Combinatorial approaches to catalysis have made an impact in targeted transformation development, including Ag-mediated carbene insertion,[1] Sc-pybox-based asymmetric cyclopropanation,[2] and Rh/Ir-based asymmetric hydrogenation.[3] Useful design elements have emerged from these studies, e.g. the value of ligand self assembly,[4] or of the inclusion of peptide-like structural elements[5],[6],[7] in building ligand arrays. Efficient screening methods are of paramount importance for such efforts. Methods based on fluorescence,[8] REMPI,[9] MS,[10] NMR,[11] and IR thermography[12] have appeared. A chromophore may be installed into the substrate[13] or product[14] of the reaction under study. Alternatively, one can exploit chromophores inherent in proteins[15] or enzyme-associated reactions,[16] and use these sensors to report back on product formation and composition.
Our group has developed an in situ enzymatic screening (ISES) approach whereby an organometallic reaction under study is coupled to an enzymatic reporting reaction in real time.[17] This screening method led to the discovery of the first asymmetric allylic amination with Ni(0)[18] and to the identification of novel salen ligands with promise for asymmetric synthesis.[19] Those approaches involved dehydrogenase enzymes[20] as sensors, utilizing the inherent nicotinamide cofactor to provide a UV signal.
This Communication describes an important new ISES-mode in which the reporting enzymes lead to a visible signal in real time. The advantages of colorimetric approaches have been articulated[13, 21] and include the ability to screen a diverse array of catalysts with the naked eye, without employing specialized equipment, increasing convenience and throughput.
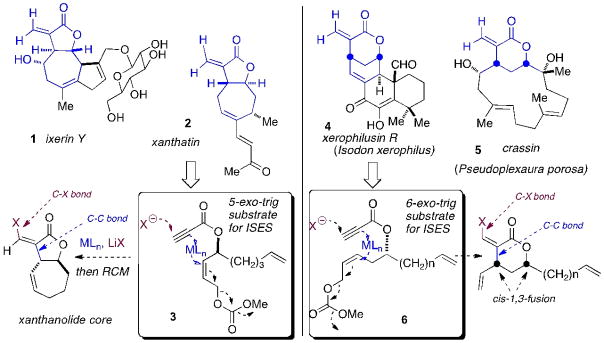
Synthetically, this study was directed at developing formal halometalation/carbocyclization transformations. One can envision this providing a rapid entry into the cores of terpenoid natural products featuring exomethylene γ-lactones (Scheme 1). The 6-exo-trig substrate 6 would lead into cores of xerophilusin and crassin, a specific modulator of STAT phosphorylation.[27] The 5-exo-trig substrate 3 is designed to give 5,7-sesquiterpenoid lactone cores.[28] Key natural products (NPs) here include the guaianolide, ixerin Y (1),[29]] and xanthatin (2), which shows anti-MRSA,[30] antifungal[31] and anti-ulcer[32] activity.
Scheme 1.
Proposed halometalation/carbocyclization routes into the core structures of terpenoid exomethylene lactone natural products.
Related NP-derived α-methylene butyrolactone moieties appear to Michael-add Cys-38[33] of the transcription factor NF-κB,[34] thereby blocking DNA binding.[35] Our synthetic approach is particularly attractive in light of such SAR postulates, as it would deliver the NP core with a β-halo-α,β-unsaturated lactone moiety, for potential target capture, in vivo, and also to tap into cross-coupling chemistry ex vivo, for chain extension/library elaboration. The drive toward streamlined methods for the construction of such NP cores is motivated by the effectiveness of NP-core based chemical biology libraries in defining studies by groups such as Schreiber,[22] Waldmann,[23] Shair, [24]Arndt [25]and Snapper.[26]
While the chemistry envisioned in Scheme 1 remained largely unexplored, there was some precedent from the work of Lu,[36] primarily with acetoxy-metalation/carbocyclization, employing Pd(II) catalysis in neat acetic acid[37] as solvent. We set out to examine a much broader spectrum of metal, (pseudo)halide and substrate space, combinatorially, using visual colorimetric-ISES for higher throughput.
We demonstrate herein that the combination of alcohol oxidase and peroxidase serves as an effective reporting duo for the title transformation (Figure 1). By utilizing ABTS [2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonate)], as peroxidase cofactor, one achieves particular sensitivity. This is because each molecule of alcohol (by)product emanating from the organic reaction of interest that is oxidized by the alcohol oxidase reporter gives rise to two equivalents of ABTS radical cation, providing for an intense (ε405-414 (2 ABTS.+) ~ 70,000 M−1cm−1) [38] colorimetric signal in the visible range (jungle green). This allows for first pass scanning of a large number of potential catalytic combinations with the naked eye (Figure 2). A more quantitative ranking (relative rates) may then be obtained by UV-visible spectrophotometry on first pass hits (Fig. 3).
Figure 1.
