Abstract
Most of the energy in the brain comes from glucose and supports glutamatergic activity. The firing rate of cortical glutamatergic neurons, as well as cortical extracellular glutamate levels, increase with time spent awake and decline throughout non rapid eye movement (NREM) sleep, raising the question whether glucose levels reflect behavioral state and sleep/wake history. Here chronic (2–3 days) electroencephalographic (EEG) recordings in the rat cerebral cortex were coupled with fixed-potential amperometry to monitor the extracellular concentration of glucose ([gluc]) on a second-by-second basis across the spontaneous sleep-wake cycle and in response to 3 hours of sleep deprivation. [Gluc] progressively increased during NREM sleep and declined during REM sleep, while during wake an early decline in [gluc] was followed by an increase 8–15 minutes after awakening. There was a significant time of day effect during the dark phase, when rats are mostly awake, with [gluc] being significantly lower during the last 3–4 hours of the night relative to the first 3–4 hours. Moreover, the duration of the early phase of [gluc] decline during wake was longer after prolonged wake than after consolidated sleep. Thus, the sleep/wake history may affect the levels of glucose available to the brain upon awakening.
Keywords: glucose, in vivo amperometry, sleep, rat, cerebral cortex, EEG, slow wave activity
Introduction
A high metabolic price is paid to sustain cerebral activity, with roughly 20% of total oxygen consumption supporting oxidative phosphorylation within the brain (Sokoloff 1960). The brain overwhelmingly relies on the delivery of glucose from the blood stream as a substrate to produce the ATP necessary to support its energetic demands (Siesjo 1978, Gjedde et al. 2002). Moreover, glucose can be diverted through the pentose phosphate shunt to produce the reducing agent NADPH and many precursor molecules for nucleotide and amino acid biosynthesis (Gaitonde et al. 1987). Consequently, sufficient glucose availability is critical for proper brain function.
About 80% of all neurons in the cerebral cortex use glutamate as a neurotransmitter (Braitenberg & Schuz 1998, Jones 2009) and most of the energy in the brain, including the cerebral cortex, is used to support glutamatergic activity (Attwell & Laughlin 2001). Indeed, glucose consumption is stoichiometrically coupled to glutamate-glutamine cycling (Sibson et al. 1998). Cortical and thalamic cells are relatively depolarized in wake and REM sleep relative to NREM sleep (Steriade & McCarley 1990, Steriade et al. 2001) and mean cortical firing rate is higher in wake and REM sleep than in NREM sleep (Desiraju 1972, Noda & Adey 1973). It is not surprising, therefore, that brain metabolism is higher in wake and REM sleep than in NREM sleep (e.g. (Kennedy et al. 1982, Madsen & Vorstrup 1991, Netchiporouk et al. 2001, Shram et al. 2002)).
It was recently shown that within each behavioral state cortical firing rates are not static, but depend on sleep/wake history, being higher following prolonged wake and lower after sleep (Vyazovskiy et al. 2009). Cortical extracellular glutamate levels also progressively increase with time spent spontaneously awake and decline throughout NREM sleep (Dash et al. 2009). Moreover, cerebral metabolism in mice is contingent upon sleep-wake history. Specifically, the uptake of 2-deoxyglucose during quiet wake is 15–20% lower in mice that had slept for ~ 2.5 hours prior to the injection of the tracer, compared to mice that had been awake prior to the injection (Vyazovskiy et al. 2008). These observations raise the question of how brain glucose levels change during behavioral states with high energy need - wake and REM sleep - and especially during sleep deprivation, when wake duration is extended beyond its physiological limits.
To address this question we performed EEG recordings in the rat cerebral cortex coupled with fixed-potential amperometry, to monitor the extracellular concentration of glucose ([gluc]) during sleep, wake, and sleep deprivation, on a second-by-second basis for up to 3 days.
Materials and Methods
Surgical procedures and monitoring [gluc]
Male Wistar Kyoto rats (n = 6; Harlan, Madison, WI; 300–350 g at time of surgery) were individually housed in recording chambers (clear Plexiglas enclosure, 36.5 × 25 × 46 cm in a sound-attenuating box) in a controlled environment (24 ± 1 °C; 12h light/dark cycle, lights on at 10:00 a.m.; food and water ad libitum). Under isoflurane anesthesia three screws serving as EEG electrodes were affixed to the scalp: one frontal (mm from bregma: anteroposterior, AP +2, mediolateral, ML +3), one parietal (AP −2.3, ML +3.8), and one cerebellar (AP −11 ML +1). Wire electrodes were implanted in the nuchal muscles to monitor the electromyogram (EMG). To enable subsequent recordings of [gluc] in various cortical regions, a guide cannula (MD-2250 Bioanalytical Systems, Inc.) was implanted in either frontal cortex (n=4; AP 3.2, ML −0.8, DV −4.5), somatosensory cortex (n=1; AP −2.3, ML −3.8), or primary visual cortex (n=1; AP −7, ML −3). Dental cement was used to affix the electrodes and the cannula to the animal’s skull. After one week of recovery from surgery, pre-fabricated glucose biosensors (Pinnacle Technologies) with an integrated Ag/AgCl reference electrode were implanted through the guide cannula. These sensors consist of a platinum/iridium recording electrode that is coated with glucose oxidase and a series of membranes to increase the selectivity and specificity of the sensors to glucose. Glucose oxidase catalyzes the oxidation of glucose, a process that also generates H202. When a potential is applied (0.6V) to the recording electrode (vs. the Ag/AgCl reference), H202 can itself oxidize at the recording electrode and the current from this electrochemical reaction can be monitored. Critically, the current produced from this reaction is linearly dependent upon [gluc] and hence can be used as a measure of [gluc] (see below). With such a system, one can reliably measure [gluc] with high temporal resolution (~1s). In one pilot experiment (see Discussion), we implanted a ceramic-based microelectrode array (Center for Microelectrode Technology, Lexington, KY), instead of the pre-fabricated glucose biosensor (Pinnacle), to record [gluc] in the frontal cortex. This microelectrode array is composed of a glucose-sensitive electrode (similar to the pre-fabricated biosensors used in the rest of the study) and also a glucose-insensitive control electrode (that lacks glucose oxidase). As previously described (Dash et al., 2009; Dash et al., 2012), the current measured by this control electrode can be subtracted from the current produced by the glucose-sensitive electrode to remove any confounding signals produced by the oxidation of potential interferents. For this pilot experiment, [gluc] in frontal cortex, the EEG, and EMG were simultaneously recorded (FAST-16, Quanteon, KY; VitalRecorder, Kissie Comtec, Nagano, Japan) for 3 days. All animal procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and facilities were reviewed and approved by the IACUC of the University of Wisconsin-Madison, and were inspected and accredited by AAALAC.
Sleep/wake recordings
To record the EEG and EMG, electrodes were connected to a headstage amplifier (Pinnacle Technologies) that provides the initial amplification (100x) and filtering (EEG: High Pass 0.5 Hz, EMG: High Pass 10 Hz). The headstage amplifier was connected to a low-torque commutator and the signals were passed into the data conditioning and acquisitions system (8401 DACS, Pinnacle Technologies). This acquisition system provided the interface with the recording computer to enable storage of all data signals (sampling rate, 250 Hz). Data were collected with commercially available software (Pal8400, Pinnacle Technologies) and stored offline. Data were then exported to Matlab (MathWorks) and SleepSign (Kissei Comtec) to enable assessment of [gluc], EEG spectra, and vigilance state. Vigilance state was determined offline by visual inspection of EEG and EMG signals and scored in 4-sec epochs as either wake (low-voltage, high-frequency EEG, high EMG), NREM sleep (high-voltage, low-frequency EEG, low EMG), or REM sleep (low-voltage, high-frequency EEG, low EMG activity). Vigilance state could be determined for all epochs.
