Abstract
Tumor necrosis factor (TNF) utilizes two receptors, TNFR1 and 2, to initiate target cell responses. We assessed expression of TNF, TNFRs and downstream kinases in cardiac allografts, and compared TNF responses in heart organ cultures from wild-type (WTC57BL/6), TNFR1-knockout (KO), TNFR2KO, TNFR1/2KO mice. In non-rejecting human heart TNFR1 was strongly expressed coincidentally with inactive apoptosis signal-regulating kinase-1 (ASK1) in cardiomyocytes (CM) and vascular endothelial cells (VEC). TNFR2 was expressed only in VEC. Low levels of TNF localized to microvessels. Rejecting cardiac allografts showed increased TNF in microvessels, diminished TNFR1, activation of ASK1, upregulated TNFR2 co-expressed with activated endothelial/epithelial tyrosine kinase (Etk), increased apoptosis and cell cycle entry in CM. Neither TNFR was expressed significantly by cardiac fibroblasts. In WTC57BL/6 myocardium, TNF activated both ASK1 and Etk, and increased both apoptosis and cell cycle entry. TNF-treated TNFR1KO myocardium showed little ASK1 activation and apoptosis but increased Etk activation and cell cycle entry, while TNFR2KO myocardium showed little Etk activation and cell cycle entry but increased ASK1 activation and apoptosis. These observations demonstrate independent regulation and differential functions of TNFRs in myocardium, consistent with TNFR1-mediated cell death and TNFR2-mediated repair.
Keywords: TNF, TNFR1, TNFR2, Cell cycle entry, Apoptosis, Cardiomyocytes
Introduction
Tumor necrosis factor (TNF, sometimes designated TNF-α) signals through two distinct TNF receptors (TNFRs), TNFR1 (CD120a) and TNFR2 (CD120b) (1). TNF blockade is effective in controlling disease activity in rheumatoid arthritis and inflammatory bowel disease (2), (3), and TNF has been implicated in a number of cardiovascular diseases (4), but clinical trials have demonstrated no benefit of TNF blockade in congestive cardiac failure (5). We hypothesize that TNFRs are independently regulated and mediate different cardiac responses, so that effects of TNF blockade will depend on TNFR expression patterns. In murine models of heart failure, signaling through TNFR1 appears detrimental, whereas TNFR2 appears to mediate cardioprotective effects (6, 7). In murine organ transplantation, TNFRs play opposing roles in acute rejection (8), but both TNFRs contribute to graft vasculopathy (9). Injury independently alters expression of the two TNFRs (10). Understanding how TNFRs are regulated and function in cardiac tissue is essential for rational use of TNF blockade.
TNF is synthesized as a membrane protein and soluble cytokine is released from the cell by the action of a secreted metalloproteinase designated TNF-α converting enzyme (TACE); TACE also cleaves TNFR1 and TNFR2 from the cell surface, both desensitizing cells to TNF action through surface receptor loss and generating soluble receptors that neutralize TNF (11). Each TNFR activates different sets of signalling pathways which vary with cell type. Both TNFRs lack intrinsic enzyme activity and require the recruitment of adaptor molecules to initiate signaling. TNFR1 uses apoptosis signal-regulating kinase 1 (ASK1), a mitogen-activated protein kinase kinase kinase (MAP3K), to activate downstream signaling molecules such as c-Jun N-terminal kinase and p38 MAPK (12). ASK-1 may mediate cardiac myocyte (CM) death and promote diabetic cardiomyopathy in transgenic mice (13, 14). TNFR2 uses endothelial/epithelial tyrosine kinase (Etk), a Btk non-receptor tyrosine kinase family member, to promote cell adhesion, migration, proliferation, survival and angiogenesis (14–17).
Here we describe in situ protein and mRNA expression of TNF, TNFRs and associated signaling molecules and assess apoptosis and cell cycle entry in normal human heart and post-transplant endomyocardial biopsies showing either normal myocardium or cardiac allograft rejection. In addition we delineate functions of both TNFRs in cardiac tissue using a murine heart organ culture model derived from wild type and TNFR knock out mice. We show that expression of TNFRs is altered in the heart by allograft rejection and that TNFR1 activates ASK1 and mediates CM cell death whereas TNFR2 activates Etk, and increases markers of cell cycle entry.
Materials and Methods
Human Heart Tissue
Patients receiving heart transplants at Papworth Hospital (Cambridge UK) gave informed consent to participate in this institutional review board-approved study. We evaluated normal (non-pathological) human cardiac biopsies (grade 0; n=45) and biopsies with histological evidence of different degrees of acute cellular rejection from 2 weeks to 20 months post cardiac transplantation. Grade 1A, n = 20 - mild rejection with focal perivascular or interstitial infiltrate but lack of myocyte damage; grade 2, n = 22 - focal moderate rejection with aggressive infiltration and/or focal myocyte damage grade 3A, n = 19 - low moderate rejection with multifocal aggressive infiltrates and myocyte damage (18). In addition, normal cardiac tissue was obtained from seven organ donors whose hearts were not used for transplantation under protocols approved by the Yale University Human Investigation Committee and the New England Organ Bank. Tissues were frozen in liquid-nitrogen or fixed in 4% paraformaldehyde for paraffin-wax embedding. Each sample was stained with hematoxylin and eosin (H&E) and classified as normal with no pathological changes or acute cellular rejection and some sections placed on poly-L-lysine coated slides for immunohistochemistry, in situ hybridization and terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) assays.
Mouse Heart Organ Cultures
Animal procedures were approved by the Institutional Animal Care and Use Committee of Yale University. WTC57BL/6, TNFR1KO (B6.129-tnfrsfla), TNFR2KO (B6.129-tnfrsflb) and TNFR1/TNFR2KO (double-knockout) mice, purchased from Jackson Laboratory (Bar Harbor, ME) and confirmed by genotyping with specific primers. Approximately 1 mm3 samples of heart were incubated in media containing fetal calf serum for 3 h at 37°C with or without recombinant murine TNF (AMS Biotechnology, Abingdon) (10). A dose-response curve showed that both TNFRs were activated in the same concentration range. 10 ng/ml, an optimal concentration, was used in all reported experiments. Harvested tissue was processed as described above.
Detection of TNFRs Proteins and Associated Signaling Molecules in Normal Human Myocardium and Acute Cellular Rejection
Sections from histologically normal and allograft hearts were immunolabeled as described previously (10) with either mouse anti-TNFR1 and rabbit anti–ASK1p-Ser967 or –pThr845 (Cell Signaling, New England BioLabs, Hertfordshire, UK) or mouse anti-TNFR2 (R&D systems, Oxford, UK) and rabbit anti-Etk or anti-phospho-EtkTyr40 (Cell Signaling Technology, Danvers, MA) and with anti-TACE (Bioclear UK Ltd., Mile Elm Calne, Wiltshire, UK). Positive cells were characterized by co-staining for a CM marker (19, 20) (mouse anti-α-sarcomeric actin, Sigma clone 5c5; a gift from Dr Mehregan, University of Cambridge) or a VEC marker (mouse anti-CD31 or rabbit anti-vWF) (Dakocytomation, Ely, UK) or a fibroblast marker (rat anti-human or rabbit anti-mouse pro-collagen type 1 (Millipore, Watford, UK). Although expression of pro-collagen type I has been reported in smooth muscle cells (21), we did not detect it nor α-sarcomeric actin on these cells which are readily distinguished from CM and fibroblasts by morphology and anatomical distribution. Primary antibodies were detected using anti-mouse-FITC and anti-rabbit-Alexa Fluor568 (Vector Laboratories Ltd., Peterborough, UK) and nuclei detected with To-PRO-3'iodide (Molecular Probes, Eugene, Oregon, USA).. Sections were mounted in Vectashield Mountant and examined using a TCS-NT Confocal Laser Scanning Microscope (CLSM, Leica Microsystems, Milton Keynes, UK). No signal was observed when primary antibody was replaced by either non-immune serum or isotype-specific antisera.
TUNEL assay for Apoptosis on Normal Human Myocardium and Acute Cellular Rejection
4% formaldehyde-fixed paraffin-wax sections were incubated with Proteinase-K (50 µg/mL) (Roche Diagnostics, NJ, USA) for 4 min at room temperature (RT), washed in PBS and incubated for 30 min at 37°C with TUNEL Label mix containing Fluorescein-11dUTP and 0.2 U/ul TdT enzyme (Roche). This was followed by staining with anti-CD31 and anti-mouse-AlexaFluor568 (Molecular Probes), each for 1 hr at RT, and mounting in Vectashield for examination with CLSM.
In situ hybridization on Normal Human Myocardium and Acute Cellular Rejection
For in situ hybridization (10), cardiac tissue was incubated overnight at 37°C with hybridization solution containing single-stranded antisense DNA oligonucleotide probe 5'-end labeled with digoxigenin specific for TNFR1, TNFR2, ASK1 (gb/NM_005923/HUMMAP3K, 82–121), Etk (gb/NM_203281/HUMBMX, 115–141), TACE (gb/NM_033274/HUMADAM19, 701–741) (MWG-Biotech, UK) or human TNFα (R&D), followed by anti-digoxigenin-11-dUTP conjugated-alkaline phosphatase (Roche) for 2 hr at RT and visualized with BCIP-NBT (Sigma). Digoxigenin-labeled Oligo-dT (R&D) was used as a positive control and specific sense probes served as negative controls. No mRNA signal was detected with sense probe to TNFRs, ASK1, Etk, TNF or TACE (data not shown). Sections were visualized using Nikon OPTIPHOT-2 with a Polaroid DMC 2.0 Digital Microscope Camera.
TUNEL assay for Apoptosis on Mouse Heart Organ Cultures
4% formaldehyde-fixed paraffin sections were incubated with Proteinase-K and washed in PBS as described above. TUNEL label mix was replaced with digoxigenin-11-dUTP and 0.2 U/ul TdT enzyme for 30 min at 37°C, followed by anti-digoxigenin-11-dUTP conjugated-alkaline phosphatase for 1 hr at RT and colour developed with Fast-Red (Dako). Negative controls included omission of the TdT enzyme.
Co-Immunolocalization of Cell Cycle Activation in Human and Mouse Heart Organ Cultures
Cell cycle activity in mouse and human heart was assessed using mouse anti-PCNA (22); (Millipore); rabbit anti-Ki-67 (23) and mouse anti-phospho-histone H3-S10 (24) (Abcam, Cambridge, UK). Positive cells were characterized by co-staining for a CM (19), VEC, or fibroblast marker. Untreated or TNF-treated WTC5BL/6, TNFR1KO, TNFR2KO and TNFR1/2KO cultures were subjected to pressure cooker containing 0.01 M citric acid buffer pH 6.0 for 5 min, followed by overnight incubation at 4°C with primary antibodies. Antibodies were detected with anti-rabbit-AlexaFlour488 and anti-mouse-AlexaFlour568 or anti-mouseFITC and anti-rabbitTexas Red followed by To-PRO-3'iodide for nuclei detection. Sections were mounted in Vectashield and examined by CLSM. Control sections, which omitted the first or second antibody, showed negative results.