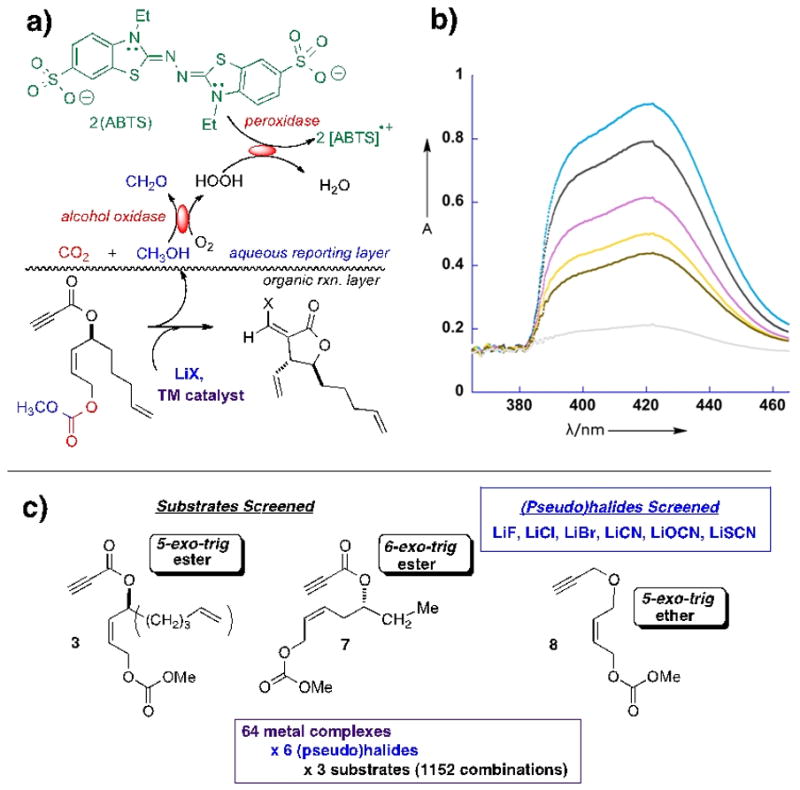
a) - Configuration of this in situ screen; b) – UV spectrum for the formation of ABTS radical cation with time; c) – potential catalytic combinations screened
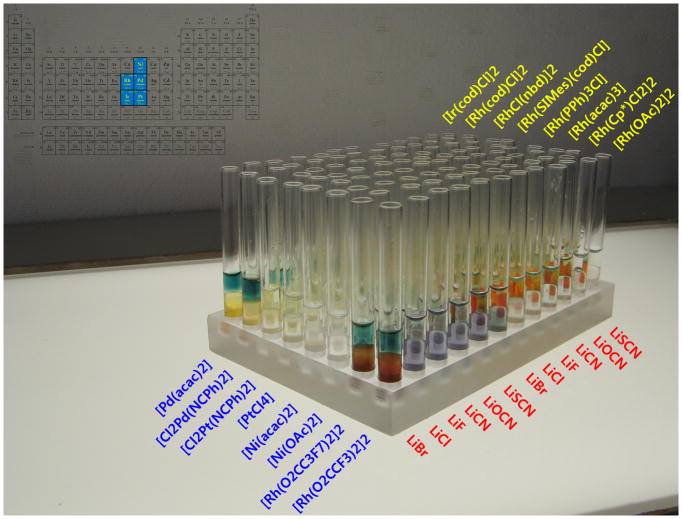
Figure 2.
Example of a d9-d10 screening array for substrate 3 –sixteen metal complexes are screened across six (pseudo)halides with propiolate ester 3 (5-exo-trig substrate).
Figure 3.
