Abstract
In the renal tubules, ATP released from epithelial cells stimulates purinergic receptors, regulating salt and water reabsorption. However, the mechanisms by which ATP is released into the tubular lumen are multifaceted. Pannexin1 (Panx1) is a newly identified. ubiquitously expressed protein that forms connexin-like channels in the plasma membrane, which have been demonstrated to function as a mechanosensitive ATP conduit. Here, we report on the localization of Panx1 in the mouse kidney. Using immunofluorescence, strong Panx1 expression was observed in renal tubules, including proximal tubules, thin descending limbs, and collecting ducts, along their apical cell membranes. In the renal vasculature, Panx1 expression was localized to vascular smooth muscle cells in renal arteries, including the afferent and efferent arterioles. Additionally, we tested whether Panx1 channels expressed in renal epithelial cells facilitate luminal ATP release by measuring the ATP content of urine samples freshly collected from wild-type and Panx1−/− mice. Urinary ATP levels were reduced by 30% in Panx1−/− compared with wild-type mice. These results suggest that Panx1 channels in the kidney may regulate ATP release and via purinergic signaling may participate in the control of renal epithelial fluid and electrolyte transport and vascular functions.
Keywords: ATP release, channel, immunofluorescence, kidney
purinergic signaling is involved in several major regulatory mechanisms in the kidney, including tubuloglomerular feedback, control of renin release, and tubular fluid and electrolyte transport (17). ATP concentrations consistent with P2 receptor activation have been found in the tubular lumen (40). Additionally, the identity of several P2X and P2Y receptors along the renal epithelium, as well as their effect on ion transport, has already been determined (17). However, the mechanisms by which ATP, the ligand, is released into the tubular lumen remain contentious.
In the renal tubules, ATP may be released by both vesicular and channel-based release mechanisms. Several different transporters have been proposed as ATP conduits, including the cystic fibrosis transmembrane conductance regulator and the P2X7 receptor, but subsequently their ATP permeability has been challenged (24). It has also been suggested that connexin (Cx) proteins can form hemichannels, or connexons, which allow ATP release to the extracellular fluid (8, 10, 12, 24). Recently, a study from our laboratory provided evidence that Cx30 is required for luminal ATP release in the cortical collecting duct and that this mechanism is involved in pressure-natriuresis (35). However, the issue of functional hemichannels formed solely by connexins under physiological conditions is controversial (37).
A recently identified plasma membrane ATP channel is the pannexin channel (3–7). Pannexins are a class of proteins that also form hexameric transmembrane channels, similar to those formed by connexins (4, 5, 7). Despite having similar structures, connexins and pannexins have no sequence homology. There is consensus that pannexin channels are not gap junction hemichannels (16, 36). Overwhelming evidence suggests that pannexins form functional channels under physiological conditions, allowing the passage of small molecules between the intra- and extracellular environment (3, 5, 7, 9, 30, 33). Three pannexin isoforms have been identified, i.e., Panx1, Panx2, and Panx3, and all three have been reported to form functional single-membrane channels (1, 23). Panx1 channels are mechanosensitive, open at physiological extracellular Ca2+ concentration ([Ca2+]), and are activated by increased intracellular [Ca2+] (3–5). As such, Panx1 channels have been shown to facilitate ATP release in several cell types, including epithelial cells in the lungs and taste buds (3, 15, 18, 26). Additionally, studies have found Panx1 mRNA expression in the kidney (4, 27).
Therefore, in the present study we aimed to determine the intrarenal localization and cell-specific expression of Panx1 protein using immunofluorescence in the mouse kidney. In addition, urinary ATP levels were measured in wild-type and Panx1−/− mice to test whether, similar to in other organs, Panx1 plays a role in ATP release in renal epithelial cells.
MATERIALS AND METHODS
Animals.
Male C57BL/6 mice (8–10 wk of age, bred in house) were used for the immunolocalization studies and were fed standard diets (Harlan, Madison, WI) and drinking water ad libitum. Mice were anesthetized with a combination of ketamine (100 mg/kg) and xylazine (10 mg/kg) and, after kidney extraction, euthanasia was performed using cervical dislocation. Immunoblotting and ATP measurements were performed using samples from Panx1−/− mice and their wild-type littermates. The generation of Panx1−/− mice used for these studies was described previously (29, 39). All animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Southern California.
Antibodies.
The chicken polyclonal Panx1 antibodies (4515) for immunofluorescence and immunoblotting studies were provided by Dr. Gerhard Dahl (University of Miami Miller School of Medicine, Miami, FL) and have been characterized in previous publications (18). Rabbit polyclonal aquaporin-1 (AQP1) and aquaporin-2 (AQP2) antibodies were kind gifts from Dr. Dennis Brown (Harvard University) and Dr. Mark Knepper (National Institutes of Health), respectively. The rabbit polyclonal sodium-chloride cotransporter (NCC) antibody was a kind gift from Dr. David Ellison (Oregon Health Sciences University). Mouse monoclonal α-smooth muscle actin was purchased from Sigma. The antibodies were characterized in previous publications (22, 28, 31).
