Abstract
Nε-acetylation occurs on select lysine residues in α-crystallin of the human lens and alters its chaperone function. In this study, we investigated the effect of Nε-acetylation on advanced glycation end product (AGE) formation and consequences of the combined Nε-acetylation and AGE formation on the function of α-crystallin. Immunoprecipitation experiments revealed that Nε-acetylation of lysine residues and AGE formation co-occurs in both αA- and αB-crystallin of the human lens. Prior acetylation of αA- and αB-crystallin with acetic anhydride (Ac2O) before glycation with methylglyoxal (MGO) resulted in significant inhibition of the synthesis of two AGEs, hydroimidazolone (HI) and argpyrimidine. Similarly, synthesis of ascorbate-derived AGEs, pentosidine and Nε-carboxymethyl lysine (CML), was inhibited in both proteins by prior acetylation. In all cases, inhibition of AGE synthesis was positively related to the degree of acetylation. While prior acetylation further increased the chaperone activity of MGO-glycated αA-crystallin, it inhibited the loss of chaperone activity by ascorbate-glycation in both proteins. BioPORTER-mediated transfer of αA- and αB-crystallin into CHO cells resulted in significant protection against hyperthermia-induced apoptosis. This effect was enhanced in acetylated and MGO-modified αA- and αB-crystallin. Caspase-3 activity was reduced in α-crystallin transferred cells. Glycation of acetylated proteins with either MGO or ascorbate produced no significant change in the anti-apoptotic function. Collectively, these data demonstrate that lysine acetylation and AGE formation can occur concurrently in α-crystallin of human lens, and that lysine acetylation improves anti-apoptotic function of α-crystallin and prevents ascorbate-mediated loss of chaperone function.
Keywords: Acetylation, α-Crystallin, Chaperone, Glycation, Apoptosis
1. Introduction
Crystallins account for more than 90% of vertebrate lens proteins, and α-crystallin accounts for 40–50% of lens crystallins. α-Crystallin is composed of two sub units, αA- and αB-crystallin [1]. They exist as large oligomers of 30–40 subunits in a preferred ratio of 3:1 (αA:αB) in the human lens [2]. α-Crystallin is essential for both structural integrity of the lens and for keeping other lens proteins soluble [3]. While αA-crystallin is predominantly present in the lens with small amounts in the retina, αB-crystallin is present, in addition to the lens, in a wide variety of tissues, such as skeletal muscle, heart, kidney, brain, and retina [4–6]. Both αA- and αB-crystallin are chaperone proteins. They bind to structurally perturbed proteinsand prevent their denaturation [7]. In the lens, α-crystallin binds to other crystallins (β- and γ-crystallins) and cytoskeletal proteins, and prevents their denaturation from age-associated stresses, such as oxidation [8]. α-Crystallin is a robust anti-apoptotic protein, and it inhibits apoptosis of cells through different mechanisms: by binding to procaspase-3 and Bax, inhibiting cytochrome-C release from the mitochondria, by activating PI3 kinase, by promoting the activation of PDK-1 and AKT, and by inhibiting PTEN [9–12].
The lens grows throughout life with very little protein turnover; this entails accumulation of post-translational modifications of lens proteins throughout life [13]. The major modifications include glycation, acetylation, deamidation, truncation, phosphorylation, oxidation, and carbamylation [14–18]. Previous reports indicate that the N-terminal residue in α-crystallin can be readily acetylated, and acetylation leads to alterations in protein structure [19–22]. Nε-acetylation of epsilon group of lysine is another modification. Aspirin has been used as an anti-cataract agent [23]; it inhibits cataract development in experimental animals possibly through Nε-acetylation of lysine residues in lens proteins. In fact, one previous study has shown Nε-acetylation of specific lysine residues in aspirin-treated lens proteins [24]. We previously reported that major lysine acetylation sites in αA-crystallin were K70 and K99, and in αB-crystallin were K92 and K166 [25]. We showed that in vitro acetylation of αA-crystallin alters its chaperone function.
