Abstract
The exact mechanisms of CD4 help in the generation of memory cytotoxic T lymphocytes (CTLs) remain largely illusive. We propose that dendritic cells (DCs) first interact with CD4+ T cells, resulting in DC licensing and CD4+ T-cell priming. Thereafter, CD8+ T cells can receive stimulatory signals from DC-CD4+ T-cell clusters and as well as individually from licensed DCs and primed CD4+ T cells.
Keywords: CD4 help, CD40-CD40L, CTL response, dendritic cells, two-photon
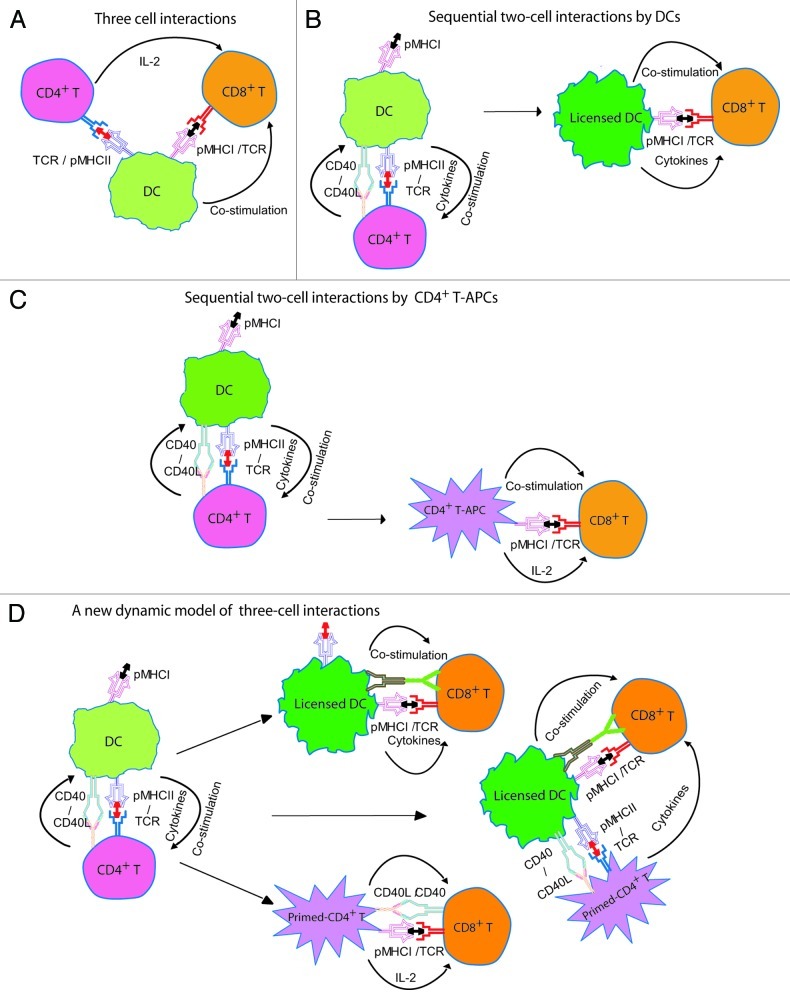
In the immune system, antigen-presenting cells (APCs) such as dendritic cells (DCs) function by engulfing antigens, processing them, and subsequently presenting them to CD4+ and CD8+ T cells via MHC class II and MHC class I molecules, respectively, leading to CD8+ cytotoxic T lymphocyte (CTL) responses. However, how these three cell populations precisely interact and how CD4+ T cell signals are delivered to CD8+ T cells in vivo remain controversial. Based on the observation that the antigen recognized by CD4+ and CD8+ T cells must be carried by the same DC, several distinct models have been proposed. A “three-cell interaction model” was originally proposed for CD4+ T cell-dependent CTL responses,1 according to which an antigen-specific DC must simultaneously interact with single antigen-specific CD4+ and CD8+ T-cell precursors (Fig. 1A). However, as both antigen-specific CD4+ and CD8+ T-cell precursors are relatively rare, this three-cell interaction appears to be almost impossible. Thus, Ridge et al. proposed a “dynamic model of sequential two-cell interactions by APCs,” according to which APCs must be first activated (licensed) by CD4+ T cells via CD40/CD40L interactions and then can directly stimulate memory CTL responses (Fig. 1B).2 However, this model has been challenged by the observation that efficient memory CTL responses require CD40 expression on CD8+ T cells but not on DCs,3 highlighting the importance of direct CD4+-CD8+ T-cell interactions in the development of memory CTLs.
