Abstract
Background
Low bone mineral density (BMD) has been reported among 10–54% of HIV-infected adolescents in developed countries. We studied the prevalence and predictors of low BMD among HIV-infected Thai adolescents receiving antiretroviral therapy.
Methods
A cross-sectional study of lumbar spine (L2–L4) BMD as measured by dual-energy X-ray absorptiometry (DXA) in Thai HIV-infected adolescents aged 12–20 years was performed. The BMD Z-score was analyzed using age-matched healthy Thai children as a reference. Serum 25-hydroxyvitamin D (25-OHD) was performed. Osteopenia was defined as BMD Z-score≤ −2.
Results
From October 2010 to February 2011, 101 adolescents, 50% male, with a median age of 14.3 (range, 13.0–15.7) years were enrolled. The median (IQR) current CD4 T-cell count was 646 (506–796) cells/mm3 and 90% had plasma HIV-1 RNA <50 copies/ml. The mean BMD among HIV-infected adolescents and controls were 0.855 and 0.980 g/cm2(P<0.001). The median (IQR) L2–L4 spine BMD Z-score was −1.0 (−1.9 to −0.1), of which 24% had BMD Z-score ≤ −2.0. The median (IQR) of 25-OHD level was 24.8 (20.0–31.4) ng/ml, of which 25% had vitamin D level < 20 ng/ml. In multivariate analysis, the height for age Z-score <−1.5 (adjusted odds ratio (aOR) 6.2; 95% CI 2.2–17.7) and history of WHO clinical stage 4 prior to ART (aOR 3.7; 95%CI 1.3–10.7) were significantly associated with osteopenia.
Conclusion
One-fourth of HIV-infected Thai adolescents have osteopenia. Children with history of advanced- staging or having low height for age are at risk of osteopenia. Preventive measures to prevent osteopenia should be incorporated in routine care for these adolescents.
Keywords: Bone mineral density, HIV-infected adolescents, vitamin D deficiency, osteopenia
Introduction
Antiretroviral therapy (ART) increases life expectancy of HIV-infected patients. However, many long term complications, including low bone mineral density (BMD), are concerning. A meta-analysis of HIV-infected adults showed that two-third of patients had low BMD, of whom 15% had osteoporosis1. In addition, the fracture rate in the HIV-infected population was 30–70% higher than uninfected control subjects2. Many studies reported lower bone mass in perinatally HIV-infected children and adolescents compared to healthy peers3–6. A study from the US showed that 20% of HIV-infected children had BMD Z-score < −1.5 at any site of measurement7. A great deal of accumulation of bone mass occurs during the adolescent years, and peak attainment is seen at age 18 8, 9. Therefore, loss of bone deposition during adolescence could lead to an increased risk of osteoporosis and fractures later of life10.
The predictors of reduced BMD in HIV-infected adults are many and include traditional risk factors and HIV-related metrics. Traditional risk factors include older age, female sex, menopause, corticosteroid therapy, smoking, alcohol use, low calcium intake, low vitamin D level, limited physical activity, low body mass index (BMI) and white race11. HIV-related factors such as duration of HIV infection12–14, uncontrolled viremia2, and exposure to ART are also related. Antiretroviral drugs have variable effects on bone density. For example, protease inhibitors (PIs) are associated with reduced BMD and osteoporosis and tenofovir disoproxil fumarate (TDF) is associated with decreased bone mass15–20.
Many factors related with lower peak bone mass in children including delayed growth and puberty, low lean body mass, hormone and inflammation cytokine, vitamin D deficiency, malabsorption and physical inactivity6,8,9. However, HIV-related factors are important contributing factors to bone loss in children such as advanced HIV disease, uncontrolled viremia, and specific ART 2,6.
The objective of this study is to investigate the prevalence of low bone mass in HIV-infected Thai adolescents and characterize the associated risk factors in our populations. We conducted a cross-sectional study to measure the bone mineral density among HIV-infected adolescents using a dual-energy X-ray absorptiometry (DXA).
Materials and Methods
Participants
We performed a cross-sectional study to measure BMD in perinatally-HIV infected Thai adolescents aged 12–20 years who have been followed up at two referral centers in Bangkok, Siriraj and King Chulalongkorn Memorial hospitals from October 2010 to February 2011. As BMD may be affected by gender, ART regimens, and Tanner stage, we enrolled 10–15 subjects for each of eight strata: male or female, receiving protease inhibitor (PI) regimen or nonnucleoside reverse-transcriptase inhibitor (NNRTI) regimen, and Tanner stage 1–2 or 3–5. The exclusion criteria were having conditions that might affect bone mass such as endocrine disorders, renal impairment (serum creatinine > 2 mg/dl), liver impairment (ALT > 100 IU/L) within 6 months prior to enrollment, underlying malignancy, and taking any non-ARV medications that probably impact bone mass such as corticosteroids or anticonvulsants. The protocol was approved by the institutional review boards at both centers. Written informed assent and consent were obtained from adolescents and their caregivers. The study procedure included physical examination, anthropometric measurement, Tanner stage evaluation, blood sample collection for 25-hydroxyvitamin D (25-OHD), intact parathyroid hormone level (iPTH), alkaline phosphatase (ALK), calcium, phosphorus, and DXA scanning. The clinical data collected from the medical record were WHO clinical stage prior to ART, HIV-1 RNA levels, CD4 T-cell count within the past 6 months, nadir CD4 T-cell count, and ART history. History of fracture was taken from direct questioning of participants and guardians.