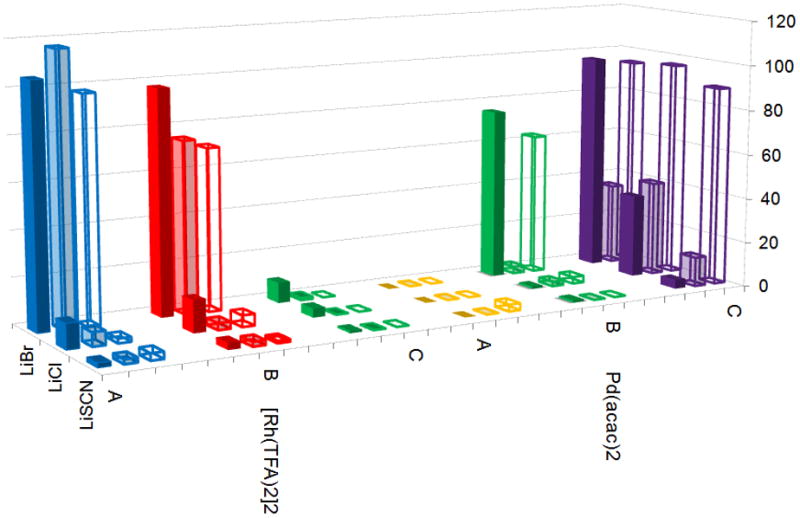
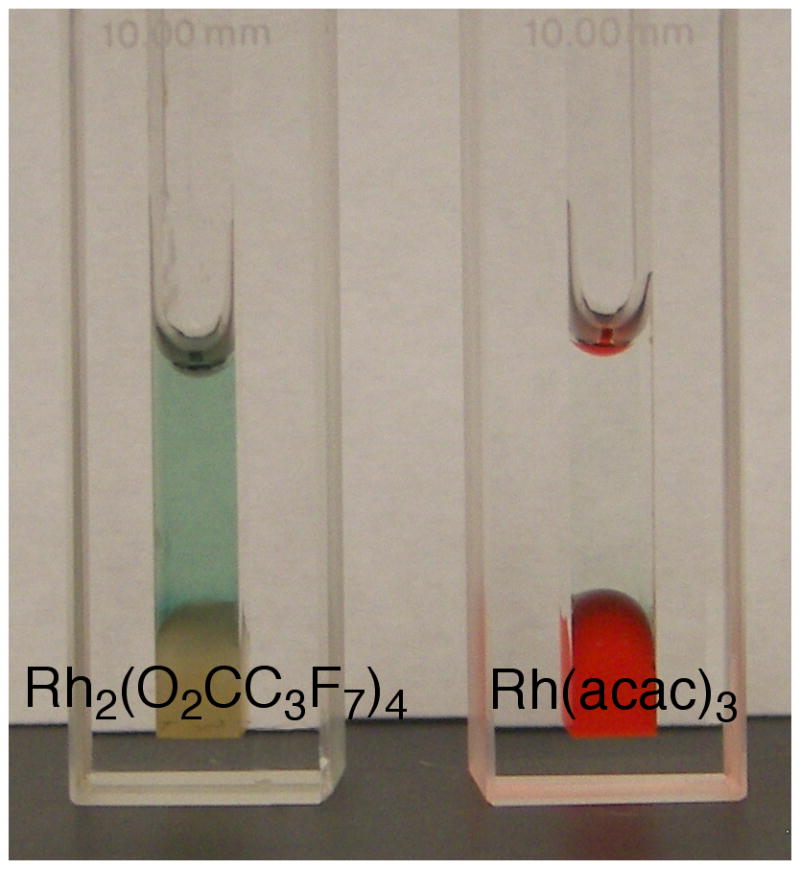
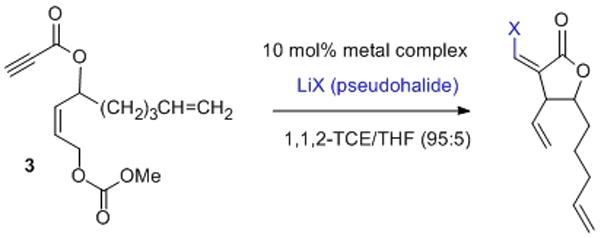
Initial hits from visual colorimetric-ISES are ranked spectrophotometrically (Abs405 in mAbs min−1; A, B and C are respectively substrates 3, 7 and 8-see SI for conditions). Right side: example of the cuvette-ISES experiment. Note: The ABTS indicator shows turnover with Rh(II)-perfluorobutyrate, while the highly colored Rh(III) catalyst fails.
A broad array of 64 metal catalyst candidates was chosen; subdivided into 4 groups of 16 catalysts each, as detailed in the SI. These were screened against 6 (pseudo)halides (LiF, LiCl, LiBr, LiCN, LiOCN, LiSCN) and 3 candidate substrates (3, 7, 8), creating a 64x6x3 =1152 combinatorial array. Figure 2 shows a 96 well tray for the metal set III vs. substrate 3. These were run in a convenient 300 μL format (200 μL organic/100 μL aq. enz). One sees clear positive readouts for the combination of LiBr with both Rh(II)-perfluorocarboxylates (in contrast to the Rh(II) carboxylate catalyst), as well as with Pd(II)(acac) [but not Ni(II)(acac)] and Cl2Pd(NCPh)2 [but not Cl2Pt(NCPh)2].
The most interesting hits in the colorimetric tray screen were next “cherry-picked” visually, and then ranked more quantitatively by spectrophotometric analysis in the cuvette (Fig. 3). As can be seen, for Pd(II), the cyclization chemistry proceeds efficiently with (PhCN)2PdCl2 and LiBr for both the 5-exo-trig-ester and –ether substrates. Acetic acid clearly is not necessary for these cyclizations. Among other Pd(II) catalysts screened, Pd(acac)2, gave the next fastest rates.