Statistics
Human tissue
Statistical significance, p<0.01, was analysed using ANOVA followed by Boneferroni’s comparison test on GraphPad Prism 5.0. The number of positive cells were counted in 3 random regions from 3 random biopsies from each grade (n = 9). For apoptotic and proliferative indices, TUNEL- and pH3-positive VEC and CM were counted in 10 representative fields at a ×40 magnification on 5 random biopsies from normal myocardium and from 5 of each rejection grade. A similar method was used on WTC57BL/6, TNFR1KO and TNFR2KO mice to compare the effects of TNF treatment within and between experimental groups. Statistical analysis was performed using a 2-tailed Student’s t-test assuming unequal variance (*=p<0.01, **=p<0.01, ***=p<0.001). Values are given as mean ± SEM.
In addition, in mouse heart organ cultures, the number of pH3-positive CM and VEC were quantified by counting on average 30 cells in 10 separate fields of vision under the microscope in 3 different organ cultures from each of the 2 treatments. The stimulation index (SI) value was calculated and significant differences analysed by ANOVA followed by Newman-Keuls multiple comparison test as presented in table 3. The results obtained with each marker in each strain were comparable.
Table 3.
Immunostaining results of the three different molecular markers of cell cycle in cardiomyocytes (CM) and VEC (vascular endothelial cells). Data is expressed as stimulation index (SI), defined by the (% labeled nuclei +TNF) − (% labeled nuclei −TNF)] / (% labeled nuclei −TNF) for each strain. TNF-treated TNFR1KO cultures show the highest SI index as compared to WTC56B/L controls or TNFR2KO. Data are from three independent experiments.
| WTC57BL/6 | TNFR1KO | TNFR2KO | TNFR1/TNFR2KO | |
|---|---|---|---|---|
| Ki-67 CM | 3.37±0.62 | 8.60±1.92 | 0.37±0.12 | 0.38±0.27 |
| Ki-67 VEC | 2.87±1.11 | 8.03±1.77 | 0.81±0.49 | 0.70±0.36 |
| pH3 CM | 3.22±0.52 | 7.78±2.10 | 0.80±0.45 | 0.18±0.24 |
| pH3 VEC | 3.39±0.98 | 6.55±1.30 | 0.48±0.37 | 0.43±0.36 |
| PCNA CM | 2.80±0.63 | 9.97±1.99 | 0.52±0.26 | 0.38±0.30 |
| PCNA VEC | 3.85±1.21 | 7.01±1.87 | 0.43±0.28 | 0.71±0.49 |
Evaluation was carried out independently by 2 persons who were unaware of the experimental protocols.
Results
Protein and mRNA Expression of TNF Pathway Components in Normal Myocardium
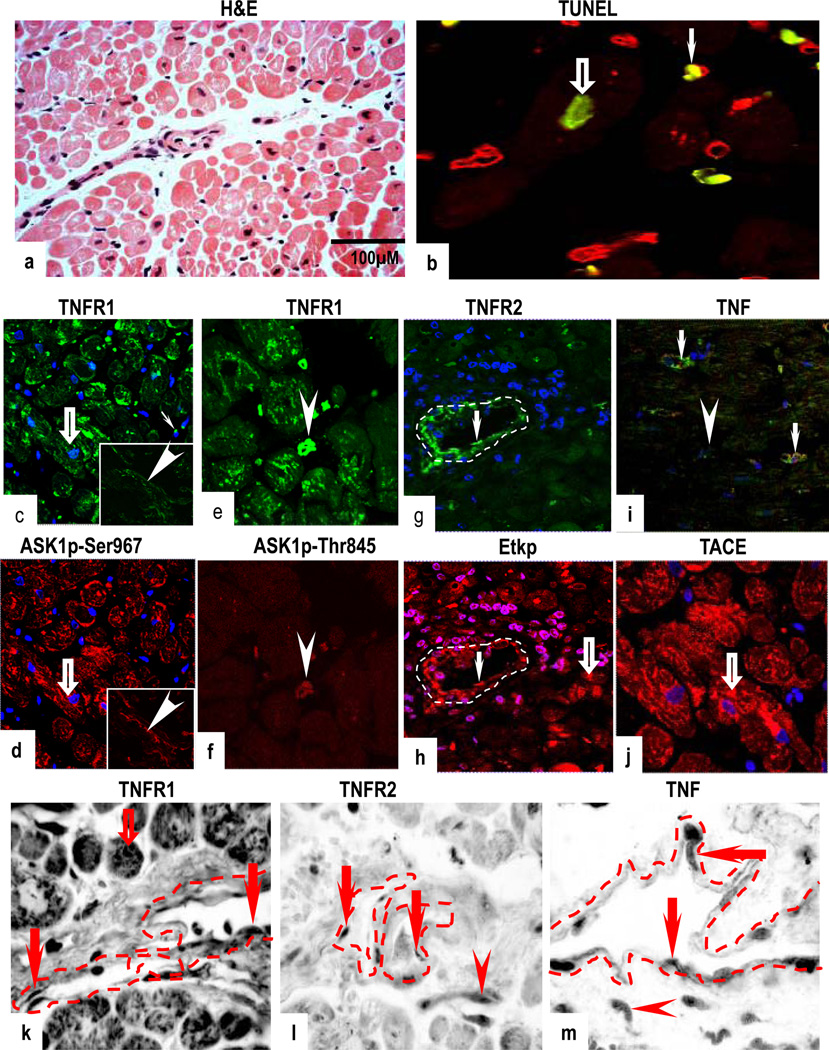
H&E sections of histologically normal myocardium showed cross striations of healthy CM and normal vasculature (Fig 1a). Rare TUNEL staining was observed in CM (Fig 1b). TNFR1 was predominantly in CM and in VEC, where it co-localized with inactive ASK1p-Ser967 (Figs 1c–d and Supplementary Figs 1a & 1b) and rare fibroblasts (data not shown). Co-expression of TNFR1 and activated ASK1p-Thr845 was confined to rare leukocytic mononuclear cells (MNC) (Figs 1e & 1f). TNFR2 was confined to VEC, where it co-localized mostly with inactive Etk (reactive with antibody to total Etk but not activated Etkp, Supplementary Figs 1c & 1d). TNFR2 and Etkp was observed in some VEC and in MNC (Figs 1g & 1h), and Etkp was also detected in a few CM, negative for TNFR2. TNF was confined to microvessels (possibly VEC and/or perivascular cells) and MNC (Fig 1i). TACE was detected in CM and in microvessels (Fig 1j). mRNA expression for TNF, TNFRs and their associated signalling molecules was concordant with protein expression (Figs 1k–1m and Supplementary Figs 1e–g).
Figure 1.
Normal human myocardium; H&E section shows normal histology (a) with negligible TUNEL staining in a few CM (open arrow) and in CD31-positive VEC (arrow) (b). A marked co-expression for TNFR1 and ASK1Ser967 is seen in (open arrows), in resident MNC and in VEC (inset; arrowheads) (c,d). Co-expression of TNFR1 and ASK1pThr845 is seen in resident MNC (arrowheads) (e,f). TNFR2 and activation of Etkp is demonstrated in VEC (arrows) and Etkp is also detected in a few CM (open arrow) (g,h). TNF is confined to microvessels (arrows) and in MNC (arrowhead) and TACE is present in CM (open arrow) (I,j). TNFR1 mRNA is present in CM (open arrow) and in VEC (arrows) (k) while mRNA for TNFR2 and TNF is confined to VEC (arrows) and in MNC (arrowheads) (l,m). Dotted lines mark out the vessels. Images are representative of 10 different samples, each of which gave similar results. Cardiomyocytes (CM); Vascular endothelial cells (VEC); Mononuclear cells (MNC). (Original Mags: a, ×112; b; ×63; c–d; ×40; e–f; ×63; g–I; ×40; j; ×63; k–m, ×235).
Protein and mRNA Expression of TNF Pathway Components in Acute Cellular Rejection
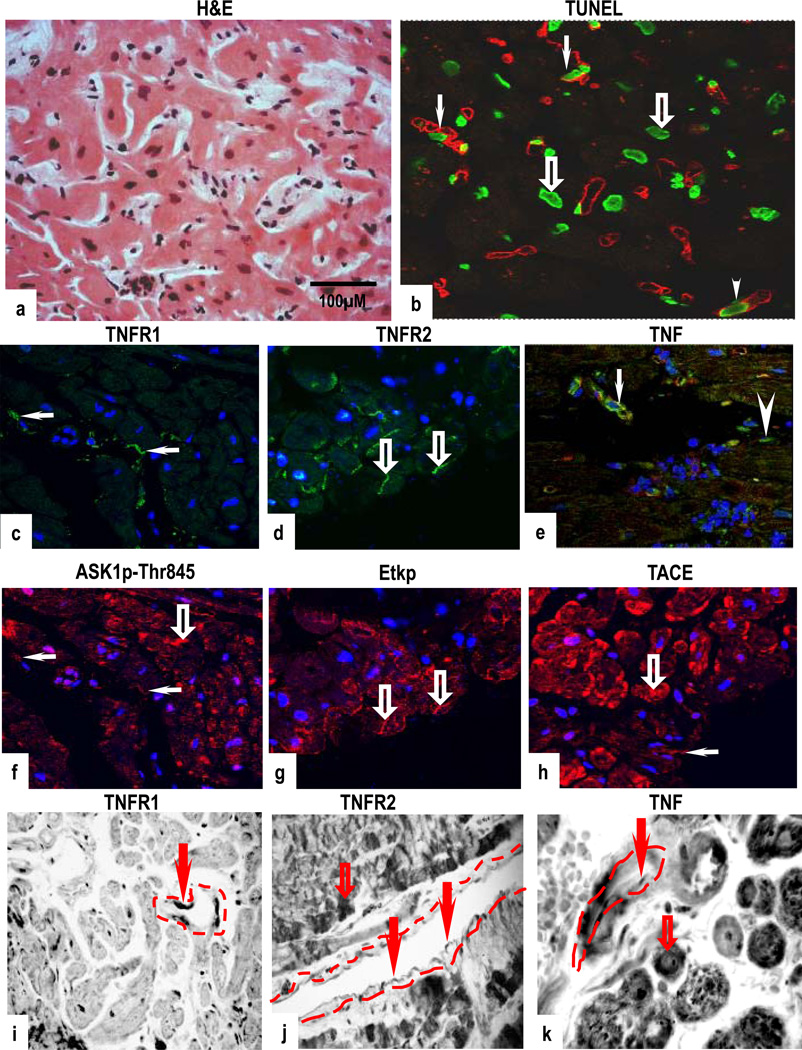
Biopsies with grade 1A rejection showed mild focal perivascular and interstitial MNC without overt CM damage (Fig 2a). However, increased TUNEL-positive nuclei were detected in both CM and in VEC (Fig 2b). Strong co-expression of TNFR1 and ASK1p-Ser967 (Supplementary Figs 2a & 2b) or ASK1p-Thr845 was evident in VEC, with some CM also positive for ASK1p-Thr845 but negative for TNFR1 (Figs 2c & 2f). TNFR2 and activation of Etkp was strongly induced in CM, with staining detected in the region of the intercalated discs (Figs 2d & 2g). TNFR2 also co-localized with Etk in VEC (Supplementary Figs 2c & 2d). Similar to normal myocardium, TNF was confined to microvessels and MNC (Fig 2e) and TACE was markedly expressed in CM and in VEC (Fig 2h). TNFR1 mRNA was present in VEC but absent in CM (Fig 2i) while TNFR2, TNF and associated molecules were strongly detected in CM and in VEC (Figs 2j–2k and supplementary Figs 2e–2g).
