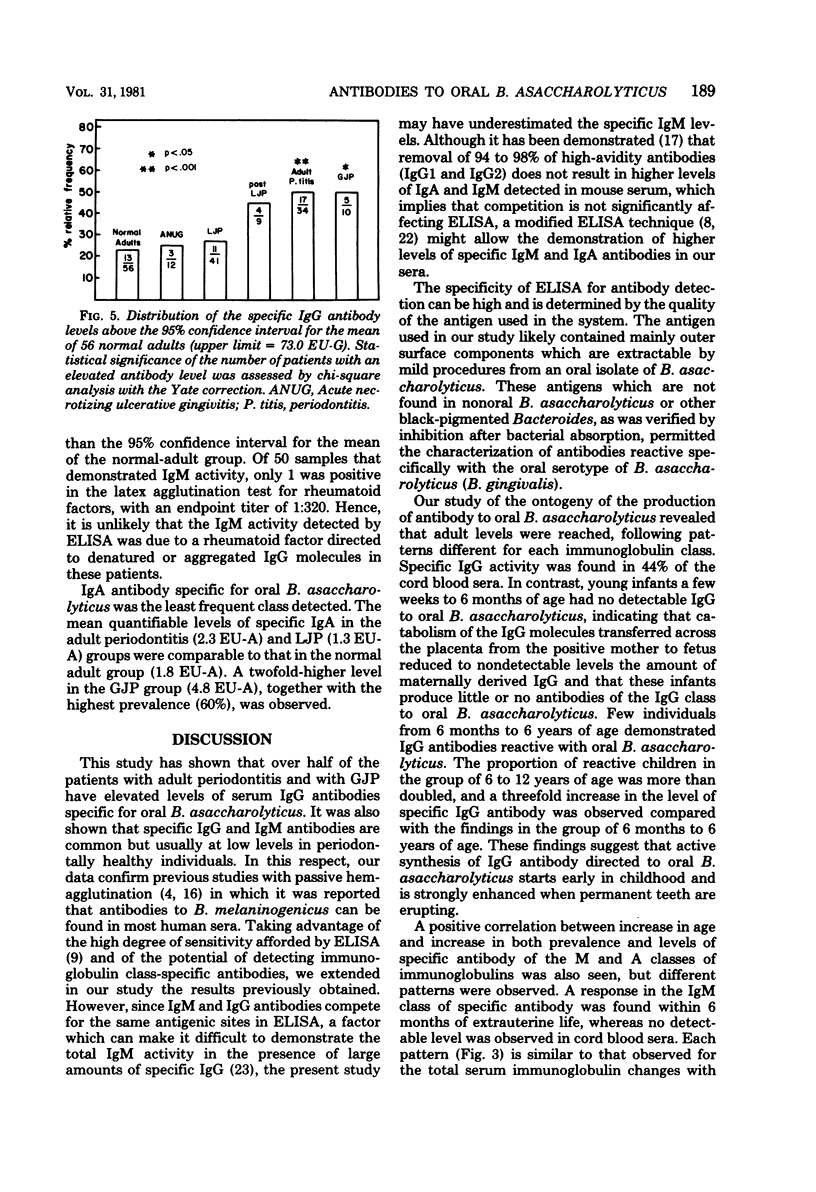
Abstract
An enzyme-linked immunosorbent assay microplate method was used for measuring levels of antibody specific for the oral serotype of Bacteroides asaccharolyticus (Bacteroides gingivalis) in serum samples obtained from umbilical cords, infants, children, periodontally normal adults, and edentulous adults. Serum from patients with various periodontal diseases, including adult periodontitis, localized juvenile periodontitis, generalized juvenile periodontitis, post-localized juvenile periodontitis, and acute necrotizing ulcerative gingivitis, were also studied. A positive correlation between increase in age and increase in both prevalence and level of specific antibody in the G, A, and M classes of immunoglobulins was observed. This indicates that antibodies reactive with oral B. asaccharolyticus found in up to 84% of normal adults are natural antibodies, presumably with a protective role. Among the patient groups, those with adult periodontitis were found to have levels of immunoglobulin G antibodies to oral B. asaccharolyticus that were five times higher than the antibody levels found in control subjects. The levels of IgG antibodies to this organism in the other patient groups were comparable to the levels found in the control group. However, 50% of the individuals in the generalized juvenile periodontitis group had high levels of immunoglobulin G antibodies to B. asaccharolyticus, suggesting heterogeneity with respect to immune response in these patients. These results indicate that antibodies to oral B. asaccharolyticus (B. gingivalis) occur at low levels in most normal children and adults and that the rise in titer of the specific antibodies of each major class of immunoglobulins parallels the ontogenic change in serum levels of that isotype. In contrast, there is a marked increase in titer of immunoglobulin G antibodies to oral B. asaccharolyticus in the group of patients with adult periodontitis and in patients with the generalized form of juvenile periodontitis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAILIT H. L., BALDWIN D. C., HUNT E. E., Jr THE INCREASING PREVALENCE OF GINGIVAL BACTEROIDES MELANINOGENICUS WITH AGE IN CHILDREN. Arch Oral Biol. 1964 Jul-Aug;9:435–438. doi: 10.1016/0003-9969(64)90028-7. [DOI] [PubMed] [Google Scholar]
- Clem T. R., Yolken R. H. Practical colorimeter for direct measurement of microplates in enzyme immunoassay systems. J Clin Microbiol. 1978 Jan;7(1):55–58. doi: 10.1128/jcm.7.1.55-58.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courant P. R., Gibbons R. J. Biochemical and immunological heterogeneity of Bacteroides melaninogenicus. Arch Oral Biol. 1967 Dec;12(12):1605–1613. doi: 10.1016/0003-9969(67)90194-x. [DOI] [PubMed] [Google Scholar]
- DE ARAUJO W. C., MACDONALD J. B. THE GINGIVAL CREVICE MICROBIOTA IN FIVE PRESCHOOL CHILDREN. Arch Oral Biol. 1964 Mar-Apr;9:227–228. doi: 10.1016/0003-9969(64)90013-5. [DOI] [PubMed] [Google Scholar]