However, the most generally effective catalytic combination found was LiBr with the Rh(II)-perfluorocarboxylates, providing efficient formal bromorhodiation/carbocyclization, across all three test substrates, in stark contrast to Rh(II)-acetate dimer, and all Rh(I)- and Rh(III)-complexes examined. This reactivity was verified under standard RB flask conditions, through which product identity, stereochemistry and yield were established (Figure 4). Note that the cyclizations are highly diastereoselective, giving the 1,2-trans stereochemistry for the xanthatin core from 3, and the 1,3-cis stereochemistry for the crassin-type core from 7. Also of interest, catalyst loading could be lowered to 2.5 mol%, without compromise in yield, by gentle heating or sonication (SI).
Figure 4.
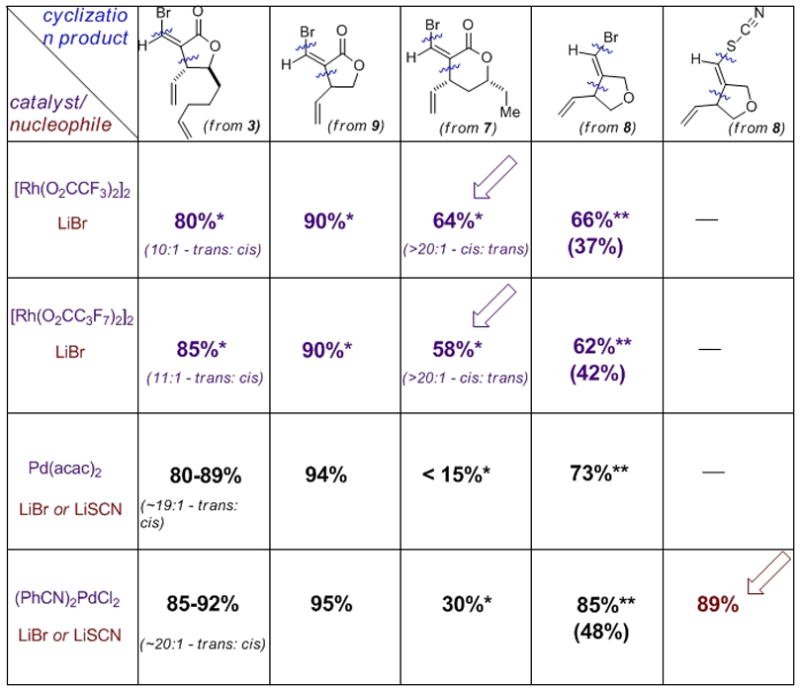
Chart showing success of catalytic metal-(pseudo)halide combinations as a function of substrate. Isolated yields for homogeneous material, following standard RB flask reactions (*reaction carried out at 60°C; **GC yield)
This would appear to constitute a new reaction mode for the Rh(II)/LiX combination. Control experiments established that this reactivity is not a function of stray TFA (see SI). The disparate reactivity of Rh(II)-perfluorocarboxylates vs. Rh(II)-carboxylates is reminiscent of observations by Padwa and Doyle, the tendency of only the former to promote electrophilic aromatic substitution over carbene insertion.[39] Clearly this unusual and valuable reactivity warrants further exploration.
Perhaps of equal significance, the combination of (PhCN)2PdCl2 with LiSCN yields an unprecedented formal thiocyano-palladation/carbocyclization transformation. As such, this reaction manifold assembles a cyclic NP-core bearing a terminal vinyl thiocyanate in one operation [Note: product structure verified both spectroscopically and chemically-SI]. Given the importance of the thiocyanate functionality for elegant vibrational Stark effect studies or active site environmnet by Boxer,[40] this transformation will likely be of real value to chemical biologists.
We next utilized the new Rh(II)-perfluorocarboxylate chemistry to fashion a small xanthatin-core library, based upon stereo-controlled synthesis, followed by tailoring chemistry (Scheme 2). Alpine-Borane-mediated ynone reduction establishes the absolute stereochemistry,[41] (see SI), while the bromometallation/carbocyclization sets the relative stereochemistry. Finally, RCM yields the desired xanthanolide core, with the bromomethylene lactone undergoing Pd-mediated chain extension reactions as projected (Scheme 3).
Scheme 2.
