Abstract
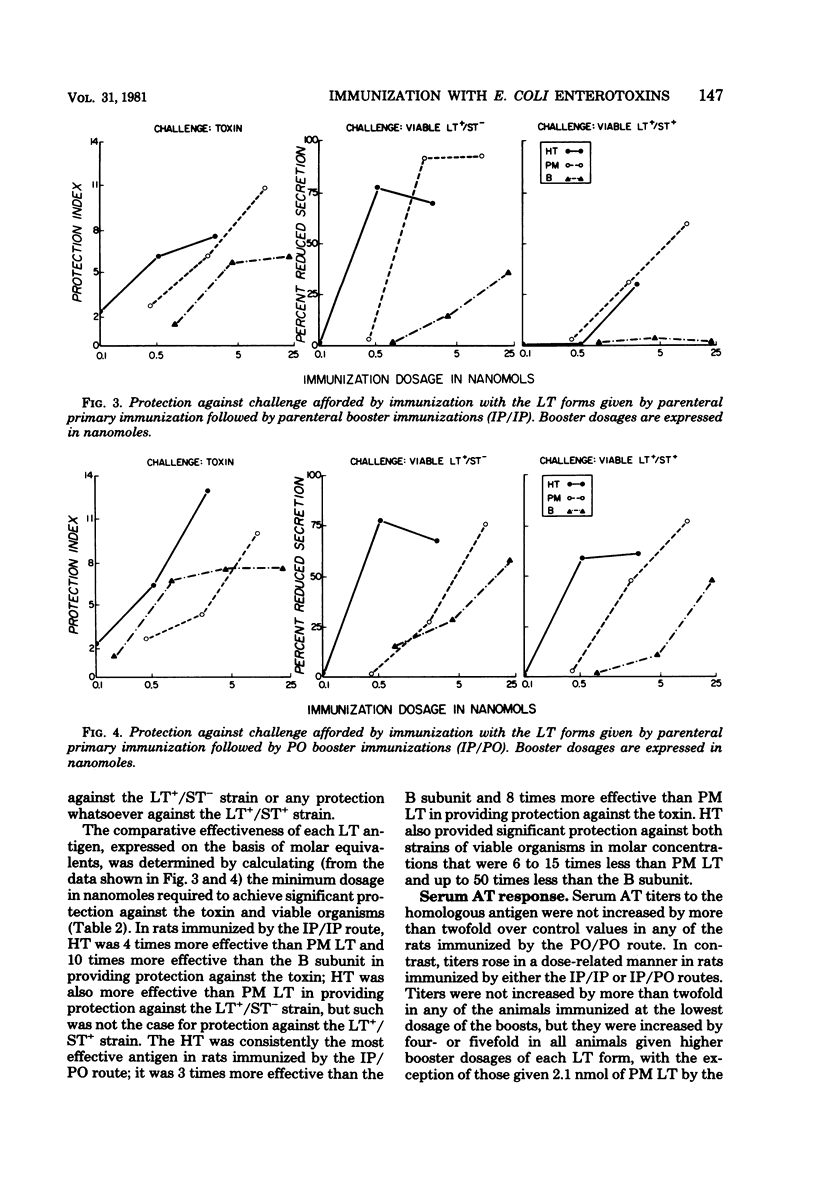
The relative immunogenicities of three forms of the Escherichia coli heatlabile enterotoxin (LT), the holotoxin, its B subunit, and the polymyxin-release form (PM LT) were compared by immunizing rats with various dosages of each given exclusively by the parenteral (IP/IP) or peroral (PO/PO) routes or by a combination of the two (IP/PO). The degree of protection was evaluated by challenge in ligated ileal loops, and the serum antitoxin response was determined by an enzyme-linked immunosorbent assay with homologous antigens. When given by the PO/PO route, each LT antigen provided only weak protection against the toxin and virtually none against viable LT-producing strains; serum antitoxin titers were not significantly increased. When the toxins were given after a parental primary immunization by either the IP/IP or the IP/PO routes, each LT antigen provided a dose-related increase in serum antitoxin titers and in the degree of protection against the toxin as well as against viable strains which produce LT alone (LT+/ST−) or in combination with the heat-stable toxin (LT+/ST+). The degree of protection against viable bacteria, particularly the LT+/ST+ strain, was stronger in animals which received booster immunizations by the PO route. When expressed on the basis of molar equivalents, holotoxin provided significant protection (a protection index of >5 against toxin challenge and >50% reduced secretion with bacterial challenge) with 4 to 15 times fewer moles than PM LT and up to 50 times fewer moles than the B subunit. These observations indicate that, on the basis of molar equivalents, the holotoxin (which contains one A plus five or six B subunits) is a more potent immunogen than either PM LT (which contains one A and probably one B subunit) or the B subunit.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brimblecombe R. W., Duncan W. A., Durant G. J., Emmett J. C., Ganellin C. R., Leslie G. B., Parsons M. E. Characterization and development of cimetidine as a histamine H2-receptor antagonist. Gastroenterology. 1978 Feb;74(2 Pt 2):339–347. [PubMed] [Google Scholar]
- Clegg S., Evans D. G., Evans D. J., Jr Enzyme-linked immunosorbent assay for quantitating the humoral immune response to the colonization factor antigen of enterotoxigenic Escherichia coli. Infect Immun. 1980 Feb;27(2):525–531. doi: 10.1128/iai.27.2.525-531.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Finkelstein R. A. Demonstration of shared and unique immunological determinants in enterotoxins from Vibrio cholerae and Escherichia coli. Infect Immun. 1978 Dec;22(3):709–713. doi: 10.1128/iai.22.3.709-713.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Finkelstein R. A. Isolation and characterization of homogeneous heat-labile enterotoxins with high specific activity from Escherichia coli cultures. Infect Immun. 1979 Jun;24(3):760–769. doi: 10.1128/iai.24.3.760-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Yancey R. J., Finkelstein R. A. Properties of homogeneous heat-labile enterotoxin from Escherichia coli. Infect Immun. 1980 Jul;29(1):91–97. doi: 10.1128/iai.29.1.91-97.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas W. S., Falkow S. The molecular nature of heat-labile enterotoxin (LT) of escherichia coli. Nature. 1979 Feb 1;277(5695):406–407. doi: 10.1038/277406a0. [DOI] [PubMed] [Google Scholar]
