Abstract
Sleep reduction is associated with increased energy intake and weight gain, though few studies have explored the relationship between sleep architecture and energy balance measures in the context of experimental sleep restriction. Fourteen males and 13 females (body mass index: 22–26 kg/m2) participated in a crossover sleep curtailment study. Participants were studied under two sleep conditions: short (4 h/night; 0100–0500 h) and habitual (9 h/night; 2200–0700 h), for 5 nights each. Sleep was polysomnographically recorded nightly. Outcome measures included resting metabolic rate (RMR), feelings of appetite-satiety, and ad libitum food intake. Short sleep resulted in reductions in stage 2 sleep and rapid eye movement (REM) sleep duration (P < 0.001), as well as decreased percentage of stage 2 sleep and REM sleep and increased slow wave sleep (SWS) percentage (P < 0.05). Linear mixed model analysis demonstrated a positive association between stage 2 sleep duration and RMR (P = 0.051). Inverse associations were observed between REM sleep duration and hunger (P = 0.031) and between stage 2 sleep duration and appetite for sweet (P = 0.015) and salty (P = 0.046) foods. Stage 2 sleep percentage was inversely related to energy consumed (P = 0.024). Stage 2 sleep (P = 0.005), SWS (P = 0.008), and REM sleep (P = 0.048) percentages were inversely related to fat intake, and SWS (P = 0.040) and REM sleep (P = 0.050) were inversely related to carbohydrate intake. This study demonstrates that changes in sleep architecture are associated with markers of positive energy balance and indicate a means by which exposure to short sleep duration and/or an altered sleep architecture profile may lead to excess weight gain over time.
Keywords: sleep duration, sleep architecture, sleep deprivation, energy expenditure, appetite, food intake
the prevalence of obesity has risen drastically over the last few decades and has been mirrored by concomitant decreases in sleep episode length. Indeed, results from epidemiological studies indicate an association between short sleep duration and weight gain (20). These cross-sectional studies have been supported by laboratory-based research, which demonstrate that experimental sleep length curtailment leads to decreased physical activity (25), increased feelings of hunger (4, 27) and food intake (4, 18, 29), and changes in appetite-regulating hormones (24, 27).
While the aforementioned studies demonstrate an association between sleep duration per se and markers of energy balance, none considered the role of specific sleep architecture changes in response to experimental sleep restriction. This is important since sleep is not a uniform process, and the amount and presence of specific sleep stages throughout the night is not constant. Sleep is regulated by an interaction between homeostatic and circadian mechanisms (3). Accordingly, in response to the build-up of homeostatic sleep pressure during the waking episode, the expression of slow wave sleep (SWS) is highest at the start of the sleep episode, whereas rapid eye movement (REM) sleep, under a circadian drive, shows maximal propensity during the early morning hours (5). Thus, under conditions of experimental and/or real-life sleep restriction, the amount of REM sleep may be disproportionately reduced compared with SWS, which is expected to be conserved.
Various sources indicate a link between REM sleep and energy balance-related parameters (8). An inverse relationship was found between REM sleep and orexinergic neuronal activity (12), and REM sleep-deprived rats show increased and decreased hypothalamic gene expression of neuropeptide Y and pro-opiomelanocortin, respectively (14), which may account for the hyperphagia observed in rats under REM sleep deprivation (15). Such an altered hormonal profile in response to REM sleep loss may lead to overeating and weight gain in humans as well. Compared with other sleep stages, only REM sleep was found to be significantly and independently associated with overweight in a group of children and adolescents, and a 1-h decrease in REM sleep was associated with an approximately threefold increased odds of overweight (16). Thus, when discussing the sleep-obesity link, sleep architecture should be considered in addition to sleep duration.
The goal of the current study was to further elucidate the mechanisms by which reductions in sleep duration lead to obesity. We aimed to explore how specific alterations in sleep architecture in response to experimental sleep restriction affect energy balance-related parameters. We hypothesized that a sleep architecture profile characterized by reductions in stage 2 sleep and REM sleep, which would likely occur in response to the experimental sleep restriction, would be associated with indicators of positive energy balance, namely increased appetite and food intake, and lower energy expenditure, which can lead to excess weight gain over time.
METHODS
Participants.
Thirty participants were enrolled in the study. One man was dismissed after phase 1 for previously undiagnosed periodic leg movement (PLM) disorders, one woman was dismissed before the start of the study for previously undisclosed use of antidepressant medication (an exclusion factor for the study), and one woman withdrew for personal reasons after completing phase 1. Twenty-seven (14 men, 13 women) healthy participants, age 30–45 yr [mean (SD): 35.3 (5.2) yr] and body mass index (BMI) 22–26 kg/m2 [23.5 (1.1) kg/m2], completed both experimental phases. Before entry into the study, a history of habitual sleep duration between 7 and 9 h/night was confirmed with the use of waist-worn actigraphy (19) and sleep diary for 2 wk. Inclusion criteria required mean sleep duration during the 2-wk screening period of 7–9 h/night, with at least 10 nights of sleep with a duration ≥7 h and less than 4 nights of sleep with a duration <6 h. Exclusion criteria for the study included smoking, Type 2 diabetes, history of alcohol or substance abuse, excessive caffeine intake, shift work, and transmeridian travel within the last 4 wk. Other exclusion criteria included the presence of any eating, sleeping, or neurological disorder and use of antidepressant medications, among others. Each participant provided written informed consent before being enrolled in the study.
Experimental design.