Glycation is another significant post-translational modification in proteins [26]. Both intracellular and extracellular proteins are targets for this modification. The glycation reaction occurs between carbonyl compounds and free amino groups in proteins. The Amadori product, the first stable product of the reaction, is transformed by a series of reactions into a variety of stable products that are collectively known as advanced glycation end products or AGEs. Some AGEs are fluorescent and some are formed as amino acid crosslinking adducts in proteins [17]. Formation of AGEs due to glycation alters the surface charge on the protein leading to conformational changes, which in turn affect protein–protein and protein–water interactions [27]. These properties of AGEs have detrimental effects on the transparency of the lens.
Methylglyoxal (MGO) has been identified as one of the major precursors of AGEs in the human lens. MGO is primarily derived from triose phosphate intermediates of glycolysis [28–31]. Glycation by MGO occurs mainly on arginine residues. Hydroimidazolone and argpyrimidine are two of such AGEs, and both are present in human lenses. Ascorbic acid is a major antioxidant in lens, but its oxidation products are strong precursors of AGEs [32–34]. K2P, vesperlysine, pentosidine, carboxymethyl lysine (CML), and OP-lysine are some of the AGEs in the human lens produced from ascorbate oxidation products [35]. These AGEs accumulate with age in the lens, and their accumulation occurs at a higher rate in cataractous lenses [35].
The fact that α-crystallin is the most abundant protein in the lens, coupled with the fact that it does not turnover during the lifespan of an individual, suggest that post translational modifications of this protein not only affect transparency of the lens, but could also affect its two important functions, chaperoning and anti-apoptosis. With this in mind, we investigated the effects of acetylation and glycation on the chaperone and anti-apoptotic function of αA- and αB-crystallin.
2. Materials and Methods
Acetic anhydride, MGO, ascorbic acid and lysozyme were obtained from Sigma-Aldrich Chemical Co. LLC (St. Louis, MO, USA). All chemicals were of analytical grade. Non-cataractous human lenses were obtained from Heartland Lions Eye Bank, St. Louis, MO. Senile cataractous lenses (age: 65–78 years) were obtained from Illadevi Cataract and IOL Research Centre, Ahmedabad, India.
2.1. Nε-acetyllysine in human lens αB-crystallin
Previously we reported the occurrence of Nε-acetyllysine in human lens αA-crystallin [25]. Here we determined Nε-acetyllysine in αB-crystallin of aging and cataractous lenses. Lenses were stored at −80°C until use. The water-soluble fraction was prepared from these lenses as previously described [25]. Senile cataractous lenses (classified based on pigmentation according to the Pirie classification [36]) were homogenized in 20 mM sodium phosphate buffer, pH 7.4 containing 200 µM EDTA (2.0 ml/lens), dialyzed against PBS, and lyophilized. The lyophilized protein was subjected to sonication (amplitude = 30%, three 40 second cycles) in 0.5 ml PBS-EDTA (200 µM). The sonicated sample was centrifuged at 20,000 g for 30 min. Protein concentration in the resulting supernatant were determined using the BCA Protein Assay Kit (Thermo Scientific, Rockford, IL) and BSA as the standard. Samples (20 µg protein) were separated by polyacrylamide gel electrophoresis, and Nε-acetyllysine and αB-crystallin were detected by Western blotting using a monoclonal antibody against Nε-acetyllysine (1:50,000 dilution, Cell Signaling Technologies, Danvers, MA) or a polyclonal antibody against αB-crystallin (1:1,000 dilution, Enzo life Sciences, Farmingdale, NY).
2.2. Immunoprecipitation studies to determine acetylation and glycation in human lens αA and αB-crystallin
Water soluble protein from three donor lenses was used. For the detection of Nε-acetyllysine in αA- and αB-crystallin, 500 µg protein was incubated with 5 µg of monoclonal antibody to Nε-acetyllysine. For CML and argpyrimidine detection in αA- and αB-crystallin, 500 µg of protein was incubated with 5 µg of either mouse monoclonal anti-CML (from Jes Thorn Clausen, Novo Nordisk, Germany) or argpyrimidine antibody (from Koji Uchida, Nagoya University, Japan) for 3 h at 37°C followed by addition of protein A/G-Sepharose (Santa Cruz Biotechnology, Santa Cruz, CA) and overnight incubation at 4°C with shaking. The sample mixture was centrifuged, and the pellet was washed three times with PBS. The gel pellet was dissolved in 2 X SDS-PAGE sample buffer, and proteins were subjected to electrophoresis on a 12% gel. Western blot was carried out with antibodies to αA-crystallin, and αB-crystallin (Enzo life Sciences, Farmingdale, NY).
