Abstract
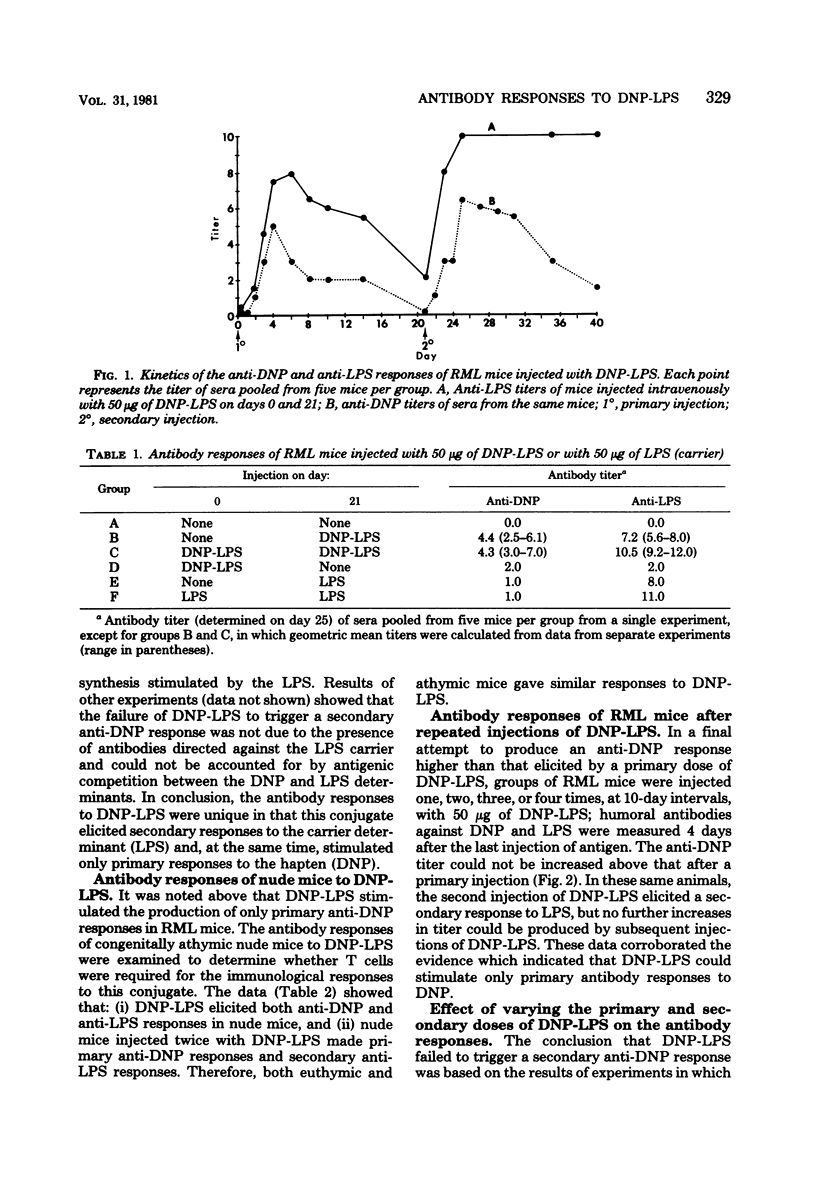
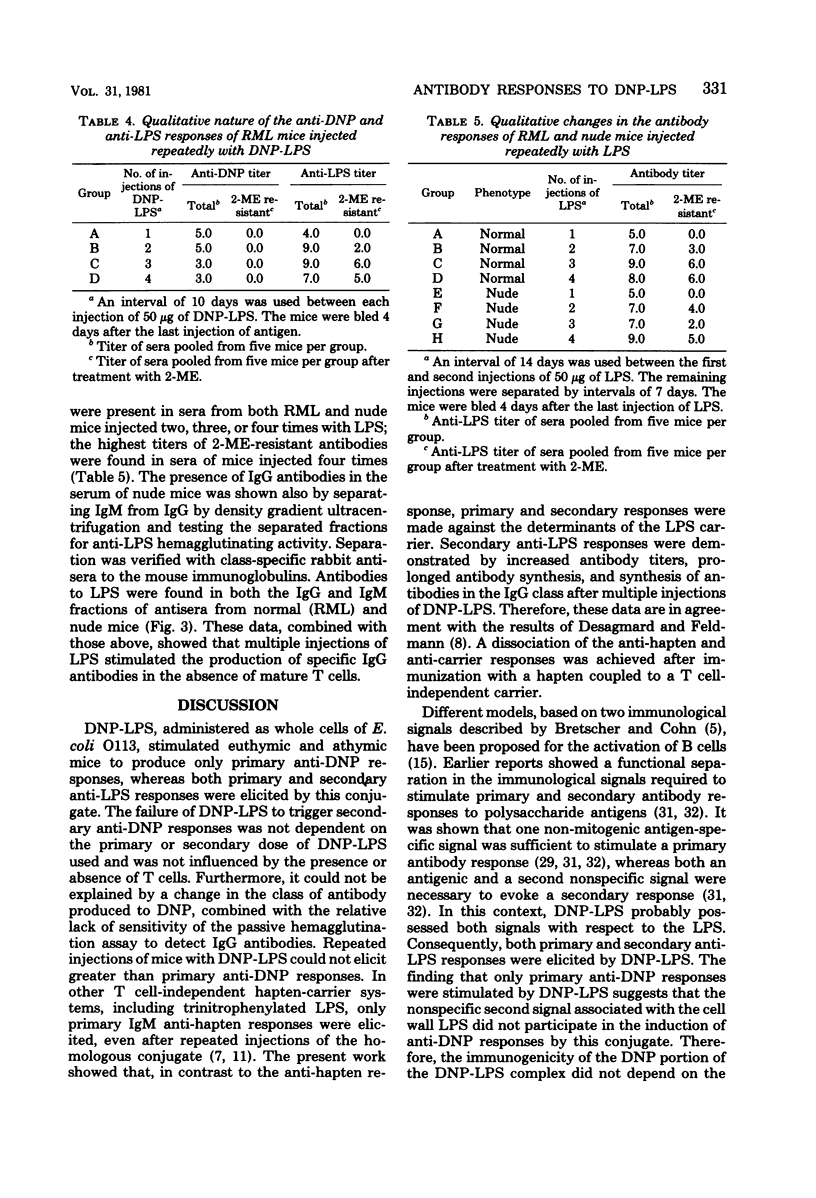
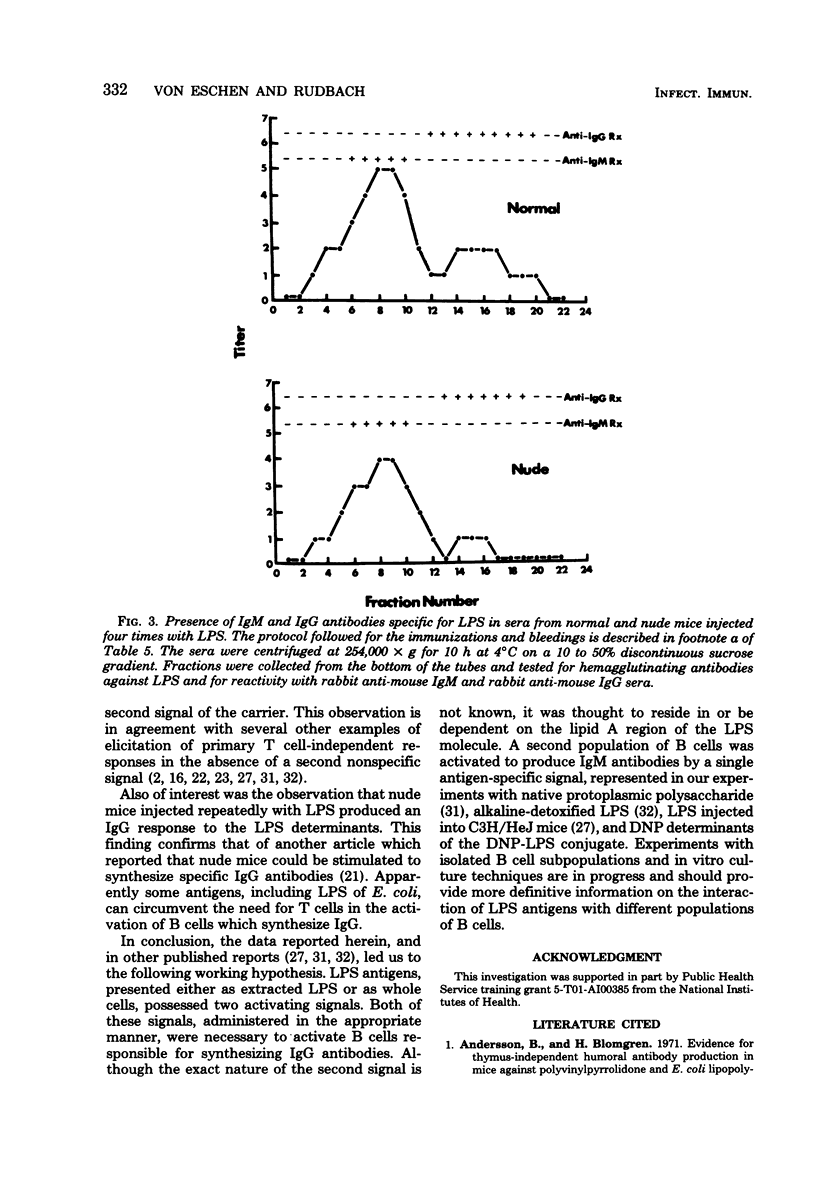
The quantitative and qualitative nature of the antibody responses of euthymic (normal, RML) and athymic (nude) mice injected with dinitrophenylated (DNP) lipopolysaccharide (LPS) was evaluated. Antibody responses to both the haptenic (DNP) and carrier (LPS) determinants were measured. On a quantitative basis, RML and nude mice stimulated with DNP-LPS produced only primary anti-DNP responses, whereas both primary and secondary anti-LPS responses were elicited by this conjugate. The failure of DNP-LPS to trigger secondary anti-DNP responses was not dependent on the amount of DNP-LPS given in the primary or secondary doses and could not be overcome by repeated injections of DNP-LPS. Also, the anti-DNP responses of RML mice injected repeatedly with DNP-LPS were restricted to immunoglobulin M antibodies whereas both immunoglobulin M and G anti-LPS responses were elicited. Nude mice also produced immunoglobulin G antibodies to the LPS determinants. These data showed a dissociation of the anti-hapten and anti-carrier antibody responses and suggested that different immunological signals were functioning in the respective anti-DNP and anti-LPS responses.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Melchers F. Maturation of mitogen-activated bone marrow-derived lymphocytes in the absence of proliferation. Eur J Immunol. 1974 Aug;4(8):533–539. doi: 10.1002/eji.1830040803. [DOI] [PubMed] [Google Scholar]
- BAUER D. C., MATHIES M. J., STAVITSKY A. B. Sequences of synthesis of gamma-1 macroglobulin and gamma-2 globulin antibodies during primary and secondary responses to proteins, salmonella antigens, and phage. J Exp Med. 1963 Jun 1;117:889–907. doi: 10.1084/jem.117.6.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer D. C., Stavitsky A. B. ON THE DIFFERENT MOLECULAR FORMS OF ANTIBODY SYNTHESIZED BY RABBITS DURING THE EARLY RESPONSE TO A SINGLE INJECTION OF PROTEIN AND CELLULAR ANTIGENS. Proc Natl Acad Sci U S A. 1961 Oct;47(10):1667–1680. doi: 10.1073/pnas.47.10.