In almost all eukaryotes, mitochondrial genes are inherited mainly from the maternal parent (1). The mechanisms by which this occurs, and their evolutionary advantages, have remained largely elusive. Two reports in this issue, by Sato and Sato (2) on page 1141 and Al Rawi et al. (3) on page 1144, show that paternal mitochondria are eliminated from the fertilized oocyte (2, 3) by autophagy, a degradation process that removes structures that may threaten cell survival (4).
Uniparental (maternal) mitochondrial DNA (mtDNA) inheritance is commonly thought to be caused by simple dilution of paternal mtDNA (5); paternal gametes (sperm) are much smaller than maternal gametes (eggs) and contribute a limited amount of mitochondria to progeny. Indeed, paternally inherited mtDNA has been detected at a low frequency in mice (6). However, this theory is challenged by evidence that sperm mtDNA are actively digested (5), which in mammals may involve a protein modification process called ubiquitination (7).
Selective autophagy degrades various cellular structures including protein aggregates, damaged organelles, and microbes (8). Such targets may be modified with ubiquitin, a tag that is recognized by adaptor proteins that also bind to an autophagosomal membrane protein (called LC3 in mammals, Atg8 in yeast, or LGG-1/2 in the nematode Caenorhabditis elegans). This molecular link delivers tagged cargo to the autophagosome, which then fuses with a lysosome, wherein cargo is destroyed. Selective autophagy degrades mitochondria (mitophagy) either as part of differentiation programs (such as erythroid maturation), damage control responses (such as when mitochondrial membranes become depolarized), or during normal cellular homeostasis (9).
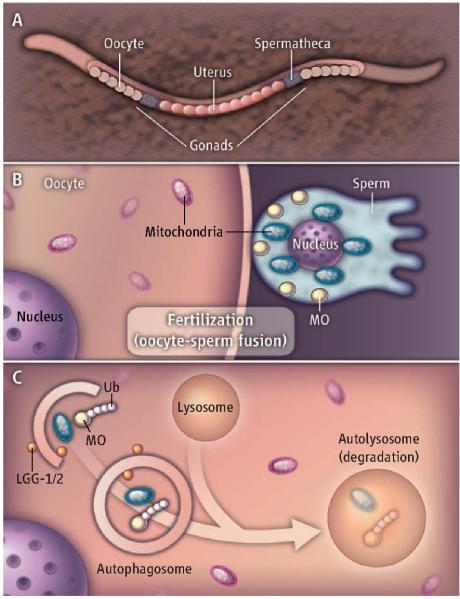
To determine whether active digestion of paternal mitochondria in the newly fertilized oocyte is by selective autophagy, Al Rawi et al. and Sato and Sato crossed male C. elegans containing fluorescently labeled mitochondria (or harboring a deletion in mtDNA) with hermaphrodites lacking such markers. Paternal mitochondria and paternal mtDNA disappeared by the 16-cell-stage embryo. Within minutes after oocyte fertilization, autophagosomes (LGG-1/2–labeled membranes) surrounded paternal mitochondria and vesicular structures called membranous organelles (MOs), but not maternal mitochondria (see the figure). The MOs are specific to C. elegans and have been compared functionally to the mammalian sperm acrosome (structure at the head of sperm containing enzymes that break down the outer membrane of the mature oocyte during fertilization). Subsequent degradation of paternal mitochondria and MOs required the oocyte autophagy machinery. Unexpectedly, both groups show that ubiquitin associates with paternally derived MOs but not with paternal mitochondria, although autophagosomes degrade both structures. Thus, at least in C. elegans, degradation of paternal mitochondria and paternal mtDNA is mediated by autophagy. An important implication is that autophagy of sperm mitochondria after fertilization may underlie the uniparental, maternal mtDNA inheritance that occurs in most eukaryotic species.
What is the precise basis for selectively targeting paternally derived organelles for destruction? It is unlikely that in C. elegans, ubiquitin is the only signal, as there is yet no evidence for ubiquitin association with sperm mitochondria in C. elegans (2, 3) [unlike the case for mammalian sperm mitochondria (7)]. The role of paternal MOs in this process remains unclear. Ubiquitination of lysine-63 appears to precede recruitment of autophagosomal markers (LGG-1/2) to MOs. One possibility is that these vesicles could supply membrane for autophagosome formation. Or perhaps they may initiate an autophagy signal in the vicinity of the paternal mitochondria.
