Abstract
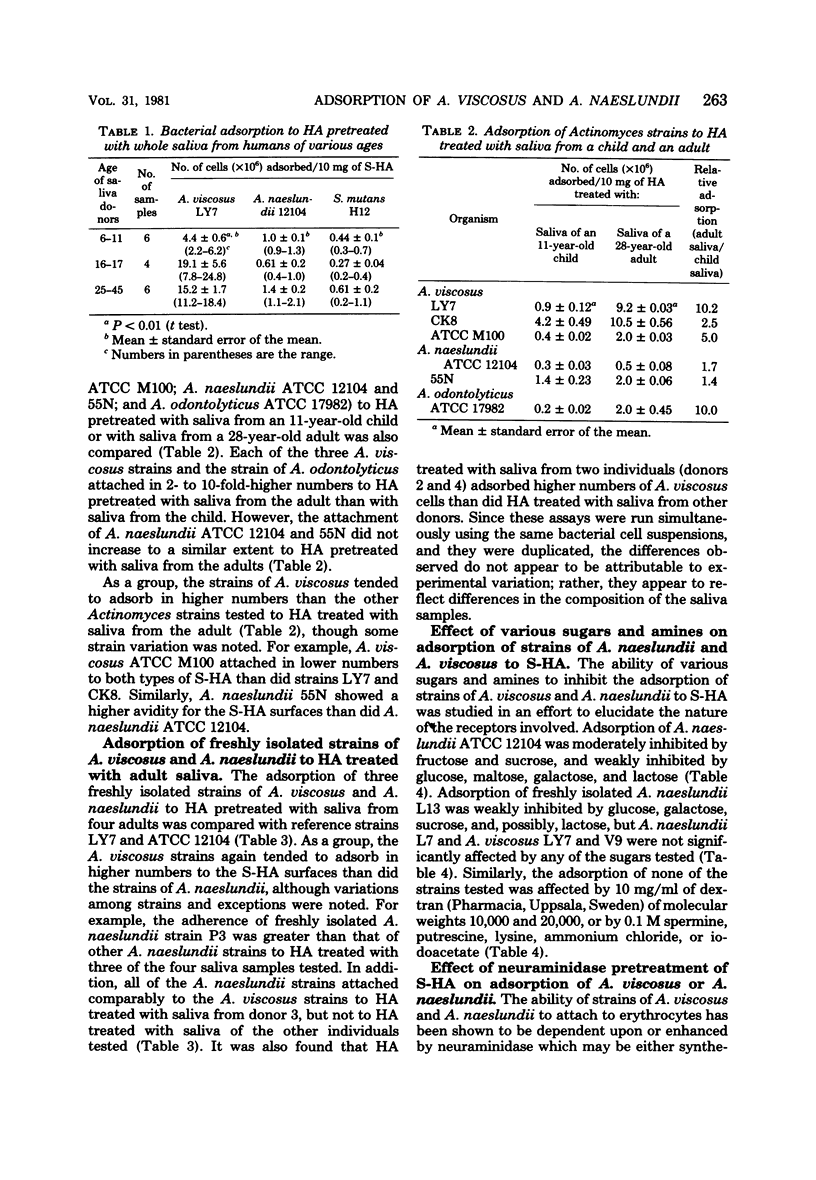
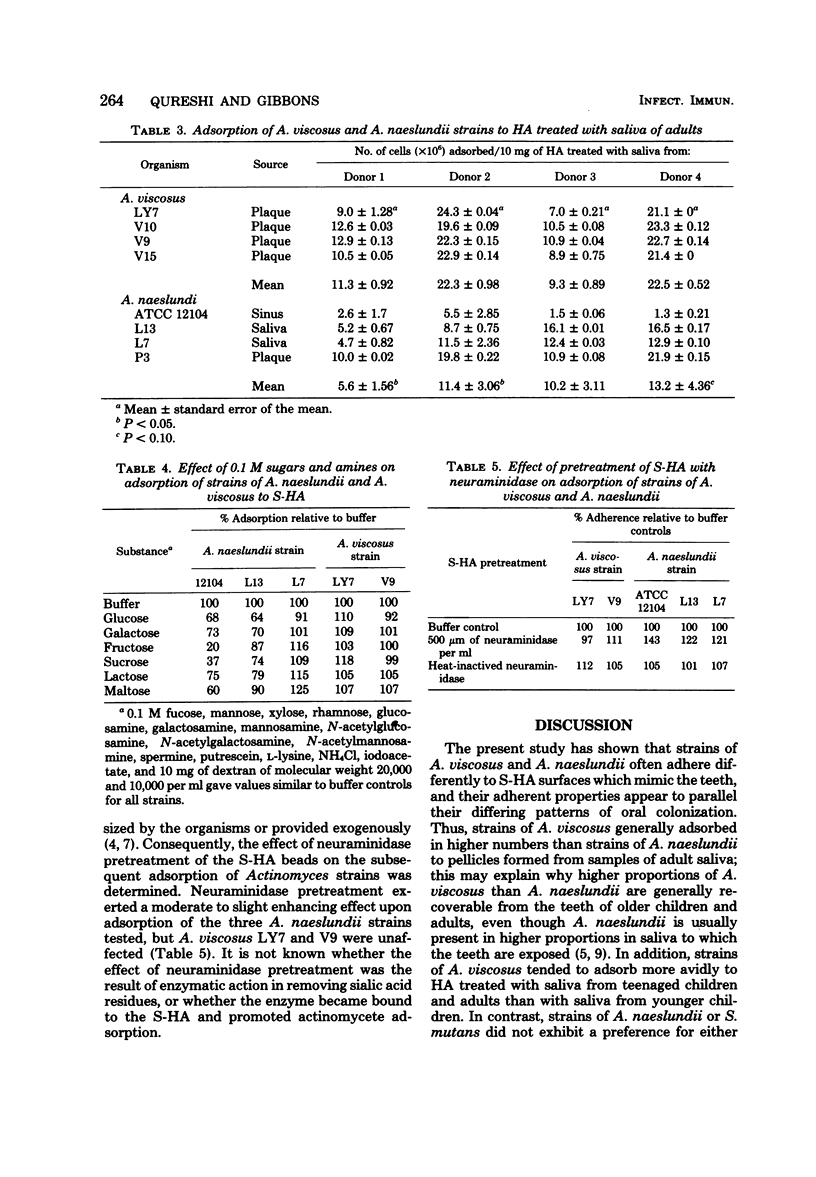
Human strains of Actinomyces viscosus and A. naeslundii differ in the time of their appearance and in their patterns of colonization in the mouth. Strains of these organisms were found to differ in their abilities to adsorb to saliva-treated hydroxyapatite (S-HA) surfaces, thought to mimic the teeth, and these differences parallel their patterns of colonizing the dentition. Thus, strains of A. viscosus tended to adsorb in higher numbers to hydroxyapatite (HA) treated with saliva of older children and adults than with saliva of younger children (ages 6 to 11). These salivary changes may account for the increased frequency with which this organism can be isolated from the mouths of children as they grow older. In contrast, strains of A. naeslundii and Streptococcus mutans did not show a preference for attaching to either type of S-HA. Strains of A. viscosus also generally adsorbed in higher numbers than A. naeslundii to HA treated with adult saliva; this may explain why higher proportions of A. viscosus are usually recoverable from the teeth of adults, even though A. naeslundii is generally present in higher proportions in saliva. Significant variation was noted between strains and between saliva samples collected from different donors. The differences in adsorptive behavior of strains of these species suggests that they are binding to different receptors in the salivary glycoprotein coating on HA surfaces. Adsorption of A. naeslundii ATCC 12104 was enhanced when S-HA was pretreated with neuraminidase, but this had little effect upon the adsorption of other Actinomyces strains tested. Adsorption of strain ATCC 12104 to S-HA was also strongly inhibited by fructose and sucrose and weakly inhibited by glucose, maltose, galactose, and lactose. However, other strains of A. naeslundii tested were affected less, or not at all, by these sugars. Adsorption of two strains of A. viscosus was not affected by any of the sugars or amines tested.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brecher S. M., van Houte J. Relationship between host age and susceptibility to oral colonization by Actinomyces viscosus in Sprague-Dawley rats. Infect Immun. 1979 Dec;26(3):1137–1145. doi: 10.1128/iai.26.3.1137-1145.