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher P., Cohn M. A theory of self-nonself discrimination. Science. 1970 Sep 11;169(3950):1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Gronowicz E., Bullock W. W., Möller G. Mechanism of thymus-independent immunocyte triggering. Mitogenic activation of B cells results in specific immune responses. J Exp Med. 1974 Jan 1;139(1):74–92. doi: 10.1084/jem.139.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Guercio P., Thobie N., Poirier M. F. IgM anamnestic immune response to the haptenic determinant DNP on a thymus-independent carrier. J Immunol. 1974 Jan;112(1):427–429. [PubMed] [Google Scholar]
- Desaymard C., Feldmann M. Lack of requirement for cell cooperation in the antibody response to DNP conjugated to levan. Cell Immunol. 1975 Mar;16(1):106–114. doi: 10.1016/0008-8749(75)90189-6. [DOI] [PubMed] [Google Scholar]
- FUDENBERG H. H., KUNKEL H. G. Physical properties of the red cell agglutinins in acquired hemolytic anemia. J Exp Med. 1957 Nov 1;106(5):689–702. doi: 10.1084/jem.106.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUKUSHI K., ANACKER R. L., HASKINS W. T., LANDY M., MILNER K. C., RIBI E. EXTRACTION AND PURIFICATION OF ENDOTOXIN FROM ENTEROBACTERIACEAE: A COMPARISON OF SELECTED METHODS AND SOURCES. J Bacteriol. 1964 Feb;87:391–400. doi: 10.1128/jb.87.2.391-400.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M., Easten A. The relationship between antigenic structure and the requirement for thymus-derived cells in the immune response. J Exp Med. 1971 Jul 1;134(1):103–119. doi: 10.1084/jem.134.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M. Induction of immunity and tolerance in vitro by hapten protein conjugates. II. Carrier independence of the response to dinitrophenylated polymerized flagellin. Eur J Immunol. 1972 Apr;2(2):130–137. doi: 10.1002/eji.1830020208. [DOI] [PubMed] [Google Scholar]