Experimental procedures for the current study have been previously described (29). This was a laboratory-based randomized, crossover study composed of two separate phases, including a short and a habitual sleep duration condition. Each experimental phase lasted 6 days, and each phase was separated by a 3-wk washout period to ensure recuperation from sleep deprivation and that women were studied in both conditions during the same menstrual cycle phase. Both inpatient phases took place at Clinilabs, a research sleep laboratory (New York, NY). Experimental procedures during each phase were identical except for the duration of the nocturnal sleep episodes. Specifically, during the short sleep phase, participants had the opportunity to sleep from 0100 to 0500 h. During the habitual sleep phase, participants had the opportunity to sleep from 2200 to 0700 h. While the in-lab sleep length opportunity for this phase was in fact longer than the actual mean duration spent asleep (∼7.5 h; see results), we chose to refer to the 9-h sleep opportunity as “habitual” as opposed to “long” because this length ensured that all participants would achieve their habitual sleep length. It has also been recommended that sleep restriction studies utilize sleep opportunities of >8 h time in bed for baseline sleep periods (32). Daytime naps were not permitted, and study personnel ensured that all participants remained awake during the entire duration of scheduled wake episodes. Participants were inpatients but were allowed to leave the laboratory under study personnel supervision.
During the first 4 days of each experimental phase, participants were fed a controlled diet based on their weight maintenance energy requirements, estimated by the Harris-Benedict equation with an activity factor of 1.3. Meals were served at 0800, 1200, and 1900 h, with a snack at 1600 h. Each meal provided 30% of daily energy requirements, and the snack provided the remaining 10%. Starting on the morning of day 5 and continuing until discharge at 2000 h on day 6, participants underwent the ad libitum feeding portion of the study in which they self-selected their food intake (see Measures below).
All experimental procedures were approved by the Institutional Review Boards of St. Luke's-Roosevelt Hospital Center and Columbia University (New York, NY). Participants were given the opportunity to ask questions about the study protocol prior to providing informed consent.
Measures.
Sleep duration and composition for each sleep episode was assessed via polysomnographic (PSG) recording (Aurora Recording Systems, Gamma, version 4.9; Grass Technologies, West Warwick, RI). PSG recordings, including electroencephalogram, electrooculogram, chin electromyogram (EMG), and electrocardiogram, were visually scored in 30-s epochs according to standard criteria (22). The amount of time in each sleep stage was determined and expressed in minutes and as a percentage of total sleep time (TST). TST was the sum of stage 1 and stage 2 sleep, SWS (stage 3 and stage 4 combined), and REM sleep. The number of REM sleep periods during the sleep episode was also determined. PLM disorders and sleep-disordered breathing were ruled out during the first two nights' recordings. For PLM, EMGs of the left and right anterior tibialis were recorded. Respiratory events were monitored with nasal and oral airflow via thermistor and oxygen saturation via pulse oximetry, and participants were excluded if an apnea/hypopnea index >5 events/h was observed.
Resting metabolic rate (RMR) and respiratory quotient (RQ) were measured in the fasted state on the morning of day 5 at the Body Composition Unit of St. Luke's-Roosevelt Hospital. Each participant rested for ≥30 min before beginning the test. RMR was measured using a ventilated-hood indirect calorimetry system (Delta-Trac II Metabolic Monitor; SensorMedics, Yorba Linda, CA). On a same day test-retest, the coefficient of variation for the system was 2.3%. Participants were asked to remain supine while the ventilated hood was placed over their head and respiration gases were collected over the 30-min recording period. Oxygen consumed and carbon dioxide produced were analyzed to calculate RMR and RQ according to the equations of Jequier et al. (10). All participants reached a steady state when measured.
On day 4, during the last day of the controlled feeding period and before the start of the ad libitum feeding portion of the study, participants filled out Likert scales assessing their feelings of appetite and satiety. The participants rated their feelings, on a scale of 0 to 10, to the following questions: 1) How hungry do you feel right now?, 2) How satisfied do you feel right now?, 3) How full do you feel right now?, 4) How much do you think you could eat right now?, 5) How much would you like to eat something sweet right now?, 6) How much would you like to eat something salty right now?, 7) How much would you like to eat something savory right now?, and 8) How much would you like to eat fruits and vegetables right now? A rating of 0 corresponded to “not at all” and a rating of 10 corresponded to “very much so.” Appetite-satiety Likert scales were completed hourly from 0700–2200 h, and a mean waking episode score spanning from 0800 to 2200 h was calculated for each participant.
During the ad libitum feeding portion of the study, participants were allowed to self-select meal times and food quantity and type. Various foods were available at the laboratory, and participants were also given a monetary allowance of $25 to purchase food and beverages of their choice outside the lab. For purchased items, nutrient information must have been available, and beverages were all nonalcoholic. Caffeinated beverages were limited to 1 per day. Food intake during this period was weighed and recorded by study personnel and entered into Diet Analysis Plus Software version 8.0 (Wadsworth, Florence, KY). Only ad libitum food intake from day 5 was considered for the current report, as previously reported (29).
Statistical analyses.
Data from 27 participants were included in initial PSG analyses. A total of 26 participants were included in the food intake analyses, the appetite-satiety scale analyses, and the RMR and RQ analyses. One male participant was considered an outlier for food intake measurements because his food intake during the ad libitum feeding portion of the habitual phase was almost twice his estimated energy requirements, and his consumption level was 3.6 times the SD of the intake of all other participants. Data from this participant were accordingly also not included in appetite-satiety scale analyses. Data from one woman were not included in the RMR and RQ analyses because she exercised before the measurement in the short sleep phase. Data from the aforementioned male outlier for food intake were included in the RMR and RQ analyses because his measurements were taken before the ad libitum feeding began.