Electrode calibration and in vivo amperometry
Prior to implantation in freely moving rats, each microelectrode was calibrated in vitro to ensure proper selectivity and sensitivity for glucose by assessing the response of the electrode to glucose and ascorbate acid, the primary interferent in the brain. Glucose-sensitive electrodes responded robustly to the presence of glucose (8.09 ± 0.55 nA/1mM glucose) and hence were suitable for use in vivo. Once calibrated, the glucose-sensitive electrodes were implanted in freely behaving rats and fixed-potential amperometry (0.6 V) was performed (PAL8400, Pinnacle Technologies). Chronic recordings (3 days) of amperometric, EEG, and EMG signals were performed continuously. [Gluc] was measured throughout the undisturbed sleep/wake cycle as well as during and following a 3-hr sleep deprivation period. Sleep deprivation was performed as previously described (Vyazovskiy et al. 2009). Briefly, rats were given novel objects or activated by mild acoustic stimuli (e.g. tapping on the cage) whenever a sleep posture or an electrophysiological sign of drowsiness was observed. Rats were never touched or handled directly and were left undisturbed during spontaneous wake behaviors such as grooming, exploring, feeding, or drinking. In a subset of animals, separate control experiments were performed to assess the response of cortical [gluc] to intraperitoneal injections of ketamine/xylazine anesthesia (87 mg/kg and 13 mg/kg, respectively; n=2). In another subset of animals (n=3), the response of cortical [gluc] to both an intraperitoneal injection of glucose (2 mL, 30% glucose) and an intraperitoneal injection of saline (2mL) was recorded. Glucose and saline injections were counterbalanced and a minimum of 3 hours separated the two injections. Following glucose and saline injections, wakefulness was enforced for 30 minutes to control for behavioral-state dependent effects on cortical [gluc]. All injections occurred on the third and final day of recording and were performed in the rat’s home cage.
After 3 days of chronic recordings, electrodes were removed and recalibrated to ensure that sensitivity to glucose was maintained throughout the prolonged in vivo recording. Despite a small loss of sensitivity between the original calibrations and post-experiment recalibrations (−14.65 ± 3.16 %), all glucose electrodes responded robustly to glucose (7.01 ± 0.74 nA/1mM glucose), confirming that they are suitable for monitoring [gluc] across several days in vivo. In some recordings, this decay in sensitivity could also be observed in vivo as a small but constant decline in the overall magnitude of current produced by the electrode. To account for this decay and facilitate comparison between experimental days, the linear component of this decay was removed before analysis of [gluc] (see (Dash et al. 2009)).
The glucose-sensitive electrodes that we used are specifically designed to measure second-by-second changes in [gluc] across days, rather than absolute values. Theoretically, the absolute concentration of an analyte can be determined from a calibration curve of known standards. In practice, however, reporting absolute concentrations can be confounded by technical shortcomings and introduces unwarranted variability because the brain tissue surrounding the implanted electrode may cause differences in the diffusion coefficients of the analyte (Benoit-Marand et al. 2007), partially occlude the implanted electrode and thereby alter the recording surface area (Wang 2008) and/or alter the shape and size of the diffusion layer at the electrodes surface (Robinson et al. 2008). Consequently, the current produced in vivo may not directly equate to that produced during an in vitro calibration (Wilson & Gifford 2005). Therefore, throughout this paper [gluc] are reported as changes from the daily mean, not as absolute concentrations, consistent with previous reports (Dash et al. 2012, Wisor et al. 2012).
Data Processing
Recorded signals were imported into Matlab (MathWorks) and processed with custom scripts. Amperometric signals were low-pass filtered (chebyshev type II, passband = 18 Hz, stopband = 19 Hz) to remove any high-frequency noise and were subsequently binned and averaged over 4-sec epochs to facilitate comparisons with scored behavioral state. Using a 30-min moving window across each day, artifacts (<5% of total recording time) were identified (data points > or < 5 standard deviations from the mean) and removed. The calibration curve established for each glucose electrode prior to implantation was used to convert the signals recorded in vivo from current (nA) to concentration changes (mM). All statistical tests were performed using Matlab or Statistica (Statsoft) and all data are presented as mean ± the standard error of the mean.
Histology
At the end of the experiment rats were deeply anesthetized with isoflurane anesthesia (3% in oxygen) and perfused transcardially with saline immediately followed by a 4% solution of paraformaldehyde in 0.1 M sodium phosphate, pH 7.2. Brains were post-fixed for 48 hours in 4% PFA, then cut into 50 μm serial coronal sections using a vibratome, and subjected to cresyl-violet (Nissl) staining. Placement of glucose and EEG electrodes was determined using light microscopy. In all cases electrodes were in the infragranular layers of the targeted cortical regions and did not extend to the white matter below layer VI. No excessive or unexpected tissue damage was noted.
Results
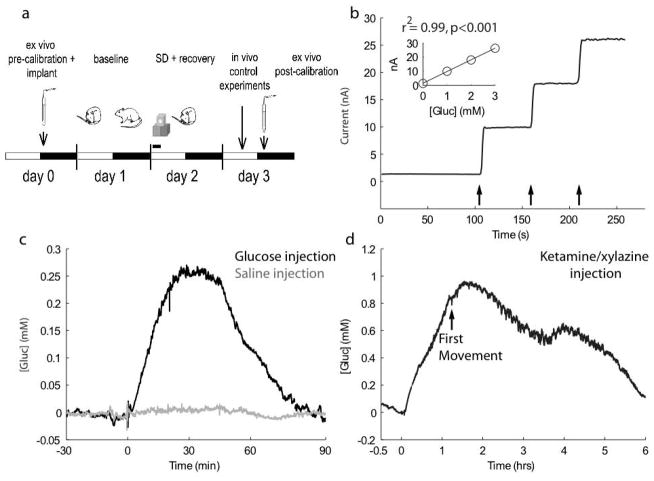
A schematic of the experimental design is shown in Figure 1a. After in vitro calibration to ensure proper selectivity and sensitivity for glucose (Fig. 1b), electrodes were implanted in cortex and then polarized in the evening prior to the first day of recording to ensure that a stable baseline was achieved. For all animals, Day 1 of recording consisted of 24 hours of undisturbed baseline while Day 2 began with an acute (3 hours) sleep deprivation followed by 21 hours of recovery. All data presented describing the relationship between behavioral state and cortical [gluc] were obtained on these two days. On Day 3, in vivo control experiments were conducted to confirm that these glucose-sensitive electrodes respond to changes in [gluc] following manipulations known to affect glucose supply and/or demand. Specifically, in one set of experiments (n = 3) we measured [gluc] in frontal cortex after intraperitoneal injection of glucose (2 mL, 30% glucose). As expected, we found a large increase in [gluc] (maximum increase: 0.37 ± 0.11 mM ) when compared to an injection of 2 mL saline (Fig. 1c). Importantly, we controlled for behavioral state-dependent effects on [gluc] by ensuring that animals were awake for at least 30 minutes following each injection. In another set of experiments (n = 2) we assessed changes in cortical [gluc] under ketamine-xylazine anesthesia (87 mg/kg and 13 mg/kg, respectively). At this depth of anesthesia, the EEG showed rhythmic, high voltage, low frequency activity. Also as expected, we found that [gluc] in frontal cortex increased very significantly during anesthesia (maximum increase: 0.73 ± 0.15mM; Fig. 1d), in agreement with previous evidence showing that anesthesia increases [gluc] in the brain (Dedrick et al. 1975, Canal et al. 2005), likely by increasing [gluc] in the blood (Canal et al. 2005). Together, these control experiments confirmed that the electrodes were responsive to changes in [gluc] in vivo. Of note, glucose oxidase utilizes oxygen as a co-substrate. Consequently, the relatively low concentrations of molecular oxygen in the cortex (~50uM; (Nair et al. 1987, Murr et al. 1994, Bazzu et al. 2009)) may disrupt the otherwise linear response of the electrodes to glucose and cause the rate of glucose oxidation to become dependent upon changes in either glucose or oxygen (Silver & Erecinska 1994, Dixon et al. 2002). Before their use in vivo, we tested the dependence of the current produced by our electrodes on oxygen by additionally calibrating them in vitro when molecular oxygen in the solution was displaced by vigorous bubbling of nitrogen gas. We found that the response of these electrodes to glucose was not affected by the amount of oxygen present in the calibration solution (data not shown).