2.3. Cloning and purification of human αA and αB-crystallin
Cloning and purification of αA- and αB-crystallin were done as previously described [25].
2.4. In vitro acetylation of αA and αB-crystallin
Acetylation of αA- and αB-crystallin using lysine:Ac2O molar ratios of 1:0, 1:0.05, 1:0.5, 1:1, and 1:10 was performed as previously described [25].
2.5. Incubation of αA and αB-crystallin with MGO and ascorbate
Recombinant human αA- and αB-crystallin, either acetylated or unacetylated (1 mg/ml) were incubated for 7 days at 37°C in 0.1 M sodium phosphate buffer (pH 7.4) with glycating agents: 100 µM MGO or 10 mM ascorbate. All reaction mixtures were sterile-filtered using 0.2 µm syringe filters (Millipore Corp, MA) before incubation. After incubation, samples were dialyzed against 2 L of PBS for 24 hrs, and protein concentration was measured by the BCA method (Thermo Scientific, Rockford, IL).
2.6. Chaperone assay with lysozyme
The chaperone assay was carried out as previously described [25] using a ratio of αA- and αB-crystallin to lysozyme of 1:4. Briefly, assays were carried out at 37°C in 50 mM phosphate buffer, pH 7.4 that had 150 mM sodium chloride. The aggregation of lysozyme was initiated by 2 mM Tris(2-carboxyethyl)phosphine (TCEP) (Thermo scientific, Rockford, IL) and light scattering was monitored at 360 nm. Lysozyme incubated alone in the absence of α-crystallin served as the control.
2.7. Direct ELISA for CML, HI, and argpyrimidine
Microplate wells were coated with 5 µg protein/well in 50 µl of 50 mM sodium carbonate buffer, pH 9.6, overnight at 4°C followed by washing three times with PBST. The wells were blocked with 5% non-fat dry milk (NFDM) in PBST for 2 h in a humid chamber. Following washing, mouse monoclonal antibody to CML, HI (HI monoclonal antibody was made in mice by immunizing HI coupled to KLH) or argpyrimidine (in PBST-NFDM) (all 1:5000 dilutions) were added and incubated for 1 h at 37°C. The wells were then washed three times with PBST and incubated with goat anti-mouse antibody (1:5000) conjugated to horseradish peroxidase (Promega, Madison, WI). Following incubation for 1 h and washing three times with PBST, the wells were incubated with 100 µl of 3,3′,5,5′-tetramethylbenzidine dihydrochloride (Sigma-Aldrich) substrate for 45 min, 37°C. The enzymatic reaction was stopped at 45 min by the addition of 50 µl of 2 N H2SO4and the optical density of the wells was read at 450 nm in a microplate reader.
2.8. Measurement of pentosidine
Protein samples (250 µg/ml) were subjected to acid hydrolysis. Dried samples were reconstituted in 200 µl of water and filtered through 0.45 µm centrifugal filters. Pentosidine was measured by HPLC using a reversed-phase C18 column as previously described [37]. The amino acid content of the acid hydrolysates was estimated by the ninhydrin assay [17]. Pentosidine concentrations in the protein samples were calculated based on standard curves generated using synthetic compound (synthesized by the previously described procedure [38]), and were expressed as pmoles/µmole of amino acid.
2.9. Measurement of apoptosis
α-Crystallin was mixed in a cationic lipid, BioPORTER, according to the manufacture’s instructions (Gene Therapy Systems, Inc., San Diego, CA). PBS (100 µl) containing 7.5 µg of protein was added to each tube, thoroughly mixed by vortexing for 15s, and then incubated at room temperature for 5 min. Chinese hamster ovary (CHO) cells were cultured in Ham’s F12 media supplemented with 10% fetal bovine serum. When cells were 80% confluent, adherent cells were treated with BioPORTER alone or BioPORTER containing proteins in serum free media. Cells were incubated for 4 hrs at 37°C in a humidified 5% CO2 atmosphere, and then washed with PBS. The cells were incubated at 43°C for 1 h, followed by incubation at 37°C for 24 h. The percentage of apoptotic cells was determined by staining the cells with Hoechst 33342.