Paired-samples t-tests were used to compare PSG sleep parameters obtained from the final night's sleep recording (day 5) between sleep duration conditions. Linear mixed model analysis was used to assess the relationships between sleep stage parameters and RMR, RQ, food intakes, and appetite-satiety ratings, after controlling for age, sex, body weight, and sleep phase condition as covariates. For all linear mixed model analyses, sleep stages (minutes and percentage of stage 2, SWS, and REM sleep) and number of REM periods were designated as independent predictor variables, with RMR, RQ, appetite-satiety ratings, and food intake designated as outcome variables. Analyses were adjusted for age, sex, weight, and sleep phase condition. Subject was used as a grouping variable in the mixed model. To align sleep data with energy balance-related parameters, PSG data for linear mixed model analyses with RMR, RQ, and food intakes were taken from the last night's sleep recording (day 5). We used night 5 PSG data instead of night 4 data (which precede the RMR, RQ, and food intake measures) because an in-dwelling antecubital vein catheter that was inserted to draw blood samples, including throughout the night 4 sleep episode, could have affected sleep outcomes. PSG data for linear mixed model analysis with appetite-satiety ratings were taken from the third night's sleep recording, which corresponded to the night before the assessment of appetite-satiety. Data are expressed as means (SD). A P value of <0.05 was used to define statistical significance. We have also tested for potential carryover effects by testing the significance of phase X treatment in the linear mixed model. It was not found to be significant and subsequently we dropped the interaction term for further analyses.
RESULTS
PSG sleep.
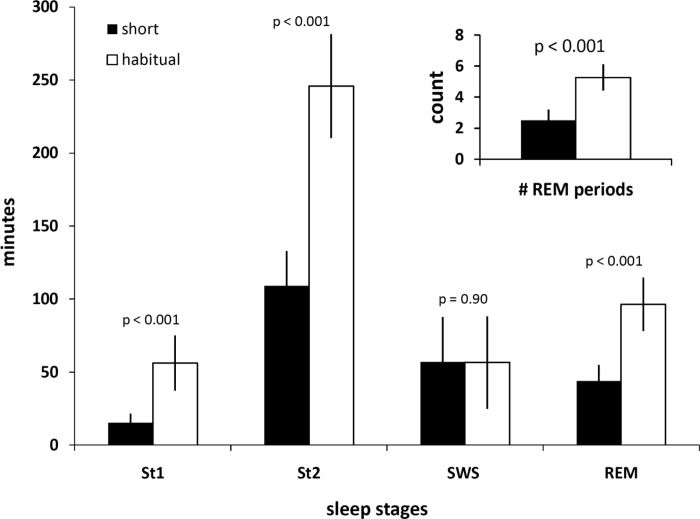
Sleep duration during the short sleep condition was 225.8 (6.5) min (∼3 h 46 min) compared with 455.1 (30.2) min (∼7 h 35 min) for the habitual sleep condition (P < 0.001). During short sleep compared with habitual sleep duration, significant decreases were observed in the amount of time spent in stage 1 sleep [15.3 (6.3) min vs. 56.2 (18.8) min; P < 0.001], stage 2 sleep [109.1 (23.8) min vs. 245.8 (35.5) min; P < 0.001], and REM sleep [43.9 (11.0) min vs. 96.4 (18.2) min; P < 0.001] (Fig. 1). The amount of time spent in SWS was not different in short sleep compared with habitual sleep conditions [57.1 (30.5) min vs. 56.6 (31.6) min; P = 0.90]. The number of REM sleep periods during the sleep period was significantly decreased in short sleep compared with habitual sleep [2.5 (0.7) vs. 5.3 (0.8); P < 0.001].
Fig. 1.
Duration of sleep stages and number of rapid eye movement sleep (REM) periods (inset) under short (4 h in bed; filled bars) and habitual (9 h in bed; open bars) sleep. St1, stage 1 sleep; St2, stage 2 sleep; SWS, slow wave sleep. P values indicate level of significance, by paired-samples t-test. Values are means ± SD.
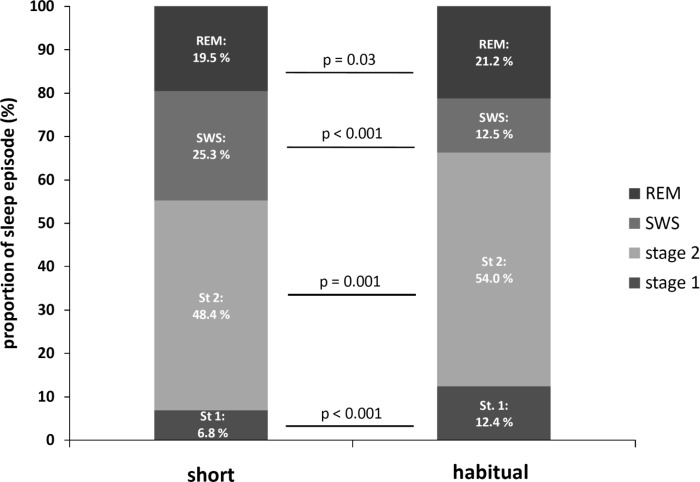
When data were expressed as a percentage of TST, significant reductions were observed for the percentage of time spent in stage 1 sleep [6.8 (2.8)% vs. 12.4 (4.1)%; P < 0.001], stage 2 sleep [48.4 (10.5)% vs. 54.0 (6.3)%; P = 0.001], and REM sleep [19.5 (4.9)% vs. 21.2 (3.7)%; P = 0.03] during short sleep compared with habitual sleep (Fig. 2). The percentage of time spent in SWS was significantly increased during short compared with habitual sleep [25.3 (13.4)% vs. 12.5 (7.0)%; P < 0.001].