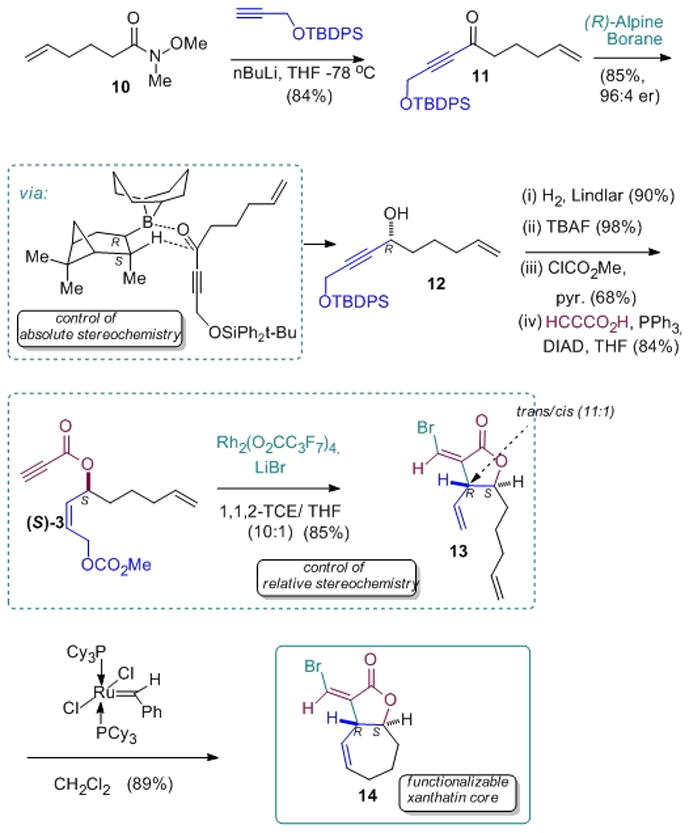
Application of the new halometalation/carbocyclization route to the stereocontrolled synthesis of the xanthatin core.
Scheme 3.
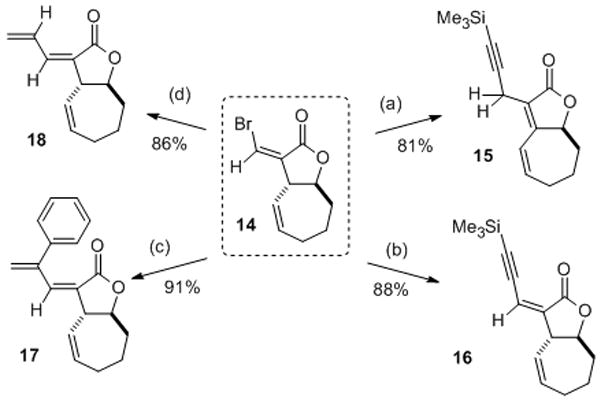
Exploitation of the bromomethylene lactone functionality for transition metal-mediated tailoring chemistry upon the bicyclic xanthanolide core: (a) HCC-TMS, Cui, Cl2Pd (PPh3)2, Et2NH; (b) Bu3SnCC-TMS, cat. Pd2dba3, Pfur3, Δ (c) BrZnC(Ph)C=CH2, Pd(PPh3)4 Δ. (d) Bu3SnCH=CH2, Pd2dba3, Pfur3, Δ.
Note that the standard Sonagashira coupling proceeds with double bond migration, yielding bicyclic dienoate in 15. Use of modified Stille conditions (i.e. stannylated acetylene) prevents this to give ynenoate 16. All analogues feature more extended Michael acceptors of potential interest, given the mechanistic hypothesis that has been advanced (vide supra). In summary, the first application of visible, colorimetric ISES has uncovered both a generalizable Rh(II)-mediated halometalation/carbocyclization and the first formal thiocyanometalation/carbocyclization. Current efforts are to explore the scope of these new tandem bond constructions, and of the colorimetric screen that led to their discovery.
Supplementary Material
Acknowledgments
DBB currently serves as a Program Director at the National Science Foundation. This research was facilitated by the Individual Research & Development (IR/D) program associated with that appointment. The authors acknowledge NSF-CHE-0911732 for research support. We thank the NIH (SIG-1-510-RR-06307) and NSF (CHE-0091975, MRI-0079750) for NMR instrumentation, and the NIH (RR016544) for facilities renovation.
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.Burgess K, Lim HJ, Porte AM, Sulikowski GA. Angew Chem, Int Ed. 1996;35:220–222. [Google Scholar]
- 2.Moye-Sherman D, Welch MB, Reibenspies J, Burgess K. Chem Commun. 1998:2377–2378. [Google Scholar]
- 3.a) Ager DJ, Lefort L, de Vries JG. ACS Symp Ser. 2009;1009:239–250. [Google Scholar]; b) Reetz MT, Bondarev O. Angew Chem, Int Ed. 2007;46:4523–4526. doi: 10.1002/anie.200700533. [DOI] [PubMed] [Google Scholar]; c) de Vries JG, Lefort L. Chemistry--A European Journal. 2006;12:4722–4734. doi: 10.1002/chem.200500819. [DOI] [PubMed] [Google Scholar]
- 4.a) Weis M, Waloch C, Seiche W, Breit B. J Am Chem Soc. 2006;128:4188–4189. doi: 10.1021/ja058202o. [DOI] [PubMed] [Google Scholar]; b) Takacs JM, Chaiseeda K, Moteki SA, Reddy DS, Wu D, Chandra K. Pure Appl Chem. 2006;78:501–509. [Google Scholar]
- 5.a) Fowler BS, Mikochik PJ, Miller SJ. J Am Chem Soc. 2010;132:2870–2871. doi: 10.1021/ja9107897. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Gustafson JL, Lim D, Miller SJ. Science. 2010;328:1251–1255. doi: 10.1126/science.1188403. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Jordan PA, Kayser-Bricker KJ, Miller SJ. Proc Natl Acad Sci U S A. 2010;107:20620–20624. doi: 10.1073/pnas.1001111107. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Agarkov A, Greenfield S, Xie D, Pawlick R, Starkey G, Gilbertson SR. Biopolymers. 2006;84:48–73. doi: 10.1002/bip.20395. [DOI] [PubMed] [Google Scholar]