Figure 1. In vitro and in vivo assessment of glucose-sensitive microelectrodes.
a) Schematic of experimental design. White and black bars indicate the light and dark phase, respectively. b) A typical in vitro calibration conducted following removal of electrode from a chronic in vivo recording. Arrows depict 1mM additions of glucose to the calibration solution. After 3 days of in vivo recordings, this electrode still responds robustly (8.27 nA/mM) and linearly to glucose, as particularly evident in the inset. c) [Gluc] in frontal cortex largely increases following an i.p. injection of glucose (black; 2mL of 30% glucose) but is largely unaffected by an equivalent volume injection of saline (grey). d) [Gluc] in frontal cortex increases during ketamine/xylazine anesthesia (87 mg/kg and 13 mg/kg). Following recovery from anesthesia, [gluc] remains elevated for ~5 hours.
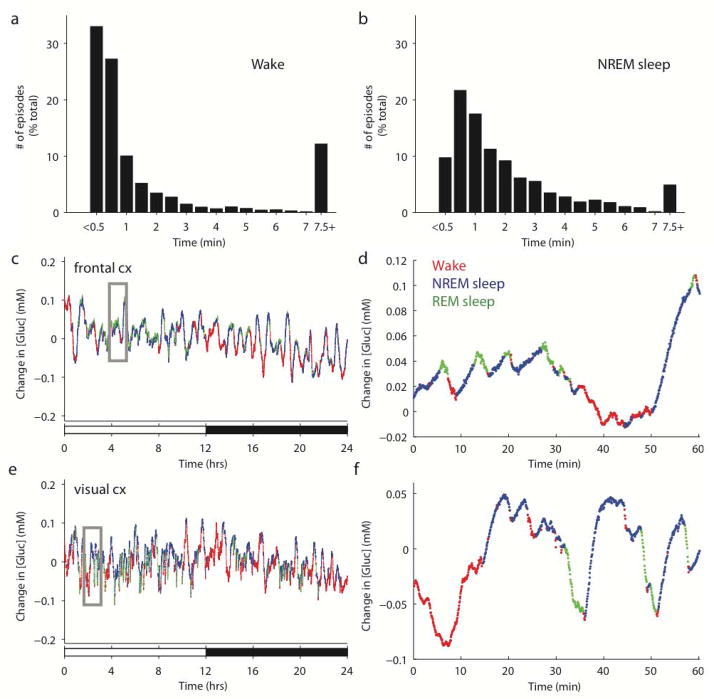
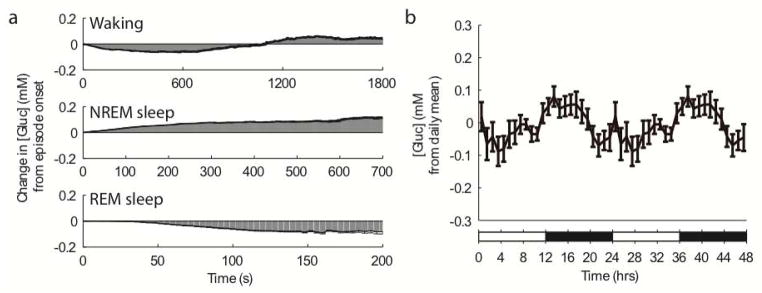
Changes in cortical [gluc] across the sleep/wake cycle were measured continuously for 48 hours in six freely behaving rats. Rats are nocturnal animals, spending more time awake during the dark phase and more time asleep during the light phase. Accordingly, rats in the present study exhibited a clear circadian rhythmicity and were awake for 58.83 ± 2.29% of the dark period, compared to 38.21 ± 2.63% of the light period. Percentages of wake, NREM and REM sleep across the 24-hour cycle were also within the published norms (Alfoldi et al. 1991, Fisher & Sugden 2010), with 48.54 ± 2.01% of total recording time spent awake, 44.37 ± 1.76% spent in NREM sleep, and 7.09 ± 0.60% spent in REM sleep. Of note, the sleep/wake cycle in rats is polyphasic, with numerous quick transitions from sleep to wake and vice versa. Indeed, average bout duration (min) was 3.60 ± 0.17 for wake, 2.40 ± 0.29 for NREM sleep, and 1.49 ± 0.13 for REM sleep. Bout durations were not normally distributed, however, and similar to previous reports (McShane et al. 2010) could be characterized by a large proportion of short episodes and a small proportion of long ones (Fig. 2a–b). Crucially, fixed potential amperometry has high temporal resolution (~1Hz sampling rate), and thus is ideally suited to resolve changes in [gluc] between and within each behavioral state. Visual inspection of the individual recordings from the four rats with glucose electrodes implanted in the frontal cortex showed that second-by second [gluc] was not static, but on average was declining during wake and REM sleep and increasing during NREM sleep (Fig. 2c–d). The same dynamic fluctuations were observed in two rats whose glucose electrodes were located either in somatosensory cortex (not shown), or in visual cortex (Fig. 2e–f), with [gluc] decreasing during wake and REM sleep and increasing during NREM sleep. To quantify the magnitude of these changes we focused on consolidated episodes of wake, NREM and REM sleep (minimum bout duration of 20 sec for wake/NREM sleep and 8 sec for REM sleep), and obtained a profile of the average changes in [gluc] as a function of behavioral state (Fig. 3a). This analysis demonstrated that dynamic changes in [gluc] were not confined to transitions between behavioral states, but occurred across the entire duration of individual wake, NREM and REM sleep episodes. [Gluc] increased progressively throughout the entirety of the majority of NREM sleep episodes (7.99 ± 1.32 uM/min) and decreased progressively throughout REM sleep episodes (−28.25 ± 4.69 uM/min). These state-dependent changes in [gluc] closely align with a recent report detailing behavioral state-dependent changes in [gluc] in mice (Naylor et al., 2012). That study, however, observed that [gluc] did not continue to increase throughout long NREM sleep episodes (>10–12 min), but rather began to decline as a NREM sleep episode continued. Only 4.51% of episodes in the present study lasted longer than 12 minutes and of those episodes, only 41% exhibited a late decline in [gluc]. Consequently, in rats, [gluc] appears to increase through the entirety of the vast majority of NREM sleep episodes. By contrast, during wake, [gluc] first showed a “decline phase”, in which it progressively decreased (−15.19 ± 3.65 uM/min) for 7.55 ± 2.69 minutes, then reversed course and progressively increased (8.87 ± 2.31 uM/min) for the remainder of the wake episode. Due to the natural variability of episode duration, 65.0 ± 2.48% of wake bouts ended with [gluc] still lower than at wake onset, while in the remaining episodes [gluc] ended higher. Thus, the ultimate effect of a single wake episode on [gluc] was heterogeneous and depended on wake duration. However, as a whole, wake resulted in a large average daily reduction in cortical [gluc] of −9.06 ± 1.65 mM. REM sleep also resulted in a pronounced daily reduction of cortical [gluc] of −3.90 ± 1.87 mM. During NREM sleep, by contrast, [gluc] increased by 12.05 ± 2.86 mM per day.