Fig. 2.
Distribution of sleep stages under short (4 h in bed; left column) and habitual (9 h in bed; right column) sleep episodes. P values indicate level of significance, by paired-samples t-test.
Linear mixed model analysis.
Our prior publication detailed the absolute values and differences in RMR, RQ, appetite-satiety ratings, and ad libitum energy and macronutrient intakes between short and habitual sleep durations (29). In general, there was no difference between sleep periods on RMR, RQ, and appetite-satiety ratings but participants ate more calories, particularly from fat, during the period of short sleep relative to habitual sleep.
Time spent in stage 2 sleep was positively and marginally significantly associated (P = 0.051) with RMR but not with RQ (Table 1). Time spent in other sleep stages was not associated with RMR or RQ. In the model considering percentage of each sleep parameter, none were significantly associated with RMR or RQ.
Table 1.
Results of the linear mixed model analysis for sleep stages with energy expenditure
| Minutes |
Percent |
|||||
|---|---|---|---|---|---|---|
| Coefficient | Standard error | P value | Coefficient | Standard error | P value | |
| RMR | ||||||
| Stage 2 | 1.7 | 0.85 | 0.051 | 1.11 | 5.53 | 0.84 |
| SWS | 0.81 | 0.99 | 0.42 | −2.06 | 4.98 | 0.68 |
| REM | 0.06 | 1.29 | 0.96 | −4.90 | 6.47 | 0.45 |
| REM periods | −29.87 | 25.77 | 0.25 | −26.30 | 25.749 | 0.31 |
| RQ | ||||||
| Stage 2 | 1.06E-04 | 2.48E-04 | 0.67 | −2.14E-03 | 1.59E-03 | 0.19 |
| SWS | 1.78E-04 | 2.90E-04 | 0.54 | −1.70E-03 | 1.44E-03 | 0.24 |
| REM | 3.21E-04 | 3.78E-04 | 0.40 | −1.37E-03 | 1.86E-03 | 0.47 |
| REM periods | 4.66E-03 | 7.55E-03 | 0.54 | 5.46E-03 | 7.42E-03 | 0.46 |
Sleep stages were expressed as minutes and percent total sleep time. Analyses were adjusted for age, sex, weight, and sleep phase condition. RMR, resting metabolic rate; RQ, respiratory quotient; SWS, slow wave sleep; REM, rapid eye movement sleep.
Minutes of REM sleep showed a significant inverse relationship with ratings on question 1 (“How hungry do you feel right now?”; P = 0.031; Table 2) and a marginally significant inverse relationship with question 4 (“How much do you think you could eat right now?”; P = 0.052). Minutes of stage 2 sleep showed a significant inverse relationship with ratings on question 5 (“How much would you like to eat something sweet right now?”; P = 0.015) and question 6 (“How much would you like to eat something salty right now?”; P = 0.046), and percent TST in stage 2 showed a significant inverse relationship with question 5 ratings (P = 0.04; Table 2). A trend for a negative association was observed between percent TST in SWS and question 3 (“How full do you feel right now?”; P = 0.056).
Table 2.
Results of linear mixed model analysis for sleep stages with appetite-satiety ratings
| Minutes |
Percent |
|||||
|---|---|---|---|---|---|---|
| Coefficient | Standard error | P value | Coefficient | Standard error | P value | |
| Q1: How hungry do you feel right now?† | ||||||
| Stage 2 | −0.02 | 0.01 | 0.069 | −0.04 | 0.06 | 0.55 |
| SWS | −0.01 | 0.01 | 0.27 | −0.01 | 0.05 | 0.82 |
| REM | −0.03 | 0.01 | 0.031 | −0.07 | 0.07 | 0.32 |
| REM periods | 0.10 | 0.24 | 0.66 | −0.13 | 0.22 | 0.58 |
| Q2: How satisfied do you feel right now? | ||||||
| Stage 2 | 0.00 | 0.01 | 0.77 | −0.07 | 0.06 | 0.29 |
| SWS | 0.00 | 0.01 | 0.79 | −0.06 | 0.05 | 0.27 |
| REM | 0.02 | 0.01 | 0.25 | −0.01 | 0.07 | 0.91 |
| REM periods | −0.06 | 0.25 | 0.81 | −0.03 | 0.22 | 0.89 |
| Q3: How full do you feel right now? | ||||||
| Stage 2 | −0.02 | 0.01 | 0.095 | −0.08 | 0.05 | 0.15 |
| SWS | −0.02 | 0.01 | 0.12 | −0.09 | 0.05 | 0.056 |
| REM | 0.02 | 0.01 | 0.079 | 0.02 | 0.06 | 0.75 |
| REM periods | 0.16 | 0.22 | 0.46 | 0.08 | 0.19 | 0.67 |
| Q4: How much do you think you could eat right now? | ||||||
| Stage 2 | −0.01 | 0.01 | 0.54 | −0.03 | 0.07 | 0.70 |
| SWS | 0.00 | 0.02 | 0.83 | −0.01 | 0.06 | 0.87 |
| REM | −0.03 | 0.02 | 0.052 | −0.10 | 0.08 | 0.25 |
| REM periods | 0.07 | 0.29 | 0.82 | −0.06 | 0.26 | 0.81 |
| Q5: How much would you like to eat something sweet right now?† | ||||||
| Stage 2 | −0.04 | 0.02 | 0.015 | −0.24 | 0.11 | 0.040 |
| SWS | −0.02 | 0.02 | 0.35 | −0.15 | 0.09 | 0.11 |
| REM | −0.03 | 0.03 | 0.31 | −0.17 | 0.13 | 0.20 |
| REM periods | −0.11 | 0.43 | 0.79 | −0.49 | 0.39 | 0.22 |
| Q6: How much would you like to eat something salty right now? | ||||||
| Stage 2 | −0.03 | 0.02 | 0.046 | −0.17 | 0.10 | 0.083 |
| SWS | 0.01 | 0.02 | 0.74 | −0.10 | 0.08 | 0.22 |