- Fidler J. M. In vivo immune response to TNP hapten coupled to thymus-independent carrier lipopolysaccharide. Cell Immunol. 1975 Apr;16(2):223–236. doi: 10.1016/0008-8749(75)90114-8. [DOI] [PubMed] [Google Scholar]
- Gander J. E., Rudbach J. A. Immunological investigations of Penicillium. II. Primary binding of glycopeptides and glycopeptide derivatives to specific antibodies. Immunochemistry. 1973 Feb;10(2):81–92. doi: 10.1016/0019-2791(73)90234-6. [DOI] [PubMed] [Google Scholar]
- Jacobs D. M., Morrison D. C. Stimulation of a T-independent primary anti-hapten response in vitro by TNP-lipopolysaccharide (TNP-LPS). J Immunol. 1975 Jan;114(1 Pt 2):360–364. [PubMed] [Google Scholar]
- Jacobs M. D., Morrison D. C. Dissociation between mitogenicity and immunogenicity of TNP-lipopolysaccharide, a T-independent antigen. J Exp Med. 1975 Jun 1;141(6):1453–1458. doi: 10.1084/jem.141.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong D. L., Rudbach J. A. Antigenic competition between and endotoxic adjuvant and a protein antigen. Infect Immun. 1971 Feb;3(2):308–317. doi: 10.1128/iai.3.2.308-317.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda J. J. Studies on immunological paralysis. IX. The immunogenicity and tolerogenicity of levan (polyfructose) in mice. Immunology. 1972 Dec;23(6):829–842. [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E., Johnson B. M., Paul W. E., McMaster P. R. Cellular requirements for the primary in vitro antibody response to DNP-ficoll. J Exp Med. 1974 May 1;139(5):1354–1360. doi: 10.1084/jem.139.5.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe W. J., Michael J. G. Separation of the mitogenic and antigenic responses to bacterial lipopolysaccharide. Immunology. 1976 Feb;30(2):241–248. [PMC free article] [PubMed] [Google Scholar]
- Poe W. J., Michael J. G. The effect of serum inhibitor on the antigenic and mitogenic responses to E. coli bacteria. J Immunol. 1976 Apr;116(4):1129–1133. [PubMed] [Google Scholar]