Figure 2. Changes in [gluc] associated with behavioral state.
Characterization of all episodes of wake (a) and NREM sleep (b) from all rats as a function of their duration. The final bin (7.5+) includes all episodes with durations greater than 7.5 minutes. [Gluc] in frontal (c-d) or visual (e-f) cortex is depicted for each 4-sec epoch of wake (red), NREM sleep (blue), or REM sleep (green) across 24 hours. Panels d and f show at higher resolution the boxed regions indicated in c and e, respectively. For all graphs, [gluc] is depicted as a change in concentration relative to the 24-hr mean. White and black bars indicate the light and dark phase, respectively.
Figure 3. Effects of duration of sleep/wake episodes on [gluc].
a) State-dependent changes in [gluc] are depicted for wake, NREM, and REM sleep. Each panel depicts the average change in [gluc] across all episodes of a particular behavioral state. Unidirectional error bars (black) depict½ the standard error. b) Changes in [gluc] across the light/dark cycle. Data are double plotted, revealing that [gluc] typically increases across the light period and decreases across the dark period. Values are mean ± SEM.
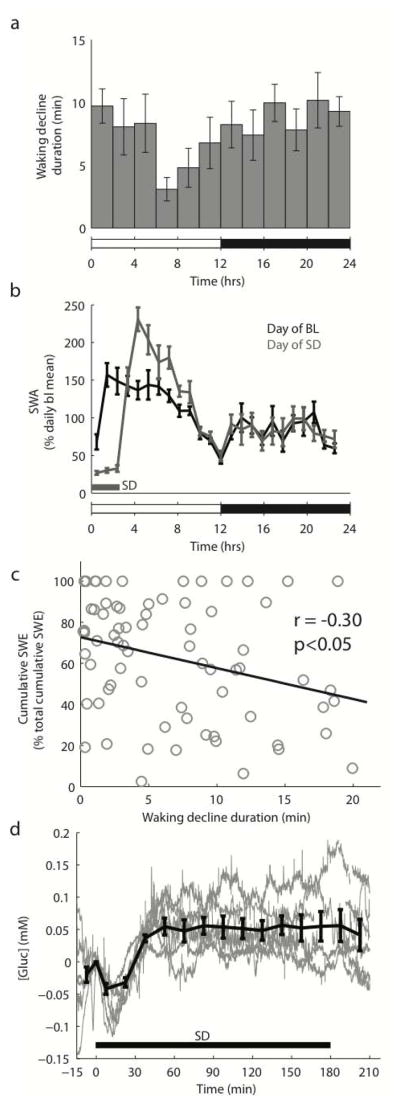
Overall, the net deficits in cortical [gluc] that occurred during wake and REM sleep were opposed by the net increase in [gluc] during NREM sleep, resulting in an almost perfect balance across 24 hours. By examining the average [gluc] (1-hr bins) across the 12 hours of the dark period for each rat (n=6), however, we found that [gluc] was significantly affected by time of day across the dark period (F(11,55) = 2.43, p<0.05) and more specifically, that [gluc] was significantly lower during the last 4 hours of the dark period than during the first 4 hours (t(5) = 2.78, p < 0.05; Fig. 3b). To determine whether time of day also affected the duration of the decline phase during wake, we calculated the average duration of the decline phase (2-hr bins) across the entire day for each rat (Fig. 4a). We found that time of day significantly affected the duration of the decline phase during wake (F(11,55) = 2.27, p < 0.05), with longer declines at the end of the dark phase and at the beginning of the light phase, and shorter declines 6–10 hours after light onset, when most of the daily consolidated sleep had occurred (Fig. 4a). We determined therefore whether the [gluc] decline phase during wake depended on sleep quantity and quality by first measuring mean hourly values of NREM slow wave activity (SWA) and then calculating slow wave energy (SWE = SWA x time), i.e. the accumulation of SWA over time. SWA is defined as the EEG power between 0.5 and 4 Hz during NREM sleep, and is the best established electrophysiological marker of sleep intensity: the higher is SWA, the deeper is sleep (Vyazovskiy et al. 2011). As expected, SWA was significantly higher during the first four hours than during the last four hours of the light phase, i.e. during early as compared to late sleep, consistent with a decline in sleep pressure in the course of sleep (SWA, % of daily mean, 129.01 ± 5.90% vs. 99.37 ± 1.68%, t(5) = 4.67, p<0.01; Fig. 4b). We found a significant negative correlation between the duration of the [gluc] decline phase during wake and SWE across the light phase (r = −0.30; p<0.05; Fig. 4c). Thus, the longer and/or deeper the animals had slept during the day, the shorter was the phase of [gluc] decline when they woke up.
Figure 4. Effects of sleep/wake history on [gluc].
a) 24-hr profile of the duration of the early decline phase during wake (2-hr bins). b) Average (n=6 rats) 24-hr profile (1-hr bins) of NREM SWA during undisturbed baseline days (BL) and on days that began with a 3-hr sleep deprivation (SD). c) Negative correlation between the duration of the decline phase during wake and SWE across the light phase during baseline. Each point depicts the average wake decline duration and SWE across 2-hr bins of the light period. d) Changes in [gluc] during a 3-hr sleep deprivation beginning at light onset. Grey lines refer to individual rats (n = 6), while the black line is the group average. Values are mean ± SEM.
To further examine the relationships between [gluc] and wake duration we measured changes in [gluc] during 3 hours of sleep deprivation starting at light onset. Thus, after being spontaneously awake for most of the night, all six rats were kept awake in the first part of the light phase by exposure to novel objects. As expected, SWA during the first four hours of recovery sleep after sleep deprivation increased by 149.41 ± 13.05% over the same time period during baseline (t(5) = 4.70, p<0.01; Fig. 4b). Consistent with the previous results, we found that during sleep deprivation there was an initial decline in [gluc] that persisted for 14.76 ± 1.52 minutes, a decline significantly longer than during spontaneous wake (t(5) = 3.58, p<0.05). Following this decline, [gluc] began to increase and subsequently remained at or above its initial concentration for the duration of the sleep deprivation (Fig. 4d). Consequently, by the end of the 3 hours of extended wake [gluc] was not significantly different from its levels at the onset of sleep deprivation (t(5) = 1.92, p = 0.11), and for most of the 3 hours it was actually higher (average [gluc] between 30 and 240 minutes as compared to initial [gluc]: t(5) = 3.86, p<0.05). Overall, the data during baseline and after sleep deprivation show that the sleep/wake history can affect the duration of the [gluc] decline phase during wake.