| REM | −0.01 | 0.02 | 0.71 | −0.11 | 0.11 | 0.31 |
| REM periods | 0.23 | 0.37 | 0.53 | 0.01 | 0.34 | 0.97 |
| Q7: How much would you like to eat something savory right now? | ||||||
| Stage 2 | −0.03 | 0.02 | 0.11 | −0.12 | 0.10 | 0.23 |
| SWS | 0.00 | 0.02 | 0.84 | −0.06 | 0.08 | 0.52 |
| REM | −0.04 | 0.02 | 0.095 | −0.14 | 0.11 | 0.22 |
| REM periods | 0.23 | 0.38 | 0.55 | −0.03 | 0.35 | 0.93 |
| Q8: How much would you like to eat fruits & vegetables right now? | ||||||
| Stage 2 | −0.01 | 0.02 | 0.37 | −0.02 | 0.10 | 0.85 |
| SWS | 0.00 | 0.02 | 0.94 | 0.04 | 0.08 | 0.63 |
| REM | −0.04 | 0.02 | 0.065 | −0.08 | 0.11 | 0.50 |
| REM periods | 0.03 | 0.39 | 0.94 | −0.17 | 0.35 | 0.63 |
Sleep stages were expressed as minutes and percent total sleep time. Analyses were adjusted for age, sex, weight, and sleep phase condition. Q, question;
Under Minutes heading, significant effect of sleep phase covariate (Q1: P = 0.009; Q5: P = 0.017).
A statistically significant inverse relationship was observed between percentage of stage 2 sleep and calories consumed (P = 0.024, Table 3). Percent time spent in stage 2 (P = 0.005), SWS (P = 0.008), and REM sleep (P = 0.048) was significantly and inversely associated with fat intake (Table 3). Percent SWS showed a marginally significant inverse association with saturated fat intake (P = 0.054). Sleep stages and protein were not associated, although REM sleep percentage showed a tendency for a positive association with protein intake (P = 0.093). Percent time spent in stage 2 (P = 0.057) and REM sleep (P = 0.050) was marginally, and SWS (P = 0.040) was significantly inversely related to carbohydrate intake (Table 3).
Table 3.
Results of linear mixed model analysis for sleep stages with energy and macronutrient intakes
| Minutes |
Percent |
|||||
|---|---|---|---|---|---|---|
| Coefficient | Standard error | P value | Coefficient | Standard error | P value | |
| Calories | ||||||
| Stage 2 | −4.27 | 4.51 | 0.35 | −60.23 | 25.78 | 0.024 |
| SWS | −0.55 | 5.16 | 0.92 | −43.43 | 23.87 | 0.076 |
| REM | 6.29 | 6.81 | 0.36 | −17.42 | 28.55 | 0.54 |
| REM periods | −154.73 | 132.01 | 0.25 | −149.30 | 123.27 | 0.23 |
| Fat | ||||||
| Stage 2 | −0.41 | 0.27 | 0.13 | −4.55 | 1.54 | 0.005 |
| SWS | −0.26 | 0.30 | 0.40 | −3.93 | 1.42 | 0.008 |
| REM | −0.04 | 0.40 | 0.92 | −3.47 | 1.70 | 0.048 |
| REM periods | 0.83 | 7.76 | 0.92 | −0.27 | 7.36 | 0.97 |
| Saturated fat | ||||||
| Stage 2 | −0.13 | 0.13 | 0.35 | −1.43 | 0.81 | 0.084 |
| SWS | −0.22 | 0.16 | 0.17 | −1.50 | 0.75 | 0.054 |
| REM | −0.03 | 0.21 | 0.89 | −1.05 | 0.89 | 0.25 |
| REM periods | −4.81 | 4.01 | 0.24 | −5.25 | 3.85 | 0.18 |
| Protein | ||||||
| Stage 2 | −0.20 | 0.16 | 0.21 | −0.35 | 0.90 | 0.70 |
| SWS | −0.07 | 0.18 | 0.69 | 0.15 | 0.84 | 0.86 |
| REM | 0.28 | 0.24 | 0.25 | 1.72 | 1.00 | 0.093 |
| REM periods | 6.98 | 4.59 | 0.14 | 6.37 | 4.32 | 0.15 |
| Carbohydrate | ||||||
| Stage 2 | 0.17 | 1.28 | 0.89 | −8.87 | 7.39 | 0.057 |
| SWS | 1.46 | 1.46 | 0.33 | −3.43 | 6.84 | 0.040 |
| REM | 1.51 | 1.93 | 0.44 | −1.81 | 8.18 | 0.050 |
| REM periods | −65.85 | 37.38 | 0.085 | −58.20 | 35.33 | 0.11 |
Sleep stages were expressed as minutes and percent total sleep time. Analyses were adjusted for age, sex, weight, and sleep phase condition.
DISCUSSION
Few studies have examined the association between sleep architecture changes in response to experimental restriction of sleep duration and resulting markers of energy balance, including energy expenditure, feelings of hunger, and food intake. This line of investigation is important, since laboratory-based research on the link between sleep and weight gain has frequently only considered short sleep duration, per se, and not resultant alterations in sleep stage duration and distribution. Furthermore, as was pointed out in a recent review (13), methodological variations in prior sleep restriction studies, which can differentially and uniquely alter the expression of sleep stages, may account for discrepancies in the literature regarding how sleep affects energy homeostasis. Our data show a consistent pattern of lower RMR, increased subjective appetite for sweet and salty foods, and increased energy and fat intake with lower TST in stage in 2 sleep, specifically. Stage 2 sleep may be critical in the maintenance of energy balance regulation. SWS and REM sleep, although not related to energy expenditure, were also found to be related to food choice: increased intakes of fat and carbohydrates were observed with lower SWS and REM sleep.