Figure 2.
Mild rejection (grade 1A); H&E show focal perivascular or interstitial infiltrates with mild intensity and lack of CM damage (a) and an increased TUNEL-reactive CM (open arrows) and in CD31-positive VEC (arrows) (b). Co-expression of TNFR1 and ASK1p-Thr845 is seen in VEC (arrows), with ASK1p-Thr845 also detected in some CM, negative for TNFR1 (open arrow) (c,f). TNFR2 and activation of Etkp is strongly demonstrated in CM with some staining present in regions of the intercalated discs (open arrows) (d,g). TNF is confined to microvessels (arrow) and MNC (arrowhead) and TACE is present in CM (open arrow) and in VEC (arrow) (e,h). TNFR1 mRNA is seen in VEC (arrow) (i) while TNFR2 and TNF mRNA is present in CM (open arrows) and in VEC (arrows) (j,k). Dotted lines mark out the vessels. Images are representative of 6 experiments different samples with similar results (Original Mags; a; ×112; c–h; ×63; I–j; ×112; k; ×235).
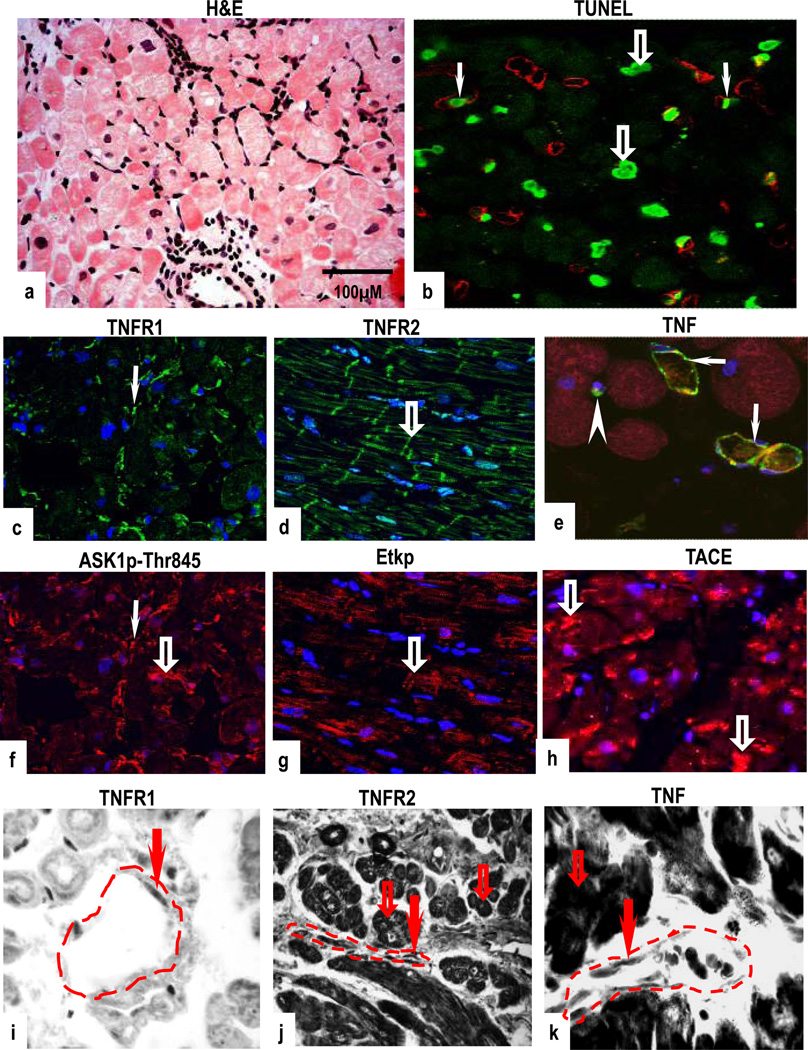
Biopsies with grade 2 rejection showed aggressive MNC infiltration and/or focal CM damage (Fig 3a). The pattern and frequency of TUNEL-positive nuclei was comparable to that in sections of mild rejection with staining detected in some CM and in VEC (Fig 3b). Parallel sections showed strong co-expression of TNFR1 and ASK1p-Ser967 (Supplementary Figs 3a & 3b) or ASK1p-Thr845 in VEC and in MNC (Figs 3c & 3f). Co-expression of TNFR2 and Etk (Supplementary Figs 3c & 3d) or Etkp was seen in VEC and in MNC (Figs 3d & 3g). Within CM, Etkp was concentrated in the region of the intercalated discs. TNF was expressed in microvessels and in MNC, and TACE in CM and in VEC (Figs 3e & 3h). mRNA expression for TNFRs and their signalling molecules mirrored that of protein expression (Figs 3i & 3k). There were no overt differences in the pattern or intensity of ASK1, Etk and TACE mRNA expression between normal and allograft biopsies (Supplementary Figs 3e–g).
Figure 3.
Focal moderate rejection (grade 2); H&E stained section show aggressive infiltration and/or focal CM damage (a) with increased TUNEL-reactive CM (open arrows) and VEC (arrows) (b). TNFR1 and ASK1p-Thr845 co-expression in VEC (arrows) with ASK1p-Thr845 also detected in some CM (open arrow) (c,f). TNFR2 and Etkp activation is evident in CM with some staining in regions of the intercalated discs (open arrows) (d,g). TNF is confined to microvessels (arrows) and MNC (arrowhead) (e) and TACE is demonstrated in CM (open arrows) (h). TNFR1 mRNA is confined to VEC (arrows) and TNFR2 and TNF mRNA are strongly expressed in CM (open arrows) and in VEC (arrow). Dotted lines mark out the vessels. Images are representative of 6 independent experiments with similar results. (Original Mags: a, ×112; b–h, ×63; i–k, ×235).
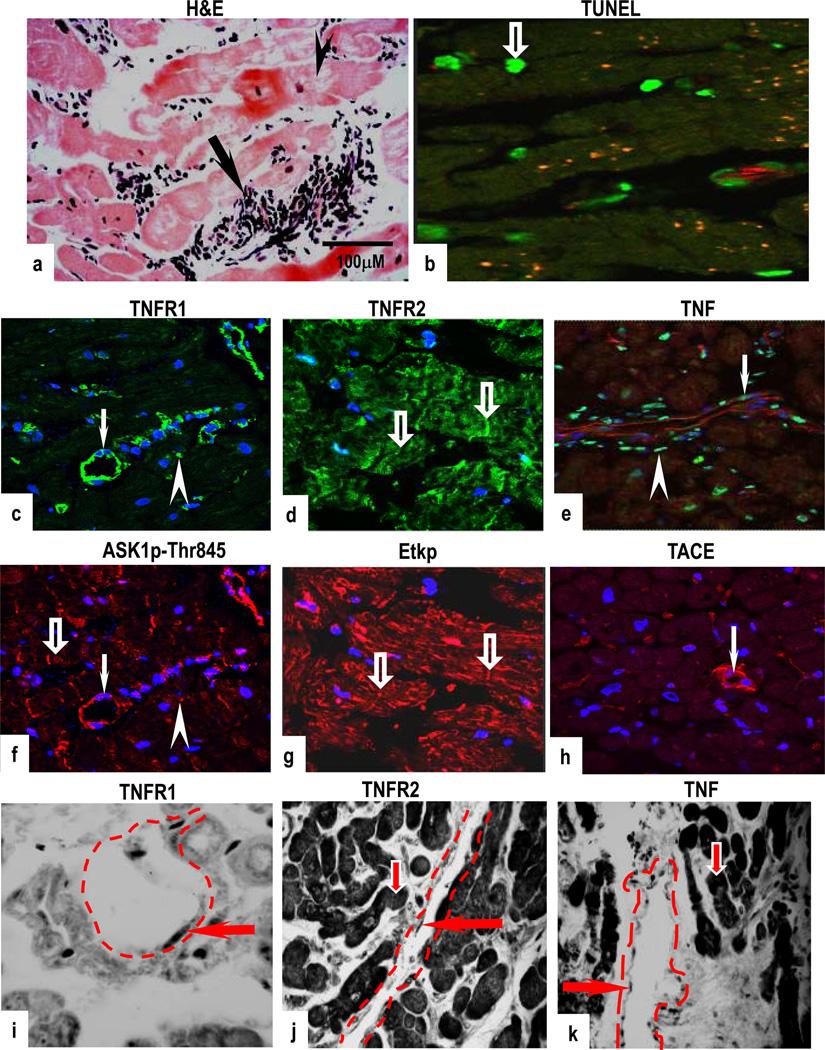
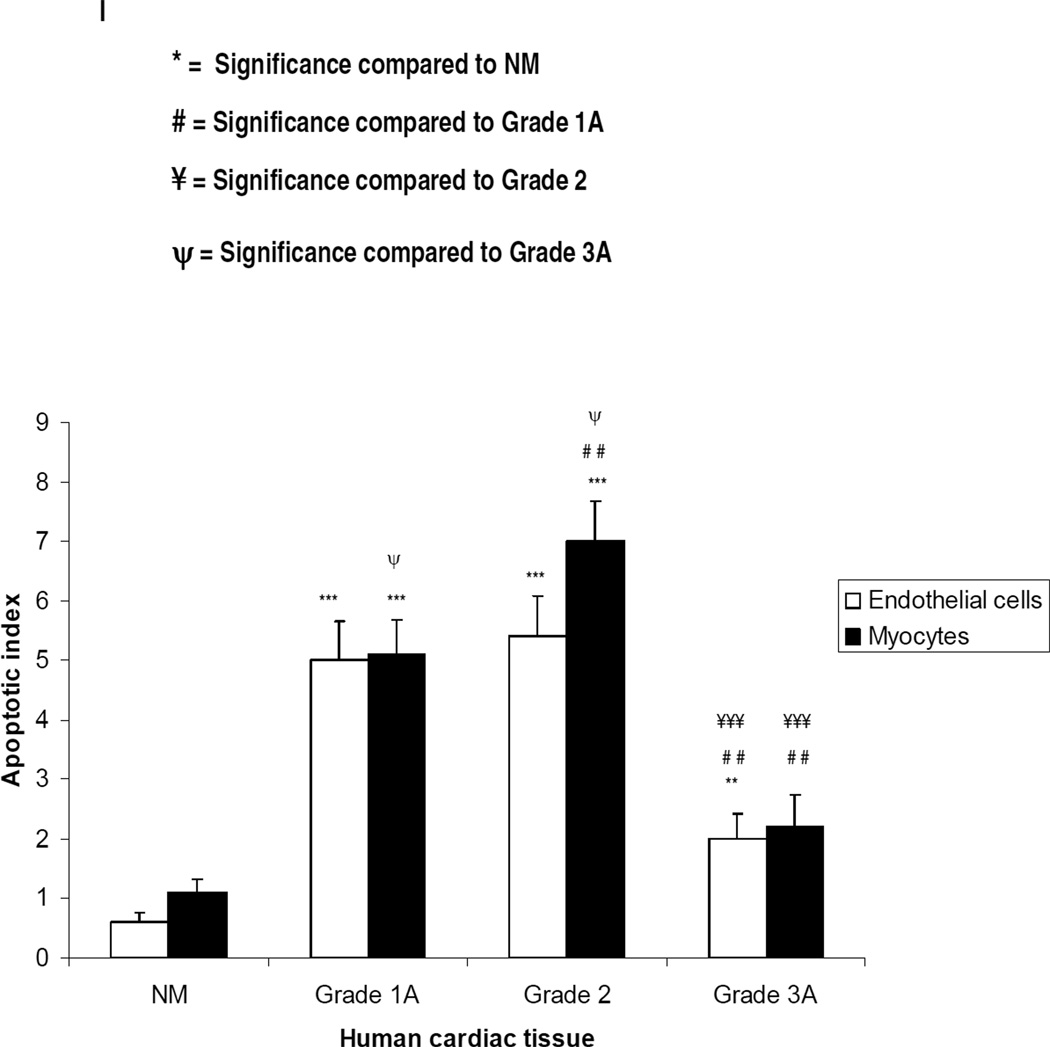
Biopsies with grade 3A rejection showed multifocal aggressive MNC infiltrates and/or CM damage (Fig 4a) but contained fewer TUNEL-positive CM and VEC compared to sections with mild and focal moderate rejection (Fig 4b). Co-expression of TNFR1 and ASK1p-Ser967 (Supplementary Figs 4a & 4b) or ASK1p-Thr845 was demonstrated in VEC and in MNC, and TNFR2 and Etk or Etkp was strongly detected in VEC (Figs 4d & 4g and Supplementary Figs 4c & 4d). TNF was confined to microvessels and MNC while TACE was markedly diminished in CM but strongly detected in VEC (Figs 4e & 4h). Both TNFRs were rarely detected in fibroblasts (data not shown). TNFR1 mRNA was confined to VEC while TNFR2, TNF, ASK1, Etk and TACE mRNA expression was evident in CM and in VEC (Figs 4i–4k and Supplementary Figs 4e–g). No signal was detected in sections labelled with sense probes (data not shown). TUNEL findings are summarized in Fig 4l. There was no obvious variability of intensity of staining within individual samples but a noticeable and significant variability in staining was seen between each grade as shown in Table 1 and Supplementary Fig 5.
