Abstract
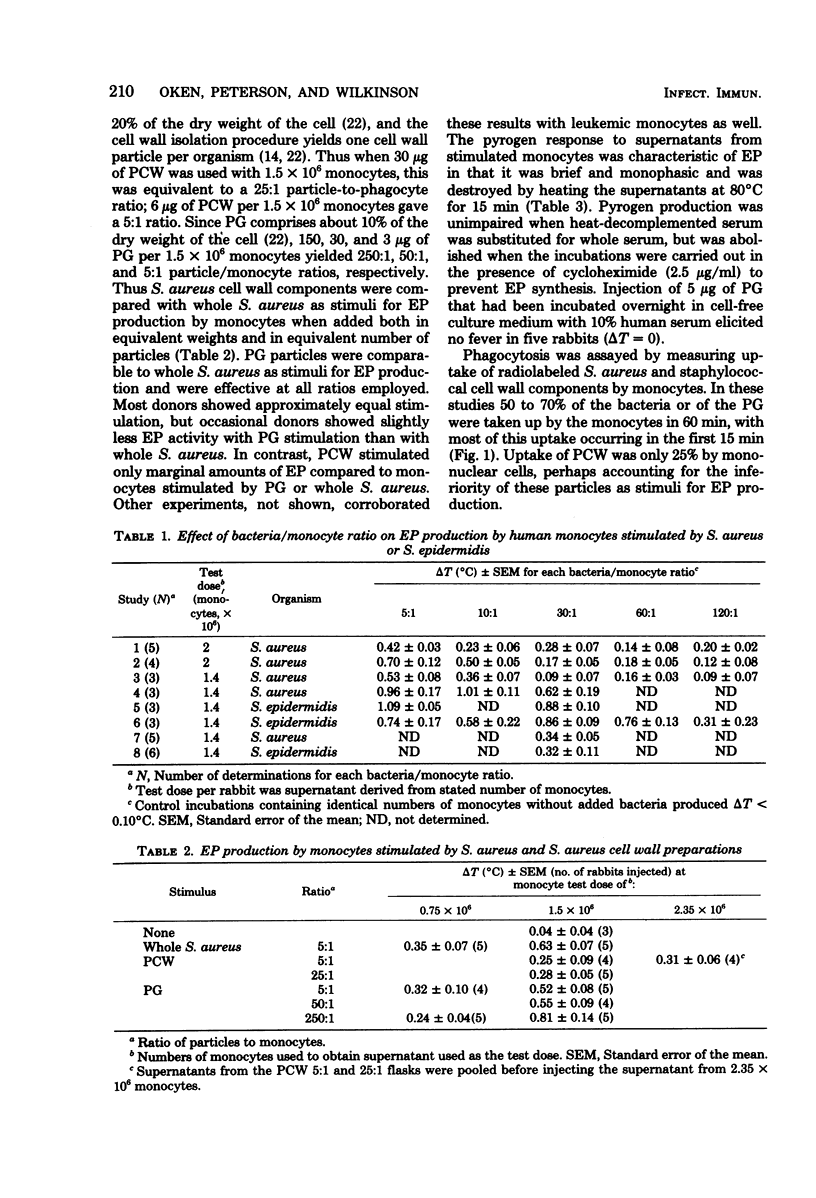
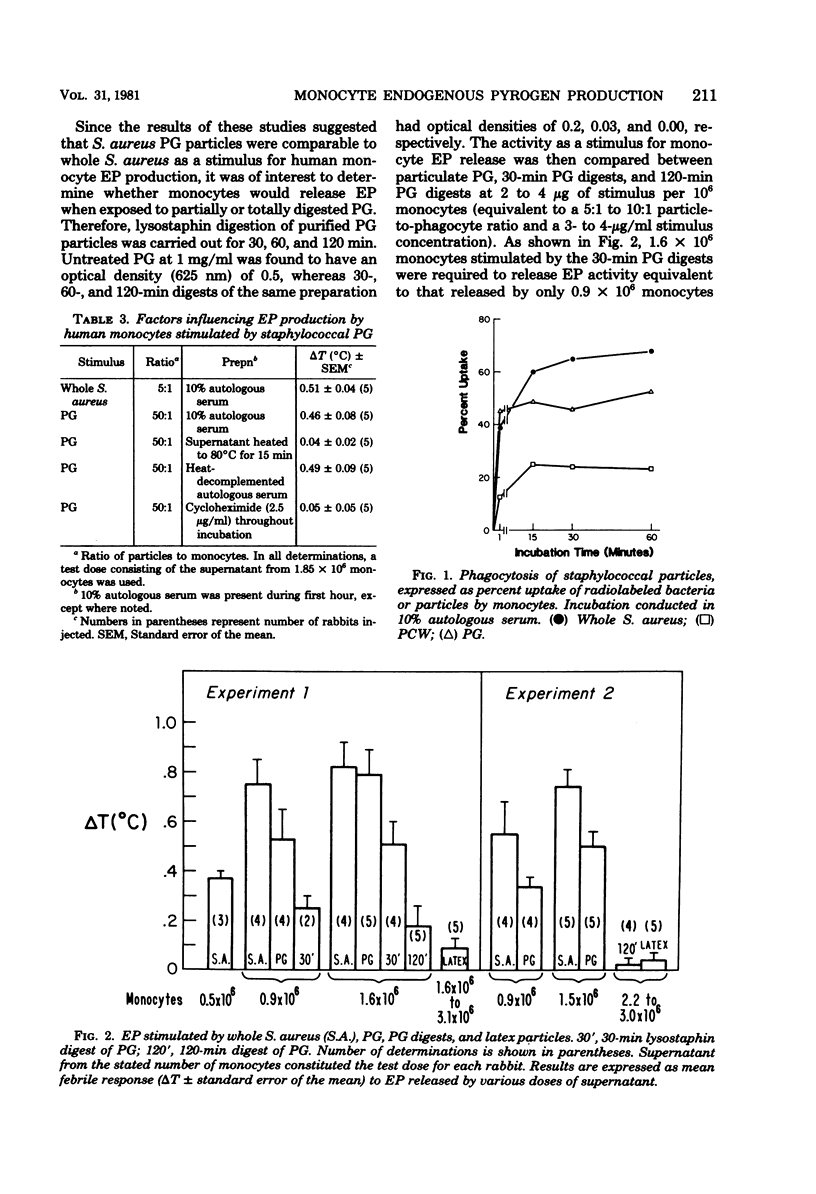
To determine the properties of Staphylococcus aureus contributing to its pyrogenicity, we compared, in human monocytes, endogenous pyrogen production stimulated by heat-killed S. aureus with that stimulated by purified S. aureus cell walls or by particulate peptidoglycan prepared from the same strain. Peptidoglycan, but not the purified cell wall preparation, was found comparable to S. aureus as an endogenous pyrogen stimulus. This finding was associated with a more effective monocyte phagocytosis of S. aureus and peptidoglycan as compared with that of purified cell walls. Lysostaphin digestion of peptidoglycan markedly reduced its pyrogenicity. To test whether the chemical composition of the ingested particles is important, latex particles were tested as possible stimuli for monocyte endogenous pyrogen release. Although 40 to 68% of monocytes ingested latex particles during the first hour, there was no evidence of endogenous pyrogen activity in the supernatant even when supernatants equivalent to 5.2 X 10(6) monocytes were tested. This study demonstrates that the pyrogenic moiety of the S. aureus cell wall resides in the peptidoglycan component. Phagocytosis is not in itself a pyrogenic stimulus, but rather serves as an effective mechanism to bring about contact between the chemical stimulus and the monocyte.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins E., Morse S. I. Studies in staphylococcal fever. VI. Responses induced by cell walls and various fractions of staphylococci and their products. Yale J Biol Med. 1967 Apr;39(5):297–311. [PMC free article] [PubMed] [Google Scholar]