2.10. Measurement of Caspase-3 activity
Protein lysates (100 µg) were incubated with 0.2 mM Ac-DEVD-AFC (acetyl-DEVD-7-amino-4-trifluoromethylcoumarin) at 37°C for 60 min in HEPES-Buffer [20 mM HEPES, pH 7.5, 10% glycerol, and 2 mM DTT (dithiothreitol)] after thermal stress (43°C for 60 min). AFC (7-amino-4-trifluoromethylcoumarin) released from the substrates was measured using a microtiter plate reader with an excitation wavelength of 400 nm and an emission wavelength of 505 nm.
2.11. Statistics
Results are given as the mean ± SD. Statistical significance was determined by two-tailed t test. Values of p <0.05 were considered to be significant.
3. Results
3.1. Acetylation of αB-crystallin in human lens
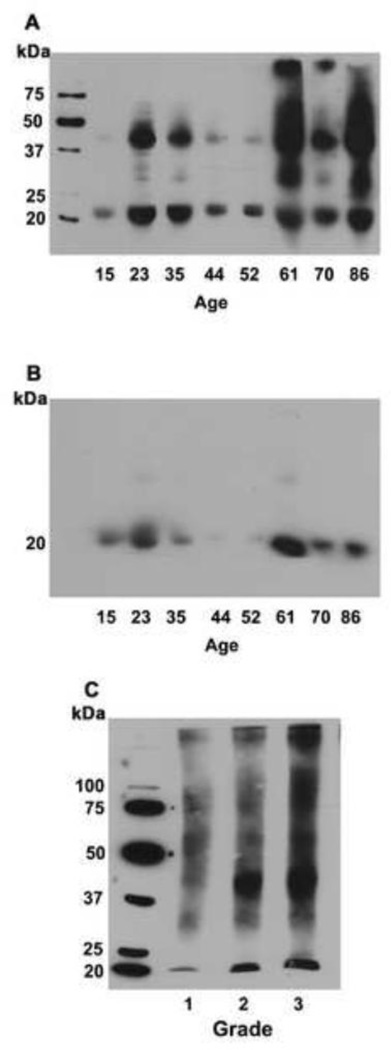
The effect of age and cataract on lysine acetylation in human lens αB-crystallin was investigated. Normal lenses of varying age along with cataractous lenses were analyzed. Immunoblotting with an antibody to Nε-acetyllysine showed considerable heterogeneity in the reaction with the antibody, revealing multiple immunoreactive protein bands with strong reactions in the 20–25 and the 40–45 kDa regions in all normal lenses (Fig. 1A). In aged lenses (>60 years) high molecular weight proteins (> 50 kDa) also reacted with the antibody. The immunoblotted membrane was stripped and probed again with an antibody to αB-crystallin. The results showed immunoreactivity for Nε-acetyllysine coinciding with that of αB-crystallin, suggesting that αB-crystallin is acetylated in human lens proteins (Fig. 1B). We then investigated the effect of cataract on Nε-acetylation of α-crystallin. Western blot analysis showed immunoreactivity in proteins of molecular weight ~20 kDa along with high molecular weight aggregates (Fig. 1C).
Fig. 1. Acetylation of αB-crystallin in human lens.
Human lens water soluble fractions were subjected to Western blotting with a monoclonal antibody to N -acetyllysine (A). The same membrane was re-probed with a monoclonal antibody for αB-crystallin (B). Cataractous lens proteins from grade 1–3 were sonicated and soluble protein fraction was subjected to Western blotting with Nε-acetyllysine antibody (C).