The Z-score for weight for age (WAZ) and height for age (HAZ) was calculated based on Thai reference21. The BMI percentile was based on US-CDC reference22. The 25-OHD level and iPTH were measured by chemiluminescent microparticle immunoassay (ARCHITECT 25-OH vitamin D assay and ARCHITECT Intact PTH assay, Abbott Diagnostics Division, Wiesbaden, Germany). Vitamin D deficiency defined as 25-OHD level < 20 ng/ml23. The upper normal limit of serum iPTH was 65 pg/ml24. Serum calcium, phosphorus, and ALK were measured by the VITROS Ca Slide method, VITROS PHOS Slide method, and VITROS ALKP Slide method (Ortho-Clinical Diagnostics Inc., Rochester, New York, USA), respectively. The normal range of calcium and phosphorus were 8.6–10.0 mg/dl and 4.0–7.0 mg/dl, respectively. The normal range of alkaline phosphatase level for male and female were 100–390 U/L and 100–320 U/L, respectively.
Dual-energy X-ray absorptiometry (DXA) and interpretation
BMD was evaluated by DXA scanners, Lunar Prodigy (General Electric Healthcare, Madison, Wisconsin, USA) at Siriraj hospital and Hologic Discovery A (Hologic, Inc., Bedford, Massachusetts, USA) at King Chulalongkorn Memorial Hospital. The scanner machines were calibrated on a daily basis according to the manufacturer’s instructions. The subjects were scanned at the second to fourth lumbar spines (L2–L4).
We compared the bone mineral density data of HIV-infected adolescents with data from a cohort of 199 HIV-negative aged from 12–18 years measured by Lunar Prodigy scanner. The inclusion criteria of healthy control was adolescent who has weight and height within 2 standard deviation from Thai normative data, has no underlying disease, and does not receive medications that may affect BMD such as glucocorticoid. At least 10 children per gender per year of age from 12–18 years old were enrolled at Siriraj hospital. Mean (SD) weight for age and height for age Z-score of control were 0.2 (0.8) and 0.2 (1.0), respectively. For adolescents who reached 18 years of age, the Thai adult normative reference was used25.
Because of variations in BMD values measured by different machines and the normative reference BMD were available only by Lunar scanner, the BMD values measured by Hologic scanner were converted to the values measured by Lunar scanner using the equation previously published (GE-Lunar=1.195×Hologic−0.023) 26 before calculating Z-score. This equation was developed from the Hologic Delphi (using Hologic Apex software) and GE-Lunar Prodigy DXA systems (using GE-Lunar EnCore software). Before adopting this equation, our first 15 adolescents were scanned by both machines. We then compared the results of BMD from Lunar with the converted BMD from Hologic scanner, using the equation, and found no difference (P=0.669). The limits of agreement between 2 scanner types was −0.062 to 0.074 (P=0.861) by Bland -Altman analysis. The BMD Z-score was calculated based on the reference values for normal Thai adolescents using the equation: BMD Z-score = (BMD measured−BMDreference for age and sex)/SDreference for age and sex. According to International Society for Clinical Densitometry (ISCD), low BMD was defined as spine BMD Z-score less than or equal to −2.0 27.
Statistical analysis
Descriptive analysis of BMD among HIV-infected adolescents was stratified by age and gender. BMD values and BMD Z-score of HIV-infected adolescents were compared with normal healthy Thai adolescents by Student t-test. Categorical variables were compared between the adolescents with BMD Z-score ≤ −2 and >−2.0 by the chi-square test or Fisher exact test as appropriate. Continuous variables were compared by the Mann-Whitney rank sum test, Student t-test or One-Way ANOVA analysis as appropriate. Univariate logistic regression was performed to evaluate the association between reported factors related to osteopenia including body mass index, weight for age Z-score, height for age Z-score, tanner stage, WHO staging, antiretroviral therapy regimen, HIV-1 RNA level, and vitamin D level. Multivariate analyses for factors associated with low BMD were performed by stepwise logistic regression model including factors that had p-value less than 0.2 in univariate analyses. Data were analyzed with the IBM SPSS Statistics 20.0.