- BERLIN R. D., WOOD W. B., Jr STUDIES ON THE PATHOGENESIS OF FEVER. 13. THE EFFECT OF PHAGOCYTOSIS ON THE RELEASE OF ENDOGENOUS PYROGEN BY POLYMORPHONUCLEAR LEUKOCYTES. J Exp Med. 1964 May 1;119:715–726. doi: 10.1084/jem.119.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodel P., Miller H. Differences in pyrogen production by mononuclear phagocytes and by fibroblasts or HeLa cells. J Exp Med. 1977 Mar 1;145(3):607–617. doi: 10.1084/jem.145.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodel P. Studies on the mechanism of endogenous pyrogen production. III. Human blood monocytes. J Exp Med. 1974 Oct 1;140(4):954–965. doi: 10.1084/jem.140.4.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Dinarello C. A., Elin R. J., Chedid L., Wolff S. M. The pyrogenicity of the synthetic adjuvant muramyl dipeptide and two structural analogues. J Infect Dis. 1978 Dec;138(6):760–767. doi: 10.1093/infdis/138.6.760. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Goldin N. P., Wolff S. M. Demonstration and characterization of two distinct human leukocytic pyrogens. J Exp Med. 1974 Jun 1;139(6):1369–1381. doi: 10.1084/jem.139.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Narita T., Kotani S., Kato K. Studies on cell walls of group A Streptococcus pyogenes, type 12. II. Pyrogenic and related biological activities of the higher molecular weight fraction of an enzymatic digest of the cell walls. Biken J. 1971 Sep;14(3):217–231. [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Shimono T., Harada K., Shiba T. Correlation between the immunoadjuvant activities and pyrogenicities of synthetic N-acetylmuramyl-peptides or -amino acids. Biken J. 1976 Mar;19(1):9–13. [PubMed] [Google Scholar]
- Mitchell R. H., Gander G. W., Goodale F. The role of phagocytosis in the production of endogenous pyrogen by polymorphonuclear leukocytes. Adv Exp Med Biol. 1976;73(PT-A):257–266. doi: 10.1007/978-1-4684-3297-8_22. [DOI] [PubMed] [Google Scholar]