Our prior report (29), like others (18), found no change in overall energy expenditure, including RMR, between short and habitual sleep duration conditions, though we did observe a tendency toward reduced RMR after short sleep (29). However, RMR was found to be significantly reduced in response to a night of total sleep deprivation (2). Our current description of a differential effect of sleep stages, and specifically a role of stage 2 on RMR, may explain some of the discrepancies between various studies. Stage 2 is the predominant sleep stage constituting up to 50% of the sleep episode. Thus its elimination likely contributes to the reduction in RMR observed after a full night spent awake. Furthermore, throughout a sleep episode, energy expenditure is significantly reduced during stage 2 sleep compared with wakefulness after sleep onset (11). Together with the finding that energy expenditure was increased by ∼32% during a night of total sleep deprivation compared with baseline sleep (11), stage 2 sleep seems to play a role in energy conservation during the sleep episode. Thus periods of restricted sleep and the resulting substantial loss of stage 2 sleep are likely to result in large increases in energy expenditure during the night. It is theoretically possible that, in an attempt to restore energy homeostasis, a compensatory decrease in RMR upon awakening is observed, though this speculative explanation needs experimental confirmation. The current study did not record energy expenditure during the sleep episode. However, future studies relating metabolic rate during the sleep episode to daytime RMR under baseline sleep conditions and comparing this to the relationship between metabolic rate during the time spanning the habitual sleep episode and daytime RMR under sleep restriction conditions can explore the aforementioned hypothesis. In a study by Hursel and colleagues (9), experimentally induced sleep fragmentation did not affect RMR or sleeping metabolic rate but did result in increased RQ and carbohydrate oxidation as well as reduced fat oxidation. A direct comparison with our present report is difficult, however, since sleep fragmentation in the prior study caused a simultaneous reduction in minutes of SWS, REM sleep, and stage 2 sleep (9). Consistently reduced energy expenditure combined with increased energy intake, and possibly RQ, in response to reduced stage 2 sleep, may therefore be a mechanism by which sleep restriction leads to positive energy balance and weight gain over time.
Some prior studies have demonstrated an increase in hunger ratings (4) and food intake (4, 18, 29) in response to experimental sleep restriction. Moreover, increased hunger was observed in connection with increased appetite for sweet and salty foods (27). While sleep architecture was not reported in the aforementioned study by Spiegel and colleagues (26, 27), sleep restriction was achieved by reducing sleep from 10 h/night (2200–0800 h) to 4 h/night (0100–0500 h), which would imply a significant reduction in stage 2 sleep and REM sleep. Our current findings of an inverse association between REM sleep and hunger, as well as inverse relationships between stage 2 sleep and appetite for both sweet and salty foods may explain this. Similarly, the study by Nedeltcheva and colleagues (18) reported significant reductions in stage 2 and REM sleep when bedtimes were reduced to 5.5 h/night from 8.5 h/night. That investigation also reported increased energy intake from snacks without concomitant changes in the leptin and ghrelin concentrations (18), implying a mechanism of increased energy intake that is more associated with altered sleep architecture than altered appetite-regulating hormones. A study by Gonnissen and colleagues (7) that utilized experimental sleep fragmentation to reduce REM sleep while maintaining SWS and TST reported an increase in postdinner desire-to-eat during fragmented compared with undisturbed sleep, which is in line with our present findings. These and our findings reinforce a role of sleep architecture in appetite and food intake regulation.
Prior studies have demonstrated relationships between sleep stages and overweight. An important epidemiological study reported that REM sleep is the sleep stage most strongly associated with overweight in children and adolescents and attributed the short sleep-obesity association to reductions in REM sleep (16). Our current data are consistent with this hypothesis and support a pathway whereby reductions in REM sleep are related to weight gain via increased hunger and intakes of fat and carbohydrate.
Evidence for a relationship between sleep and body weight and metabolism has also been demonstrated for SWS. An innovative study demonstrating a role of SWS in metabolism found that selective suppression of SWS without concomitant reductions in TST resulted in decreased insulin sensitivity, reduced glucose tolerance, and increased sympathovagal balance (30). SWS has also been found to be inversely related to BMI (21), waist circumference (21), and hypertension (6) in older men. Our data further show an inverse relationship between SWS and intakes of fat and carbohydrates, presenting a possible mechanism relating changes in this sleep stage with weight gain and adverse metabolic outcomes. The inverse association described here may be surprising since our sleep manipulation did not reduce SWS duration and increased percent time spent in SWS. However, others have described significant reductions in both REM sleep and SWS in response to reducing sleep time to 4 h/night from 8 h/night (26). Interestingly, in the same study, sleep extension to 12 h/night was associated with a decrease in percent SWS (26), and some epidemiological evidence links long sleep with obesity (17).