- 6.Francis MB, Jacobsen EN. Angew Chem, Int Ed. 1999;38:937–941. doi: 10.1002/(SICI)1521-3773(19990401)38:7<937::AID-ANIE937>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 7.Wieland LC, Vieira EM, Snapper ML, Hoveyda AH. J Am Chem Soc. 2009;131:570–576. doi: 10.1021/ja8062315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a) Biswas R, Maillard N, Kofoed J, Reymond JL. Chem Commun. 2010;46:8746–8748. doi: 10.1039/c0cc02700f. [DOI] [PubMed] [Google Scholar]; b) Becker S, Hoebenreich H, Vogel A, Knorr J, Wilhelm S, Rosenau F, Jaeger KE, Reetz MT, Kolmar H. Angew Chem, Int Ed. 2008;47:5085–5088. doi: 10.1002/anie.200705236. [DOI] [PubMed] [Google Scholar]; c) Lewis WG, Magallon FG, Fokin VV, Finn MG. J Am Chem Soc. 2004;126:9152–9153. doi: 10.1021/ja048425z. [DOI] [PubMed] [Google Scholar]; d) Stauffer SR, Hartwig JF. J Am Chem Soc. 2003;125:6977–6985. doi: 10.1021/ja034161p. [DOI] [PubMed] [Google Scholar]; e) Jarvo ER, Evans CA, Copeland GT, Miller SJ. J Org Chem. 2001;66:5522–5527. doi: 10.1021/jo015803z. [DOI] [PubMed] [Google Scholar]
- 9.Senkan SM. Nature. 1998;394:350–353. [Google Scholar]
- 10.a) Lichtor PA, Miller SJ. ACS Combinatorial Science. 2011;13:321–326. doi: 10.1021/co200010v. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ebner C, Muller CA, Markert C, Pfaltz A. J Am Chem Soc. 2011;133:4710–4713. doi: 10.1021/ja111700e. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Wassenaar J, Jansen E, van Zeist WJ, Bickelhaupt FM, Siegler MA, Spek AL, Reek JNH. Nature: Chemistry. 2010;2:417–421. doi: 10.1038/nchem.614. [DOI] [PubMed] [Google Scholar]; d) Mueller CA, Pfaltz A. Angew Chem, Int Ed. 2008;47:3363–3366. doi: 10.1002/anie.200705081. [DOI] [PubMed] [Google Scholar]; e) Chen P. Angew Chem, Int Ed. 2003;42:2832–2847. doi: 10.1002/anie.200200560. [DOI] [PubMed] [Google Scholar]
- 11.a) Reetz MT, Tielmann P, Eipper A, Ross A, Schlotterbeck G. Chem Commun. 2004:1366–1367. doi: 10.1039/b403249g. [DOI] [PubMed] [Google Scholar]; b) Evans MA, Morken JP. J Am Chem Soc. 2002;124:9020–9021. doi: 10.1021/ja026703t. [DOI] [PubMed] [Google Scholar]
- 12.a) Reetz MT, Becker MH, Liebl M, Furstner A. Angew Chem, Int Ed. 2000;39:1236–1239. doi: 10.1002/(sici)1521-3773(20000403)39:7<1236::aid-anie1236>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]; b) Taylor SJ, Morken JP. Science. 1998;280:267–270. doi: 10.1126/science.280.5361.267. [DOI] [PubMed] [Google Scholar]
- 13.Loch JA, Crabtree RH. Pure Appl Chem. 2001;73:119–128. [Google Scholar]
- 14.a) Lavastre O, Morken JP. Angew Chem, Int Ed. 1999;38:3163–3165. doi: 10.1002/(sici)1521-3773(19991102)38:21<3163::aid-anie3163>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]; b) Moreira R, Havranek M, Sames D. J Am Chem Soc. 2001;123:3927–3931. doi: 10.1021/ja003786+. [DOI] [PubMed] [Google Scholar]
- 15.a) Matsushita H, Yamamoto N, Meijler MM, Wirsching P, Lerner RA, Matsushita M, Janda KD. Molecular BioSystems. 2005;1:303–306. doi: 10.1039/b511408j. [DOI] [PubMed] [Google Scholar]; b) Matsushita M, Yoshida K, Yamamoto N, Wirsching P, Lerner RA, Janda KD. Angew Chem, Int Ed. 2003;42:5984–5987. doi: 10.1002/anie.200352793. [DOI] [PubMed] [Google Scholar]; c) Taran F, Gauchet C, Mohar B, Meunier S, Valleix A, Renard PY, Creminon C, Grassi J, Wagner A, Mioskowski C. Angew Chem, Int Ed. 2002;41:124–127. doi: 10.1002/1521-3773(20020104)41:1<124::aid-anie124>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 16.a) Hamberg A, Lundgren S, Penhoat M, Moberg C, Hult K. J Am Chem Soc. 2006;128:2234–2235. doi: 10.1021/ja058474r. [DOI] [PubMed] [Google Scholar]; b) Sprout CM, Seto CT. Org Lett. 2005;7:5099–5102. doi: 10.1021/ol0521681. [DOI] [PubMed] [Google Scholar]; c) Abato P, Seto CT. J Am Chem Soc. 2001;123:9206–9207. doi: 10.1021/ja016177q. [DOI] [PubMed] [Google Scholar]