Figure 4.
Low moderate rejection (grade 3A); H&E stained section show multifocal aggressive infiltrates and/or CM damage with a few TUNEL-positive CM (b). Co-expression of TNFR1 and ASK1p-Thr845 is evident in VEC (arrows) and in MNC (arrowheads) with ASK1p-Thr845 also detected in some CM (open arrows) (c,f). TNFR2 and Etkp activation is evident in CM (open arrows) (d,g) while TNF is confined to microvessels (arrow) and in MNC (arrowhead) (e). TACE is noticeably diminished in CM with a strongly signal detected in VEC (arrow) (h). TNFR1 mRNA is confined to VEC (arrow) (i) and TNFR2 and TNF mRNA is seen in CM (open arrows) and in VEC (arrows) (j,k). Dotted lines mark out the vessels. Images are representative of 6 independent experiments with similar results (Original Mags: a,i. ×235; b–h, ×63; j,k, ×112). Apoptotic index (l) presented as means ± SEM show statistical significant differences between control (normal human myocardium) and the rejection grades; with the highest level of apoptotic cell death observed in grades 1A and 2 as compared to control. Controls Vs each rejection grade is indicated in the graph and ranges from p<0.001 to p<0.05.
Table 1.
Quantitative analysis of immunostaining for TNF, TNF receptors and, their associated molecules in normal human myocardium (NK) and in the 3 different grades of rejection. Vascular endothelial cells (VEC), leukocytic mononuclear cells (MNC) and cardiomyocytes (CM). Data represents the number of positive cells in 3 random regions from 3 random biopsies from each grade (n = 9).
| TNFR1 | TNFR2 | ASK1p967 | ASK1p845 | Etk/BMX | Etkp | TACE | TNF | ||
|---|---|---|---|---|---|---|---|---|---|
| NM | |||||||||
| VEC | 19.3 ±1.0 | 10.1 ±2.6 | 15.9 ±3.6 | 0.8 ±0.7 | 9.0 ±2.8 | 9.0 ±2.6 | 15.9 ±2.6 | 5.0 ±2.5 | |
| MNC | 6.1 ±2.6 | 3.0 ±1.7 | 9.1 ±2.7 | 9.3 ±2.0 | 7.6 ±3.3 | 2.6 ±1.3 | 13.2 ±4.8 | 4.9 ±2.4 | |
| 15.4 | |||||||||
| CM | 19.0 ±1.7 | 0.4 ±0.5 | 14.0 ±4.3 | 0.7 ±0.7 | ±3.3 | 6.7 ±3.6 | 18.9 ±1.3 | 0.6 ±0.5 | |
| Grade 1A | |||||||||
| VEC | 15.6 ±5.6 | 7.9 ±1.8 | 16.3 ±2.5 | 13.0 ±3.6 | 8.0 ±2.6 | 8.0 ±2.1 | 14.6 ±2.2 | 12.0 ±2.6 | |
| MNC | 11.2 ±4.5 | 16.1 ±2.4 | 6.9 ±2.8 | 10.0 ±1.5 | 7.4 ±2.6 | 15.0 ±2.4 | 12.6 ±3.4 | 17.0 ±2.7 | |
| CM | 1.8 ±1.6 | 13.4 ±2.9 | 1.2 ±0.8 | 6.8 ±2.0 | 7.7 ±2.8 | 13.1 ±2.9 | 18.8 ±1.1 | 0.9 ±0.6 | |
| Grade 2 | |||||||||
| VEC | 15.2 ±2.3 | 8.9 ±2.7 | 16.0 ±2.6 | 13.7 ±3.8 | 8.6 ±1.7 | 7.3 ±2.0 | 17.4 ±2.4 | 11.4 ±2.8 | |
| MNC | 10.7 ±3.0 | 14.8 ±2.6 | 8.2 ±2.1 | 12.0 ±1.9 | 7.8 ±2.2 | 12.1 ±2.8 | 15.2 ±1.9 | 17.0 ±2.7 | |
| CM | 1.3 ±1.3 | 18.4 ±1.2 | 1.1 ±0.8 | 12.4 ±3.2 | 7.9 ±2.6 | 16.7 ±3.5 | 15.0 ±4.0 | 1.0 ±0.9 | |
| Grade 3A | |||||||||
| VEC | 15.9 ±4.5 | 11.9 ±3.0 | 7.7 ±4.5 | 12.9 ±3.3 | 9.8 ±1.8 | 8.7 ±2.3 | 17.0 ±2.6 | 10.7 ±2.4 | |
| MNC | 10.7 ±4.1 | 14.9 ±3.5 | 1.0 ±0.7 | 13.6 ±2.6 | 8.2 ±1.4 | 7.2 ±1.9 | 15.2 ±1.9 | 17.0 ±2.7 | |
| CM | 1.3+/−2.2 | 18.4 ±2.9 | 0.9 ±0.6 | 11.1 ±3.1 | 6.1 ±3.5 | 16.2±3.1 | 5.9 ±2.2 | 1.0 ±0.9 |
Significant differences compared to controls (normal myocardium) are shown in bold;* p<0.05 or highlighted;*** p<0.001.
Cell Cycle Entry in Human Cardiac Allograft Biopsies
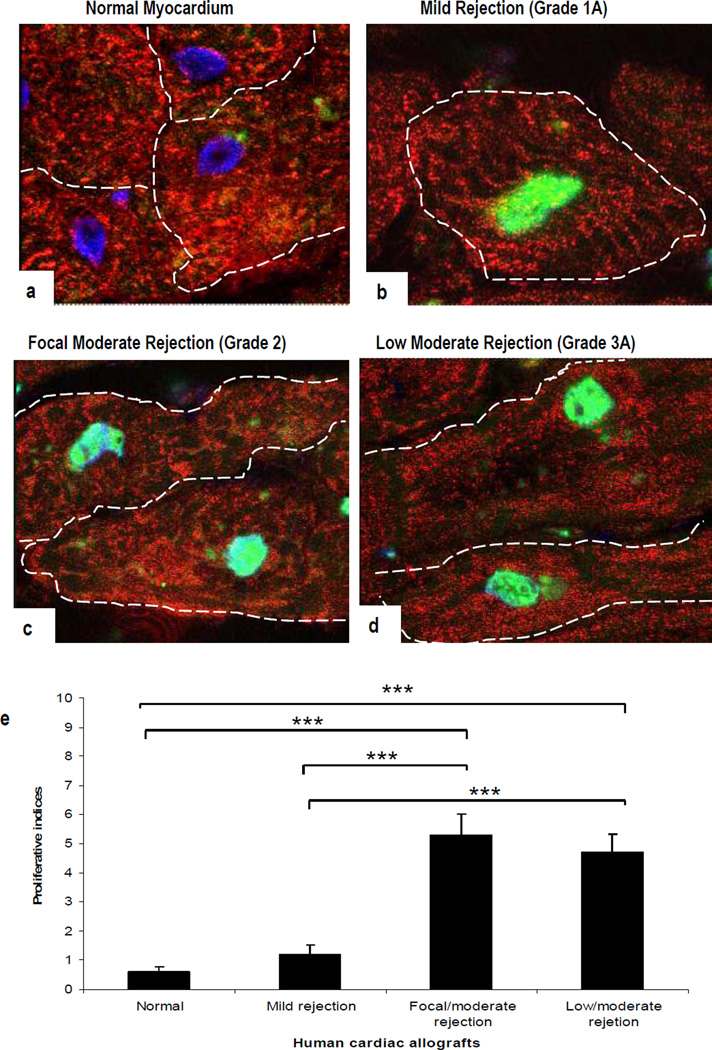
Since TNFR2 signals cause proliferative responses in VEC and renal tubular epithelial cells (10), we investigated whether allograft rejection affects cell cycle entry in human heart. Sections of normal myocardium and allograft hearts were immunostained with two different molecular markers of cell cycle entry (Ki-67 and pH3). Both the markers showed a similar pattern and intensity of staining. No signal for pH3 expression was detected in normal myocardium (Fig 5a) and only a few CM were positive in grade 1 rejection (Fig 5b). In comparison, sections of grade 2 rejection showed a marked increase in pH3 expression in CM identified with α-sarcomeric actin (Fig 5c). Compared to grade 2, grade 3 rejection showed less signal in CM (Fig 5d). Significant differences were seen between normal myocardium and grades 2/3A rejection (p<0.001) but not between grades 2 and 3A rejection (Fig 5e). We did not observe either nuclear or cell division, although such changes have been reported in CM by others (25).
Figure 5.