Another question is whether autophagy plays a conserved role in degrading paternal mitochondria and mtDNA in other eukaryotes. Indeed, Al Rawi et al. demonstrate, in fertilized mouse zygotes, increased ubiquitination and recruitment of the autophagosome protein LC3 in the mid-piece of the sperm, where the paternal mitochondria are situated. Notably, autophagy is triggered upon mouse oocyte fertilization and is essential for embryo survival beyond the four- to eight-cell stages (10). One possibility is that autophagy in preimplantation development is required to degrade maternal proteins in oocytes, thereby initiating zygotic protein synthesis (10). The findings by Al Rawi et al. and Sato and Sato raise the question of whether autophagy-dependent successful preimplantation development of mouse embryos also involves the degradation of paternal mitochondria.
The findings of Sato and Sato and Al Rawi et al. help to explain how paternal mitochondria and mtDNA are destroyed, but why they are destroyed remains a mystery. Is heteroplasmy, the occurrence of more than one mtDNA genotype, dangerous for the developing embryo? Or is the degradation of paternal mitochondria merely a primitive defense in which the fertilized oocyte views the paternal mitochondria as a potentially dangerous intruder that must be destroyed? In this scenario, the degradation of paternal mitochondria by selective autophagy may parallel the use of this pathway by somatic cells to defend themselves against invasive bacteria. It also may be that paternally derived mitochondrial factors, rather than heteroplasmy per se, are detrimental to the developing embryo. Sato an Sato found that 95% of animals lacking lgg-1 die at or before the L1 larval stage, suggesting that persisting paternal mitochondria may harm development during embryogenesis. However, this lethality may be due to other zygotic effects of loss of LGG-1.
The results of Sato and Sato and Al Rawi et al. in C. elegans should help guide investigation into how, and perhaps even why, mammalian mitochondrial DNA comes mainly from mothers. Such an understanding will have far-reaching implications for human developmental biology and for unraveling the pathogenesis of mitochondrial diseases.
Figure 1. Selective autophagy of paternal mitochondria.
(A) The C. elegans hermaphrodite reproductive tract is shown. (B) Fusion of sperm and oocyte in the C. elegans spermatheca transfers the paternal nucleus, membranous organelles (MOs), and mitochondria to the (C) cytoplasm of the fertilized oocyte. MOs in the vicinity of the paternal mitochondria are labeled with ubiquitin (Ub) chains for degradation. Both MOs and paternal mitochondria are selectively recruited to autophagosomes (LGG-1/2–labeled membranes), which fuse with lysosomes.
References
- 1.Ankel-Simons F, Cummins JM. Proc. Natl. Acad. Sci. U.S.A. 1996;93:13859. doi: 10.1073/pnas.93.24.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sato M, Sato K. Science. 2011;334:1141. doi: 10.1126/science.1210333. [DOI] [PubMed] [Google Scholar]
- 3.Al Rawi S, et al. Science. 2011;334:1144. doi: 10.1126/science.1211878. [DOI] [PubMed] [Google Scholar]
- 4.Weidberg H, Shvets E, Elazar Z. Annu. Rev. Biochem. 2011;80:125. doi: 10.1146/annurev-biochem-052709-094552. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura Y, et al. Proc. Natl. Acad. Sci. U.S.A. 2006;103:1382. [Google Scholar]
- 6.Gyllensten U, et al. Nature. 1991;352:255. doi: 10.1038/352255a0. [DOI] [PubMed] [Google Scholar]
- 7.Sutovsky P, et al. Nature. 1999;402:371. doi: 10.1038/46466. [DOI] [PubMed] [Google Scholar]
- 8.Kirkin V, et al. Mol. Cell. 2009;34:259. doi: 10.1016/j.molcel.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 9.Youle RJ, Narendra DP. Nat. Rev. Mol. Cell Biol. 2011;12:9. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsukamoto S, et al. Science. 2008;321:117. doi: 10.1126/science.1154822. [DOI] [PubMed] [Google Scholar]