- 17.Berkowitz DB, Bose M, Choi S. Angew Chem, Int Ed. 2002;41:1603–1607. doi: 10.1002/1521-3773(20020503)41:9<1603::aid-anie1603>3.0.co;2-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.a) Berkowitz DB, Shen W, Maiti G. Tetrahedron: Asymmetry. 2004;15:2845–2851. doi: 10.1016/j.tetasy.2004.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Berkowitz DB, Maiti G. Org Lett. 2004;6:2661–2664. doi: 10.1021/ol049159x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.a) Dey S, Powell DR, Hu C, Berkowitz DB. Angew Chem, Int Ed. 2007;46:7010–7014. doi: 10.1002/anie.200701280. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Dey S, Karukurichi KR, Shen W, Berkowitz DB. J Am Chem Soc. 2005;127:8610–8611. doi: 10.1021/ja052010b. [DOI] [PubMed] [Google Scholar]
- 20.For complementary examples of ADHs in asymmetric synthesis, see: Applegate GA, Cheloha RW, Nelson DL, Berkowitz DB. Chem Commun. 2011;47:2420–2422. doi: 10.1039/c0cc04585c.Friest JA, Maezato Y, Broussy S, Blum P, Berkowitz DB. J Am Chem Soc. 2010;132:5930–5931. doi: 10.1021/ja910778p.Broussy S, Cheloha RW, Berkowitz DB. Org Lett. 2009;11:305–308. doi: 10.1021/ol802464g.
- 21.a) Folmer-Andersen JF, Lynch VM, Anslyn EV. J Am Chem Soc. 2005;127:7986–7987. doi: 10.1021/ja052029e. [DOI] [PubMed] [Google Scholar]; b) Kawatsura M, Hartwig JF. Organometallics. 2001;20:1960–1964. [Google Scholar]
- 22.a) Schreiber SL. Nat Chem Biol. 2007;3:352. doi: 10.1038/nchembio0707-352. [DOI] [PubMed] [Google Scholar]; b) Burke MD, Berger EM, Schreiber SL. J Am Chem Soc. 2004;126:14095–14104. doi: 10.1021/ja0457415. [DOI] [PubMed] [Google Scholar]
- 23.a) Basu S, Ellinger B, Rizzo S, Deraeve C, Schurmann M, Preut H, Arndt HD, Waldmann H. Proc Natl Acad Sci U S A. 2011;108:6805–6810. doi: 10.1073/pnas.1015269108. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Waldmann H. Nat Chem Biol. 2009;5:76–77. doi: 10.1038/nchembio0209-76. [DOI] [PubMed] [Google Scholar]
- 24.a) Pelish HE, Peterson JR, Salvarezza SB, Rodriguez-Boulan E, Chen JL, Stamnes M, Macia E, Feng Y, Shair MD, Kirchhausen T. Nat Chem Biol. 2006;2:39–46. doi: 10.1038/nchembio751. [DOI] [PubMed] [Google Scholar]; b) Pelish HE, Westwood NJ, Feng Y, Kirchhausen T, Shair MD. J Am Chem Soc. 2001;123:6740–6741. doi: 10.1021/ja016093h. [DOI] [PubMed] [Google Scholar]
- 25.a) Hackenberger CPR, Arndt HD, Schwarzer D. Chemie in Unserer Zeit. 2010;44:198–206. [Google Scholar]; b) Walther T, Renner S, Waldmann H, Arndt HD. ChemBioChem. 2009;10:1153–1162. doi: 10.1002/cbic.200900035. [DOI] [PubMed] [Google Scholar]
- 26.a) Kim BG, Chun TG, Lee HY, Snapper ML. Bioorg Med Chem. 2009;17:6707–6714. doi: 10.1016/j.bmc.2009.07.061. [DOI] [PubMed] [Google Scholar]; b) Radeke HS, Digits CA, Bruner SD, Snapper ML. J Org Chem. 1997;62:2823–2831. doi: 10.1021/jo962292l. [DOI] [PubMed] [Google Scholar]