Confocal images of human cardiac biopsies. Nuclear staining for pH3 is illustrated in combination with α-sarcomeric-actin staining of the CM cytoplasm (green and red, respectively). (a). pH3 is absent in normal human myocardium. In comparison, a strong signal is apparent in CM in sections with mild grade 1A (b), focal moderate grade 2 (c), and low moderate grade 3A rejection (d). Proliferative indices (e); results are presented as means ± SEM. A significant difference is seen between normal myocardium and all three rejection episodes (***p<0.001) but not between grade 2 and 3A rejection. (Original Mags; ×63; Controls: n = 5; Allograft rejections, n= 5).
Effects of TNF in Mouse Heart Organ Cultures
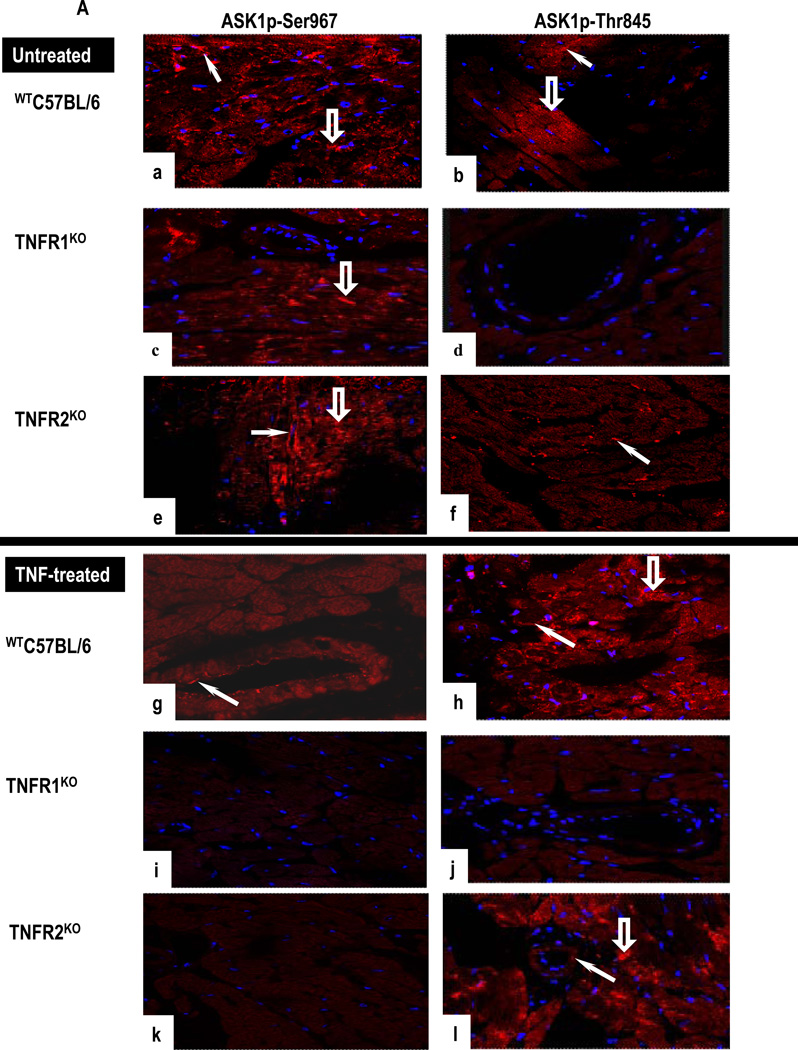
TNF signaling was examined in heart organ cultures from WTC57BL/6, TNFR1KO, TNFR2KO and TNFR1/2KO mice. As in normal human heart, TNFR1 was detected in CM and in VEC and TNFR2 only in VEC in untreated WTC57BL/6 cultures (Supplementary Figs 6a–b). A strong signal for ASK1p-Ser967 and ASK1p-Thr845 was present in CM and in VEC (Figs 6a–6b). Expression of ASK1p-Ser967 but not ASK1p-Thr845 was detected in TNFR1KO cultures (Figs 6c–6d) while TNFR2KO cultures showed strong ASK1p-Ser967 expression in CM and in VEC but ASK1p-Thr845 only in VEC (Figs 6e–6f). TNF caused upregulation of TNFR2 in CM and in VEC, but not TNFR1 in WTC57BL/6 cultures (Supplementary Figs 6c–d). TNF diminished ASK1p-Ser967 in VEC and markedly increased ASK1p-Thr845 expression in CM and in VEC (Figs 6g–6h). TNF-treated TNFR1KO cultures were negative for ASK1 (Figs 6i–6j). In contrast, TNF-treated TNFR2KO cultures showed a strong signal for ASK1p-Thr845 in CM and VEC (Figs 6k–6l). TNFR1/2KO cultures did not respond to TNF. Signals for TNFRs, ASK1 and Etk were negligible in fibroblasts identified by pro-collagen type I (data not shown).
Figure 6.
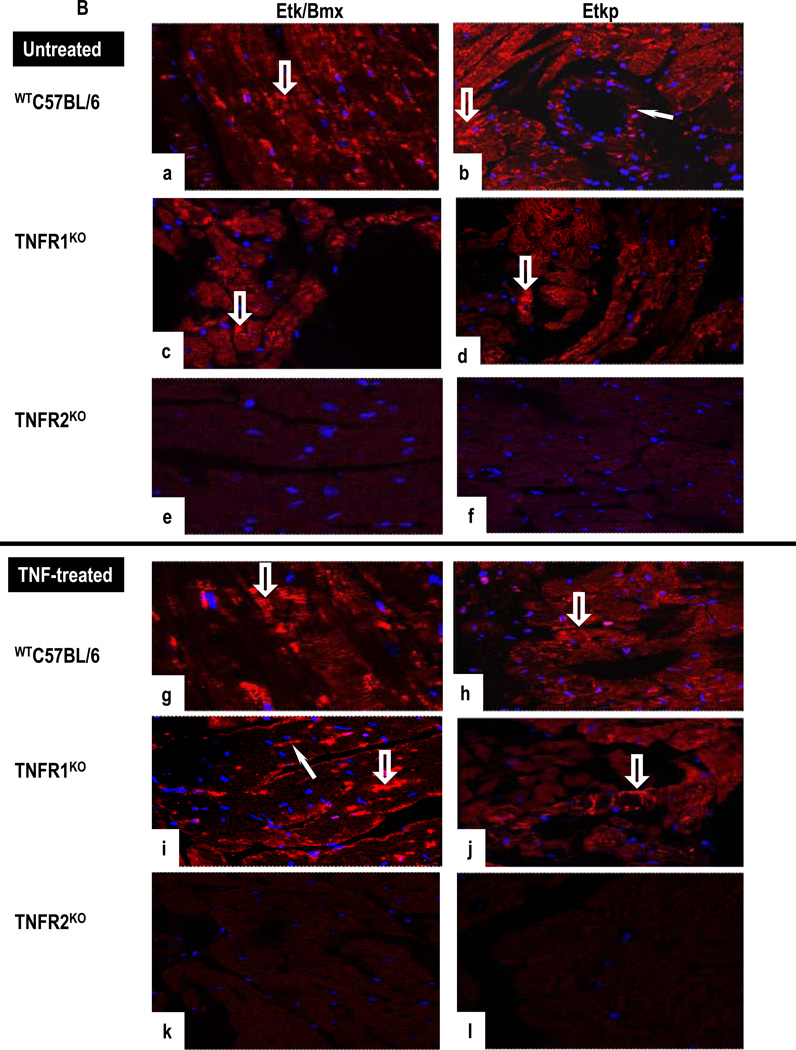
(A). Untreated WTC57BL/6 cultures show positive staining for ASK1p-Ser967 and ASK1p-Thr845 in CM (open arrows) and in VEC (arrows) (a,b). Untreated TNFR1KO cultures are positive for ASK1p-Ser967 (arrowhead) but not for ASK1p-Thr845 (c,d). In contrast, TNF-treated WTC57BL/6 cultures show a diminished ASK1p-Ser967 (g) but a strong signal for ASK1p-Thr845 in CM (open arrow) and in VEC (arrowhead) (h) while TNF-treated TNFR1KO are negative (i,j). In contrast, CM (open arrow) and VEC (arrow) are strongly positive for ASK1p-Thr845 but not for ASK1p-Ser967 in TNF-treated TNFR2KO cultures (k–l). (B). A strong signal for total Etk and phosphorylated Etk (Etkp) is seen in CM (open arrow) and in VEC (arrow) in untreated WTC57BL/6 (a,b) and in TNFR1KO cultures (c,d), while untreated TNFR2KO cultures are negative (e,f). TNF-treated WTC57BL/6 and TNFR1KO cultures show Etk and Etkp in VEC (arrows) and in CM (open arrows) (g–j). In contrast, TNF-treated TNFR2KO cultures are negative (k,l). Images are representative of 3 independent experiments with similar results (Original Mags, ×63).
Untreated WTC57BL/6 cultures showed a strong signal for Etk and Etkp in CM and in VEC (Figs 6a–6b). A similar pattern and intensity of Etk expression and activation was evident in TNFR1KO cultures (Figs 6c–6d). TNFR2KO cultures were negative for Etk (Figs 6e–6f). TNF-treated cultures of WTC57BL/6 and of TNFR1KO showed strong signals for Etk and Etkp in VEC and in CM (Figs 6g–6j) while TNFR2KO cultures were negative (Figs 6k–6l). Statistically significant effects of TNF within and between experimental groups are summarized in Tables 2A & 2B. These results suggest that TNFR1 activates ASK1 while TNFR2 activates Etk.
Table 2.