Figure 1. A new dynamic model of three cell interactions. (A) The passive “three-cell interaction” model. A CD4+ T helper cell activated by a dendritic cells (DC) delivers interleukin-2 (IL-2) required for the activation of a CD8+ T cell that recognizes the same antigen presented by the DC. (B) The dynamic model of “sequential two-cell interaction by DCs.” The cross-priming DC gets activated (licensed) by antigen-specific CD4+ T helper cell through CD40-CD40L interactions, and then acquires the ability to directly prime CTLs. (C) The dynamic model of “sequential two-cell interactions by CD4+ T cells-APCs.” CD4+ T cells can acquire molecules (particularly pMHCI) from APCs and thus can directly stimulate CTL responses. (D) A new dynamic model of three cell interactions. The cross-priming DC first interacts with antigen-specific CD4+ T helper cell (a crucial step in CTL responses), resulting in DC licensing and CD4+ T cell activation (priming). Cognate CD8+ CTLs can then receive helper or stimulatory signals not only from DC-CD4+ T-cell clusters, but also separately from fully licensed DCs and primed CD4+ T cells, even after their dissociation from DC-CD4+ T-cell clusters.
Trogocytosis (intercellular membrane protein transfer) is a common phenomenon between immune cells that plays an important role in immune modulation.4 CD8+ and CD4+ T cells have been reported to acquire the immunological synapse-composed pMHCI and pMHCII complexes from activating DCs, respectively, via internalization and recycling of synapse components.5 As we were the first to discover, in addition to pMHCII, CD4+ T cells also acquire pMHCI and CD80 molecules from DCs and can use these components of the antigen presenting machinery to directly exert helper effects onto cognate CD8+ T cells. This led us to propose a “new dynamic model of sequential two-cell interactions by CD4+ T cells-APCs” (Fig. 1C).6 This model is supported by recent evidences from other laboratories.4,7 Interestingly, we also showed that CD4+ T cell-APCs preferentially stimulate CD8+CD44+CD62LhighCD127+ cmCTL responses.8 However, one important question that still is unanswered is whether the “CD4+ T cell-APC concept” is of any physiological significance in vivo. Moreover, the mechanisms for functional delivery of CD4+ T-cell help, whether CD8+ T cells receive helper signals by forming a three-cell cluster (DC-CD4+ T cell-CD8+ T cell), indirectly through licensed DCs, or directly from primed CD4+ T cells, remain unresolved. Therefore, we sought to gain a better understanding of the mechanisms that regulate CTL responses.
Recently, we have developed novel immunization protocols, wherein antigen-presenting DCs (CD11c-diphtheria toxin receptor transgenic DCs, presenting ovalbumin as a model antigen), CD4+ and CD8+ T cells can be separately manipulated for dissecting their respective roles in the priming of CTL responses in vivo.9 In our experimental setup, CD4+ T cells and antigen-presenting DCs were allowed to engage with one another in vivo, in the absence of cognate CD8+ T cells (CD8+ T cell-depleted host). Thereafter, we selectively depleted either the previously engaged CD4+ T cells (injecting the GK1.5 antibody) or DCs (injecting diphtheria toxin) before allowing the interaction of none, either or both these populations with adoptively transferred naïve CD8+ T cells. This protocol allowed us to clearly document the importance of CD4+ T cell-licensed DCs and DC-primed CD4+ T cells in CTL responses. Our study provided direct in vivo evidence that primed CD4+ T cells or licensed DCs can stimulate CTL and memory responses, independent of DC-CD4+ T-cell clusters. Remarkably, our imaging studies, using intravital two-photon microscopy, provided unequivocal in vivo evidence for pMHCI-dependent direct CD4+-CD8+ T-cell interactions in the absence of DC-CD4+ T cell-CD8+ T cell ternary clusters.