- Duermeyer W., van der Veen J. Specific detection of IgM-antibodies by ELISA, applied in hepatitis-A. Lancet. 1978 Sep 23;2(8091):684–685. doi: 10.1016/s0140-6736(78)92802-7. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- GIBBONS R. J., SOCRANSKY S. S., SAWYER S., KAPSIMALIS B., MACDONALD J. B. The microbiota of the gingival crevice area of man. II. The predominant cultivable organisms. Arch Oral Biol. 1963 May-Jun;8:281–289. doi: 10.1016/0003-9969(63)90020-7. [DOI] [PubMed] [Google Scholar]
- GOOD R. A., PAPERMASTER B. W. ONTOGENY AND PHYLOGENY OF ADAPTIVE IMMUNITY. Adv Immunol. 1964;27:1–115. doi: 10.1016/s0065-2776(08)60706-3. [DOI] [PubMed] [Google Scholar]
- Hofstad T. Antibodies reacting with lipopolysaccharides from Bacteroides melaninogenicus, in serum from normal human subjects. J Infect Dis. 1974 Mar;129(3):349–352. doi: 10.1093/infdis/129.3.349. [DOI] [PubMed] [Google Scholar]
- Horowitz S. A., Cassell G. H. Detection of antibodies to Mycoplasma pulmonis by an enzyme-linked immunosorbent assay. Infect Immun. 1978 Oct;22(1):161–170. doi: 10.1128/iai.22.1.161-170.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leinikki P., Pässilä S. Solid phase antibody assay by means of enzyme conjugated to anti-immunoglobulin. J Clin Pathol. 1976 Dec;29(12):1116–1120. doi: 10.1136/jcp.29.12.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashimo P. A., Ellison S. A. Diffusate media for cultivation of oral anaerobic bacteria. Koku Eisei Gakkai Zasshi. 1972 Mar;22(1):38–44. doi: 10.5834/jdh.22.38. [DOI] [PubMed] [Google Scholar]
- Michael J. G. Natural antibodies. Curr Top Microbiol Immunol. 1969;48:43–62. doi: 10.1007/978-3-642-46163-7_3. [DOI] [PubMed] [Google Scholar]
- Mouton C., Hammond P., Slots J., Genco R. J. Evaluation of Fluoretec-M for detection of oral strains of Bacteroides asaccharolyticus and Bacteroides melaninogenicus. J Clin Microbiol. 1980 Jun;11(6):682–686. doi: 10.1128/jcm.11.6.682-686.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller A. M., Mathiesen L. R. Detection of immunoglobulin M antibodies to hepatitis A virus by enzyme-linked immunosorbent assay. J Clin Microbiol. 1979 Nov;10(5):628–632. doi: 10.1128/jcm.10.5.628-632.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M. G., Socransky S. S. Predominant cultivable microbiota in periodontosis. J Periodontal Res. 1977 Mar;12(2):120–128. doi: 10.1111/j.1600-0765.1977.tb00114.x. [DOI] [PubMed] [Google Scholar]
- Newman M. G., Socransky S. S., Savitt E. D., Propas D. A., Crawford A. Studies of the microbiology of periodontosis. J Periodontol. 1976 Jul;47(7):373–379. doi: 10.1902/jop.1976.47.7.373. [DOI] [PubMed] [Google Scholar]
- Reed M. J., Slots J., Mouton C., Genco R. J. Antigenic studies of oral and nonoral black-pigmented Bacteroides strains. Infect Immun. 1980 Aug;29(2):564–574. doi: 10.1128/iai.29.2.564-574.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruitenberg E. J., Steerenberg P. A., Brosi B. J., Buys J. Reliability of the enzyme-linked immunosorbent assay (ELISA) for the serodiagnosis of Trichinella spiralis infections in conventionally raised pigs. J Immunol Methods. 1976;10(1):67–83. doi: 10.1016/0022-1759(76)90008-9. [DOI] [PubMed] [Google Scholar]