- Donta S. T., Viner J. P. Inhibition of the steroidogenic effects of cholera and heat-labile Escherichia coli enterotoxins by GM1 ganglioside: evidence for a similar receptor site for the two toxins. Infect Immun. 1975 May;11(5):982–985. doi: 10.1128/iai.11.5.982-985.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Evans D. G., Gorbach S. L. Polymyxin B-Induced Release of Low-Molecular-Weight, Heat-Labile Enterotoxin from Escherichia coli. Infect Immun. 1974 Nov;10(5):1010–1017. doi: 10.1128/iai.10.5.1010-1017.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G. Inhibition of immune hemolysis: serological assay for the heat-labile enterotoxin of Excherichia coli. J Clin Microbiol. 1977 Jan;5(1):100–105. doi: 10.1128/jcm.5.1.100-105.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A. Antitoxic immunity in experimental cholera: observations with purified antigens and the ligated ileal loop model. Infect Immun. 1970 May;1(5):464–467. doi: 10.1128/iai.1.5.464-467.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K., Finkelstein R. A. Antitoxic immunity in experimental cholera: comparison of immunity induced perorally and parenterally in mice. J Infect Dis. 1972 Jun;125(6):647–655. doi: 10.1093/infdis/125.6.647. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Richardson S. H. Adenosine diphosphate-ribosylation of adenylate cyclase catalyzed by heat-labile enterotoxin of Escherichia coli: comparison with cholera toxin. J Infect Dis. 1980 Jan;141(1):64–70. doi: 10.1093/infdis/141.1.64. [DOI] [PubMed] [Google Scholar]
- Gyles C. L. Relationships among heat-labile enterotoxins of Escherichia coli and Vibrio cholerae. J Infect Dis. 1974 Mar;129(3):277–283. doi: 10.1093/infdis/129.3.277. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M., Lönnroth I., Fall-Persson M., Markman B., Lundbeck H. Development of improved cholera vaccine based on subunit toxoid. Nature. 1977 Oct 13;269(5629):602–604. doi: 10.1038/269602a0. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M., Ouchterlony O., Anderson A., Walletström G., Westerberg-Berndtsson U. Antitoxic immunity in experimental cholera: protection, and serum and local antibody responses in rabbits after enteral and parenteral immunization. Infect Immun. 1975 Dec;12(6):1331–1340. doi: 10.1128/iai.12.6.1331-1340.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F. Influence of route of administration on immediate and extended protection in rats immunized with Escherichia coli heart-labile enterotoxin. Infect Immun. 1980 Jan;27(1):81–86. doi: 10.1128/iai.27.1.81-86.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F. Protective effect of active immunization with purified Escherichia coli heat-labile enterotoxin in rats. Infect Immun. 1979 Mar;23(3):592–599. doi: 10.1128/iai.23.3.592-599.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F. Respective contributions to protection of primary and booster immunization with Escherichia coli heat-labile enterotoxin in rats. Infect Immun. 1981 Jan;31(1):252–260. doi: 10.1128/iai.31.1.252-260.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F., Short H. B. Protective effect of immunization with heat-labile enterotoxin in gnotobiotic rats monocontaminated with enterotoxigenic Escherichia coli. Infect Immun. 1980 Apr;28(1):163–170. doi: 10.1128/iai.28.1.163-170.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel S. L., Robertson D. C. Purification and chemical characterization of the heat-labile enterotoxin produced by enterotoxigenic Escherichia coli. Infect Immun. 1979 Aug;25(2):586–596. doi: 10.1128/iai.25.2.586-596.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lange S., Hansson H. A., Molin S. O., Nygren H. Local cholera immunity in mice: intestinal antitoxin-containing cells and their correlation with protective immunity. Infect Immun. 1979 Mar;23(3):743–750. doi: 10.1128/iai.23.3.743-750.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange S., Holmgren J. Protective antitoxic cholera immunity in mice: influence of route and number of immunizations and mode of action of protective antibodies. Acta Pathol Microbiol Scand C. 1978 Aug;86C(4):145–152. doi: 10.1111/j.1699-0463.1978.tb02572.x. [DOI] [PubMed] [Google Scholar]