Immunofluorescence labeling of kidney tissue.
Kidneys were fixed in situ by perfusion of 4% paraformaldehyde, and coronal tissue blocks containing all kidney zones were then placed in the same fixative overnight at 4°C. Kidney blocks were embedded in paraffin, and 4-μm sections were cut, deparaffinized in toluene, and rehydrated through xylene and graded ethanol. To retrieve antigens, slides were heated for 2 × 10 min in a microwave with medium heat in PBS and allowed to cool for 40 min. Sections were then fixed with 4% paraformaldehyde for 10 min and permeabilized for 10 min with 0.1% Triton X-100 in PBS. Ten % BlockHen (Aves, Tigard, OR) in PBS was applied to sections for 1 h to block nonspecific binding. Sections were then probed with Panx1 chicken polyclonal antibodies, at a dilution of 1:100 overnight followed by incubation with secondary Alexa Fluor 594-conjugated goat anti-chicken antibodies (Invitrogen, Carlsbad, CA) at a 1:500 dilution for 1 h. Additionally, some sections were also probed with the following antibodies: α-smooth muscle actin (1:1,000), AQP1 (1:50), AQP2 (1:500), and NCC (1:500). These sections were incubated for 1 h with either goat anti-rabbit or goat anti-mouse secondary Alexa Fluor 488-conjugated antibodies at a dilution of 1:500. Following a final wash step, all sections were mounted with Vectashield mounting media containing the nuclear stain 4,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA) and examined with a Leica TCS SP2 confocal microscope.
Immunoblotting of tissue.
Kidneys from three wild-type and three Panx1−/− mice were perfused with cold PBS to remove blood, snap frozen in liquid nitrogen, and sent to us on dry ice from the laboratory of Prof. Eliana Scemes (Department of Neuroscience, Albert Einstein College of Medicine, Bronx, NY). Brain samples were taken from mice, and all tissues were processed and homogenized as described recently (21). Protein concentrations were determined using the Pierce BCA kit (Thermo Scientific), and 80 μg of protein were then run on SDS-PAGE gels, transferred to polyvinylidene difluoride membranes (Immobilon-FL; Millipore, Temecula, CA), blocked (bløk-FL; Millipore), and then probed with chicken polyclonal antibodies to Panx1 at a dilution of 1:1,000 overnight. Reactivity of the primary antibodies was detected with IR680-labeled goat anti-chicken antibodies (1:5,000 dilution, Li-Cor). To test for protein loading and quality of transfer, the blot was reprobed with a mouse monoclonal antibody to β-actin (1:10,000 dilution, Chemicon) at room temperature for 1 h. The primary antibody was recognized by an IR800-labeled goat anti-mouse antibody (1:5,000 dilution, Li-Cor). Blots were imaged using the Odyssey Infrared Imaging System (Li-Cor) and accompanying software.
Urine analysis.
Spot urine samples were freshly harvested at the same time from 10 wild-type and 10 Panx1−/− mice, frozen immediately, and sent to us on dry ice (kind gift from Prof. Eliana Scemes). Samples were diluted eightfold with ddH2O, and urinary ATP was measured in 100-μl samples by a luciferin-luciferase ATP assay kit (Sigma) in accordance with manufacturer's instructions. Luminescence was detected using a cuvette-based spectrofluorometer (Quantamaster-8, PTI, Birmingham, NJ), and data was analyzed using FeliX32 software (PTI). A standard ATP curve was generated using ATP disodium salt (Sigma) and its serial dilutions (at 1, 2.5, 5, 10, 25, 50, and 100 nM concentrations) to determine the linear dynamic range of the assay and the spectrofluorometry system. Data were collected in relative luminescence units and calibrated to the standard curve to calculate the concentration of ATP. Each sample was measured in triplicate and then averaged. Urinary creatinine values were assessed with an improved Jaffé method using a Creatinine Companion Kit (Exocell, Philadelphia, PA), and urine osmolality was measured with a Vapro 5520 Vapor Pressure Osmometer (Wescor, Logan, UT) following the manufacturers' protocols. Statistical significance was calculated by Student's t-test (2-tailed) with data shown as means ± SE. P < 0.05 was considered significant.
RESULTS
Expression of Panx1 within the mouse kidney.