Whereas the current report focuses on different components of energy balance, in our prior publication (29), energy balance during short and habitual sleep was calculated by subtracting total energy expenditure over 5 days (determined with doubly labeled water) from total energy intake over the 5-day period (i.e., the 4-day controlled feeding + ad libitum intake). After controlling for phase order and sex, no significant effect of sleep duration was observed (29). When calculated energy balance values from day 1 to day 6 were included in the linear mixed model analyses used in the current report, no statistically significant relationships with any sleep stages, both minutes or percent, were observed (data not shown). In contrast, Rutters and colleagues (23) investigated interindividual relationships between sleep architecture during full sleep episodes recorded in the laboratory and energy balance and observed an inverse relationship between SWS and energy balance. It should be pointed out that our current study was well designed to explore how individual components of energy balance, energy expenditure, and food intake relate to changes in sleep architecture but less so in describing energy balance per se, since controlled feeding contributed the majority of energy intake, with only a short duration of time devoted to ad libitum feeding. More studies should be conducted to further explore the relationship between sleep architecture, independent of sleep duration, and energy balance.
Since up to 50% of the sleep episode is composed of stage 2 sleep, it is possible that what we interpret as associations between stage 2 sleep specifically and markers of energy balance are really simply associations with reduced sleep duration overall. Nevertheless, as is observed in this study, different aspects of sleep architecture are associated with different components of energy balance, such that whereas stage 2 sleep alone seems to be related to energy expenditure, REM sleep and SWS may be more related to hunger/satiety. Moreover, if the relationships between stage 2 sleep and energy balance components were due solely to reductions in TST, we would expect stage 2 sleep percentage, as well as the sleep phase covariate to be significant in all instances of significant relationships with stage 2 sleep, though the latter is only observed for the question of “How much would you like to eat something sweet right now?” Future investigations should utilize established techniques to selectively reduce the expression of specific sleep stages and observe the metabolic outcomes (30). Such an experimental approach would enable researchers to determine whether a more direct causal link exists between sleep architecture and energy balance outcomes, in addition to the relationships reported here.
The finding of significant relationships for appetite-satiety ratings with sleep stages when expressed in minutes (but not percentage) and for food intake with sleep stages when expressed as percent TST (but not minutes) was unexpected. Nonetheless, we believe that these results are useful to further delineate the differential effects of sleep quantity from sleep architecture in a novel way, thus indicating more clearly the specific roles of sleep architecture on energy balance-related parameters and, by extension, weight regulation. Specifically, it is possible that sleep stage as well as TST are related to energy balance, and it is not known if these effects are independent. By including comparisons of both minutes of sleep stages as well as expressing them as percentage of TST, we have gone some way into addressing these differential effects. Specifically, data expressed as percent help to distinguish sleep duration from sleep stages by incorporating sleep quantity: When expressed as a percentage, sleep duration is taken into account, as the denominator is reduced sleep time. Based on our findings, it may appear that sleep quantity plays a larger role in influencing food intake, whereas it may be playing a smaller role in subjective ratings of appetite and satiety. A physiological underpinning of the aforementioned discrepancy remains unknown. It is possible that the observed inverse association between feelings of hunger and REM sleep minutes is due to the increased signaling of orexin (12) and neuropeptide Y (14) previously found to occur in response to selective REM sleep deprivation. The observation in the current report that actual intakes of food were associated with sleep stages when expressed as percent TST but not in absolute minutes may indicate that sleep duration plays a role in reward valuation of foods (31). Indeed, using functional brain imaging techniques, we (28) and others (1) have recently presented evidence that sleep restriction affects brain regions involved in reward, decision making, and cognitive control. These explanations, however, are speculative and require further testing.
Our use of data from night 3 for some analyses and data from night 5 for others could be viewed as a weakness, since relationships may differ depending on the extent of sleep debt. However, the difference between 3 and 5 days of restricted sleep seems limited; there was no significant difference between sleep onset latency on night 3 short sleep versus night 5 short sleep (P = 0.67) or between SWS on night 3 short sleep versus night 5 short sleep (both minutes and percent, P = 0.23 and P = 0.24, respectively).
Perspectives and Significance
We demonstrated an association between sleep architecture and energy balance components. Specifically, changes in sleep architecture including reductions in stage 2 sleep, REM sleep, and SWS are associated with markers of positive energy balance, such as reduced RMR, increased hunger, and increased intakes of energy, fat, and carbohydrates. Our results give insight into the mechanisms underlying the association between sleep and body weight and indicate a means by which chronic exposure to short sleep duration or an altered sleep architecture profile can lead to excess weight gain over time. These findings also indicate that changes in sleep architecture may affect energy balance components in a pathway independent of sleep duration. Sleep architecture likely plays a role in the development and maintenance of adverse weight and energy balance outcomes, and this may be particularly relevant for obese individuals and those with obstructive sleep apnea, who often experience reductions in REM sleep and SWS (20, 29). Future studies should address the mechanisms by which sleep affects energy homeostasis, and further clarify the relative contributions of sleep quantity and architecture on these relationships.
GRANTS
This publication was supported by the New York Obesity Nutrition Research Center Grant P30 DK-26687, National Center for Research Resources and the National Center for Advancing Translational Sciences, and National Institutes of Health (NIH) Grants UL1 RR-024156, R01 HL-091352 (to M.-P. St-Onge), and T32-DK-007559 (to A. Shecter). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.S., A.R., and M.-P.S.-O. analyzed data; A.S., A.R., and M.-P.S.-O. interpreted results of experiments; A.S. prepared figures; A.S. and M.-P.S.-O. drafted manuscript; A.S., M.O., A.L.R., G.K.Z., A.R., and M.-P.S.-O. edited and revised manuscript; A.S., M.O., A.L.R., G.K.Z., A.R., and M.-P.S.-O. approved final version of manuscript; M.O., A.L.R., G.K.Z., and M.-P.S.-O. performed experiments; M.-P.S.-O. conception and design of research.