| A. Quantitative analysis of immunostaining for TNF receptors and their associated molecules in WTC57B/L6 (B6-Ctrl), TNFR1KO (R1-KO) and TNFR2KO (R2-KO). Vascular endothelial cells (VEC), leukocytic mononuclear cells (MNC) and, cardiomyocytes (CM). Data represents 1 of 3 organ culture experiments and shows a statistical significance within animals or each cell type, UT v TNF-treatment. | |||||||
|---|---|---|---|---|---|---|---|
| TNFR1 | TNFR2 | ASK1p1967 | ASK1 p845 | Etk/Bmx | Etkp | ||
| B6-Ctrl | UT | ||||||
| VEC | 14.2±5.1 | 8.8±2.0 | 12.3±2.1 | 6.4±2.4 | 11.6±1.1 | 10.8±2.3 | |
| MNC | 4.8±1.9 | 4.0±1.5 | 7.4±2.1 | 6.1±1.3 | 8.4±2.6 | 8.7±2.6 | |
| CM | 11.2±3.9 | 0.4±0.5 | 12.3±3.7 | 4.7±1.0 | 14.3±3.2 | 10.8±3.1 | |
| TNF | |||||||
| VEC | 15.7± 2.6 | 8.4±1.6 | 4.1±1.7 | 12.4±2.9 | 13±3.0 | 14.0±2.5 | |
| MNC | 8.2 ± 2.6 | 5.3±1.4 | 5.7±1.7 | 13.3±2.5 | 8.3±1.8 | 9.4±2.2 | |
| CM | 13.2 ± 2.0 | 13.4±2.9 | 2.5±1.8 | 15.3±3.0 | 12.2±3.7 | 14.6±3.7 | |
| TNFR1-KO | UT | ||||||
| VEC | 0.0±0.0 | 8.0±2.0 | 12.8±2.8 | 1.0±0.7 | 13.8±2.5 | 10.3±2.0 | |
| MNC | 0.0±0.0 | 3.1±1.6 | 7.1+/−1.2 | 0.7±0.6 | 8.4±1.7 | 7.6±3.0 | |
| CM | 0.0±0.0 | 0.6±0.5 | 15.6±1.8 | 0.7±0.6 | 17.1±2.6 | 10.5±2.2 | |
| TNFR1-KO | TNF | ||||||
| VEC | 0.0±0.0 | 8.0±1.7 | 1.2±0.9 | 0.8±0.6 | 15.2±2.6 | 16.2±2.6 | |
| MNC | 0.0±0.0 | 8.0±2.2 | 0.7±0.6 | 0.7±0.4 | 8.8+/−2.8 | 8.8±2.1 | |
| CM | 0.0±0.0 | 7.5±1.5 | 0.5±0.5 | 1.0±0.7 | 16.5±2.6 | 17.5±1.3 | |
| TNFR2-KO | UT | ||||||
| VEC | 10.0±1.6 | 0.0±0.0 | 10±1.8 | 4.6±1.4 | 0.0±0.0 | 0.0±0.0 | |
| MNC | 6.1±1.8 | 0.0±0.0 | 8.8±1.5 | 8.3±1.8 | 0.0±0.0 | 0.0±0.0 | |
| CM | 10.4±1.5 | 0.0±0.0 | 13.1±3.3 | 4.4±1.2 | 0.0±0.0 | 0.0±0.0 | |
| TNFR2-KO | TNF | ||||||
| VEC | 11.0±2.1 | 0.0±0.0 | 0.1±0.3 | 12.2±1.6 | 0.0±0.0 | 0.0±0.0 | |
| MNC | 7.7±1.2 | 0.0±0.0 | 0.7±0.4 | 9.3±1.7 | 0.0±0.0 | 0.0±0.0 | |
| CM | 12.5±3.0 | 0.0±0.0 | 0.5±0.5 | 15.2±2.7 | 0.0±0.0 | 0.0±0.0 | |
| B. Quantitative analysis of immunostaining for TNF receptors and their associated molecules in vascular endothelial cells (VEC), in leukocytic mononuclear cells (MNC) and in cardiomyocytes (CM), in WTC57B/L6 (B6-Ctrl), TNFR1KO (R1-KO) and TNFR2KO (R2-KO). Data is represents 1 of 3 organ culture experiments and shows a statistical significance between Ctrl Vs KO animals (same cells and treatments). | |||||||
|---|---|---|---|---|---|---|---|
| TNFR1 | TNFR2 | ASK1967 | ASK 1845 |
Etk/Bmx | Etkp | ||
| B6-Ctrl | UT | ||||||
| VEC | 12.2±5.1 | 8.8±2.0 | 12.3±2.1 | 6.4±2.4 | 11.6±1.1 | 10.8±2.3 | |
| MNC | 4.8±1.9 | 4.0±1.5 | 7.4±2.1 | 6.1±1.3 | 8.4±2.6 | 8.7±2.6 | |
| CM | 11.2±3.9 | 0.4±0.5 | 12.3±3.7 | 4.7±1.0 | 14.3±3.2 | 10.8±3.1 | |
| TNF | |||||||
| VEC | 15.7±2.6 | 8.4±1.6 | 4.1±1.7 | 12.4±2.9 | 13±3.0 | 14.0±2.5 | |
| MNC | 8.2±2.6 | 5.3±1.4 | 5.7±1.7 | 13.3±2.5 | 8.3±.8 | 9.4±2.2 | |
| CM | 13.2±2.0 | 13.4±2.9 | 2.5±1.8 | 15.3±3.0 | 12.2±3.7 | 14.6±3.7 | |
| TNFR1-KO | |||||||
| UT | |||||||
| VEC | 0.0±0.0 | 8.0±2.0 | 12.8±2.8 | 1.0±0.7 | 13.8±2.5 | 10.3±2.0 | |
| MNC | 0.0±0.0. | 3.1±1.6 | 7.1±1.2 | 0.7±0.6 | 8.4±1.7 | 7.6±3.0 | |
| CM | 0.0±0.0 | 0.6±0.5 | 15.6±1.8 | 0.7±0.6 | 17.1±2.6 | 10.5±2.2 | |
| TNF | |||||||
| VEC | 0.0±0.0 | 8.0±1.7 | 1.2±0.9 | 0.8±0.6 | 15.2±2.6 | 16.2±2.6 | |
| MNC | 0.0±0.0 | 8.0±2.2 | 0.7±0.6 | 0.7±0.4 | 8.8±2.8 | 8.8±2.1 | |
| CM | 0.0±0.0 | 7.5±1.5 | 0.5±0.5 | 1.0±0.7 | 16.5±2.6 | 17.5±1.3 | |
| TNFR2-KO | |||||||
| UT | VEC | 10.0±1.6 | 0.0±0.0 | 10±1.8 | 4.6±1.4 | 0.0±0.0 | 0.0±0.0 |
| MNC | 6.1±1.8 | 0.0±0.0 | 8.8±1.5 | 8.3±1.8 | 0.0±0.0 | 0.0±0.0 | |
| CM | 10.4±1.5 | 0.0±0.0 | 13.1±3.3 | 4.4±1.2 | 0.0±0.0 | 0.0±0.0 | |
| TNF | |||||||
| VEC | 11.0±2.1 | 0.0±0.0 | 0.1±0.3 | 12.2±1.6 | 0.0±0.0 | 0.0±0.0 | |
| MNC | 7.7±1.2 | 0.0±0.0 | 0.7±0.4 | 9.3±1.7 | 0.0±0.0 | 0.0±0.0 | |
| CM | 12.5±3.0 | 0.0±0.0 | 0.5±0.5 | 15.2±2.7 | 0.0±0.0 | 0.0±.0.0 | |
Bold; *p<0.05; highlighted; ***p<0.001.
Bold; *p<0.05; underlined; **p<0.01 or highlighted; ***p<0.001.
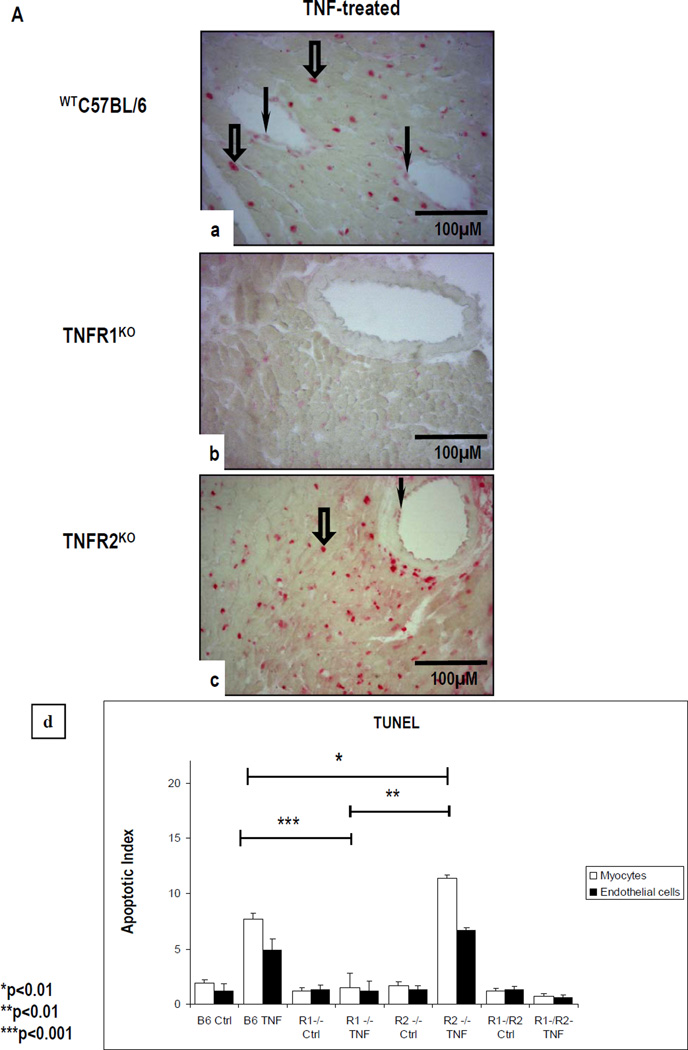
We next analyzed the mouse heart organ cultures for TUNEL staining (Fig 7A) and for the expression of Ki-67, pH3 and PCNA (Fig 7B). TUNEL staining was negligible in untreated cultures (data not shown), and in CM and in VEC in TNF-treated WTC57BL/6 cultures (Fig 7a) but not TNFR1KO cultures (Fig 7b). TNF-treated TNFR2KO cultures showed a marked increase in TUNEL-positive CM and VEC (Fig 7c). TNF-treated TNFR1/2KO cultures showed a similar level and pattern of TUNEL staining to untreated WTC57BL/6 cultures (data not shown). A statistically significant increase in TUNEL staining was seen in TNF-treated TNFR2KO cultures as compared to TNF-treated WTC57BL/6 or -TNFR1KO cultures (p<0.01) and between TNF-treated WTC57BL/6 and TNFR1KO cultures (p< 0.001). Apoptotic indices are summarized in Fig 7d.
Figure 7.