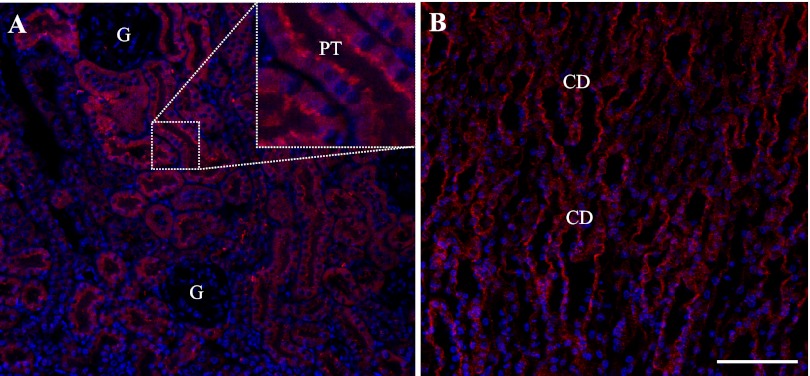
While previous studies have shown evidence of Panx1 mRNA in the kidney, no details regarding the specific renal localization of Panx1 were presented. Therefore, immunofluorescence studies were performed to establish where Panx1 protein is expressed within the kidney. Mouse kidney sections were labeled with chicken polyclonal Panx1 antibodies and imaged using confocal fluorescence microscopy (Fig. 1). Intense Panx1 labeling was observed in both cortical (Fig. 1A) and medullary (Fig. 1B) tubule segments, including proximal tubules and collecting ducts as well as thin-walled tubular structures. Labeling was present mostly at the apical membrane of tubules, in addition to weaker intracellular staining (Figs. 1 and 2). No signal was detected in the glomerulus, while Panx1 labeling was observed in both the large and small arteries, including afferent (Fig. 2D) and efferent arterioles (not shown). In these blood vessels, the immunolabeling of Panx1 colocalized with that of the vascular smooth muscle cell marker α-smooth muscle actin (Fig. 2D). The vascular endothelium was devoid of Panx1 labeling (Fig. 2D, inset).
Fig. 1.
Overview of pannexin1 (Panx1) immunofluorescence in the mouse kidney. A: among cortical tubules, Panx1 labeling was strongest in proximal tubules (PT) in the apical brush border (inset). There was no signal in the glomerulus (G). B: in the medulla, intense Panx1 immunofluorescence was observed in the merging collecting duct (CD) system. In addition, thin-walled tubular structures showed weaker labeling. Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI; blue). Bar = 50 μm.
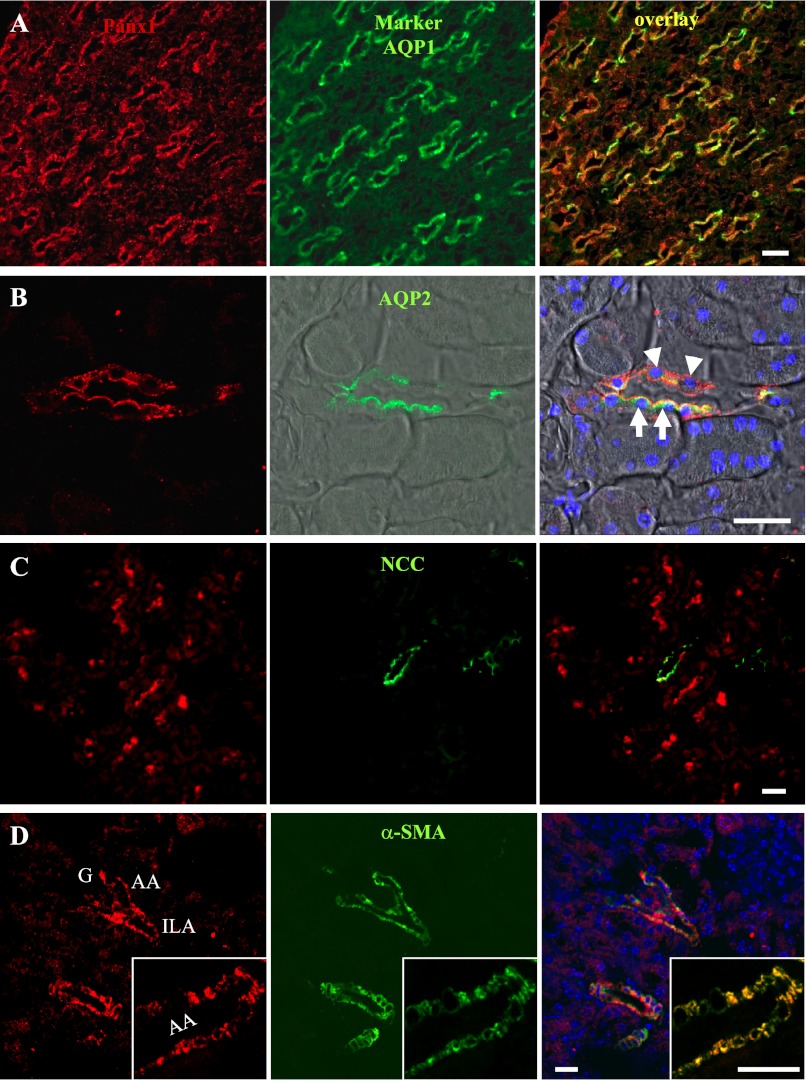
Fig. 2.