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. B., Bammann L. L., Gibbons R. J. Comparative estimates of bacterial affinities and adsorption sites on hydroxyapatite surfaces. Infect Immun. 1978 Mar;19(3):846–853. doi: 10.1128/iai.19.3.846-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello A. H., Cisar J. O., Kolenbrander P. E., Gabriel O. Neuraminidase-dependent hamagglutination of human erythrocytes by human strains of Actinomyces viscosus and Actinomyces naeslundii. Infect Immun. 1979 Nov;26(2):563–572. doi: 10.1128/iai.26.2.563-572.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Balcerzak-Raczkowski I. B. Differential medium for detecting dental plaque bacteria resembling Actinomyces viscosus and Actinomyces naeslundii. J Clin Microbiol. 1975 Oct;2(4):305–310. doi: 10.1128/jcm.2.4.305-310.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P. Establishment and distribution of Actinomyces viscosus and Actinomyces naeslundii in the human oral cavity. Infect Immun. 1976 Nov;14(5):1119–1124. doi: 10.1128/iai.14.5.1119-1124.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Fillery E. D., Chan K. H., Grove D. A. Sialidase-enhanced lectin-like mechanism for Actinomyces viscosus and Actinomyces naeslundii hemagglutination. Infect Immun. 1980 Feb;27(2):335–343. doi: 10.1128/iai.27.2.335-343.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Leung W. L., Fillery E. D., Grove D. A. Mannose-contaminating agglutinin for Actinomyces viscosus and Actinomyces naeslundii. Infect Immun. 1979 Nov;26(2):427–434. doi: 10.1128/iai.26.2.427-434.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellen R. P., Segal D. N., Grove D. A. Relative proportions of Actinomyces viscosus and Actinomyces naeslundii in dental plaques collected from single sites. J Dent Res. 1978 Apr;57(4):550–550. doi: 10.1177/00220345780570040201. [DOI] [PubMed] [Google Scholar]
- Fillery E. D., Bowden G. H., Hardie J. M. A comparison of strains of bacteria designated Actinomyces viscosus and Actinomyces naeslundii. Caries Res. 1978;12(6):299–312. doi: 10.1159/000260349. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Qureshi J. V. Inhibition of adsorption of Streptococcus mutans strains to saliva-treated hydroxyapatite by galactose and certain amines. Infect Immun. 1979 Dec;26(3):1214–1217. doi: 10.1128/iai.26.3.1214-1217.