- Oken M. M., Loch J. Corticosteroid and antihistamine modification of bleomycin-induced fever. Proc Soc Exp Biol Med. 1979 Sep;161(4):594–596. doi: 10.3181/00379727-161-40603. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Wilkinson B. J., Kim Y., Schmeling D., Douglas S. D., Quie P. G., Verhoef J. The key role of peptidoglycan in the opsonization of Staphylococcus aureus. J Clin Invest. 1978 Mar;61(3):597–609. doi: 10.1172/JCI108971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERSON B. S., SCHWAB J. H. Endotoxic properties associated with cell walls of group A streptococci. J Infect Dis. 1961 Jan-Feb;108:25–34. doi: 10.1093/infdis/108.1.25. [DOI] [PubMed] [Google Scholar]
- ROTTA J., RASKA K. Some biological properties of cellular components of A streptococci. J Hyg Epidemiol Microbiol Immunol. 1963;7:16–27. [PubMed] [Google Scholar]
- Rotta J., Bednár B. Biological properties of cell wall mucopeptide of hemolytic streptococci. J Exp Med. 1969 Jul 1;130(1):31–47. doi: 10.1084/jem.130.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotta J. Endotoxin-like properties of the peptidoglycan. Z Immunitatsforsch Exp Klin Immunol. 1975 Jul;149(2-4):230–244. [PubMed] [Google Scholar]
- Rotta J., Schleifer K. H. Pyrogenic activity of bacterial mucopeptides. J Hyg Epidemiol Microbiol Immunol. 1974;18(1):50–59. [PubMed] [Google Scholar]
- Schmeling D. J., Peterson P. K., Hammerschmidt D. E., Kim Y., Verhoef J., Wilkinson B. J., Quie P. G. Chemotaxigenesis by cell surface components of Staphylococcus aureus. Infect Immun. 1979 Oct;26(1):57–63. doi: 10.1128/iai.26.1.57-63.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyder J., Baggiolini M. Role of phagocytosis in the activation of macrophages. J Exp Med. 1978 Dec 1;148(6):1449–1457. doi: 10.1084/jem.148.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Kim Y., Peterson P. K., Quie P. G., Michael A. F. Activation of complement by cell surface components of Staphylococcus aureus. Infect Immun. 1978 May;20(2):388–392. doi: 10.1128/iai.20.2.388-392.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