Immunofluorescence colabeling of Panx1 (red, left) with cell-specific markers (green, middle) in the mouse kidney and their merged images (overlay, right). A: in the loop of Henle, Panx1 (red) and aquaporin-1 (AQP1; green) colocalized (yellow) in descending thin limbs. B: Panx1 (red) was coexpressed with aquaporin-2 (AQP2; green), a marker of the principal cells in collecting ducts (arrows), mainly at the apical cell membranes. Labeling was also observed in the AQP2-negative intercalated cells (arrowheads). DIC background was added and merged with fluorescence. Nuclei were stained with DAPI (blue). C: colabeling of the distal convoluted tubule marker the sodium-chloride cotransporter (NCC; green) and Panx1 (red) failed to show any overlap in signal. D: immunofluorescence colabeling of Panx1 (red) and α-smooth muscle actin (α-SMA; green), a marker of vascular smooth muscle cells. Panx1 expression was found in vascular smooth muscle cells of large and small renal arteries, including interlobular arteries (ILA) and afferent arterioles (AA). A single AA is shown magnified in the inset. G, glomerulus. Bar = 20 μm.
Colabeling of Panx1 with tubule segment-specific markers.
Mouse kidney sections were colabeled with antibodies against Panx1 and several tubule segment-specific markers to confirm the initially observed Panx1 labeling (Fig. 2, A–C). To ascertain the identity of the Panx1-positive region in the loop of Henle, sections were colabeled with Panx1 and AQP1, a marker of the descending thin limbs. Panx1 labeling was detected in all AQP1-positive cells in the loop of Henle (Fig. 2A). Since some, but not all, cells of the cortical or medullary collecting duct expressed Panx1, colabeling with the principal cell marker AQP2 was used. Panx1 labeling was detected in both AQP2-positive and -negative cells, indicating that Panx1 is expressed in principal as well as intercalated cells (Fig. 2B). Next, colabeling of Panx1 and NCC, a known marker of the distal convoluted tubules, was performed. Panx1-positive tubules consistently lacked any NCC expression, indicating that Panx1 is not expressed in the distal convoluted tubules (Fig. 2C).
Panx1 is necessary for ATP release into the urine.
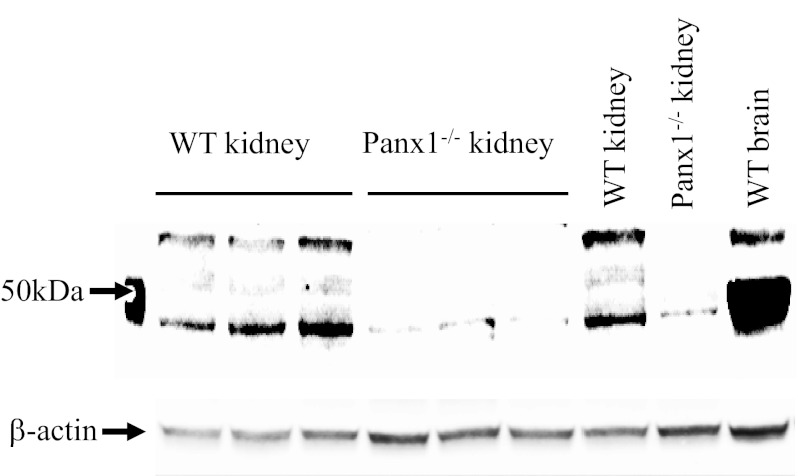
To test whether, similar to in other organs and cell types, Panx1 has a role in ATP release by renal epithelial cells, we obtained kidney tissue and urine samples from wild-type and Panx1−/− mice. First, whole kidney homogenates from wild-type and Panx1−/− mice were immunoblotted with Panx1 antibodies to confirm Panx1 deficiency. Brain tissue homogenate served as a positive control, and equal protein loading was confirmed by β-actin levels (Fig. 3). Strong bands characteristic of Panx1 (6, 18, 23) were detected around the expected size in wild-type but not in Panx1−/− mouse kidneys. There was a very weak but detectable lower band in Panx1−/− kidneys (Fig. 3).
Fig. 3.
Confirmation of kidney tissue Panx1 deficiency in Panx1−/− mice. Whole kidney homogenates collected from wild-type (WT) and Panx1−/− mice (3 animals each, 1 pair loaded twice as indicated) were immunoblotted and probed with Panx1 antibodies. Strong bands characteristic of Panx1 at ∼50 kDa were present in WT but not in Panx1−/− tissues. A very weak lower band was still detectable in Panx1−/− kidneys. A brain tissue homogenate served as a positive control. β-Actin was used as a loading control.