Results
Demographic characteristics
One hundred and one perinatally HIV-infected adolescents, median (interquartile range; IQR) age 14.3 years (13.0–15.7), were enrolled. The demographic characteristics are shown in Table 1. The mean duration of ART was 83.9 (52.2–104.2) months. The current ART regimens were 50% NNRTI-based, 48%PI-based, and 2% NNRTI plus PI-based. Fifteen (14.9%) adolescents have received TDF for mean duration of 10 months. The median (IQR) current CD4 T-cell count was 646 (506–796) cells/mm3 and 90% had plasma HIV-1 RNA < 50 copies/ml. Twenty-eight adolescents had history of WHO stage 4 prior to receiving ART.
Table 1.
Demographic characteristics among 101 HIV-infected adolescents
| Characteristics | Total (N=101) | Adolescents with BMD Z-score > −2.0 (N=77) | Adolescents with BMD Z score ≤ −2.0 (N=24) | P value |
|---|---|---|---|---|
| Male | 51 (50.5) | 39 (50.6) | 12 (50.0) | 0.956a |
|
| ||||
| Age, years | 14.3 (13.0–15.7) | 14.3 (13.0–15.7) | 14.1 (13.0–16.2) | 0.870b |
|
| ||||
| Body mass index, kg/m2 | 17.7 (16.2–19.9) | 18.0 (16.5–20.7) | 17.0 (14.8–18.5) | 0.033b |
| < 5 percentile | 26 (25.7) | 15 (19.5) | 11 (45.8) | 0.010a |
|
| ||||
| Thai weight for age Z-score (WAZ) | −0.8 (−1.7 to 0.2) | −0.6 (−1.3 to 0.4) | −1.9 (−2.3 to − 0.9) | <0.001 b |
| WAZ < −1.5 | 29 (28.7) | 14 (18.2) | 15 (62.5) | <0.001a |
|
| ||||
| Thai height for age Z-score (HAZ) | −0.9 (−1.8 to −0.3) | −0.7 (−1.4 to −0.1) | −1.8 (−2.4 to −1.3) | <0.001 b |
| HAZ < −1.5 | 33 (32.7) | 17 (22.1) | 16 (66.7) | <0.001 a |
|
| ||||
| Tanner stage | ||||
| Stage 1–2 | 32 (31.7) | 21 (27.3) | 11 (45.8) | 0.088a |
| Stage 3–5 | 69 (68.3) | 56 (72.7) | 13 (54.2) | |
|
| ||||
| Duration of ART, months | 83.9 (52.2–104.2) | 88.2 (62.0–105.9) | 56.4 (36.5–88.0) | 0.026b |
|
| ||||
| Duration of ART by regimen type, months | ||||
| NRTI | 82.9 (43.8–102.9) | 84.9 (46.5–106.8) | 53.6 (28.6–89.3) | 0.065b |
| NNRTI | 54.0 (29.9–88.7) | 60.5 (37.3–90.3) | 46.7 (24.5–53.8) | 0.041b |
| PI | 44.8 (33.2–76.3) | 54.9 (33.2–79.2) | 40.8 (32.4–63.1) | 0.260b |
|
| ||||
| Current ART regimens | ||||
| NNRTI-based | 50 (49.5) | 41 (53.2) | 9 (37.5) | 0.143a, # |
| PI-based | 49 (48.5) | 34 (44.2) | 15 (62.5) | |
| both NNRTI and PI-based | 2 (2.0) | 2 (2.6) | - | |
|
| ||||
| Receiving TDF | 15 (14.9) | 13 (16.9) | 2 (8.3) | 0.511c |
|
| ||||
| Current CD4 T-cell count, cells/mm3 | 646 (506–796) | 628 (486–804) | 717 (556–801) | 0.181b |
|
| ||||
| Nadir CD4 T-cell count, cells/mm3 | 114 (31–226) | 134 (28–245) | 99 (38–184) | 0.814b |
|
| ||||
| Proportion with plasma HIV-1 RNA viral load < 50 copies/ml | 91 (90.1) | 71 (92.3) | 20 (83.3) | 0.243c |
|
| ||||
| WHO clinical stage prior to ART | ||||
| Stage 1–2 | 34 (33.7) | 30 (39.0) | 4 (16.7) | 0.527c,† |
| Stage 3 | 39 (38.6) | 32 (41.5) | 7 (29.2) | |
| Stage 4 | 28 (27.7) | 15 (19.5) | 13 (54.2) | 0.004c,‡ |
NOTE: Continuous data presented in median(IQR). Categorical data presented in n(%).