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggenheim B., Schroeder H. E. Reactions in the periodontium to continuous antigenic stimulation in sensitized gnotobiotic rats. Infect Immun. 1974 Sep;10(3):565–577. doi: 10.1128/iai.10.3.565-577.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman J. D., Van Houte J., Gibbons R. J. Sorption of bacteria to human enamel powder. Arch Oral Biol. 1970 Sep;15(9):899–903. doi: 10.1016/0003-9969(70)90163-9. [DOI] [PubMed] [Google Scholar]
- Holmberg K., Hallander H. O. Numerical taxonomy and laboratory identification of Bacterionema matruchotii, Rothia dentocariosa, Actinomyces naeslundii, Actinomyces viscosus, and some related bacteria. J Gen Microbiol. 1973 May;76(1):43–63. doi: 10.1099/00221287-76-1-43. [DOI] [PubMed] [Google Scholar]
- Holmberg K., Nord C. E. Numerical taxonomy and laboratory identification of Actinomyces and Arachnia and some related bacteria. J Gen Microbiol. 1975 Nov;91(1):17–44. doi: 10.1099/00221287-91-1-17. [DOI] [PubMed] [Google Scholar]
- JORDAN H. V., KEYES P. H. AEROBIC, GRAM-POSITIVE, FILAMENTOUS BACTERIA AS ETIOLOGIC AGENTS OF EXPERIMENTAL PERIODONTAL DISEASE IN HAMSTERS. Arch Oral Biol. 1964 Jul-Aug;9:401–414. doi: 10.1016/0003-9969(64)90025-1. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A. Bacteriology of human experimental gingivitis: effect of plaque and gingivitis score. Infect Immun. 1978 Sep;21(3):830–839. doi: 10.1128/iai.21.3.830-839.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky S. S., Hubersak C., Propas D. Induction of periodontal destruction in gnotobiotic rats by a human oral strain of Actinomyces naeslundii. Arch Oral Biol. 1970 Oct;15(10):993–995. doi: 10.1016/0003-9969(70)90095-6. [DOI] [PubMed] [Google Scholar]
- Sumney D. L., Jordan H. V. Characterization of bacteria isolated from human root surface carious lesions. J Dent Res. 1974 Mar-Apr;53(2):343–351. doi: 10.1177/00220345740530022701. [DOI] [PubMed] [Google Scholar]
- Syed S. A., Loesche W. J. Bacteriology of human experimental gingivitis: effect of plaque age. Infect Immun. 1978 Sep;21(3):821–829. doi: 10.1128/iai.21.3.821-829.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler T. T., Clark W. B., Birdsell D. C. Adherence of Actinomyces viscosus T14V and T14AV to hydroxyapatite surfaces in vitro and human teeth in vivo. Infect Immun. 1979 Sep;25(3):1066–1074. doi: 10.1128/iai.25.3.1066-1074.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