- ROSENBLATT E., JOHNSON A. G. Size of antibody and role of autonomic nervous system in the adjuvant action of endotoxin. Proc Soc Exp Biol Med. 1963 May;113:156–161. doi: 10.3181/00379727-113-28305. [DOI] [PubMed] [Google Scholar]
- Rice M. C., O'Brien S. J. Genetic variance of laboratory outbred Swiss mice. Nature. 1980 Jan 10;283(5743):157–161. doi: 10.1038/283157a0. [DOI] [PubMed] [Google Scholar]
- Rudbach J. A. Molecular immunogenicity of bacterial lipopolysaccharide antigens: establishing a quantitative system. J Immunol. 1971 Apr;106(4):993–1001. [PubMed] [Google Scholar]
- Rudbach J. A., Reed N. D. Immunological responses of mice to lipopolysaccharide: lack of secondary responsiveness by C3H/HeJ mice. Infect Immun. 1977 May;16(2):513–517. doi: 10.1128/iai.16.2.513-517.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon R., McMaster P. R., Kask A. M., Owens J. D., Paul W. E. DNP-Lys-ficoll: a T-independent antigen which elicits both IgM and IgG anti-DNP antibody-secreting cells. J Immunol. 1975 May;114(5):1585–1589. [PubMed] [Google Scholar]
- Skidmore B. J., Chiller J. M., Morrison D. C., Weigle W. O. Immunologic properties of bacterial lipopolysaccharide (LPS): correlation between the mitogenic, adjuvant, and immunogenic activities. J Immunol. 1975 Feb;114(2 Pt 2):770–775. [PubMed] [Google Scholar]
- Trump G. N. Preparation of dinitrofluorobenzene-treated sheep erythrocytes for detection of anti-DNP plaque-forming cells. J Immunol. 1972 Oct;109(4):754–760. [PubMed] [Google Scholar]
- Von Eschen K. B., Rudbach J. A. Antibody responses of mice to alkaline detoxifield lipopolysaccharide. J Immunol. 1976 Jan;116(1):8–11. [PubMed] [Google Scholar]
- Von Eschen K. B., Rudbach J. A. Immunological responses of mice to native protoplasmic polysaccharide and lipopolysaccharide: functional separation of the two signals required to stimulate a secondary antibody response. J Exp Med. 1974 Dec 1;140(6):1604–1614. doi: 10.1084/jem.140.6.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]