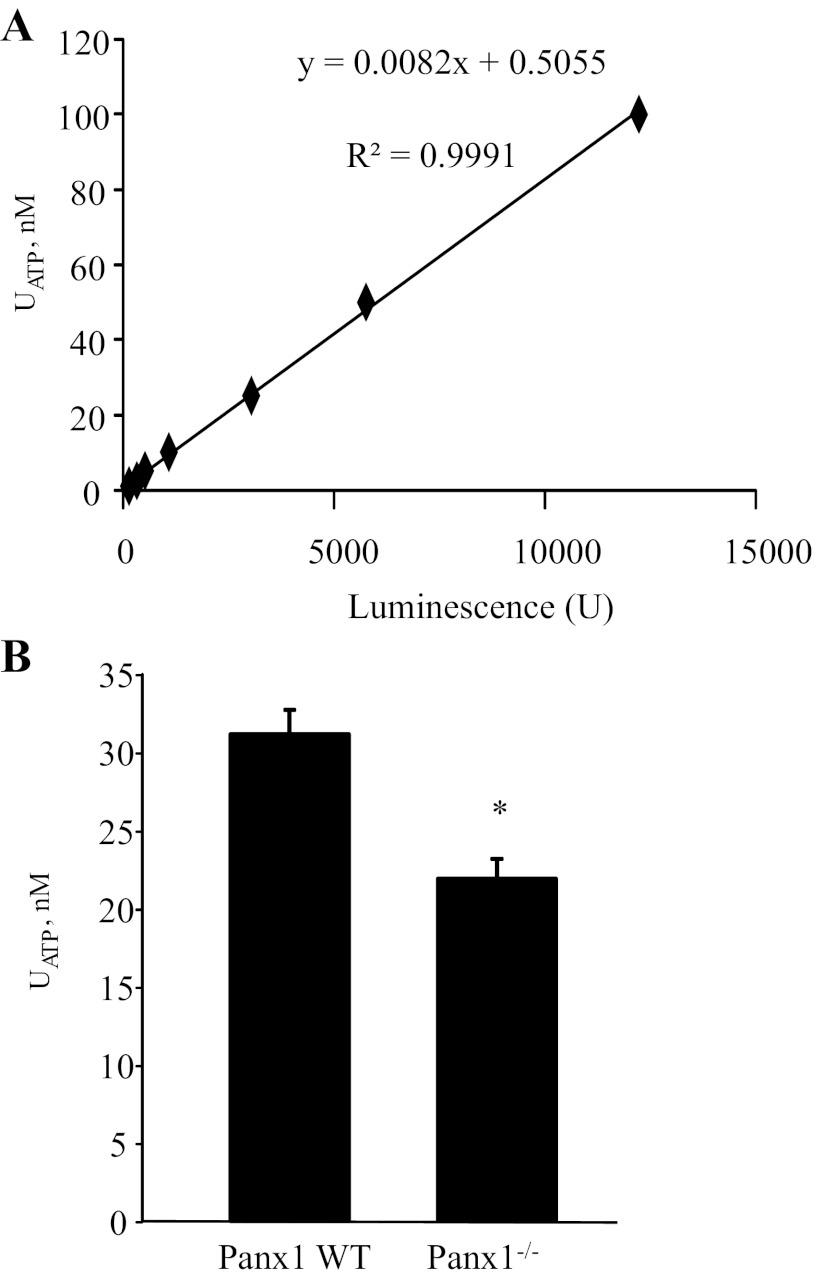
Since the plasma membrane ATP channel Panx1 was localized at the apical membrane in various renal tubule segments, we quantified the concentration of ATP in freshly collected urine. First, an ATP calibration curve was established using known ATP standards (Fig. 4A). As shown in Fig. 4B, urinary ATP concentration ([ATP]) was significantly lower in Panx1−/− mice (21.9 ± 1.3 μM) compared with wild-type mice (31.2 ± 1.6 μM, n = 10 each, P < 0.05), consistent with this channel being necessary for ATP release into the renal tubular fluid. To exclude the possibility that differences in urinary [ATP] arises from variations in glomerular filtration rate and/or urine volume, we measured urinary creatinine and osmolality in the same samples. We found no statistically significant difference between groups, although both urinary creatinine (wild-type: 76.8 ± 4.1 μM, Panx1−/−: 86.1 ± 4.7 mg/dl, P = 0.17) and urine osmolality (wild-type: 2,199 ± 91, Panx1−/−: 2,387 ± 140 mosmol/kgH2O, P = 0.28) tended to be higher in Panx1−/− mice. Therefore, urinary concentration/dilution cannot explain the lower urinary [ATP] observed in Panx1−/− mice.
Fig. 4.
Panx1 is necessary for ATP release into the urine. A: calibration curve for ATP measurements. Changes in luminescence intensity were detected in response to serial dilutions of ATP (at concentrations of 1, 2.5, 5, 10, 25, 50, and 100 nM) using a luciferin- and luciferase-based ATP bioluminescence assay. The equation and R2 value of linear regression analysis are displayed. B: summary graph of ATP concentration in freshly harvested urine in WT and Panx1−/− mice (n = 10 each). Values are means ± SE. *P < 0.05 vs. Panx1 wild-type.
