochemical characterization of a Dictyostelium myosin II heavy-chain phosphatase that promotes filament assembly. Eur. J. Biochem. 264, 582–590 10.1046/j.1432-1327.1999.00670.x [DOI] [PubMed] [Google Scholar]
- Nagasaki A., Itoh G., Yumura S., Uyeda T. Q. (2002). Novel myosin heavy chain kinase involved in disassembly of myosin II filaments and efficient cleavage in mitotic Dictyostelium cells. Mol. Biol. Cell 13, 4333–4342 10.1091/mbc.E02-04-0228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebl T., Fisher P. R. (1997). Intracellular Ca2+ signals in Dictyostelium chemotaxis are mediated exclusively by Ca2+ influx. J. Cell Sci. 110, 2845–2853 [DOI] [PubMed] [Google Scholar]
- Prevarskaya N., Skryma R., Shuba Y. (2010). Ion channels and the hallmarks of cancer. Trends Mol. Med. 16, 107–121 10.1016/j.molmed.2010.01.005 [DOI] [PubMed] [Google Scholar]
- Rai V., Egelhoff T. T. (2011). Role of B regulatory subunits of protein phosphatase type 2A in myosin II assembly control in Dictyostelium discoideum. Eukaryot. Cell 10, 604–610 10.1128/EC.00296-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redowicz M. J. (2001). Regulation of nonmuscle myosins by heavy chain phosphorylation. J. Muscle Res. Cell Motil. 22, 163–173 10.1023/A:1010552929028 [DOI] [PubMed] [Google Scholar]
- Rico M., Egelhoff T. T. (2003). Myosin heavy chain kinase B participates in the regulation of myosin assembly into the cytoskeleton. J. Cell. Biochem. 88, 521–532 10.1002/jcb.10361 [DOI] [PubMed] [Google Scholar]
- Scherer A., Kuhl S., Wessels D., Lusche D. F., Raisley B., Soll D. R. (2010). Ca2+ chemotaxis in Dictyostelium discoideum. J. Cell Sci. 123, 3756–3767 10.1242/jcs.068619 [DOI] [PubMed] [Google Scholar]
- Schlatterer C., Gollnick F., Schmidt E., Meyer R., Knoll G. (1994). Challenge with high concentrations of cyclic AMP induces transient changes in the cytosolic free calcium concentration in Dictyostelium discoideum. J. Cell Sci. 107, 2107–2115 [DOI] [PubMed] [Google Scholar]
- Soll D. R. (1995). The use of computers in understanding how animal cells crawl. Int. Rev. Cytol. 163, 43–104 10.1016/S0074-7696(08)62209-3 [DOI] [PubMed] [Google Scholar]
- Soll D., Voss E. (1998). Two and three dimensional computer systems for analyzing how cells crawl. Motion Analysis of Living Cells (ed. Soll D, Wessels D.), pp. 25–52 New York, NY: John Wiley Inc [Google Scholar]
- Soll D. R., Wessels D., Heid P. J., Zhang H. (2002). A contextual framework for characterizing motility and chemotaxis mutants in Dictyostelium discoideum. J. Muscle Res. Cell Motil. 23, 659–672 10.1023/A:1024459124427 [DOI] [PubMed] [Google Scholar]
- Soll D. R., Wessels D., Kuhl S., Lusche D. F. (2009). How a cell crawls and the role of cortical myosin II. Eukaryot. Cell 8, 1381–1396 10.1128/EC.00121-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D., Wessels D., Lusche D. F., Kuhl S., Scherer A., Grimm S. (2011). Role of extracellular cations in cell motility, polarity, and chemotaxis. Res. Rep. Biol. 2011, 89–99 [Google Scholar]
- Steimle P. A., Naismith T., Licate L., Egelhoff T. T. (2001a). WD repeat domains target Dictyostelium myosin heavy chain kinases by binding directly to myosin filaments. J. Biol. Chem. 276, 6853–6860 10.1074/jbc.M008992200 [DOI] [PubMed] [Google Scholar]
- Steimle P. A., Yumura S., Côté G. P., Medley Q. G., Polyakov M. V., Leppert B., Egelhoff T. T. (2001b). Recruitment of a myosin heavy chain kinase to actin-rich protrusions in Dictyostelium. Curr. Biol. 11, 708–713 10.1016/S0960-9822(01)00182-8 [DOI] [PubMed] [Google Scholar]
- Steimle P. A., Licate L., Côté G. P., Egelhoff T. T. (2002). Lamellipodial localization of Dictyostelium myosin heavy chain kinase A is mediated via F-actin binding by the coiled-coil domain. FEBS Lett. 516, 58–62 10.1016/S0014-5793(02)02494-8 [DOI] [PubMed] [Google Scholar]
- Stites J., Wessels D., Uhl A., Egelhoff T., Shutt D., Soll D. R. (1998). Phosphorylation of the Dictyostelium myosin II heavy chain is necessary for maintaining cellular polarity and suppressing turning during chemotaxis. Cell Motil. Cytoskeleton 39, 31–51 [DOI] [PubMed] [Google Scholar]
- Swaney K. F., Huang C. H., Devreotes P. N. (2010). Eukaryotic chemotaxis: a network of signaling pathways controls motility, directional sensing, and polarity. Annu. Rev. Biophys. 39, 265–289 10.1146/annurev.biophys.093008.131228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchik K. J., Devreotes P. N. (1981). Adenosine 3′,5′-monophosphate waves in Dictyostelium discoideum: a demonstration by isotope dilution-fluorography. Science 212, 443–446 10.1126/science.6259734 [DOI] [PubMed] [Google Scholar]