Thus, our results provided for the first time physiologically relevant in vivo evidence that primed CD4+ T cells can directly orchestrate efficient CD8+ CTL responses, proposing a “new dynamic model of three-cell interactions for CTL responses” (Fig. 1D).9 According to such a dynamic model, CTL and memory responses do not depend on an antigen-presenting DC simultaneously engaging cognate naïve CD4+ and CD8+ T cells in an environment in which both of these latter cells are extremely scarce. Primed CD4+ T cells directly locked onto their CD8+ T cell targets through acquired pMHCI/TCR and CD40/CD40L engagement would also be expected to result in a more efficient delivery of interleukin-2 (IL-2) and hence a more efficient CTL memory induction. Our study revealed the coexistence of direct and indirect mechanisms of T cell help for CTL responses in non-inflammatory situations, thereby reconciling previous conflicting studies. In addition, the CTL programming derived from licensed DCs, primed CD4+ T cells and DC-CD4+ T-cell clusters reflects microenvironments differing in signal strength, co-stimulation and cytokine levels. Such subtle differences could be one of the reasons of the T-cell heterogeneity that has been observed during memory T-cell development.10
In summary, we conclude that CTL immunity is orchestrated by dynamic interactions of antigen-presenting DCs, CD4+ T and CD8+ T cells, leading to enhanced communications between rare antigen-specific immune precursors within a short window of time and to the efficient delivery of help signals. However, the relevant signals at the DC-CD4+ T cell, DC-CD8+ T cell and CD4+-CD8+ T cell interfaces remain to be explored in details. What changes do these interactions induce at the cellular and molecular level on licensed DCs, primed CD4+ T cells or on effector and memory CD8+ T cells? Our experimental approach provides a good opportunity to dissect these issues, and our future work proceeds in this direction. These discoveries may have great impact for the design of vaccines and immunotherapy strategies against cancer and autoimmune diseases.
Acknowledgments
This study was supported by a grant of the Canadian Institute of Health Research (MOP 79415) and a Research Bridge Fund of College of Medicine, University of Saskatchewan. K.A.A. was supported by Postdoctoral Research Fellowship of the Saskatchewan Health Research Foundation.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/21175
References
- 1.Mitchison NA, O’Malley C. Three-cell-type clusters of T cells with antigen-presenting cells best explain the epitope linkage and noncognate requirements of the in vivo cytolytic response. Eur J Immunol. 1987;17:1579–83. doi: 10.1002/eji.1830171109. [DOI] [PubMed] [Google Scholar]
- 2.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–8. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 3.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–3. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed KA, Xiang J. Mechanisms of cellular communication through intercellular protein transfer. J Cell Mol Med. 2011;15:1458–73. doi: 10.1111/j.1582-4934.2010.01008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang JF, Yang Y, Sepulveda H, Shi W, Hwang I, Peterson PA, et al. TCR-Mediated internalization of peptide-MHC complexes acquired by T cells. Science. 1999;286:952–4. doi: 10.1126/science.286.5441.952. [DOI] [PubMed] [Google Scholar]
- 6.Xiang J, Huang H, Liu Y. A new dynamic model of CD8+ T effector cell responses via CD4+ T helper-antigen-presenting cells. J Immunol. 2005;174:7497–505. doi: 10.4049/jimmunol.174.12.7497. [DOI] [PubMed] [Google Scholar]
- 7.Rosenits K, Keppler SJ, Vucikuja S, Aichele P. T cells acquire cell surface determinants of APC via in vivo trogocytosis during viral infections. Eur J Immunol. 2010;40:3450–7. doi: 10.1002/eji.201040743. [DOI] [PubMed] [Google Scholar]
- 8.Umeshappa CS, Huang H, Xie Y, Wei Y, Mulligan SJ, Deng Y, et al. CD4+ Th-APC with acquired peptide/MHC class I and II complexes stimulate type 1 helper CD4+ and central memory CD8+ T cell responses. J Immunol. 2009;182:193–206. doi: 10.4049/jimmunol.182.1.193. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed KA, Wang L, Munegowda MA, Mulligan S, Gordon JR, Griebel P, et al. Direct in vivo evidence of CD4+ T cell requirement for CTL response and memory via pMHC-I targeting and CD40L signaling. J Leukoc Biol. 2012 doi: 10.1189/jlb.1211631. [DOI] [PubMed] [Google Scholar]
- 10.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–71. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]