- SOCRANSKY S. S., GIBBONS R. J., DALE A. C., BORTNICK L., ROSENTHAL E., MACDONALD J. B. The microbiota of the gingival crevice area of man. I. Total microscopic and viable counts and counts of specific organisms. Arch Oral Biol. 1963 May-Jun;8:275–280. doi: 10.1016/0003-9969(63)90019-0. [DOI] [PubMed] [Google Scholar]
- Shah H. N., Williams R. A., Bowden G. H., Hardie J. M. Comparison of the biochemical properties of Bacteroides melaninogenicus from human dental plaque and other sites. J Appl Bacteriol. 1976 Dec;41(3):473–495. doi: 10.1111/j.1365-2672.1976.tb00660.x. [DOI] [PubMed] [Google Scholar]
- Slots J., Gibbons R. J. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978 Jan;19(1):254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Hausmann E. Longitudinal study of experimentally induced periodontal disease in Macaca arctoides: relationship between microflora and alveolar bone loss. Infect Immun. 1979 Feb;23(2):260–269. doi: 10.1128/iai.23.2.260-269.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J. Microflora in the healthy gingival sulcus in man. Scand J Dent Res. 1977 May;85(4):247–254. doi: 10.1111/j.1600-0722.1977.tb00560.x. [DOI] [PubMed] [Google Scholar]
- Slots J. The predominant cultivable microflora of advanced periodontitis. Scand J Dent Res. 1977 Jan-Feb;85(2):114–121. doi: 10.1111/j.1600-0722.1977.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Slots J. The predominant cultivable organisms in juvenile periodontitis. Scand J Dent Res. 1976 Jan;84(1):1–10. doi: 10.1111/j.1600-0722.1976.tb00454.x. [DOI] [PubMed] [Google Scholar]
- Socransky S. S. Microbiology of periodontal disease -- present status and future considerations. J Periodontol. 1977 Sep;48(9):497–504. doi: 10.1902/jop.1977.48.9.497. [DOI] [PubMed] [Google Scholar]
- Sonnenwirth A. C. Antibody response to anaerobic bacteria. Rev Infect Dis. 1979 Mar-Apr;1(2):337–341. doi: 10.1093/clinids/1.2.337. [DOI] [PubMed] [Google Scholar]
- Spiegel C. A., Hayduk S. E., Minah G. E., Krywolap G. N. Black-pigmented Bacteroides from clinically characterized periodontal sites. J Periodontal Res. 1979 Sep;14(5):376–382. doi: 10.1111/j.1600-0765.1979.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Tanner A. C., Haffer C., Bratthall G. T., Visconti R. A., Socransky S. S. A study of the bacteria associated with advancing periodontitis in man. J Clin Periodontol. 1979 Oct;6(5):278–307. doi: 10.1111/j.1600-051x.1979.tb01931.x. [DOI] [PubMed] [Google Scholar]
- Voller A., Bidwell D., Huldt G., Engvall E. A microplate method of enzyme-linked immunosorbent assay and its application to malaria. Bull World Health Organ. 1974;51(2):209–211. [PMC free article] [PubMed] [Google Scholar]