- 27.Krutzik PO, Crane JM, Clutter MR, Nolan GP. Nat Chem Biol. 2008;4:132–142. doi: 10.1038/nchembio.2007.59. [DOI] [PubMed] [Google Scholar]
- 28.a) Kummer DA, Brenneman JB, Martin SF. Org Lett. 2005;7:4621–4623. doi: 10.1021/ol051711a. [DOI] [PubMed] [Google Scholar]; b) Evans MA, Morken JP. Org Lett. 2005;7:3371–3373. doi: 10.1021/ol051276k. [DOI] [PubMed] [Google Scholar]; c) Nosse B, Chhor RB, Jeong WB, Boehm C, Reiser O. Org Lett. 2003;5:941–944. doi: 10.1021/ol034141s. [DOI] [PubMed] [Google Scholar]
- 29.Ma J-Y, Wang Z-T, Xu L-S, Xu G-J. Phytochemistry. 1999;50:113–115. doi: 10.1016/s0031-9422(98)00452-x. [DOI] [PubMed] [Google Scholar]
- 30.Sato Y, Oketani H, Yamada T, Singyouchi K-I, Ohtsubo T, Kihara M, Shibata H, Higuti T. J Pharm Pharmacol. 1997;49:1042–1044. doi: 10.1111/j.2042-7158.1997.tb06038.x. [DOI] [PubMed] [Google Scholar]
- 31.Pinel B, Landreau A, Seraphin D, Larcher G, Bouchara J-P, Richomme P. J Enzyme Inhib Med Chem. 2005;20:575–579. doi: 10.1080/14756360500213231. [DOI] [PubMed] [Google Scholar]
- 32.Favier LS, Maria AOM, Wendel GH, Borkowski EJ, Giordano OS, Pelzer L, Tonn CE. J Ethnopharmacology. 2005;100:260–267. doi: 10.1016/j.jep.2005.02.042. [DOI] [PubMed] [Google Scholar]
- 33.Wagner S, Hofmann A, Siedle B, Terfloth L, Merfort I, Gasteiger J. J Med Chem. 2006;49:2241–2252. doi: 10.1021/jm051125n. [DOI] [PubMed] [Google Scholar]
- 34.Schmitz ML, Mattioli I, Buss H, Kracht M. ChemBioChem. 2004;5:1348–1358. doi: 10.1002/cbic.200400144. [DOI] [PubMed] [Google Scholar]
- 35.a) Lopez-Anton N, Hermann C, Murillo R, Merfort I, Wanner G, Vollmar AM, Dirsch VM. Apoptosis. 2007;12:41–153. doi: 10.1007/s10495-006-0331-2. [DOI] [PubMed] [Google Scholar]; b) Siedle B, Garcia-Pineres AJ, Murillo R, Schulte-Moenting J, Castro V, Ruengeler P, Klaas CA, Da Costa FB, Kisiel W, Merfort I. J Med Chem. 2004;47:6042–6054. doi: 10.1021/jm049937r. [DOI] [PubMed] [Google Scholar]
- 36.a) Xie X, Lu X, Liu Y, Xu W. J Org Chem. 2001;66:6545–6550. doi: 10.1021/jo001704u. [DOI] [PubMed] [Google Scholar]; b) Zhu G, Lu X. Organometallics. 1995;14:4899–4904. [Google Scholar]; c) Cook GR, Hayashi R. Org Lett. 2006;8:1045–1048. doi: 10.1021/ol052802a. [DOI] [PubMed] [Google Scholar]
- 37.a) Song J, Shen Q, Xu F, Lu X. Tetrahedron. 2007;63:5148–5153. [Google Scholar]; b) Zhang Q, Lu X. J Am Chem Soc. 2000;122:7604–7605. [Google Scholar]
- 38.a) Kulys J, Bratkovskaja I. Talanta. 2007;72:526–531. doi: 10.1016/j.talanta.2006.11.011. [DOI] [PubMed] [Google Scholar]; b) Solis-Oba M, Ugalde-Saldivar VM, Gonzalez I, Viniegra-Gonzalez G. J Electroanal Chem. 2005;579:59–66. [Google Scholar]; c) Scott SL, Chen WJ, Bakac A, Espenson JH. J Phys Chem. 1993;97:6710–6714. [Google Scholar]
- 39.Padwa A, Austin DJ, Price AT, Semones MA, Doyle MP, Protopopova MN, Winchester WR, Tran A. J Am Chem Soc. 1993;115:8669–8680. [Google Scholar]
- 40.a) Fafarman AT, Sigala PA, Herschlag D, Boxer SG. J Am Chem Soc. 2010;132:12811–12813. doi: 10.1021/ja104573b. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Sigala PA, Fafarman AT, Bogard PE, Boxer SG, Herschlag D. J Am Chem Soc. 2007;129:12104–12105. doi: 10.1021/ja075605a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Midland MM. Chem Rev. 1989;89:1553–1561. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.