χ2 test,
Mann-Whitney rank sum test,
Fisher’s exact test
compare between NNRTI-based and PI-based regimen,
compare between WHO clinical stage 3 and stage 1–2,
compare between WHO clinical stage 4 and stage 1–2
ART, antiretroviral therapy; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; TDF, tenofovir disoproxil fumarate
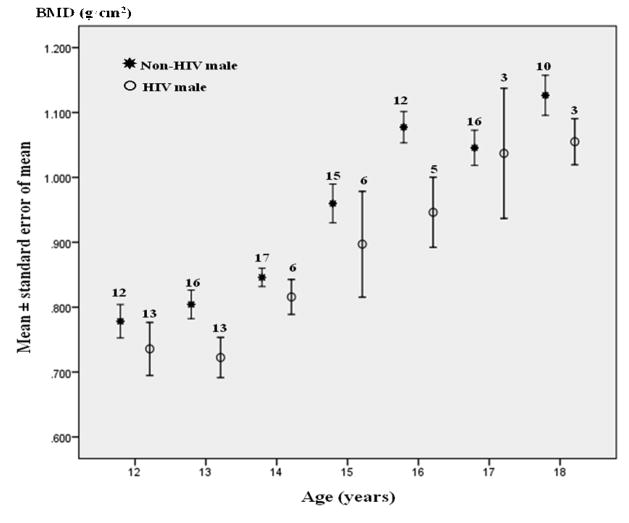
Bone mineral density at lumbar spine among HIV-infected children
The BMD data from 98 HIV-infected adolescents (not including 3 children older than 18 years of age) were compared with 199 HIV-negative age-matched healthy Thai adolescent controls. The mean BMD among HIV-infected adolescents and HIV-negative controls were 0.855 and 0.980 g/cm2, respectively (P< 0.001). HIV-infected adolescents generally had lower spine BMD than healthy Thai adolescents controls across age and gender strata (Figure 1). The mean (SD) L2–L4 spine BMD Z-score among 101 HIV-infected adolescents was −0.9 (1.4), P< 0.001 compared to zero z-score. The proportion of HIV-infected adolescents with BMD Z-score ≤ −1.5, ≤ −2.0, and ≤ −2.5 were 36 %, 24 % and 11 %, respectively. Proportion of adolescents with BMD Z-score ≤ ≤2.0 were 23.5% and 24% among children who performed BMD by Hologic and Lunar scanner, respectively (P=0.956). There was no report of fracture history.
Figure 1.
Lumbar spine bone mineral density of HIV-infected Thai adolescents compared with healthy controls
Vitamin D level and laboratory tests related to bone metabolisms
Vitamin D level and laboratory test related to bone metabolisms are shown in table 2. The median (IQR) 25-OHD level was 24.8 (20.0–31.4) ng/ml and 25% had vitamin D deficiency which defined as vitamin D level < 20 ng/ml. Median (IQR) iPTH level was 44.0 (36.4–58.7) pg/ml and 19% had iPTH level higher than upper normal limit of 65 pg/ml. Among the 25 adolescents with vitamin D deficiency, 44% had high iPTH compared to only 10% in those without vitamin D deficiency (P<0.001). The median (IQR) iPTH level was higher in adolescents with vitamin D deficiency (54.9 (38–77) vs. 40.2 (32.6–54.9, P=0.007).
Table 2.
Vitamin D level and laboratory results related to bone metabolism among HIV-infected adolescents
| Characteristics | Total (N=101) | Adolescents with BMD Z-score > −2.0 (N=77) | Adolescents with BMD Z score ≤ −2.0 (N=24) | P value |
|---|---|---|---|---|
| 25-hydroxyvitamin D level, ng/ml | 24.8 (20.0–31.4) | 24.9 (20.0–30.5) | 24.4 (17.7–31.9) | 0.618a |
| Level < 20 ng/ml | 25 (24.8) | 18 (23.4) | 7 (29.2) | 0.566b |
|
| ||||
| Parathyroid hormone level, pg/ml | 44.0 (36.4–58.7) | 44.0 (34.6–60.1) | 40.0 (29.7–52.8) | 0.271a |
| Level > 65 pg/ml | 19 (18.8) | 16 (20.8) | 3 (12.5) | 0.551c |
|
| ||||
| Alkaline phosphatase level, U/L | 198.0 (135.0–282.5) | 172.0 (130.0–278.0) | 234.5 (152.0–295.5) | 0.125a |
|
| ||||
| Calcium level, mg/dl | 9.7 (9.5–10.0) | 9.7 (9.6–10.0) | 9.8 (9.4–10.0) | 0.718a |
|
| ||||
| Phosphorus level, mg/dl | 4.6 (4.2–5.2) | 4.5 (4.1–5.1) | 5.1 (4.3–5.6) | 0.022a |
NOTE: Continuous data presented in median(IQR). Categorical data presented in n(%).
Mann-Whitney rank sum test,
χ2 test,
Fisher’s exact test
Associated factors related to low bone mineral density for age
In univariate analyses, body mass index below 5 percentile, weight for age Z-score below −1.5, height for age Z-score below < −1.5 and history of WHO clinical stage 4 prior to ART were associated with low BMD (table 3). In multivariate analysis, only HAZ <−1.5 (adjusted odds ratio (aOR) 6.19; 95%CI 2.17–17.69; P=0.001) and WHO clinical stage 4 prior to ART (aOR 3.67; 95%CI 1.26–10.67.88, P=0.017) were independently associated with low BMD.(Table 3)
Table 3.