Finally, we assessed whether the typical increase in [gluc] during NREM sleep reflected sleep intensity as measured by SWA. We found that, on the undisturbed baseline day, [gluc] increased to a similar extent (8.05 ± 1.47 uM/min vs. 11.68 ± 2.28 uM/min; t(5) = 1.40, p= 0.22) during early NREM sleep (first 4 hours following light onset, when sleep pressure is highest) as compared to late NREM sleep (last 4 hours of light period, when sleep pressure is low). To further investigate the effects of sleep pressure on changes in [gluc] during NREM sleep, we calculated the extent to which [gluc] increased during recovery sleep following sleep deprivation (first 4 hours following the conclusion of the sleep deprivation). Strikingly, despite a clear increase in sleep pressure during recovery sleep as compared to early baseline sleep (see Fig. 4b and results above), during recovery sleep [gluc] increased by 8.86 ± 1.48 uM/min, a change very similar to that observed during baseline (t(5) = 0.58, p= 0.58). Overall, there was no significant correlation between changes in SWA and changes in [gluc] during NREM sleep (r = −0.03, p = 0.87). Thus, the rate by which [gluc] increases during NREM sleep is not affected by sleep pressure.
Discussion
By using fixed potential amperometry with enzyme-coupled electrodes we found that cortical levels of extracellular glucose typically increase during NREM sleep and typically decrease during REM sleep, while changes during wake depend on wake duration, with an early decline followed by a late increase 8–15 minutes after awakening. We also found that while sleep/wake history does not affect the rate of [gluc] increase during NREM sleep, it affects the duration of the early phase of [gluc] decline in wake. Thus, upon awakening, the period of several minutes during which glucose levels keep declining despite the high energy need of wake is longer after prolonged wake than after consolidated sleep.
Enzyme-coupled electrodes have been successfully used in vivo for a variety of analytes, including glucose (i.e. (Hu & Wilson 1997, Netchiporouk et al. 2001)). Nevertheless, it remains essential to perform control experiments to ensure that the glucose-sensitive electrodes will function as intended in vivo. To this aim we run two types of control experiments. To test for sensitivity, we used intraperitoneal injections of glucose and anesthesia to confirm in vivo that our electrodes were able to detect changes in cortical [gluc], even at the end of the 48 hours of continuous recordings that were the basis of all our analyses. Moreover, to test for specificity, we measured the response of the electrodes to oxygen in vitro. This is important because glucose oxidase, whose activity underlies the ability of our electrodes to measure glucose, utilizes oxygen as a co-substrate and in a previous study we found that cortical levels of oxygen change as a function of sleep and wake, and do so in a manner almost opposite to that described here for glucose (Dash et al. 2012). We confirmed that oxygen did not affect the response to glucose, ruling out an important confounding factor.
Given that 1) the glucose biosensors rely upon the oxidation of hydrogen peroxide (H202) to monitor changes in glucose (see methods) and 2) emerging evidence indicates that endogenous H202 levels may change in response to prolonged neuronal activity with potential signaling implications (Rice 2011), state-dependent changes in endogenous H202 could conceivably confound at least some of our glucose measures. Specifically, it could be that the apparent increase in [gluc] seen with prolonged wakefulness resulted from an increase in extracellular H202, which may be expected to accumulate after a period of prolonged neuronal activity. While we cannot completely rule out this possibility, we think it is very unlikely for several reasons: 1) the extracellular concentration of H202 (e.g. (Piantadosi & Tatro 1990, Lei et al. 1998)) is at least 50–100 fold lower than that of glucose, 2) were endogenous H202 concentrations significantly contributing to the response of the biosensors, we would expect such a contribution to be manifest in the recordings of other analytes, such as glutamate or lactate, which utilize the same basic biosensor, but with different applied enzyme. However, our previous data (Dash et al. 2009, Dash et al. 2012) and those shown in a study recently published while this paper was under review (Naylor et al. 2012) demonstrate that these biosensors display different state-dependent changes as a consequence of the applied enzyme, rather than responding to a common analyte such as H202 and 3) in one pilot experiment in one rat, we performed simultaneous recordings from a glucose-sensitive biosensor and from a H202-sensitive, but glucose-insensitive control biosensor. By subtracting the current produced by the glucose-insensitive electrode from the glucose sensitive electrode, we could therefore control for current produced by any potential interferents such as H202. In this pilot experiment, we observed state-dependent alterations in [gluc] similar to those reported in the present report, including a progressive decline in [gluc] during REM sleep, a progressive increase throughout most of NREM sleep, and a biphasic pattern of changes during wake episodes characterized by an initial dip followed by a progressive increase in [gluc].
A previous study measured total tissue levels of glucose in the whole brain of rats after 4 hours of sleep deprivation by forced locomotion and after 1 hour of recovery sleep following sleep deprivation. Rats were not implanted with EEG electrodes, and behavioral states were scored based on visual observation. It was found that [gluc] was ~ 28% higher after recovery sleep than after sleep deprivation (Van den Noort & Brine 1970). The same study also used some rats with implanted EEG electrodes and found that [gluc] was ~27% higher in the whole brain of animals that were in NREM sleep just before decapitation relative to animals that were awake at that time (Van den Noort & Brine 1970). A more recent report measured extracellular glucose levels in rat somatosensory cortex with glucose oxidase-based sensors similar to those employed in our study (Netchiporouk et al. 2001). The method used, however - differential normal pulse voltammetry with removable electrodes - required the authors to average glucose levels across at least 3 minutes of uninterrupted wake or NREM sleep, and to limit recordings to a period of 8 hours. It was found that [gluc] was 13% higher in NREM sleep than in wake, and 11% lower in REM sleep as compared to wake. Moreover, [gluc] showed a trend to increase during NREM sleep, with mean values becoming significantly greater than at sleep onset after 15 minutes of NREM sleep. An opposite trend was found in wake, during which [gluc] progressively decreased, with mean values becoming significantly lower than at wake onset after 18 min of wake. While the present manuscript was under review, a report (Naylor et al. 2012) was published that used similar biosensors to record extracellular [gluc] across the sleep/wake cycle in the frontal cortex of mice. Similar to data presented herein, Naylor et al., observed 1) a decrease in [gluc] during REM sleep, 2) a decrease in [gluc] during the first ~7 minutes of wake followed by a progressive increase as wake persisted, and 3) an increase in [gluc] during the first ~10–12 minutes of NREM sleep. This study also reported that [gluc] began to decline as NREM sleep episodes persisted past ~10–12 minutes, a change that was not evident in the present study, perhaps due to the lack of prolonged NREM sleep episodes. Our results, therefore, are largely consistent with these previous findings (Netchiporouk et al. 2001, Naylor et al. 2012), and expand upon them in several ways.