DISCUSSION
Here, we present the cell-specific localization of Panx1 protein expression within the mouse kidney. Strong Panx1 immunolabeling was found in both cortical and medullary tubule segments. Specifically, Panx1 expression was detected in the proximal tubule, the thin descending limb of the loop of Henle, and the collecting duct system. Apical membrane localization was prominent in the proximal tubule and collecting ducts, suggesting that Panx1 could serve as a membrane channel in these regions. In the renal vasculature, both large and small arteries showed expression of Panx1, including the afferent and efferent arterioles. Additional in vivo studies using wild-type and Panx1−/− mice demonstrated that Panx1 channels expressed in renal epithelial cells facilitate ATP release. These localization and functional studies suggest that Panx1 channels may regulate ATP release into the tubular lumen and in the renal vasculature. Panx1 may participate in the control of renal epithelial fluid and electrolyte transport and vascular functions.
A wide range of (patho)physiological roles have been speculated for Panx1 (5, 26, 30). Based on the present findings, we speculate that one potential role of Panx1 in renal physiology is to regulate renal tubular salt and water transport and therefore body fluid homeostasis. In the proximal tubule, luminal purinergic receptor activation inhibits acidification (2), and relatively high levels of ATP have been found in the lumen (40), suggesting that an ATP release mechanism is located along the brush-border membrane of proximal tubules. The localization of Panx1 in the proximal tubule provides one possible explanation for these earlier findings (Fig. 1A). Panx1 expression was also found in the thin descending limb of the loop of Henle, where it may function similarly to Cx30.3 which is localized in the thin ascending limb, the adjacent segment of the loop of Henle (13). We previously showed that Cx30 is expressed exclusively along apical membranes in the collecting ducts of mice, presumably as Cx hemichannels (19). Additional work by Sipos et al. (35) demonstrated that Cx30 is necessary for ATP release and therefore salt and water transport regulation by purinergic signaling. Presently, however, there is no direct evidence of ATP passage via Cx30 hemichannels. Interestingly, Panx1 immunolabeling was also detected in the apical membrane of the collecting duct system (Figs. 1B and 2B). If, as we predict, Panx1 is regulating ATP release in the collecting duct similarly to Cx30, an in vivo inhibition of Panx1 (such as in Panx1−/− mice) would show a similar physiological effect as that seen in Cx30 knockout mice, namely, a blunted pressure natriuresis response resulting in a salt-retention phenotype (35). Also, it should be noted that by demonstrating the apical localization of the protein where no gap junctions can occur, the present study contributes to the list of evidence against the gap junction function of Panx1 (9, 36).
Additionally, we confirmed the ATP-releasing function of Panx1 in the kidney using freshly collected urine samples from wild-type and Panx1−/− mice (Fig. 4B). Immunoblotting confirmed Panx1 deficiency of kidney tissues obtained from Panx1−/− mice, although a very low level of remaining Panx1 expression was detected (Fig. 3). Due to the nature of the knockout strategy used to generate the Panx1-deficient mice, there is the possibility that Panx1 hypomorphism occurs in these mice, resulting in a low level of remaining Panx1 transcripts. These transcripts are currently being evaluated (personal communication from Prof. Eliana Scemes). Nevertheless, recent studies indicate that tissues and cells derived from the same Panx1 knockout mouse model function as total Panx1 knockouts (29, 39). Importantly, urinary [ATP] was ∼30% lower in Panx1−/− mice compared with wild-type mice (Fig. 4B), indicating that Panx1 is necessary for a significant portion of ATP release into the urine. This function of Panx1 is again similar to that of Cx30 since Cx30 dependence of urinary [ATP] was established recently (20). However, it is not known at this time whether the similar apical membrane localization of Cx30 and Panx1 in the collecting duct and their role in urinary ATP release represent redundant functions, or a possible interaction between Panx1 and Cx30. The partial attenuation of ATP release into the urine in both Panx1−/− (Fig. 4B) and Cx30−/− mice (20) suggests the presence of alternative ATP release pathways in renal tubules that are not Panx1 channels or Cx hemichannels. Panx1-independent, nonvesicular ATP release was described recently in mouse erythrocytes (25).