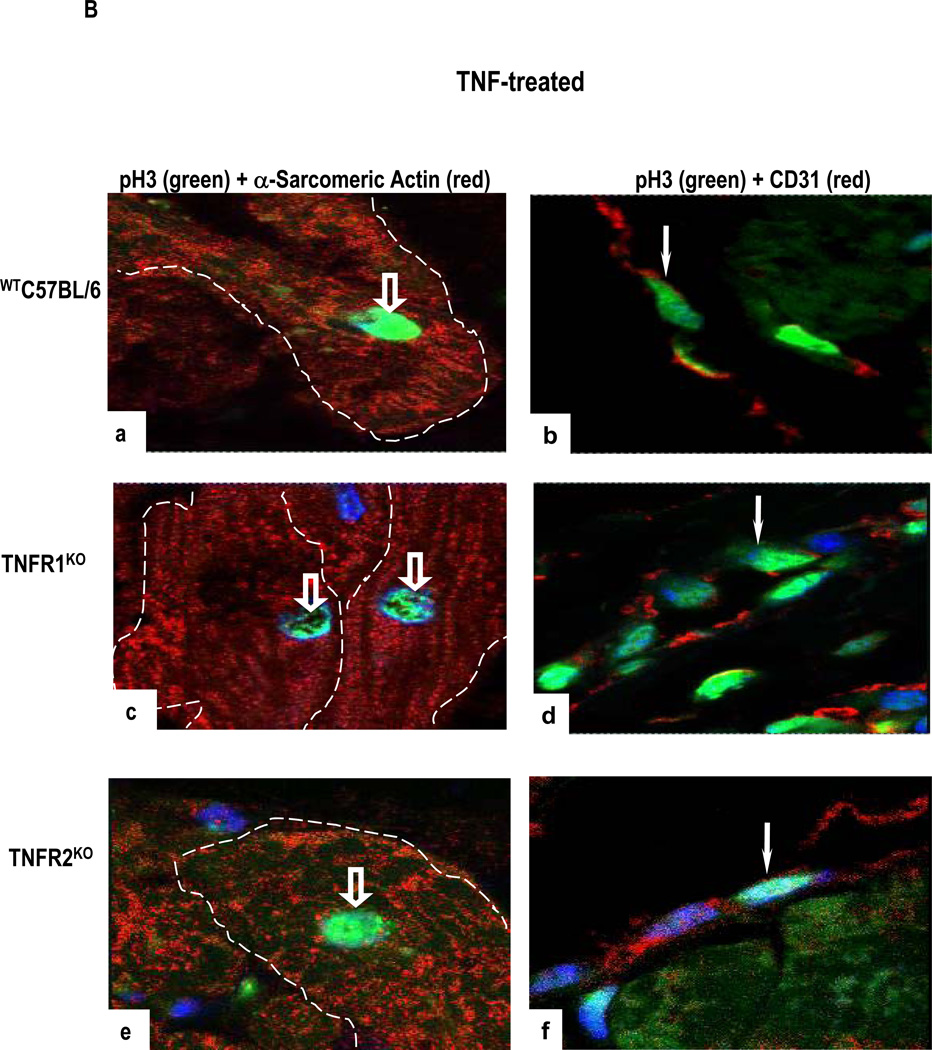
(A). TNF-treated WTC57BL/6 cultures (a) but not TNF-treated TNFR1KO cultures (b) show increased TUNEL-positive CM (open arrows) and VEC (arrows). In comparison, an increased TUNEL staining is seen in CM (arrowhead) and in VEC (arrow) in TNF-treated TNFR2KO cultures (c). Apoptotic index (d) show statistically significant difference between cultures with the highest level of apoptotic cell death observed in TNF-treated TNFR2KO cultures as compared to TNF-treated WTC57BL/6 (*p<0.01) or -TNFR1KO (**p<0.01) or TNFR1/TNFR2KO cultures. (B). A high level of pH3 expression is seen in α-sarcomeric actin-positive CM (open arrows) and in CD31-positive VEC (arrow) in TNF-treated WTC57BL/6 cultures (a,b) with a similar pattern but higher level of expression is evident in TNFR1KO cultures (c,d). In contrast, TNF-treated TNFR2KO cultures show only occasional pH3 expression (e,f). Nuclei stained with To-Pro-3-iodide. (Original Mags; A- a–c; ×112; B- a–f; ×63).
Three markers of cell cycle entry gave concordant results. Representative data from pH3 immunostaining are shown in Fig 7B. Untreated cultures showed negligible pH3 expression (data not shown) while TNF-treated WTC57BL/6 and TNFR1KO cultures showed increased pH3 expression in α-sarcomeric actin-positive CM and in CD31-positive VEC (Figs 7a–7d), suggesting that some CM and VEC are in the G1-S and G2-M stage of the cell cycle. pH3 expression in TNFR2KO cultures was negligible (Figs 7e–7f). Statistically significant more cell cycle entry was detected in TNF-treated TNFR1KO cultures compared to WTC57BL/6 cultures (Table 3).
Discussion
In this study, we report several principal new findings. First, TNFRs are differentially expressed on various cell types in normal myocardium and these patterns of expression are altered by allograft rejection. In rejection-free cardiac allograft biopsies and biopsies from normal hearts harvested for transplantation TNFR1 but not TNFR2, is predominantly and strongly expressed by CM and rarely seen in fibroblasts. During cardiac allograft rejection CM show prominent induction of TNFR2 and diminished expression of TNFR1. Activation of ASK1 largely co-localizes with TNFR1, and activation of Etk largely co-localizes with TNFR2 in VEC and CM. Second, in wild type mouse heart organ culture, TNF upregulates TNFR2, activates both ASK1 and Etk, and causes apoptosis and cell cycle entry. Third, TNF-treated TNFR1KO mouse heart organ cultures showed Etk activation, negligible TUNEL positivity and increased changes in the nucleus consistent with entrance into the cell cycle. In contrast, organ cultures from TNFR2KO showed activation of ASK1, increased TUNEL and lower levels of cell cycle entry. These observations are consistent with a report that TNFR1 is pro-apoptotic and promotes detrimental remodelling, whereas TNFR2 is anti-apoptotic and cardioprotective in the failing heart (6), and provide a mechanism through which this may occur by differential kinase activation.
CM expresses functional TNFR1 and have been shown to undergo apoptosis after stimulation with TNF in vitro (26). Furthermore, in vivo studies of ischemic reperfusion in mouse hearts from double-TNFRKO have shown that TNF binding to TNFR1 results in recruitment and activation of procaspase-8 and -3, inducing the extrinsic death pathway (27). We do not know if ASK1 activation directly contributes to apoptosis in CM during rejection or if signals other than TNF may be responsible for ASK1 activation in the biopsy samples. Early after cardiac transplantation, elevated levels of inflammatory cells and soluble molecules are associated with impaired allograft function and may contribute to activation of ASK1 (28). Nevertheless, it is useful for assessing TNFR1 signaling in the organ culture system.
Factors that result in TNFR1 down-regulation in rejection are still unclear. One possible mechanism is the TACE-mediated cleavage of the TNFR1, which may generate a soluble TNF-binding protein that modulates TNF bioactivity. Soluble TNFR1 increases in severe congestive heart failure (29), in acute myocardial inflammation (30) and in atherosclerotic lesions (31).
In contrast to a previous report (32), we did not detect TNFR2 protein or mRNA in CM in rejection-free biopsies of human heart and we observed induction rather than loss of TNFR2 with injury. The latter difference could depend on the type of injury; we studied rejection whereas Torre-Amione et al (32) studied congestive failure. Our results are consistent with previous findings in rat cardiac allografts (33, 34), demonstrating increased levels of intragraft TNF and TNFR2 mRNA but not TNFR1 mRNA expression in CM of rejecting hearts. Although the role of myocardial TNFR2 is unclear, neutralizing anti-TNFR2 antibodies exacerbate TNF-induced cell death, consistent with a protective role (27, 35). TNF expression has previously been reported in human cardiac allograft rejection (36, 37). We also detected expression of TNF protein and transcripts in microvessels of normal heart. TNF expression is dramatically increased with progression of allograft rejection, supporting a potential role for TNF as a mediator and/or marker of cardiac allograft rejection (38–40). Our mouse heart organ culture model suggests that TNF could mediate different cellular and molecular responses (injury versus repair) through altered TNFR expression. TNF has been shown to mediate protective effects (41), including hypertrophic growth response in CM, as an adaptive response to hemodynamic or environmental stress (42). We also observed marked CM and vascular TACE expression that could underlie the release of increased soluble forms of TNF as well as TNFRs (43).
Analysis of human biopsies revealed changes in CM indicative of cell cycle entry but we did not observe mitoses or cell division as previously described by others (44). Although, it is widely believed that CM do not undergo replication in the adult heart, CM cell division is reported in human adult myocardium under certain conditions (25) (45, 46). In vitro studies have shown that CM are able to re-enter the G2-M phases in the cell cycle after fusing with proliferative cell types such as EC or cardiac myofibroblast (47). Even though we have not seen cell division in our studies, the observed cell cycle changes, which can be attributed to TNFR2 signaling in the organ culture model, may be part of a mechanism of CM repair or resistance to injury as seen in renal epithelium (10). This hypothesis will require further investigation. Collectively, our results suggest that the balance of TNFR signaling is important in the outcome of the rejection process. Differential activation of ASK1 and Etk by regulated expression of TNFRs may provide an explanation for diverse and opposing responses to TNF at distinct sites in the human heart. Importantly, our functional studies in a mouse heart organ culture model identifying TNFR1 as a cell death-inducing receptor and TNFR2 as an inducer of cell cycle entry suggests that selective blockade of TNFRs may offer a better therapeutic option than global TNF blockade for patients with cardiac disease.
Supplementary Material
Acknowledgments
The authors would also like to thank Dr Aviva Tolkovsky for helpful discussion.
Funding Sources: British Heart Foundation to JRB, National Institute for Health Research Centre Cambridge Biomedical Research Centre to JRB and National Institutes of Health grant no: HL036003 and HL062188 to JSP; HL065978 and HL077357 to MW and PO1 HL70295 to GT.