- Markel D. E., Hejtmancik K. E., Peterson J. W., Kurosky A. Structure, function, and antigenicity of cholera toxin. J Supramol Struct. 1979;10(2):137–149. doi: 10.1002/jss.400100204. [DOI] [PubMed] [Google Scholar]
- Moss J., Garrison S., Fishman P. H., Richardson S. H. Gangliosides sensitize unresponsive fibroblasts to Escherichia coli heat-labile enterotoxin. J Clin Invest. 1979 Aug;64(2):381–384. doi: 10.1172/JCI109472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. W. Protection against experimental cholera by oral or parenteral immunization. Infect Immun. 1979 Nov;26(2):594–598. doi: 10.1128/iai.26.2.594-598.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr, Engel P. F. Antitoxic immunity to cholera in dogs immunized orally with cholera toxin. Infect Immun. 1980 Feb;27(2):632–637. doi: 10.1128/iai.27.2.632-637.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr, Sircar B. K. Induction of a mucosal antitoxin response and its role in immunity to experimental canine cholera. Infect Immun. 1978 Jul;21(1):185–193. doi: 10.1128/iai.21.1.185-193.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Gowans J. L. Cellular kinetics of the intestinal immune response to cholera toxoid in rats. J Exp Med. 1975 Dec 1;142(6):1550–1563. doi: 10.1084/jem.142.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Kaniecki E. A., Northrup R. S. Protection against experimental cholera by antitoxin. J Infect Dis. 1972 Dec;126(6):606–616. doi: 10.1093/infdis/126.6.606. [DOI] [PubMed] [Google Scholar]
- Pierce N. F. Protection against challenge with Escherichia coli heat-labile enterotoxin by immunization of rats with cholera toxin/toxoid. Infect Immun. 1977 Nov;18(2):338–341. doi: 10.1128/iai.18.2.338-341.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Sack R. B. Immune response of the intestinal mucosa to cholera toxoid. J Infect Dis. 1977 Aug;136 (Suppl):S113–S117. doi: 10.1093/infdis/136.supplement.s113. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Sack R. B., Sircar B. K. Immunity to experimental cholera. III. Enhanced duration of protection after sequential parenteral-oral administration of toxoid to dogs. J Infect Dis. 1977 Jun;135(6):888–896. doi: 10.1093/infdis/135.6.888. [DOI] [PubMed] [Google Scholar]
- Pierce N. F. The role of antigen form and function in the primary and secondary intestinal immune responses to cholera toxin and toxoid in rats. J Exp Med. 1978 Jul 1;148(1):195–206. doi: 10.1084/jem.148.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack R. B. Human diarrheal disease caused by enterotoxigenic Escherichia coli. Annu Rev Microbiol. 1975;29:333–353. doi: 10.1146/annurev.mi.29.100175.002001. [DOI] [PubMed] [Google Scholar]
- Sack R. B. Immunization with Escherichia coli enterotoxin protects against homologous enterotoxin challenge. Infect Immun. 1973 Oct;8(4):641–644. doi: 10.1128/iai.8.4.641-644.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith N. W., Sack R. B. Immunologic cross-reactions of enterotoxins from Escherichia coli and Vibrio cholerae. J Infect Dis. 1973 Feb;127(2):164–170. doi: 10.1093/infdis/127.2.164. [DOI] [PubMed] [Google Scholar]
- Svennerholm A., Lange S., Holmgren J. Correlation between intestinal synthesis of specific immunoglobulin A and protection against experimental cholera in mice. Infect Immun. 1978 Jul;21(1):1–6. doi: 10.1128/iai.21.1.1-6.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. L., Walker W. A. Immunological control mechanism against cholera toxin: interference with toxin binding to intestinal receptors. Infect Immun. 1976 Oct;14(4):1034–1042. doi: 10.1128/iai.14.4.1034-1042.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