3.2. Acetylation and AGE-modification co-occur in αA and αB-crystallin
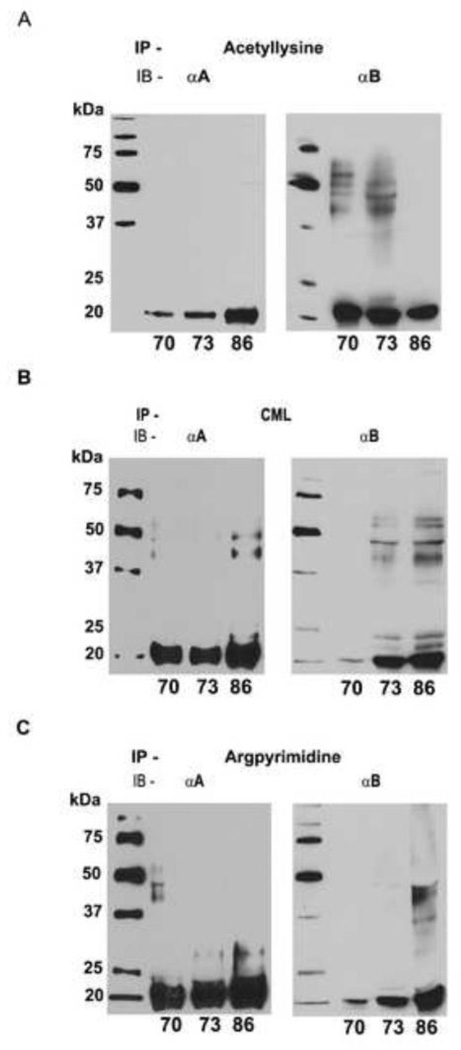
To determine if both Nε-acetylation of lysine and AGEs occur in αA- and αB-crystallin, water-soluble protein from three aged donor lenses (70, 73 and 86 year old) were immunoprecipitated with either Nε-acetyllysine or CML or argpyrimidine monoclonal antibody and Western blotted with anti αA- or an anti αB-crystallin polyclonal antibody. The results confirmed that Nε-acetylation of lysine residues occurs in both αA- and αB-crystallin (Fig. 2A). We then investigated the co-existence of AGEs and acetylated lysine in α-crystallin by Western blotting. Water-soluble protein was immunoprecipitated using CML antibody and probed with either αA- or αB-crystallin antibody. CML was identified in both αA- and αB-crystallin (Fig. 2B). Similarly, argpyrimidine was preseent in both αA- and αB-crystallin (Fig. 2C). Immunoreactions with higher molecular proteins could be due to the presence of AGEs and acetylated α-crystallin in crosslinked proteins. Together, these results suggest that AGE formation and Nε-acetylation of lysine residues concurrently occur in α-crystallin, but whether they occur in the same molecule of α-crystallin needs to be verified.
Fig. 2. Nε-acetylation of lysine residues and AGE formation occur in α-crystallin.
Water-soluble protein from aged donor lenses (70, 73 and 86 year old) was immunoprecipitated (IP) with either a Nε-acetyllysine antibody (A) or a CML antibody (B) or an argpyrimidine antibody (C) and immunoblotted (IB) with αA- and αB-crystallin polyclonal antibodies.
3.3. Nε-acetylation of lysine residues decreases AGE-mediated crosslinking in αA- and αB-crystallin
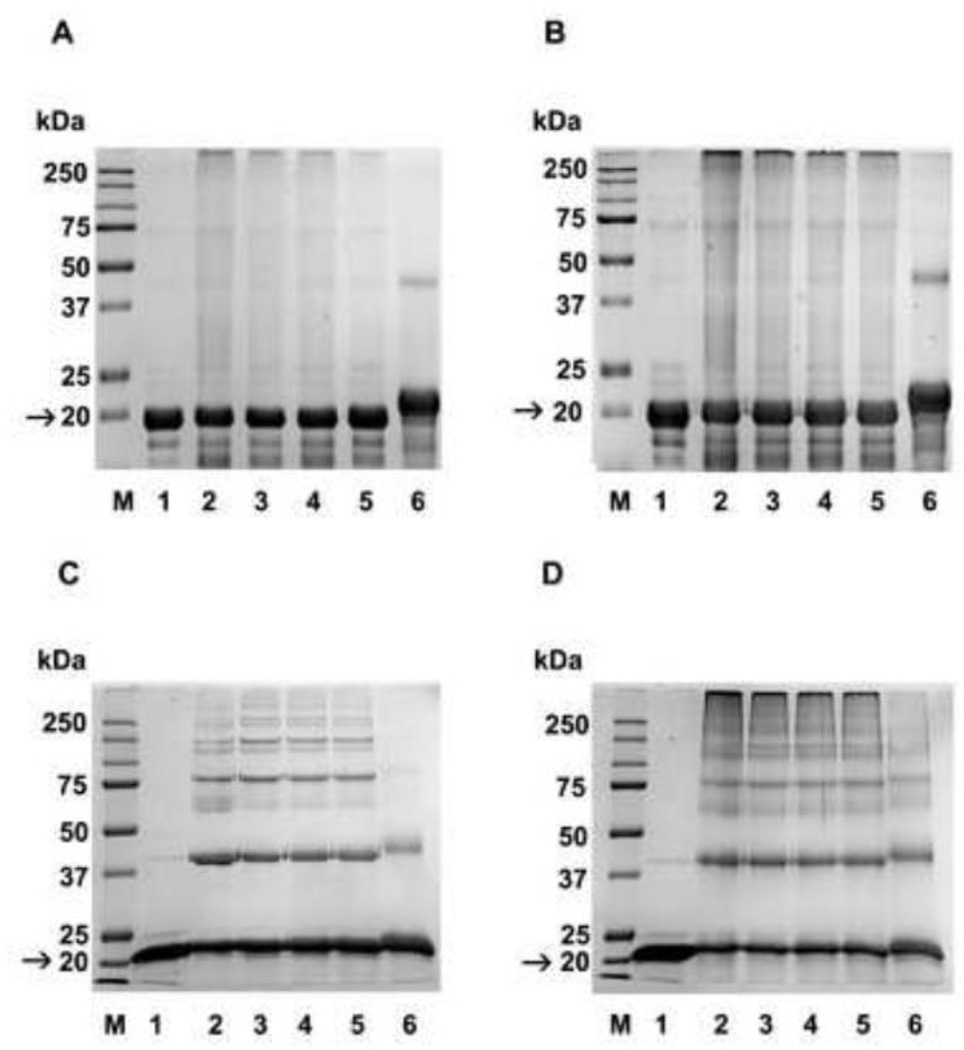