Factors associated with osteopenia (defined as lumbar spine 2nd–4th bone mineral density Z-score ≤ −2.0)
| Characteristics | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
|
| ||||
| Odds ratio (95% CI) | P value | Adjusted odds ratio (95%CI) | P value | |
| Body mass index below 5 percentile | 3.50 (1.31–9.33) | 0.012 | - | - |
|
| ||||
| Weight for age Z-score below −1.5 | 7.50 (2.73–20.57) | < 0.001 | - | - |
|
| ||||
| Height for age Z-score below −1.5 | 7.06 (2.58–19.29) | <0.001 | 6.19 (2.17–17.69) | 0.001 |
|
| ||||
| Tanner stage 1–2 (vs. stage 3–5) | 2.26 (0.88–5.82) | 0.092 | - | - |
|
| ||||
| WHO clinical classification prior to ART | ||||
| Stage 4 (vs. non stage 4) | 4.89 (1.83–13.03) | 0.002 | 3.67 (1.26–10.67) | 0.017 |
|
| ||||
| Current ART regimen (PI vs. NNRTI) | 2.01 (0.78–5.16) | 0.147 | - | - |
|
| ||||
| Duration of tenofovir (month) | 0.92 (0.77–1.09) | 0.325 | - | - |
|
| ||||
| Current plasma HIV-1 RNA < 50 copies/ml | 0.42 (0.11–1.64) | 0.214 | - | - |
|
| ||||
| 25-hydroxyvitamin D level | ||||
| < 20 ng/ml (vs. ≥ 20 ng/ml) | 1.35 (0.48–3.77) | 0.567 | - | - |
NOTE: ART, antiretroviral therapy; PI, protease inhibitors; NNRTI, non-nucleoside reverse transcriptase inhibitor
Discussion
Our study is the first study of bone mineral density in HIV-infected adolescents in Asian populations. We found one-fourth of HIV-infected Thai adolescents had low BMD. The HAZ <−1.5 and WHO clinical stage 4 prior to ART are associated with low BMD.
In this study, we found that, of Thai HIV-infected adolescents, 36% and 24% had bone mineral density for age Z-scores ≤ −1.5 and −2.0, respectively. This was higher than 10.6% reported spine BMD Z-score −1.5 in HIV-infected children and adolescents in the US7. Interpretation of BMD results should be based on the normative databases by gender, age, race/ethnicity, and should be specific for each manufacturer and model of densitometer and software27. In this study we addressed these potential confounders by comparing BMD data with data from Thai HIV-negative adolescent database and by using conversion factor to address for the differences between Lunar and Hologic machines. The results of BMD Z-score ≤ −2.0 in our study would have been as high as 43% if we used the Z-score reported by the machines using its installed software with normative reference from other ethnic groups. The International Society for Clinical Densitometry (ISCD) suggested that the BMD interpretation should be adjusted for absolute height or height age in children with linear growth or maturational delay27. Zemel B et al studied various methods to adjust BMD for height including using height age, and height for age Z-score adjustment equations28. The study suggested that substituting height age for chronological age as a means of adjusting for short stature was not a good approach because the children who are short for age will be compared with children of similar height who are younger and at an earlier stage of sexual maturation. The study proposed that using height for age Z-score adjustment equation was the least bias method. Unfortunately, the equations were developed for healthy US children based on Hologic systems, which can not apply to our study. Therefore, the higher rate of low BMD in our study compared to US data may be partly attributable to an inability to correct the effect of short stature on bone mineral density Z-score calculation.
We found WHO clinical stage 4 prior to ART and HAZ < −1.5 to be associated with low bone mass. The study in HIV-infected adults also found advanced clinical stage of HIV disease associated with higher risk for low bone mass 29. Advanced HIV disease prior to ART resulting in poor growth may be the major factor of low bone mass in our adolescent population. The association between HAZ < −1.5 and low bone mass may be a true association due to low height-age reflecting a poor long term health status or may be confounded by an inability to correct the effect of short stature on bone mineral density Z-score calculation. Other factors reportedly associated with low BMD were low BMI and uncontrolled HIV-1 RNA7, 15, 16, 30. Many studies found an association between low bone mass with PI 1, 6, 7, 15, 29, 31 and TDF use 15–20, 32–34. We found lower spine BMD Z-scores among HIV-infected adolescents receiving PI but not reached statistical significance in multivariate analysis. We found no association with the use of TDF and low bone mass, this probably due to short period of TDF exposure in this cohort with mean exposure of 10.0 months.