First, due to better temporal resolution (seconds rather than minutes), we could show that the progressive build up of [gluc] during NREM sleep occurs from the very onset of the sleep episode. Although we could measure directly neither glucose supply nor glucose utilization, our results support previous conclusions (Netchiporouk et al. 2001) that the ratio between these two processes remains positive, and actually increases in the course of NREM sleep, suggesting that the longer is NREM sleep, the more glucose is available in the brain. It is thought that sleep, and especially NREM sleep that accounts for most of sleep, may be especially important for restoring the brain and provide something not afforded by quiet wake. Specifically, the many molecular changes that have been identified between wakefulness and sleep suggest that sleep could counteract synaptic fatigue by favoring the replenishment of calcium in presynaptic stores, the replenishment of glutamate vesicles, the resting of mitochondria, the synthesis of proteins, or the trafficking and recycling of membranes (Cirelli et al. 2004, Mackiewicz et al. 2007, Mongrain et al. 2010). Our findings support the notion that high levels of glucose during NREM sleep could be beneficial for such restorative processes.
Second, by performing 2–3 days of continuous recordings we found that the build up of [gluc] during NREM sleep is not affected by sleep/wake history, i.e. it does not differ in early sleep relative to late sleep, or during recovery sleep after sleep deprivation relative to baseline sleep. This finding should be put in the context of recent studies showing that, in contrast to glucose, cortical levels of glutamate and lactate decline faster when sleep pressure is high (early sleep, recovery sleep) than when is low (late sleep, baseline sleep) (Dash et al. 2009, Dash et al. 2012, Wisor et al. 2012). As previously discussed (Dash et al. 2012), it is likely that changes in extrasynaptic glutamate concentration mainly reflect activity-induced release of glutamate, with the progressive increase during wake resulting from the increase in glutamatergic transmission and firing rate occurring during wake, and the faster decline during high pressure NREM sleep reflecting the presence of longer periods of neuronal silence (Vyazovskiy et al. 2009). Changes in lactate, we also speculated (Dash et al. 2012), primarily arise from changes in astrocytic glycolysis that follow the uptake of extracellular glutamate by astrocytes (Pellerin & Magistretti 1994, Bittner et al. 2011). As shown in the present study, overall glucose levels did not monotonically decline during wake but actually increased after extended wake. Thus, since overall glucose levels do not reliably reflect wake duration, it is perhaps not surprising that they should not reflect sleep homeostasis. A similar argument could be used for oxygen levels, which we recently found to increase during wake in relation to the type of wake (e.g. active wake) more than to its duration, and did not reflect sleep homeostasis (Dash et al. 2012).
Third, we found that upon awakening an early decline in [gluc] was followed by an increase ~8–15 minutes afterwards. Again, our method cannot determine whether the late increase was due to increased glucose supply, decreased glucose utilization, or a combination of the two. However, a delayed increase in glucose supply due to increased blood flow seems unlikely, because neurovascular coupling in humans typically increases glucose availability in a matter of seconds (Raichle et al. 1976, Lin et al. 2010). Moreover, we recently found in rats that cortical oxygen concentrations (and thus presumably cerebral blood flow) begin to increase ~30 seconds after the transition to wake (Dash et al. 2012). Changes in neurobarrier coupling, for instance the glucose transporter capacity at the blood brain barrier (Leybaert et al. 2007), may provide a more plausible mechanism to account for the time course of cortical [gluc] at wake onset. Glucose transporter 1 (Glut1) predominantly carries glucose across the blood brain barrier (Vannucci et al. 1997, Simpson et al. 2007) and mRNA encoding for Glut1 increases following spontaneous wake and sleep deprivation (Cirelli & Tononi 2000, Petit et al. 2010). Although it is unclear if Glut1 expression in the endothelium can rapidly increase or decrease as a consequence of change in behavioral state, Glut1 expression has been shown to change in response to chronic changes in glucose consumption (Duelli et al. 1998a, Duelli et al. 1998b).
Finally, an intriguing finding of this study is that, in contrast to the overall glucose levels, the duration of the early phase of [gluc] decline during wake was affected by the sleep/wake history, being longer after prolonged wake than after consolidated sleep. Notably, towards the end of the dark period, after the majority of daily wake has occurred, cortical [gluc] was significantly lower than at the start of the dark period, suggesting that when the need for sleep is at its highest the restoration of [gluc] is either insufficient to fully mitigate the costs of wake or less efficacious. Although the mechanisms linking the duration of the glucose ‘dip’ to the duration of previous wake are unknown, a possible candidate is the release of glycogen from the astrocytic stores, which occurs rapidly upon awakening and triggers long-term increases in glycogen synthesis (Karnovsky et al. 1983, Sorg & Magistretti 1992, Kong et al. 2002). Consistent with this idea, a recent study found that sleep deprivation affects the expression of the major enzymes responsible for glycogen synthesis and degradation, likely changing the balance between synthesis and degradation of cortical glycogen in favor of its synthesis (Petit et al. 2010).
The average decline in [gluc] observed upon awakening was ~ 65uM, representing ~ 11% of the mean absolute values of extracellular glucose reported in the rat somatosensory cortex (0.59mM; (Netchiporouk et al. 2001)). A similar decline (11%) has been reported in rat hippocampus during training on an easy spatial memory task, while a more complex task causes [gluc] to drop by 30% (McNay et al. 2000). As shown in Fig. 2a, given the polyphasic nature of rodent sleep, most wake episodes in the rat are of similar or smaller duration. Thus, a rat wakes up many times across the 24-hour cycle, and each time, for several minutes, there is a negative balance between glucose supply and glucose utilization. The functional significance of this decline remains to be determined. As discussed above, two important energy sources – lactate and glycogen – become quickly available upon awakening (within the first minute), and may be sufficient to meet the high energy demand of wake. On the other hand, photic and somatosensory stimulations increase glucose consumption in activated regions, often causing a decrease in [gluc] (e.g. (Ueki et al. 1988, Merboldt et al. 1992); reviewed in (Dienel & Hertz 2001)). Notably, this glucose utilization is most pronounced within the synapse-rich neuropil rather than near cell-bodies (reviewed in (Sokoloff 1999)), consistent with the notion that the majority of energetic demands are associated with the restoration of ionic gradients following action potentials and postsynaptic depolarization (Attwell & Laughlin 2001, Hallermann et al. 2012). Moreover, in rats, glucose administration during training not only prevents the decline in [gluc], but also improves performance and retention of the task (McNay et al. 2000, Suzuki et al. 2011). Thus, it is possible that the [gluc] decline observed during learning, acute neuronal stimulation, and now upon awakening, may negatively affect brain function, especially after sleep deprivation.
Acknowledgments
We thank Dr. Erik Naylor from Pinnacle Technologies for his technical advice. This work was funded by the National Institute of Mental Health (P20 MH077967 to CC), by the NIH Director’s Pioneer award (to GT), and by a grant from the James S. McDonnell Foundation.
Abbreviations
- EEG
electroencephalogram
- EMG
electromyogram
- [gluc]
extracellular concentration of glucose
- GLUT1
glucose transporter 1
- NREM sleep
non rapid eye movement sleep
- REM sleep
rapid eye movement sleep
- SWA
slow wave activity
Footnotes
All authors have indicated no conflicts of interest.
