Abstract
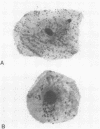
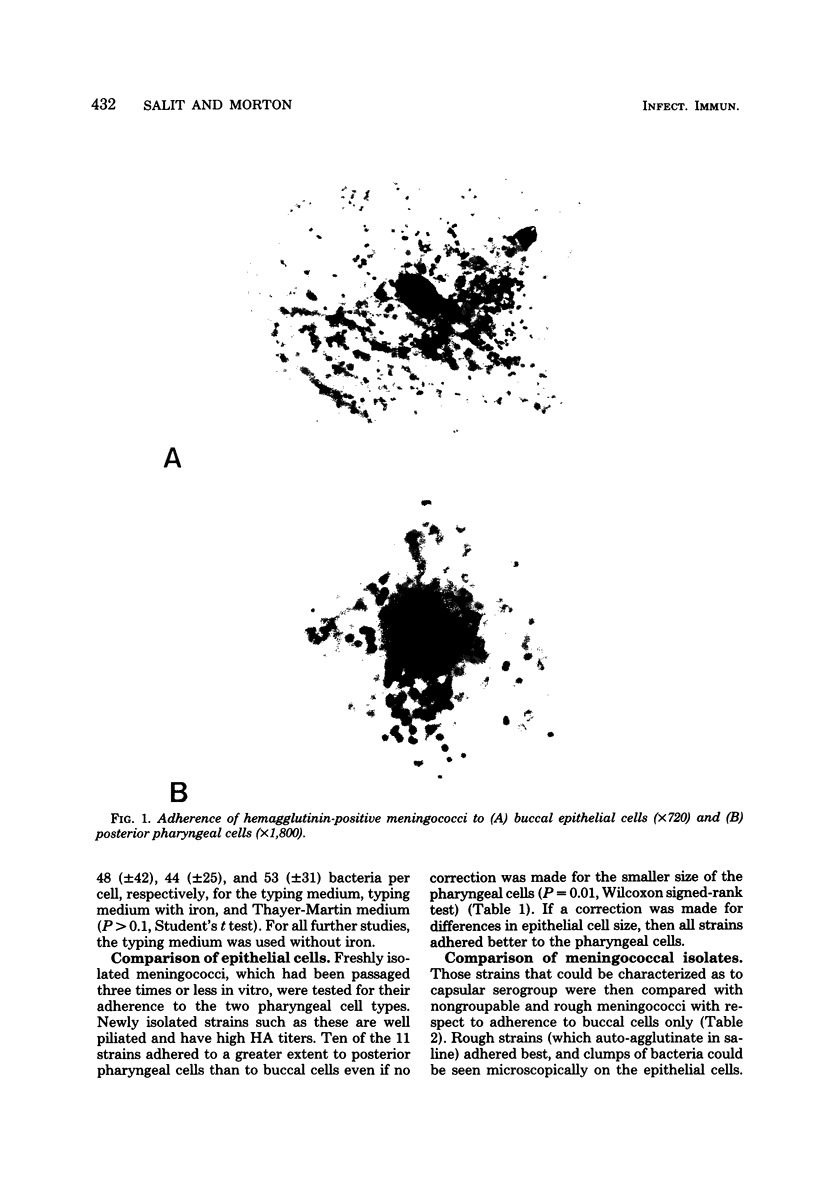
Carrier strains of Neisseria meningitidis are recovered almost solely from the posterior pharynx and they are often nongroupable or rough. Invasive strains can be serogrouped (encapsulated). We studied adherence of both carrier- and patient-derived serogroupable and nongroupable meningococci to buccal epithelial and posterior pharyngeal cells. Fresh meningococcal isolates attached significantly better to pharyngeal than to buccal cells (P = 0.01). Strains that could be serogrouped adhered less than nongroupable strains (P less than 0.05). Meningococci passed in vitro became hemagglutinin negative and nonpiliated. Hemagglutinin-negative meningococci always adhered less to both epithelial cell types than the hemagglutinin-positive variants of the same strain. These results indicate that meningococcal pili probably mediate attachment to oropharyngeal cells, but encapsulation may reduce adherence. Localization of meningococci in the posterior pharynx is in part explained by the receptivity of the epithelial cells in this area for meningococci.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkan M., Ofek I., Beachey E. H. Adherence pharyngeal and skin strains of group A streptococci to human skin and oral epithelial cells. Infect Immun. 1977 Nov;18(2):555–557. doi: 10.1128/iai.18.2.555-557.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly R., Shinefield H. I., Strauss W. G., Maibach H. I. Bacterial adherence to nasal mucosal cells. Infect Immun. 1977 Sep;17(3):546–549. doi: 10.1128/iai.17.3.546-549.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M., Pearce W. A. Pili as a mediator of the attachment of gonococci to human erythrocytes. Infect Immun. 1976 May;13(5):1483–1489. doi: 10.1128/iai.13.5.1483-1489.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edén C. S., Hanson L. A., Jodal U., Lindberg U., Akerlund A. S. Variable adherence to normal human urinary-tract epithelial cells of Escherichia coli strains associated with various forms of urinary-tract infection. Lancet. 1976 Sep 4;1(7984):490–492. [PubMed] [Google Scholar]
- Edén C. S., Hansson H. A. Escherichia coli pili as possible mediators of attachment to human urinary tract epithelial cells. Infect Immun. 1978 Jul;21(1):229–237. doi: 10.1128/iai.21.1.229-237.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faur Y. C., Weisburd M. H., Wilson M. E. Isolation of Neisseria meningitidis from the Genito-urinary tract and anal canal. J Clin Microbiol. 1975 Sep;2(3):178–182. doi: 10.1128/jcm.2.3.178-182.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch C. E., Chapman S. S. Classification of Neisseria meningitidis group B into distinct serotypes. 3. Application of a new bactericidal-inhibition technique to distribution of serotypes among cases and carriers. J Infect Dis. 1973 Feb;127(2):149–154. doi: 10.1093/infdis/127.2.149. [DOI] [PubMed] [Google Scholar]