To determine the effect of prior acetylation on glycation, chaperone, and anti-apoptotic function of α-crystallins, recombinant αA- and αB-crystallin were acetylated using Ac2O. We used a 0.05, 0.5, 1, and 10 fold molar excess of Ac2O relative to the lysine content (considering 7 lysine residues per molecule of αA-crystallin and 10 lysine residues in αB-crystallin) for the acetylation studies. To confirm acetylation in α-crystallin, Western blotting was carried out with the Nε-acetyllysine antibody. Results showed strong reaction with αA-and αB-crystallin, and the intensity of the reaction increased with increasing concentrations of Ac2O (Fig. S1B and D). The acetylated and unacetylated proteins were glycated with MGO or ascorbate. In the case of αA-crystallin, glycation by MGO resulted in high molecular weight crosslinked proteins at the top of the running gel, which poorly penetrated the gel (Fig. 3A, lane 2). Extensive crosslinking was also observed in proteins glycated with ascorbate, but the crosslinked proteins were resolved in the running gel (Fig. 3B, lane 2). With αB-crystallin, glycation by MGO resulted in less severe crosslinking (compared to αA-crystallin) (Fig. 3C, lane 2). Similarly, glycation by ascorbate resulted in extensive crosslinking of αB-crystallin (Fig. 3D, lane 2). Prior Nε-acetylation of lysine residues before glycation with either MGO or ascorbate inhibited protein crosslinking in both proteins in a manner dependent on the degree of acetylation (Fig. 3A and B); the greatest inhibition was seen in proteins acetylated with a 10 fold molar excess of Ac2O (lanes 6 in each panel).
Fig. 3. Nε-acetylation of lysine residues decreases AGE-mediated crosslinking in α-crystallin.
Human αA- and αB-crystallin were acetylated with 0.05, 0.5, 1, and 10 fold molar excesses (relative to lysine) of Ac2O. Unacetylated and acetylated αA and αB-crystallin were then glycated with MGO or ascorbate and subjected to SDS-PAGE analysis. A, αA-crystallin glycated with MGO; B, αA-crystallin glycated with ascorbate; C, αB-crystallin glycated with MGO; D, αB-crystallin glycated with ascorbate. In all panels, lane 1=unacetylated protein, lane 2=glycated protein, lanes 3 to 6 are glycated and acetylated, respectively, with 0.05, 0.5, 1, and 10 fold molar excess Ac2O. M=molecular weight markers. Arrow indicates αA or αB-crystallin in samples.