References
- Alfoldi P, Franken P, Tobler I, Borbely AA. Short light-dark cycles influence sleep stages and EEG power spectra in the rat. Behavioral Brain Research. 1991;43:125–131. doi: 10.1016/s0166-4328(05)80062-2. [DOI] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Bazzu G, Puggioni GG, Dedola S, et al. Real-time monitoring of brain tissue oxygen using a miniaturized biotelemetric device implanted in freely moving rats. Anal Chem. 2009;81:2235–2241. doi: 10.1021/ac802390f. [DOI] [PubMed] [Google Scholar]
- Benoit-Marand M, Suaud-Chagny MF, Gonon F. Presynaptic Regulation of Extracellular Dopamine as Studied by Continuous Amperometry in Anesthetized Animals. In: Michael A, Borland L, editors. Electrochemical Methods for Neuroscience. CRC Press; Boca Raton: 2007. [PubMed] [Google Scholar]
- Bittner CX, Valdebenito R, Ruminot I, et al. Fast and reversible stimulation of astrocytic glycolysis by K+ and a delayed and persistent effect of glutamate. J Neurosci. 2011;31:4709–4713. doi: 10.1523/JNEUROSCI.5311-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braitenberg V, Schuz A. Cortex: Statistics and Geometry of Neuronal Connectivity. Springer; New York: 1998. [Google Scholar]
- Canal CE, McNay EC, Gold PE. Increases in extracellular fluid glucose levels in the rat hippocampus following an anesthetic dose of pentobarbital or ketamine-xylazine: an in vivo microdialysis study. Physiol Behav. 2005;84:245–250. doi: 10.1016/j.physbeh.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Gutierrez CM, Tononi G. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron. 2004;41:35–43. doi: 10.1016/s0896-6273(03)00814-6. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Gene expression in the brain across the sleep-waking cycle. Brain Res. 2000;885:303–321. doi: 10.1016/s0006-8993(00)03008-0. [DOI] [PubMed] [Google Scholar]
- Dash MB, Douglas CL, Vyazovskiy VV, Cirelli C, Tononi G. Long-term homeostasis of extracellular glutamate in the rat cerebral cortex across sleep and waking states. J Neurosci. 2009;29:620–629. doi: 10.1523/JNEUROSCI.5486-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash MB, Tononi G, Cirelli C. Extracellular levels of lactate, but not oxygen, reflect sleep homeostasis in the rat cerebral cortex. Sleep. 2012;35:909–919. doi: 10.5665/sleep.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedrick DF, Sherer YD, Biebuyck JF. Use of a rapid brain-sampling technique in a physiologic preparation: effects of morphine, ketamine, and halothane on tissue energy intermediates. Anesthesiology. 1975;42:651–657. doi: 10.1097/00000542-197506000-00002. [DOI] [PubMed] [Google Scholar]
- Desiraju T. Discharge properties of neurons of the parietal association cortex during states of sleep and wakefulness in the monkey. Brain Res. 1972;47:69–75. doi: 10.1016/0006-8993(72)90252-1. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Hertz L. Glucose and lactate metabolism during brain activation. J Neurosci Res. 2001;66:824–838. doi: 10.1002/jnr.10079. [DOI] [PubMed] [Google Scholar]
- Dixon BM, Lowry JP, O’Neill RD. Characterization in vitro and in vivo of the oxygen dependence of an enzyme/polymer biosensor for monitoring brain glucose. J Neurosci Methods. 2002;119:135–142. doi: 10.1016/s0165-0270(02)00170-x. [DOI] [PubMed] [Google Scholar]
- Duelli R, Maurer MH, Kuschinsky W. Decreased glucose transporter densities, rate constants and glucose utilization in visual structures of rat brain during chronic visual deprivation. Neurosci Lett. 1998a;250:49–52. doi: 10.1016/s0304-3940(98)00457-1. [DOI] [PubMed] [Google Scholar]
- Duelli R, Staudt R, Grunwald F, Kuschinsky W. Increase of glucose transporter densities (Glut1 and Glut3) during chronic administration of nicotine in rat brain. Brain Res. 1998b;782:36–42. doi: 10.1016/s0006-8993(97)01264-x. [DOI] [PubMed] [Google Scholar]
- Fisher SP, Sugden D. Endogenous melatonin is not obligatory for the regulation of the rat sleep-wake cycle. Sleep. 2010;33:833–840. doi: 10.1093/sleep/33.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitonde MK, Jones J, Evans G. Metabolism of glucose into glutamate via the hexose monophosphate shunt and its inhibition by 6-aminonicotinamide in rat brain in vivo. Proc R Soc Lond B Biol Sci. 1987;231:71–90. doi: 10.1098/rspb.1987.0036. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Marrett S, Vafaee M. Oxidative and nonoxidative metabolism of excited neurons and astrocytes. J Cereb Blood Flow Metab. 2002;22:1–14. doi: 10.1097/00004647-200201000-00001. [DOI] [PubMed] [Google Scholar]
- Hallermann S, de Kock CP, Stuart GJ, Kole MH. State and location dependence of action potential metabolic cost in cortical pyramidal neurons. Nat Neurosci. 2012;15:1007–1014. doi: 10.1038/nn.3132. [DOI] [PubMed] [Google Scholar]
- Hu Y, Wilson GS. Rapid changes in local extracellular rat brain glucose observed with an in vivo glucose sensor. J Neurochem. 1997;68:1745–1752. doi: 10.1046/j.1471-4159.1997.68041745.x. [DOI] [PubMed] [Google Scholar]
- Jones EG. The Origins of Cortical Interneurons: Mouse versus Monkey and Human. Cerebral Cortex. 2009;19:1953–1956. doi: 10.1093/cercor/bhp088. [DOI] [PubMed] [Google Scholar]
- Karnovsky ML, Reich P, Anchors JM, Burrows BL. Changes in brain glycogen during slow-wave sleep in the rat. J Neurochem. 1983;41:1498–1501. doi: 10.1111/j.1471-4159.1983.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Kennedy C, Gillin JC, Mendelson W, et al. Local cerebral glucose utilization in non-rapid eye movement sleep. Nature London. 1982;297:325–327. doi: 10.1038/297325a0. [DOI] [PubMed] [Google Scholar]
- Kong J, Shepel PN, Holden CP, Mackiewicz M, Pack AI, Geiger JD. Brain glycogen decreases with increased periods of wakefulness: implications for homeostatic drive to sleep. J Neurosci. 2002;22:5581–5587. doi: 10.1523/JNEUROSCI.22-13-05581.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei B, Adachi N, Arai T. Measurement of the extracellular H2O2 in the brain by microdialysis. Brain Res Brain Res Protoc. 1998;3:33–36. doi: 10.1016/s1385-299x(98)00018-x. [DOI] [PubMed] [Google Scholar]
- Leybaert L, De Bock M, Van Moorhem M, Decrock E, De Vuyst E. Neurobarrier coupling in the brain: adjusting glucose entry with demand. J Neurosci Res. 2007;85:3213–3220. doi: 10.1002/jnr.21189. [DOI] [PubMed] [Google Scholar]
- Lin AL, Fox PT, Hardies J, Duong TQ, Gao JH. Nonlinear coupling between cerebral blood flow, oxygen consumption, and ATP production in human visual cortex. Proc Natl Acad Sci U S A. 2010;107:8446–8451. doi: 10.1073/pnas.0909711107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackiewicz M, Shockley KR, Romer MA, et al. Macromolecule biosynthesis - a key function of sleep. Physiol Genomics. 2007;31:441–457. doi: 10.1152/physiolgenomics.00275.2006. [DOI] [PubMed] [Google Scholar]