Footnotes
Disclosure
There are no conflicts of interests
References
- 1.Tartaglia LA, Weber RF, Figari IS, Reynolds C, Palladino MA, Jr, Goeddel DV. The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc Natl Acad Sci U S A. 1991;88(20):9292–9296. doi: 10.1073/pnas.88.20.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandborn WJ, Hanauer SB. Antitumor necrosis factor therapy for inflammatory bowel disease: a review of agents, pharmacology, clinical results, and safety. Inflamm Bowel Dis. 1999;5(2):119–133. doi: 10.1097/00054725-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Nikolaus S, Schreiber S. Anti-TNF biologics in the treatment of chronic inflammatory bowel disease. Internist (Berl) 2008;49(8):947–943. doi: 10.1007/s00108-008-2058-3. [DOI] [PubMed] [Google Scholar]
- 4.Meldrum DR. Tumor necrosis factor in the heart. Am J Physiol. 1998;274(3 Pt 2):R577–R595. doi: 10.1152/ajpregu.1998.274.3.R577. [DOI] [PubMed] [Google Scholar]
- 5.Muller-Ehmsen J, Schwinger RH. TNF and congestive heart failure: therapeutic possibilities. Expert Opin Ther Targets. 2004;8(3):203–209. doi: 10.1517/14728222.8.3.203. [DOI] [PubMed] [Google Scholar]
- 6.Hamid T, Gu Y, Ortines RV, Bhattacharya C, Wang G, Xuan YT, et al. Divergent tumor necrosis factor receptor-related remodeling responses in heart failure: role of nuclear factor-kappaB and inflammatory activation. Circulation. 2009;119(10):1386–1397. doi: 10.1161/CIRCULATIONAHA.108.802918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higuchi Y, McTiernan CF, Frye CB, McGowan BS, Chan TO, Feldman AM. Tumor necrosis factor receptors 1 and 2 differentially regulate survival, cardiac dysfunction, and remodeling in transgenic mice with tumor necrosis factor-alpha-induced cardiomyopathy. Circulation. 2004;109(15):1892–1897. doi: 10.1161/01.CIR.0000124227.00670.AB. [DOI] [PubMed] [Google Scholar]
- 8.Yamada J, Streilein JW, Dana MR. Role of tumor necrosis factor receptors TNFR-I (P55) and TNFR-II (P75) in corneal transplantation. Transplantation. 1999;68(7):944–949. doi: 10.1097/00007890-199910150-00008. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki J, Cole SE, Batirel S, Kosuge H, Shimizu K, Isobe M, et al. Tumor necrosis factor receptor -1 and -2 double deficiency reduces graft arterial disease in murine cardiac allografts. Am J Transplant. 2003;3(8):968–976. doi: 10.1034/j.1600-6143.2003.00164.x. [DOI] [PubMed] [Google Scholar]
- 10.Al-Lamki RS, Wang J, Vandenabeele P, Bradley JA, Thiru S, Luo D, et al. TNFR1- and TNFR2-mediated signaling pathways in human kidney are cell type-specific and differentially contribute to renal injury. FASEB J. 2005;19(12):1637–1645. doi: 10.1096/fj.05-3841com. [DOI] [PubMed] [Google Scholar]
- 11.Black RA. Tumor necrosis factor-alpha converting enzyme. Int J Biochem Cell Biol. 2002;34(1):1–5. doi: 10.1016/s1357-2725(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 12.Ichijo H, Nishida E, Irie K, Ten DP, Saitoh M, Moriguchi T, et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275(5296):90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 13.Taniike M, Yamaguchi O, Tsujimoto I, Hikoso S, Takeda T, Nakai A, et al. Apoptosis signal-regulating kinase 1/p38 signaling pathway negatively regulates physiological hypertrophy. Circulation. 2008;117(4):545–552. doi: 10.1161/CIRCULATIONAHA.107.710434. [DOI] [PubMed] [Google Scholar]
- 14.Thandavarayan RA, Watanabe K, Ma M, Veeraveedu PT, Gurusamy N, Palaniyandi SS, et al. 14-3-3 protein regulates Ask1 signaling and protects against diabetic cardiomyopathy. Biochem Pharmacol. 2008;75(9):1797–1806. doi: 10.1016/j.bcp.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Qiu Y, Kung HJ. Signaling network of the Btk family kinases. Oncogene. 2000;19(49):5651–5661. doi: 10.1038/sj.onc.1203958. [DOI] [PubMed] [Google Scholar]
- 16.Qiu Y, Robinson D, Pretlow TG, Kung HJ. Etk/Bmx, a tyrosine kinase with a pleckstrin-homology domain, is an effector of phosphatidylinositol 3'-kinase and is involved in interleukin 6-induced neuroendocrine differentiation of prostate cancer cells. Proc Natl Acad Sci U S A. 1998;95(7):3644–3649. doi: 10.1073/pnas.95.7.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan S, An P, Zhang R, He X, Yin G, Min W. Etk/Bmx as a tumor necrosis factor receptor type 2-specific kinase: role in endothelial cell migration and angiogenesis. Mol Cell Biol. 2002;22(21):7512–7523. doi: 10.1128/MCB.22.21.7512-7523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Billingham ME, Cary NR, Hammond ME, Kemnitz J, Marboe C, McCallister HA, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. The International Society for Heart Transplantation. J Heart Transplant. 1990;9(6):587–593. [PubMed] [Google Scholar]
- 19.Skalli O, Gabbiani G, Babai F, Seemayer TA, Pizzolato G, Schurch W. Intermediate filament proteins and actin isoforms as markers for soft tissue tumor differentiation and origin. II. Rhabdomyosarcomas. Am J Pathol. 1988;130(3):515–531. [PMC free article] [PubMed] [Google Scholar]
- 20.Babai F, Musevi-Aghdam J, Schurch W, Royal A, Gabbiani G. Coexpression of alpha-sarcomeric actin, alpha-smooth muscle actin and desmin during myogenesis in rat and mouse embryos I. Skeletal muscle. Differentiation. 1990;44(2):132–142. doi: 10.1111/j.1432-0436.1990.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 21.Focht RJ, Adams SL. Tissue specificity of type I collagen gene expression is determined at both transcriptional and post-transcriptional levels. Mol Cell Biol. 1984;4(9):1843–1852. doi: 10.1128/mcb.4.9.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bravo R, Frank R, Blundell PA, Donald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987;326(6112):515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- 23.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182(3):311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 24.Chadee DN, Hendzel MJ, Tylipski CP, Allis CD, Bazett-Jones DP, Wright JA, et al. Increased Ser-10 phosphorylation of histone H3 in mitogen-stimulated and oncogene-transformed mouse fibroblasts. J Biol Chem. 1999;274(35):24914–24920. doi: 10.1074/jbc.274.35.24914. [DOI] [PubMed] [Google Scholar]
- 25.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344(23):1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 26.Krown KA, Page MT, Nguyen C, Zechner D, Gutierrez V, Comstock KL, et al. Tumor necrosis factor alpha-induced apoptosis in cardiac myocytes. Involvement of the sphingolipid signaling cascade in cardiac cell death. J Clin Invest. 1996;98(12):2854–2865. doi: 10.1172/JCI119114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang M, Crisostomo PR, Markel TA, Wang Y, Meldrum DR. Mechanisms of sex differences in TNFR2-mediated cardioprotection. Circulation. 2008;118(14 Suppl):S38–S45. doi: 10.1161/CIRCULATIONAHA.107.756890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng MC, Erren M, Roeder N, Dreimann V, Gunther F, Kerber S, et al. T-cell and monocyte subsets, inflammatory molecules, rejection, and hemodynamics early after cardiac transplantation. Transplantation. 1998;65(9):1255–1261. doi: 10.1097/00007890-199805150-00018. [DOI] [PubMed] [Google Scholar]
- 29.Ferrari R, Bachetti T, Confortini R, Opasich C, Febo O, Corti A, et al. Tumor necrosis factor soluble receptors in patients with various degrees of congestive heart failure. Circulation. 1995;92(6):1479–1486. doi: 10.1161/01.cir.92.6.1479. [DOI] [PubMed] [Google Scholar]
- 30.Wang M, Markel T, Crisostomo P, Herring C, Meldrum KK, Lillemoe KD, et al. Deficiency of TNFR1 protects myocardium through SOCS3 and IL-6 but not p38 MAPK or IL-1beta. Am J Physiol Heart Circ Physiol. 2007;292(4):H1694–H1699. doi: 10.1152/ajpheart.01063.2006. [DOI] [PubMed] [Google Scholar]
- 31.Canault M, Peiretti F, Kopp F, Bonardo B, Bonzi MF, Coudeyre JC, et al. The TNF alpha converting enzyme (TACE/ADAM17) is expressed in the atherosclerotic lesions of apolipoprotein E-deficient mice: possible contribution to elevated plasma levels of soluble TNF alpha receptors. Atherosclerosis. 2006;187(1):82–91. doi: 10.1016/j.atherosclerosis.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 32.Torre-Amione G, Kapadia S, Lee J, Durand JB, Bies RD, Young JB, et al. Tumor necrosis factor-alpha and tumor necrosis factor receptors in the failing human heart. Circulation. 1996;93(4):704–711. doi: 10.1161/01.cir.93.4.704. [DOI] [PubMed] [Google Scholar]
- 33.Pulkkinen VP, Sihvola RK, Koskinen PK, Lemstrom KB. Inhibition of tumor necrosis factor-alpha does not prevent cardiac allograft arteriosclerosis in the rat. Transplant Proc. 2001;33(1–2):347. doi: 10.1016/s0041-1345(00)02041-8. [DOI] [PubMed] [Google Scholar]
- 34.Sihvola RK, Koskinen PK, Pulkkinen VP, Tikkanen JM, Lemstrom KB. Inhibition of tumor necrosis factor-alpha attenuates myocardial remodeling in rat cardiac allografts. J Heart Lung Transplant. 2006;25(5):569–578. doi: 10.1016/j.healun.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Defer N, Azroyan A, Pecker F, Pavoine C. TNFR1 and TNFR2 signaling interplay in cardiac myocytes. J Biol Chem. 2007;282(49):35564–35573. doi: 10.1074/jbc.M704003200. [DOI] [PubMed] [Google Scholar]
- 36.Alvarez CM, Fernandez D, Builes M, Zabaleta J, Restrepo LM, Villegas A, et al. Intragraft cytokine expression in heart transplants with mild or no histological rejection. Clin Transplant. 2001;15(4):228–235. doi: 10.1034/j.1399-0012.2001.150402.x. [DOI] [PubMed] [Google Scholar]
- 37.Arbustini E, Grasso M, Diegoli M, Bramerio M, Foglieni AS, Albertario M, et al. Expression of tumor necrosis factor in human acute cardiac rejection. An immunohistochemical and immunoblotting study. Am J Pathol. 1991;139(4):709–715. [PMC free article] [PubMed] [Google Scholar]
- 38.Azzawi M, Hasleton PS, Hutchinson IV. TNF-alpha in acute cardiac transplant rejection. Cytokines Cell Mol Ther. 1999;5(1):41–49. [PubMed] [Google Scholar]
- 39.Azzawi M, Hasleton PS, Turner DM, Yonan N, Deiraniya AK, Sinnott PJ, et al. Tumor necrosis factor-alpha gene polymorphism and death due to acute cellular rejection in a subgroup of heart transplant recipients. Hum Immunol. 2001;62(2):140–142. doi: 10.1016/s0198-8859(00)00235-4. [DOI] [PubMed] [Google Scholar]
- 40.Irwin MW, Mak S, Mann DL, Qu R, Penninger JM, Yan A, et al. Tissue expression and immunolocalization of tumor necrosis factor-alpha in postinfarction dysfunctional myocardium. Circulation. 1999;99(11):1492–1498. doi: 10.1161/01.cir.99.11.1492. [DOI] [PubMed] [Google Scholar]
- 41.Kurrelmeyer KM, Michael LH, Baumgarten G, Taffet GE, Peschon JJ, Sivasubramanian N, et al. Endogenous tumor necrosis factor protects the adult cardiac myocyte against ischemic-induced apoptosis in a murine model of acute myocardial infarction. Proc Natl Acad Sci U S A. 2000;97(10):5456–5461. doi: 10.1073/pnas.070036297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yokoyama H, Kreft B, Kelley VR. Biphasic increase in circulating and renal TNF-alpha in MRL-lpr mice with differing regulatory mechanisms. Kidney Int. 1995;47(1):122–130. doi: 10.1038/ki.1995.14. [DOI] [PubMed] [Google Scholar]
- 43.Nozaki N, Yamaguchi S, Shirakabe M, Nakamura H, Tomoike H. Soluble tumor necrosis factor receptors are elevated in relation to severity of congestive heart failure. Jpn Circ J. 1997;61(8):657–664. doi: 10.1253/jcj.61.657. [DOI] [PubMed] [Google Scholar]
- 44.Beltrami CA, Di LC, Finato N, Rocco M, Artico D, Cigola E et al. Proliferating cell nuclear antigen (PCNA), DNA synthesis and mitosis in myocytes following cardiac transplantation in man. J Mol Cell Cardiol. 1997;29(10):2789–2802. doi: 10.1006/jmcc.1997.0514. [DOI] [PubMed] [Google Scholar]
- 45.Kanoh M, Takemura G, Misao J, Hayakawa Y, Aoyama T, Nishigaki K, et al. Significance of myocytes with positive DNA in situ nick end-labeling (TUNEL) in hearts with dilated cardiomyopathy: not apoptosis but DNA repair. Circulation. 1999;99(21):2757–2764. doi: 10.1161/01.cir.99.21.2757. [DOI] [PubMed] [Google Scholar]
- 46.Nadal-Ginard B, Kajstura J, Anversa P, Leri A. A matter of life and death: cardiac myocyte apoptosis and regeneration. J Clin Invest. 2003;111(10):1457–1459. doi: 10.1172/JCI18611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsuura K, Wada H, Nagai T, Iijima Y, Minamino T, Sano M, et al. Cardiomyocytes fuse with surrounding noncardiomyocytes and reenter the cell cycle. J Cell Biol. 2004;167(2):351–363. doi: 10.1083/jcb.200312111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.