3.4. Effect of lysine acetylation and glycation on the chaperone function of α-crystallin
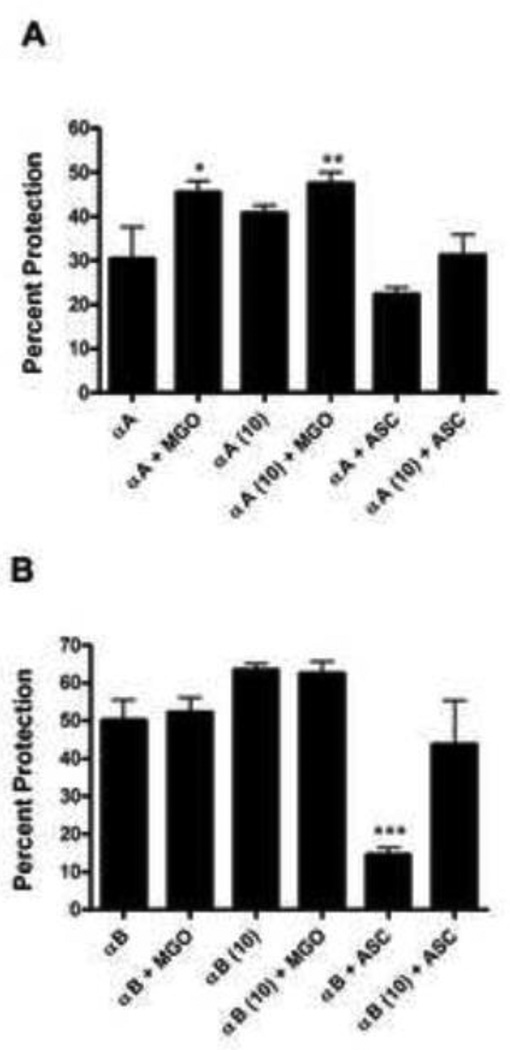
We investigated the effect of prior lysine acetylation on glycation-induced changes in the chaperone function of αA- and αB-crystallin in the thermal aggregation assay of lysozyme. In these experiments we used proteins that were acetylated with 10 fold molar excess of Ac2O. Glycation with ascorbate resulted in decreased chaperone function in both αA- and αB-crystallin compared to the unmodified protein (Fig. 4A and B). Glycation of αA-crystallin with MGO increased the chaperone function of αA-crystallin by ~40% (Fig. 4A), as previously reported [39], but did not affect the chaperone function of αB-crystallin (Fig. 4B). Prior acetylation further improved the chaperone function of MGO-modified αA-crystallin by ~20% and partially prevented the loss of chaperone function in αA-crystallin glycated with ascorbate. The chaperone function of αB-crystallin increased by ~25% after acetylation similar to αA-crystallin, and remained unchanged by MGO modification. Ascorbate-mediated loss in chaperone function (~40%) was partially corrected by prior acetylation (Fig. 4B). Taken together, these results suggest that lysine acetylation improves the chaperone function in both proteins, and MGO-glycation further improves the chaperone function of αA-crystallin, and prior lysine acetylation inhibits the loss of chaperone function by ascorbate-glycation in both αA- and αB-crystallin.
Fig. 4. Effect of Nε-acetylation and glycation on the chaperone function of α-crystallin.
The chaperone function of unacetylated and acetylated αA-crystallin (A) and αB-crystallin (B) after glycation with either 100 µM MGO or 10 mM ascorbate (ASC) for one week was assessed using lysozyme as the client protein. Acetylation was carried out with 10 molar excess of Ac2O in relation to lysine content in α-crystallin. MGO=methylglyoxal, ASC=ascorbate. Each bar represents the mean ± SD of three experiments. *p<0.05, **p<0.005 and ***p<0.0005 compared to unmodified α-crystallin.