Panx1 dependence of urinary [ATP] found in the present study adds to the growing evidence that Panx1 is an ATP conduit that plays a role in purinergic signaling throughout the body. This role of Panx1 has been demonstrated in several organs and cell types, including other epithelia (9, 15, 18). In airway epithelial cells, Panx1 expression was found at the apical membrane (similar to the localization we observed in the proximal tubule and collecting duct), and inhibition of Panx1 reduced ATP release (26). Interestingly, airway epithelia and renal epithelia share additional key characteristics. Both respond to mechanical stress by releasing ATP and have ciliated cells which may act as a flow sensor. P2 receptor activation by the released ATP regulates transport in both regions (14, 17, 32, 40). In the collecting duct specifically, purinergic signaling inhibits salt reabsorption via the epithelial Na channel, water reabsorption via AQP2, and potassium secretion by the renal outer medullary K+ channel (17). There is also a flow-dependent component to the regulation of this transport, suggesting an ATP release mechanism that can be mechanically activated (17). Our study suggests that Panx1, a mechanosensitive ATP channel, could regulate purinergic signaling in renal tubular epithelial cells. The recent discoveries that Panx1 channels serve as K+ sensors for changes in the extracellular milieu (29, 34, 39) may help establish further links between renal tubular K+, ATP, and purinergic signaling (11). Therefore, via ATP release and purinergic inhibition of renal epithelial salt and water transport, Panx1 may participate in important renal physiological mechanisms such as high-K+ diet-induced elevations in tubular flow, the aldosterone paradox, and aldosterone escape (11, 38).
In summary, our results identified the expression and cell-specific localization of Panx1 channels in the kidney. Panx1 channel activity may be a mechanism by which ATP is released into the tubular lumen or within renal resistance arteries. As has been shown with Cx30, this points to a role for Panx1 in the regulation of renal tubular transport mechanisms by purinergic signaling. Future in vivo studies using genetic modification of Panx1 expression are needed to establish the role Panx1 plays in renal (patho)physiology.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK64324, American Heart Association (AHA) Established Investigator Award 0640056N, and Grant 1-11-BS-121 from the American Diabetes Association to J. Peti-Peterdi. F. Hanner was supported by an AHA Western Affiliate Predoctoral Research Fellowship during these studies.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: F.H., M.T.X.N., A.Y., and J.P.-P. provided conception and design of research; F.H., L.L., and M.T.X.N. performed experiments; F.H., L.L., and J.P.-P. analyzed data; F.H., A.Y., and J.P.-P. interpreted results of experiments; F.H. and J.P.-P. prepared figures; F.H. drafted manuscript; F.H., L.L., and J.P.-P. edited and revised manuscript; F.H., L.L., M.T.X.N., A.Y., and J.P.-P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Eliana Scemes (Dept. of Neuroscience, Albert Einstein College of Medicine, Bronx, NY) for providing kidney tissue and urine samples from wild-type and Panx1−/− mice and for helpful comments.
REFERENCES
- 1.Ambrosi C, Gassmann O, Pranskevich JN, Boassa D, Smock A, Wang J, Dahl G, Steinem C, Sosinsky GE. Pannexin1 and pannexin2 channels show quaternary similarities to connexons and different oligomerization numbers from each other. J Biol Chem 285: 24420–24431, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey MA. Inhibition of bicarbonate reabsorption in the rat proximal tubule by activation of luminal P2Y1 receptors. Am J Physiol Renal Physiol 287: F789–F796, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett 572: 65–68, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Baranova A, Ivanov D, Petrash N, Pestova A, Skoblov M, Kelmanson I, Shagin D, Nazarenko S, Geraymovych E, Litvin O, Tiunova A, Born TL, Usman N, Staroverov D, Lukyanov S, Panchin Y. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics 83: 706–716, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Barbe MT, Monyer H, Bruzzone R. Cell-cell communication beyond connexins: the pannexin channels. Physiology 21: 103–114, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Boassa D, Ambrosi C, Qiu F, Dahl G, Gaietta G, Sosinsky G. Pannexin1 channels contain a glycosylation site that targets the hexamer to the plasma membrane. J Biol Chem 282: 31733–31743, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci USA 100: 13644–13649, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotrina ML, Lin JHC, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CCG, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci USA 95: 15735–15740, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahl G, Locovei S. Pannexin: to gap or not to gap, is that a question? IUBMB Life 58: 409–419, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Ebihara L. New roles for connexons. Physiology 18: 100–103, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Eladari D, Chambrey R, Peti-Peterdi J. A new look at electrolyte transport in the distal tubule. Annu Rev Physiol 74: 325–349, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomes P, Srinivas SP, Van Driessche W, Vereecke J, Himpens B. ATP release through connexin hemichannels in corneal endothelial cells. Invest Ophthalmol Vis Sci 46: 1208–1218, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Hanner F, Schnichels M, Zheng-Fischhöfer Q, Yang LE, Toma I, Willecke K, McDonough AA, Peti-Peterdi J. Connexin 30.3 is expressed in the kidney but not regulated by dietary salt or high blood pressure. Cell Commun Adhes 15: 219–230, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Hovater M, Olteanu D, Hanson E, Cheng NL, Siroky B, Fintha A, Komlosi P, Liu W, Satlin L, Bell P, Yoder B, Schwiebert E. Loss of apical monocilia on collecting duct principal cells impairs ATP secretion across the apical cell surface and ATP-dependent and flow-induced calcium signals. Purinergic Signal 4: 155–170, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci USA 104: 6436–6441, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Y, Grinspan JB, Abrams CK, Scherer SS. Pannexin1 is expressed by neurons and glia but does not form functional gap junctions. Glia 55: 46–56, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Leipziger J. Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol 284: F419–F432, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci USA 103: 7655–7659, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCulloch F, Chambrey R, Eladari D, Peti-Peterdi J. Localization of connexin 30 in the luminal membrane of cells in the distal nephron. Am J Physiol Renal Physiol 289: F1304–F1312, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Mironova E, Peti-Peterdi J, Bugaj V, Stockand JD. Diminished paracrine regulation of the epithelial Na+ channel by purinergic signaling in mice lacking connexin 30. J Biol Chem 286: 1054–1060, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen MTX, Yang LE, Fletcher NK, Lee DH, Kocinsky H, Bachmann S, Delpire E, McDonough AA. Effects of K+-deficient diets with and without NaCl supplementation on Na+, K+, and H2O transporters' abundance along the nephron. Am J Physiol Renal Physiol 303: F92–F104, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen S, DiGiovanni SR, Christensen EI, Knepper MA, Harris HW. Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc Natl Acad Sci USA 90: 11663–11667, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Penuela S, Bhalla R, Nag K, Laird DW. Glycosylation regulates pannexin intermixing and cellular localization. Mol Biol Cell 20: 4313–4323, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Praetorius H, Leipziger J. ATP release from non-excitable cells. Purinergic Signal 5: 433–446, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu F, Wang J, Spray DC, Scemes E, Dahl G. Two non-vesicular ATP release pathways in the mouse erythrocyte membrane. FEBS Lett 585: 3430–3435, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M. Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol 41: 525–534, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ray A, Zoidl G, Weickert S, Wahle P, Dermietzel R. Site-specific and developmental expression of pannexin1 in the mouse nervous system. Eur J Neurosci 21: 3277–3290, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Sabolic I, Valenti G, Verbavatz JM, Van Hoek AN, Verkman AS, Ausiello DA, Brown D. Localization of the CHIP28 water channel in rat kidney. Am J Physiol Cell Physiol 263: C1225–C1233, 1992 [DOI] [PubMed] [Google Scholar]
- 29.Santiago MF, Veliskova J, Patel NK, Lutz SE, Caille D, Charollais A, Meda P, Scemes E. Targeting pannexin1 improves seizure outcome. PLoS ONE 6: e25178, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scemes E, Suadicani SO, Dahl G, Spray DC. Connexin and pannexin mediated cell-cell communication. Neuron Glia Biol 3: 199–208, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmitt R, Ellison DH, Farman N, Rossier BC, Reilly RF, Reeves WB, Oberbäumer I, Tapp R, Bachmann S. Developmental expression of sodium entry pathways in rat nephron. Am J Physiol Renal Physiol 276: F367–F381, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Schwiebert EM, Wallace DP, Braunstein GM, King SR, Peti-Peterdi J, Hanaoka K, Guggino WB, Guay-Woodford LM, Bell PD, Sullivan LP, Grantham JJ, Taylor AL. Autocrine extracellular purinergic signaling in epithelial cells derived from polycystic kidneys. Am J Physiol Renal Physiol 282: F763–F775, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Shestopalov V, Panchin Y. Pannexins and gap junction protein diversity. Cell Mol Life Sci 65: 376–394, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silverman WR, de Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, Keane RW, Dahl G. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem 284: 18143–18151, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sipos A, Vargas SL, Toma I, Hanner F, Willecke K, Peti-Peterdi J. Connexin 30 deficiency impairs renal tubular ATP release and pressure natriuresis. J Am Soc Nephrol 20: 1724–1732, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sosinsky GE, Boassa D, Dermietzel R, Duffy HS, Laird DW, MacVicar B, Naus CC, Penuela S, Scemes E, Spray DC, Thompson RJ, Zhao HB, Dahl G. Pannexin channels are not gap junction hemichannels. Channels 5: 193–197, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spray DC, Ye ZC, Ransom BR. Functional connexin “hemichannels”: a critical appraisal. Glia 54: 758–773, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Stockand JD, Mironova E, Bugaj V, Rieg T, Insel PA, Vallon V, Peti-Peterdi J, Pochynyuk O. Purinergic inhibition of ENaC produces aldosterone escape. J Am Soc Nephrol 21: 1903–1911, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suadicani SO, Iglesias R, Wang J, Dahl G, Spray DC, Scemes E. ATP signaling is deficient in cultured pannexin1-null mouse astrocytes. Glia 60: 1106–1116, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vekaria RM, Unwin RJ, Shirley DG. Intraluminal ATP concentrations in rat renal tubules. J Am Soc Nephrol 17: 1841–1847, 2006 [DOI] [PubMed] [Google Scholar]