Several studies have reported high rates of vitamin D deficiency in HIV-infected individuals, ranging from 23–92%35. The vitamin D level in HIV-infected adults in the US, whether on ART or not, was also lower than in the uninfected population29. The prevalence of vitamin D deficiency in children and adolescents varies from 0–42% depending on season, latitude, and ethnicity35. A previous study in Thailand found none of the 600 Thai healthy children and adolescents (aged 9–18 years) had vitamin D deficiency (in process of publication). We found 25% of HIV-infected adolescents had vitamin D deficiency (< 20 ng/ml). Vitamin D deficiency may play role for lower bone mass in HIV-infected children. Fifty percent of HIV-infected children in the US, and 30% of adolescents in our cohort with BMD Z-score <−1.5 had low levels of 25-OHD7. HIV-infected children were in chronic illness condition and may have less habit of sun-exposure particularly in the tropical countries. These children also have poorer nutritional status and lower intake of vitamin containing food. HIV-infected patients may have impaired 1α-hydroxylation that decreases production and action of the active vitamin D metabolite 1,25(OH2D3) despite normal vitamin D levels36. Some ART such as PI and efavirenz, suppress the activity of 25 and 1α-hydroxylase or activates the destruction of 25-OHD and 1,25(OH2D3) to inactivate calcitroic acid23, 29. Higher rate of vitamin D deficiency may be explained by exposure to antiretroviral drugs especially efavirenz 37 On the other hand, PTH levels could be lower than expected in HIV-infected patients because PTH secretion is inhibited by pro-inflammatory cytokines29, 36 and PTH resistance has been reported in HIV infection36. ART also altered the PTH secretion in both directions 29, 34, 38. These findings suggested alteration of vitamin D and PTH system in HIV-infection, which may affect bone health. However, we found no association between low BMD and vitamin D deficiency. Taken together, our findings suggest that BMD in our adolescents is affected by factors other than low vitamin D. An alternate theory, previous data demonstrated pro-inflammatory cytokines such as interleukin-6, elevated in HIV patients, adversely affecting BMD36, 39.
Most of HIV-infected patients had normal serum calcium29. Similar to other studies, we did not find association between serum calcium and bone mass7, 40. Interestingly, we found phosphorus levels higher in the adolescents with low BMD, however, the levels found were mostly within normal range, and not reached statistical significance in the multivariate analysis. The ALK is not a sensitive or specific index of pathology in osteoporosis41 and was not found to associated with BMD. The bone specific alkaline phosphatase (BAP), on the other hand, is more sensitive and specific for bone formation41. Serum BAP was found to be elevated in HIV-infected children and adults suggesting high bone turnover state, especially in the patients receiving TDF 4, 12, 17, 29, 33, 34. The bone metabolic markers may not be useful for a cross sectional study as alteration of these markers may no longer exist while the decreased bone mass from previous long-term process was still evident.
Our study had several limitations. It was cross-sectional and so unable to evaluate the change of bone mass over a period of treatment. Prospective longitudinal study is therefore warranted. The use of different scanners between the two study sites may cause variability of the results despite the conversion formula. The fact that the adolescents in our cohort had been taking ART for an extended period of time, it is not possible to evaluate acute effect of initiation of ART on BMD. Many adolescents had experienced various ART regimens and therefore our ability to evaluate the impact of specific antiretroviral medications was limited.
In conclusion, we found low bone mass in a quarter of HIV-infected Thai adolescents. Poor growth and advanced HIV stage before ART were the independent associated risk factors. The results of this study underscored the importance of early ART initiation to preserve growth and bone health in children.
Acknowledgments
Funding sources: Grant from TREAT Asia Supplemental Funding, the TREAT Asia Pediatric HIV Observational Database is an initiative of TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Cancer Institute as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA; U01AI069907), and the Austrian AIDS Life Association.
We would like to thank participants and families who participated in the study. Grant from TREAT Asia Supplemental Funding, the TREAT Asia Pediatric HIV Observational Database is an initiative of TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Cancer Institute as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA; U01AI069907), and the Austrian AIDS Life Association. The National Research University Project of Commission of Higher Education and the Ratchadapiseksomphot Endowment Fund (HR 1161A-55), (Puthanakit T,).We would like to thank Frank Benjamin Steven, MD for his help in preparing the English manuscript.
Footnotes
Data has been presented at 3rd International Workshop on HIV Pediatrics, Rome, Italy, July, 15-16, 2011.