- Madsen PL, Vorstrup S. Cerebral blood flow and metabolism during sleep. Cerebrovascular and Brain Metabolism Reviews. 1991;3:281–296. [PubMed] [Google Scholar]
- McNay EC, Fries TM, Gold PE. Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proc Natl Acad Sci U S A. 2000;97:2881–2885. doi: 10.1073/pnas.050583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShane BB, Galante RJ, Jensen ST, Naidoo N, Pack AI, Wyner A. Characterization of the bout durations of sleep and wakefulness. J Neurosci Methods. 2010;193:321–333. doi: 10.1016/j.jneumeth.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merboldt KD, Bruhn H, Hanicke W, Michaelis T, Frahm J. Decrease of glucose in the human visual cortex during photic stimulation. Magn Reson Med. 1992;25:187–194. doi: 10.1002/mrm.1910250119. [DOI] [PubMed] [Google Scholar]
- Mongrain V, Hernandez SA, Pradervand S, Dorsaz S, Curie T, Hagiwara G, Gip P, Heller HC, Franken P. Separating the contribution of glucocorticoids and wakefulness to the molecular and electrophysiological correlates of sleep homeostasis. Sleep. 2010;33:1147–1157. doi: 10.1093/sleep/33.9.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murr R, Berger S, Schurer L, Peter K, Baethmann A. A novel, remote-controlled suspension device for brain tissue PO2 measurements with multiwire surface electrodes. Pflugers Arch. 1994;426:348–350. doi: 10.1007/BF00374792. [DOI] [PubMed] [Google Scholar]
- Nair PK, Buerk DG, Halsey JH., Jr Comparisons of oxygen metabolism and tissue PO2 in cortex and hippocampus of gerbil brain. Stroke. 1987;18:616–622. doi: 10.1161/01.str.18.3.616. [DOI] [PubMed] [Google Scholar]
- Naylor E, Aillon DV, Barrett BS, Wilson GS, Johnson DA, Harmon HP, Gabbert S, Petillo PA. Lactate as a biomarker for sleep. Sleep. 2012;35:1209–1222. doi: 10.5665/sleep.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netchiporouk L, Shram N, Salvert D, Cespuglio R. Brain extracellular glucose assessed by voltammetry throughout the rat sleep-wake cycle. Eur J Neurosci. 2001;13:1429–1434. doi: 10.1046/j.0953-816x.2001.01503.x. [DOI] [PubMed] [Google Scholar]
- Noda H, Adey WR. Neuronal activity in the association cortex of the cat during sleep, wakefulness and anesthesia. Brain Res. 1973;54:243–259. doi: 10.1016/0006-8993(73)90047-4. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit JM, Tobler I, Kopp C, Morgenthaler F, Borbely AA, Magistretti PJ. Metabolic response of the cerebral cortex following gentle sleep deprivation and modafinil administration. Sleep. 2010;33:901–908. doi: 10.1093/sleep/33.7.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantadosi CA, Tatro LG. Regional H2O2 concentration in rat brain after hyperoxic convulsions. J Appl Physiol. 1990;69:1761–1766. doi: 10.1152/jappl.1990.69.5.1761. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Grubb RL, Jr, Gado MH, Eichling JO, Ter-Pogossian MM. Correlation between regional cerebral blood flow and oxidative metabolism. In vivo studies in man. Archives of Neurology. 1976;33:523–526. doi: 10.1001/archneur.1976.00500080001001. [DOI] [PubMed] [Google Scholar]
- Rice ME. H2O2: a dynamic neuromodulator. Neuroscientist. 2011;17:389–406. doi: 10.1177/1073858411404531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Hermans A, Seipel AT, Wightman RM. Monitoring rapid chemical communication in the brain. Chem Rev. 2008;108:2554–2584. doi: 10.1021/cr068081q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shram N, Netchiporouk L, Cespuglio R. Lactate in the brain of the freely moving rat: voltammetric monitoring of the changes related to the sleep-wake states. Eur J Neurosci. 2002;16:461–466. doi: 10.1046/j.1460-9568.2002.02081.x. [DOI] [PubMed] [Google Scholar]
- Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci U S A. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesjo B. Brain Energy Metabolism. John Wiley & Sons; New York: 1978. [Google Scholar]
- Silver IA, Erecinska M. Extracellular glucose concentration in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J Neurosci. 1994;14:5068–5076. doi: 10.1523/JNEUROSCI.14-08-05068.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff L. Metabolism of the central nervous system in vivo. In: Field J, Magoun HW, editors. Handbook of Physiology. Neurophysiology. III. American Physiological Society; Washington, DC: 1960. pp. 1843–1864. [Google Scholar]
- Sokoloff L. Energetics of functional activation in neural tissues. Neurochem Res. 1999;24:321–329. doi: 10.1023/a:1022534709672. [DOI] [PubMed] [Google Scholar]
- Sorg O, Magistretti PJ. Vasoactive intestinal peptide and noradrenaline exert long-term control on glycogen levels in astrocytes: blockade by protein synthesis inhibition. J Neurosci. 1992;12:4923–4931. doi: 10.1523/JNEUROSCI.12-12-04923.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, McCarley RW. Brainstem Control of Wakefulness and Sleep. Plenum Press; New York: 1990. [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 2001;85:1969–1985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki M, Linn F, Hossmann KA. Functional activation of cerebral blood flow and metabolism before and after global ischemia of rat brain. Journal of Cerebral Blood Flow and Metabolism. 1988;8:486–494. doi: 10.1038/jcbfm.1988.89. [DOI] [PubMed] [Google Scholar]
- Van den Noort S, Brine K. Effect of sleep on brain labile phosphates and metabolic rate. Am J Physiol. 1970;218:1434–1439. doi: 10.1152/ajplegacy.1970.218.5.1434. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Maher F, Simpson IA. Glucose transporter proteins in brain: delivery of glucose to neurons and glia. Glia. 1997;21:2–21. doi: 10.1002/(sici)1098-1136(199709)21:1<2::aid-glia2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Cirelli C, Tononi G. Electrophysiological correlates of sleep homeostasis in freely behaving rats. Prog Brain Res. 2011;193:17–38. doi: 10.1016/B978-0-444-53839-0.00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy VV, Cirelli C, Tononi G, Tobler I. Cortical metabolic rates as measured by 2-deoxyglucose-uptake are increased after waking and decreased after sleep in mice. Brain Res Bull. 2008;75:591–597. doi: 10.1016/j.brainresbull.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy VV, Olcese U, Lazimy YM, Faraguna U, Esser SK, Williams JC, Cirelli C, Tononi G. Cortical firing and sleep homeostasis. Neuron. 2009;63:865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Electrochemical glucose biosensors. Chem Rev. 2008;108:814–825. doi: 10.1021/cr068123a. [DOI] [PubMed] [Google Scholar]
- Wilson GS, Gifford R. Biosensors for real-time in vivo measurements. Biosens Bioelectron. 2005;20:2388–2403. doi: 10.1016/j.bios.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Wisor JP, Rempe MJ, Schmidt MA, Moore ME, Clegern WC. Sleep Slow-Wave Activity Regulates Cerebral Glycolytic Metabolism. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs189. [DOI] [PMC free article] [PubMed] [Google Scholar]