ACKNOWLEDGMENTS
We thank Dr. Robert Basner for helpful discussions and comments on the manuscript. Current affiliation of A. L. Roberts is School of Public Health, University of North Carolina-Chapel Hill, Chapel Hill, NC.
REFERENCES
- 1. Benedict C, Brooks SJ, O'Daly OG, Almen MS, Morell A, Aberg K, Gingnell M, Schultes B, Hallschmid M, Broman JE, Larsson EM, Schioth HB. Acute sleep deprivation enhances the brain's response to hedonic food stimuli: an fMRI study. J Clin Endocrinol Metab 97: E443–E447, 2012 [DOI] [PubMed] [Google Scholar]
- 2. Benedict C, Hallschmid M, Lassen A, Mahnke C, Schultes B, Schioth HB, Born J, Lange T. Acute sleep deprivation reduces energy expenditure in healthy men. Am J Clin Nutr 93: 1229–1236, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms 14: 557–568, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr 91: 1550–1559, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci 15: 3526–3538, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fung MM, Peters K, Redline S, Ziegler MG, Ancoli-Israel S, Barrett-Connor E, Stone KL. Decreased slow wave sleep increases risk of developing hypertension in elderly men. Hypertension 58: 596–603, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gonnissen HK, Hursel R, Rutters F, Martens EA, Westerterp-Plantenga MS. Effects of sleep fragmentation on appetite and related hormone concentrations over 24 h in healthy men. Br J Nutr (Published ahead of print June 8): 1–9, 2012 [DOI] [PubMed] [Google Scholar]
- 8. Horne J. REM sleep, energy balance and “optimal foraging”. Neurosci Biobehav Rev 33: 466–474, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Hursel R, Rutters F, Gonnissen HK, Martens EA, Westerterp-Plantenga MS. Effects of sleep fragmentation in healthy men on energy expenditure, substrate oxidation, physical activity, and exhaustion measured over 48 h in a respiratory chamber. Am J Clin Nutr 94: 804–808 [DOI] [PubMed] [Google Scholar]
- 10. Jequier E, Acheson K, Schutz Y. Assessment of energy expenditure and fuel utilization in man. Ann Rev Nutr 7: 187–208, 1987 [DOI] [PubMed] [Google Scholar]
- 11. Jung CM, Melanson EL, Frydendall EJ, Perreault L, Eckel RH, Wright KP. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol 589: 235–244, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kitka T, Adori C, Katai Z, Vas S, Molnar E, Papp RS, Toth ZE, Bagdy G. Association between the activation of MCH and orexin immunorective neurons and REM sleep architecture during REM rebound after a three day long REM deprivation. Neurochem Int 59: 686–694, 2011 [DOI] [PubMed] [Google Scholar]
- 13. Klingenberg L, Sjodin A, Holmback U, Astrup A, Chaput JP. Short sleep duration and its association with energy metabolism. Obes Rev 13: 565–577 [DOI] [PubMed] [Google Scholar]
- 14. Koban M, Le WW, Hoffman GE. Changes in hypothalamic corticotropin-releasing hormone, neuropeptide Y, and proopiomelanocortin gene expression during chronic rapid eye movement sleep deprivation of rats. Endocrinology 147: 421–431, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Koban M, Sita LV, Le WW, Hoffman GE. Sleep deprivation of rats: the hyperphagic response is real. Sleep 31: 927–933, 2008 [PMC free article] [PubMed] [Google Scholar]
- 16. Liu X, Forbes EE, Ryan ND, Rofey D, Hannon TS, Dahl RE. Rapid eye movement sleep in relation to overweight in children and adolescents. Arch Gen Psychiatry 65: 924–932, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marshall NS, Glozier N, Grunstein RR. Is sleep duration related to obesity? A critical review of the epidemiological evidence. Sleep Med Rev 12: 289–298, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr 89: 126–133, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paavonen EJ, Fjallberg M, Steenari MR, Aronen ET. Actigraph placement and sleep estimation in children. Sleep 25: 235–237, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 16: 643–653, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rao MN, Blackwell T, Redline S, Stefanick ML, Ancoli-Israel S, Stone KL. Association between sleep architecture and measures of body composition. Sleep 32: 483–490, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring Systems for Sleep Stages af Human Subjects. Los Angeles, CA: Brain Information Service, Brain Research Institute, UCLA, 1968 [Google Scholar]
- 23. Rutters F, Gonnissen HK, Hursel R, Lemmens SG, Martens EA,. and Westerterp-Plantenga M.S.. Distinct associations between energy balance and the sleep characteristics slow wave sleep and rapid eye movement sleep. Int J Obes (Lond) 2012 [DOI] [PubMed] [Google Scholar]
- 24. Schmid SM, Hallschmid M, Jauch-Chara K, Born J, Schultes B. A single night of sleep deprivation increases ghrelin levels and feelings of hunger in normal-weight healthy men. J Sleep Res 17: 331–334, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Benedict C, Lehnert H, Born J, Schultes B. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr 90: 1476–1482, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab 89: 5762–5771, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Annal Intern Med 141: 846–850, 2004 [DOI] [PubMed] [Google Scholar]
- 28. St-Onge MP, McReynolds A, Trivedi ZB, Roberts AL, Sy M, Hirsch J. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. Am J Clin Nutr 95: 818–824, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. St-Onge MP, Roberts AL, Chen J, Kelleman M, O'Keeffe M, RoyChoudhury A, Jones PJ. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr 94: 410–416, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci USA 105: 1044–1049, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Van der Zee EA, Boersma GJ, Hut RA. The neurobiology of circadian rhythms. Curr Opin Pulm Med 15: 534–539, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 26: 117–126, 2003 [DOI] [PubMed] [Google Scholar]