Conflicts of Interest: Puthanakit T is funded in part by the National Research University Project of Commission of Higher Education and the Ratchadapiseksomphot Endowment Fund (HR 1161A-55)
References
- 1.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20:2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 2.McComsey GA, Tebas P, Shane E. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis. 2010;51:937–946. doi: 10.1086/656412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Brien KO, Razavi M, Henderson RA, et al. Bone mineral content in girls perinatally infected with HIV. Am J Clin Nutr. 2001;73:821–826. doi: 10.1093/ajcn/73.4.821. [DOI] [PubMed] [Google Scholar]
- 4.Mora S, Zamproni I, Beccio S, et al. Longitudinal changes of bone mineral density and metabolism in antiretroviral-treated human immunodeficiency virus-infected children. J Clin Endocrinol Metab. 2004;89:24–28. doi: 10.1210/jc.2003-030767. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson DL, Spiegelman D, Duggan C, et al. Predictors of bone mineral density in human immunodeficiency virus-1 infected children. J Pediatr Gastroenterol Nutr. 2005;41:339–346. doi: 10.1097/01.mpg.0000174468.75219.30. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson DL, Lindsey JC, Gordon CM, et al. Pediatric AIDS Clinical Trials Group P1045 team. Total body and spinal bone mineral density across Tanner stage in perinatally HIV-infected and uninfected children and youth in PACTG 1045. AIDS. 2010;24:687–696. doi: 10.1097/QAD.0b013e328336095d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobson DL, DiMeglio LA, Hazra R, et al. Clinical determinants of bone mineral density (BMD) in perinatally HIV-infected children [911]. Presented at: the 16th Conference on Retroviruses and Opportunistic Infections; 2009; Montreal. [Google Scholar]
- 8.Theintz G, Buchs B, Rizzoli R, et al. Longitudinal monitoring of bone mass accumulation in healthy adolescents: evidence for a marked reduction after 16 years of age at the levels of lumbar spine and femoral neck in female subjects. J Clin Endocrinol Metab. 1992;75:1060–1065. doi: 10.1210/jcem.75.4.1400871. [DOI] [PubMed] [Google Scholar]
- 9.Soyka LA, Fairfield WP, Klibanski A. Clinical review 117: hormonal determinants and disorders of peak bone mass in children. J Clin Endocrinol Metab. 2000;85:3951–3963. doi: 10.1210/jcem.85.11.6994. [DOI] [PubMed] [Google Scholar]
- 10.Hansen MA, Overgaard K, Riis BJ, et al. Role of peak bone mass and bone loss in postmenopausal osteoporosis: 12 year study. BMJ. 1991;303:961–964. doi: 10.1136/bmj.303.6808.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mallon PW. HIV and bone mineral density. Curr Opin Infect Dis. 2010;23:1–8. doi: 10.1097/QCO.0b013e328334fe9a. [DOI] [PubMed] [Google Scholar]
- 12.Mondy K, Yarasheski K, Powderly WG, et al. Longitudinal evolution of bone mineral density and bone markers in human immunodeficiency virus-infected individuals. Clin Infect Dis. 2003;36:482–490. doi: 10.1086/367569. [DOI] [PubMed] [Google Scholar]
- 13.Dolan SE, Kanter JR, Grinspoon S. Longitudinal analysis of bone density in human immunodeficiency virus-infected women. J Clin Endocrinol Metab. 2006;91:2938–2945. doi: 10.1210/jc.2006-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruera D, Luna N, David DO, et al. Decreased bone mineral density in HIV-infected patients is independent of antiretroviral therapy. AIDS. 2003;17:1917–1923. doi: 10.1097/00002030-200309050-00010. [DOI] [PubMed] [Google Scholar]
- 15.Bonjoch A, Figueras M, Estany C, et al. High prevalence of and progression to low bone mineral density in HIV-infected patients: a longitudinal cohort study. AIDS. 2010;24:2827–2833. doi: 10.1097/QAD.0b013e328340a28d. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson DL, Spiegelman D, Knox TK, et al. Evolution and predictors of change in total bone mineral density over time in HIV-infected men and women in the nutrition for healthy living study. J Acquir Immune Defic Syndr. 2008;49:298–308. doi: 10.1097/QAI.0b013e3181893e8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calmy A, Fux CA, Norris R, et al. Low bone mineral density, renal dysfunction, and fracture risk in HIV infection: a cross-sectional study. J Infect Dis. 2009;200:1746–1754. doi: 10.1086/644785. [DOI] [PubMed] [Google Scholar]
- 18.McComsey GA, Kitch D, Daar ES, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis. 2011;203:1791–1801. doi: 10.1093/infdis/jir188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stellbrink HJ, Orkin C, Arribas JR, et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis. 2010;51:963–972. doi: 10.1086/656417. [DOI] [PubMed] [Google Scholar]
- 20.Martin A, Bloch M, Amin J, et al. Simplification of antiretroviral therapy with tenofovir-emtricitabine or abacavir-lamivudine: a randomized, 96-week trial. Clin Infect Dis. 2009;49:1591–1601. doi: 10.1086/644769. [DOI] [PubMed] [Google Scholar]
- 21.Working Group on Using Weight and Height References in Evaluating the Growth Status of Thai Children. Manual on Using Weight and Height References in Evaluation in Growth Status of Thai Children. Bangkok: Department of Health, Ministry of Public Health; 2000. [Google Scholar]
- 22.Centers for disease control and prevention. BMI percentile calculator for child and teen english version. Available at: http://apps.nccd.cdc.gov/dnpabmi/
- 23.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 24.Esoterix Laboratory Services Inc. Expected values and SI Unit conversion pocket book (monograph online) Available at: http://www.esoterix.com/files/expected_values.pdf.