- Traynor D., Milne J. L., Insall R. H., Kay R. R. (2000). Ca(2+) signalling is not required for chemotaxis in Dictyostelium. EMBO J. 19, 4846–4854 10.1093/emboj/19.17.4846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves S., Vukcevic M., Griesser J., Armstrong C-F., Zhu M. X., Zorzato F. (2010). Agonist-activated Ca2+ influx occurs at stable plasma membrane and endoplasmic reticulum junctions. J. Cell Sci. 123, 4170–4181 10.1242/jcs.068387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood J., Greene J., Steimle P. A. (2010). Identification of a new mechanism for targeting myosin II heavy chain phosphorylation by Dictyostelium myosin heavy chain kinase B. BMC Res. Notes 3, 56 10.1186/1756-0500-3-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum B., Soll D. R. (1981). Chemoresponsiveness to cAMP and folic acid during growth, development, and dedifferentiation in Dictyostelium discoideum. Differentiation 18, 151–160 10.1111/j.1432-0436.1981.tb01116.x [DOI] [PubMed] [Google Scholar]
- Varnum B., Soll D. R. (1984). Effects of cAMP on single cell motility in Dictyostelium. J. Cell Biol. 99, 1151–1155 10.1083/jcb.99.3.1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum B., Edwards K. B., Soll D. R. (1985). Dictyostelium amebae alter motility differently in response to increasing versus decreasing temporal gradients of cAMP. J. Cell Biol. 101, 1–5 10.1083/jcb.101.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum B., Edwards K. B., Soll D. R. (1986). The developmental regulation of single-cell motility in Dictyostelium discoideum. Dev. Biol. 113, 218–227 10.1016/0012-1606(86)90124-7 [DOI] [PubMed] [Google Scholar]
- Vicente–Manzanares M., Ma X., Adelstein R. S., Horwitz A. R. (2009). Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 10, 778–790 10.1038/nrm2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Philipsborn A., Bastmeyer M. (2007). Mechanisms of gradient detection: a comparison of axon pathfinding with eukaryotic cell migration. Int. Rev. Cytol. 263, 1–62 10.1016/S0074-7696(07)63001-0 [DOI] [PubMed] [Google Scholar]
- Wang Y., Chen C-L., Iijima M. (2011). Signaling mechanisms for chemotaxis. Dev. Growth Differ. 53, 495–502 10.1111/j.1440-169X.2011.01265.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels D. J., Zhang H., Reynolds J., Daniels K., Heid P., Lu S., Kuspa A., Shaulsky G., Loomis W. F., Soll D. R. (2000). The internal phosphodiesterase RegA is essential for the suppression of lateral pseudopods during Dictyostelium chemotaxis. Mol. Biol. Cell 11, 2803–2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels D., Lusche D. F., Kuhl S., Heid P., Soll D. R. (2007). PTEN plays a role in the suppression of lateral pseudopod formation during Dictyostelium motility and chemotaxis. J. Cell Sci. 120, 2517–2531 10.1242/jcs.010876 [DOI] [PubMed] [Google Scholar]
- Wessels D. J., Kuhl S., Soll D. R. (2009). Light microscopy to image and quantify cell movement. Methods Mol. Biol. 571, 455–471 10.1007/978-1-60761-198-1_30 [DOI] [PubMed] [Google Scholar]
- Yumura S., Fukui Y. (1985). Reversible cyclic AMP-dependent change in distribution of myosin thick filaments in Dictyostelium. Nature 314, 194–196 10.1038/314194a0 [DOI] [PubMed] [Google Scholar]
- Yumura S., Uyeda T. Q. (1997). Myosin II can be localized to the cleavage furrow and to the posterior region of Dictyostelium amoebae without control by phosphorylation of myosin heavy and light chains. Cell Motil. Cytoskeleton 36, 313–322 [DOI] [PubMed] [Google Scholar]
- Yumura S., Mori H., Fukui Y. (1984). Localization of actin and myosin for the study of ameboid movement in Dictyostelium using improved immunofluorescence. J. Cell Biol. 99, 894–899 10.1083/jcb.99.3.894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumura S., Furuya K., Takeuchi I. (1996). Intracellular free calcium responses during chemotaxis of Dictyostelium cells. J. Cell Sci. 109, 2673–2678 [DOI] [PubMed] [Google Scholar]
- Yumura S., Yoshida M., Betapudi V., Licate L. S., Iwadate Y., Nagasaki A., Uyeda T. Q., Egelhoff T. T. (2005). Multiple myosin II heavy chain kinases: roles in filament assembly control and proper cytokinesis in Dictyostelium. Mol. Biol. Cell 16, 4256–4266 10.1091/mbc.E05-03-0219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H. (1977). Ability of polymorphonuclear leukocytes to orient in gradients of chemotactic factors. J. Cell Biol. 75, 606–616 10.1083/jcb.75.2.606 [DOI] [PMC free article] [PubMed] [Google Scholar]