- GREAVES R. I. Preservation of living cells by freeze-drying. Ann N Y Acad Sci. 1960 Apr 13;85:723–728. doi: 10.1111/j.1749-6632.1960.tb49992.x. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Selective bacterial adherence to oral epithelial surfaces and its role as an ecological determinant. Infect Immun. 1971 Apr;3(4):567–573. doi: 10.1128/iai.3.4.567-573.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold R., Goldschneider I., Lepow M. L., Draper T. F., Randolph M. Carriage of Neisseria meningitidis and Neisseria lactamica in infants and children. J Infect Dis. 1978 Feb;137(2):112–121. doi: 10.1093/infdis/137.2.112. [DOI] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969 Jun 1;129(6):1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969 Jun 1;129(6):1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K., Ramirez-Ronda C. H., Holmes R. K., Sanford J. P. Adherence of bacteria to heart valves in vitro. J Clin Invest. 1975 Dec;56(6):1364–1370. doi: 10.1172/JCI108216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. The association of K88 antigen with haemagglutinating activity in porcine strains of Escherichia coli. J Gen Microbiol. 1974 Sep;84(1):135–144. doi: 10.1099/00221287-84-1-135. [DOI] [PubMed] [Google Scholar]
- Koransky J. R., Scales R. W., Kraus S. J. Bacterial hemagglutination by Neisseria gonorrhoeae. Infect Immun. 1975 Sep;12(3):495–498. doi: 10.1128/iai.12.3.495-498.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Hemagglutination by purified type I Escherichia coli pili. J Exp Med. 1977 Nov 1;146(5):1169–1181. doi: 10.1084/jem.146.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Type I Escherichia coli pili: characterization of binding to monkey kidney cells. J Exp Med. 1977 Nov 1;146(5):1182–1194. doi: 10.1084/jem.146.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinger D. S., Reed W. P. Pneumococcal adherence to human epithelial cells. Infect Immun. 1979 Feb;23(2):545–548. doi: 10.1128/iai.23.2.545-548.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1973 Mar 1;137(3):571–589. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houte J., Gibbons R. J., Banghart S. B. Adherence as a determinant of the presence of Streptococcus salivarius and Streptococcus sanguis on the human tooth surface. Arch Oral Biol. 1970 Nov;15(11):1025–1034. doi: 10.1016/0003-9969(70)90115-9. [DOI] [PubMed] [Google Scholar]