- 25.Sriussadaporn SSN, Ploybutr S, Tanlakij M, et al. Assessment, of bone mineral density and factors influencing bone mineral density in Thalassemic diseases in Thailand; 2000. Report to the National Research Council of Thailand [Google Scholar]
- 26.Fan B, Lu Y, Genant H, et al. Does standardized BMD still remove differences between Hologic and GE-Lunar state-of-the-art DXA systems? Osteoporos Int. 2010;21:1227–1236. doi: 10.1007/s00198-009-1062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon CM, Bachrach LK, Carpenter TO, et al. Dual energy X-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom. 2008;11:43–58. doi: 10.1016/j.jocd.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Zemel BS, Leonard MB, Kelly A, et al. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010;95:1265–1273. doi: 10.1210/jc.2009-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madeddu G, Spanu A, Solinas P, et al. Bone mass loss and vitamin D metabolism impairment in HIV patients receiving highly active antiretroviral therapy. Q J Nucl Med Mol Imaging. 2004;48:39–48. [PubMed] [Google Scholar]
- 30.Bolland MJ, Grey AB, Gamble GD, et al. CLINICAL Review: low body weight mediates the relationship between HIV infection and low bone mineral density: a meta-analysis. J Clin Endocrinol Metab. 2007;92:4522–4528. doi: 10.1210/jc.2007-1660. [DOI] [PubMed] [Google Scholar]
- 31.Zuccotti G, Viganò A, Gabiano C, et al. Antiretroviral therapy and bone mineral measurements in HIV-infected youths. Bone. 2010;46:1633–1638. doi: 10.1016/j.bone.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 32.Hazra R, Gafni RI, Maldarelli F, et al. Tenofovir disoproxil fumarate and an optimized background regimen of antiretroviral agents as salvage therapy for pediatric HIV infection. Pediatrics. 2005;116:e846–854. doi: 10.1542/peds.2005-0975. [DOI] [PubMed] [Google Scholar]
- 33.Purdy JB, Gafni RI, Reynolds JC, et al. Decreased bone mineral density with off-label use of tenofovir in children and adolescents infected with human immunodeficiency virus. J Pediatr. 2008;152:582–584. doi: 10.1016/j.jpeds.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gafni RI, Hazra R, Reynolds JC, et al. Tenofovir disoproxil fumarate and an optimized background regimen of antiretroviral agents as salvage therapy: impact on bone mineral density in HIV-infected children. Pediatrics. 2006;118:e711–718. doi: 10.1542/peds.2005-2525. [DOI] [PubMed] [Google Scholar]
- 35.Huh SY, Gordon CM. Vitamin D deficiency in children and adolescents: epidemiology, impact and treatment. Rev Endocr Metab Disord. 2008;9:161–170. doi: 10.1007/s11154-007-9072-y. [DOI] [PubMed] [Google Scholar]
- 36.Kühne CA, Heufelder AE, Hofbauer LC. Bone and mineral metabolism in human immunodeficiency virus infection. J Bone Miner Res. 2001;16:2–9. doi: 10.1359/jbmr.2001.16.1.2. [DOI] [PubMed] [Google Scholar]
- 37.Dao CN, Patel P, Overton ET, et al. Low vitamin D among HIV-infected adults: prevalence of and risk factors for low vitamin D levels in a cohort of HIV-infected adults and comparison to prevalence among adults in the US general population. Clin Infect Dis. 2011;52:396–405. doi: 10.1093/cid/ciq158. [DOI] [PubMed] [Google Scholar]
- 38.van Vonderen MG, Lips P, van Agtmael MA, et al. First line zidovudine/lamivudine/lopinavir/ritonavir leads to greater bone loss compared to nevirapine/lopinavir/ritonavir. AIDS. 2009;23:1367–1376. doi: 10.1097/QAD.0b013e32832c4947. [DOI] [PubMed] [Google Scholar]
- 39.Arpadi S, Horlick M, Shane E. Metabolic bone disease in human immunodeficiency virus-infected children. J Clin Endocrinol Metab. 2004;89:21–23. doi: 10.1210/jc.2003-031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramayo E, González-Moreno MP, Macías J, et al. Relationship between osteopenia, free testosterone, and vitamin D metabolite levels in HIV-infected patients with and without highly active antiretroviral therapy. AIDS Res Hum Retroviruses. 2005;21:915–921. doi: 10.1089/aid.2005.21.915. [DOI] [PubMed] [Google Scholar]
- 41.Camozzi V, Moro L, Luisetto G, et al. Bone turnover markers in clinical practice and their potencial use in HIV-related bone disease. HAART and correlated pathologies. 2009 Available at: http://www.mnlpublimed.com/public/HA02-A01.pdf.