3.5. Effect of lysine acetylation on AGE formation in α-crystallin
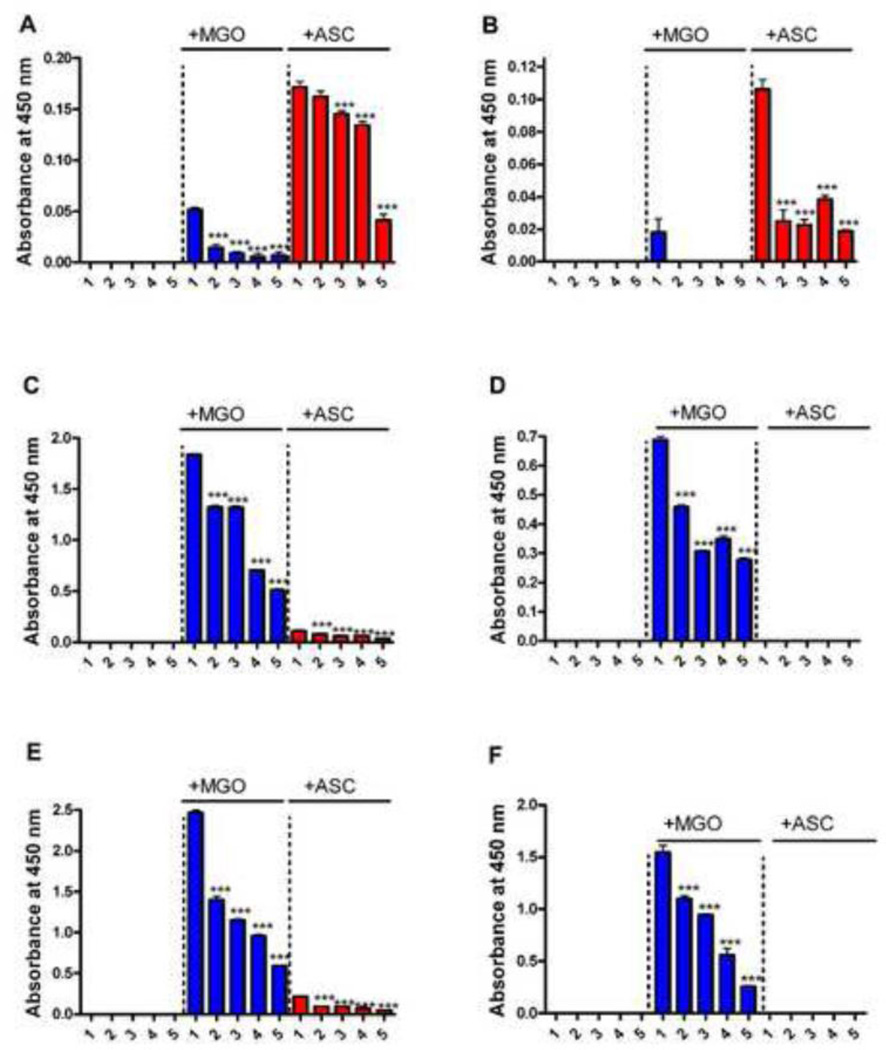
To test the effect of lysine acetylation on AGE formation, we measured CML, argpyrimidine, HI, and pentosidine in acetylated+glycated protein preparations. CML, HI, and argpyrimidine were measured by direct ELISA. While HI is specific for MGO, CML and argpyrimidine are formed from both MGO and ascorbate. In αA-crystallin, lysine acetylation decreased CML formation from ascorbate in a progressive manner; with a 10 fold molar excess of Ac2O there was a ~40% decrease (Fig. 5A). However, in αB-crystallin there was a significant (~80%) decrease with mildly acetylated protein (0.05 molar excess Ac2O) (Fig. 5B), which slightly further decreased (~85%) with 10 fold molar excess Ac2O acetylated protein.
Fig. 5. Nε-acetylation inhibits AGE formation in α-crystallin.
AGE synthesis of unacetylated and acetylated αA- and αB-crystallins after glycation with either MGO or ascorbate was determined by direct ELISAs. The effect on the CML formation in αA and αB-crystallin is shown in A and B. The effect on HI formation in αA- and αB-crystallin is shown in C and D. The effect on argpyrimidine formation in αA- and αB-crystallin is shown in E and F. In each panel 1= unacetylated protein and 2 to 5 = protein acetylated with 0.05, 0.5, 1.0, and 10 fold molar excess Ac2O. Each bar represents the mean ± SD of three experiments. ***p<0.0005 (compared to lane 1 in each case).
HI levels were high in both αA- and αB-crystallin glycated with MGO, and HI levels progressively decreased with increasing acetylation in both proteins (Fig. 5C and D). The effect on argpyrimidine formation was similar to that of HI (Fig. 5E and F); the degree of inhibition was directly proportional to the degree of lysine acetylation. Glycation with ascorbate produced very low levels of argpyrimidine in αA-crystallin, and produced none in αB-crystallin.
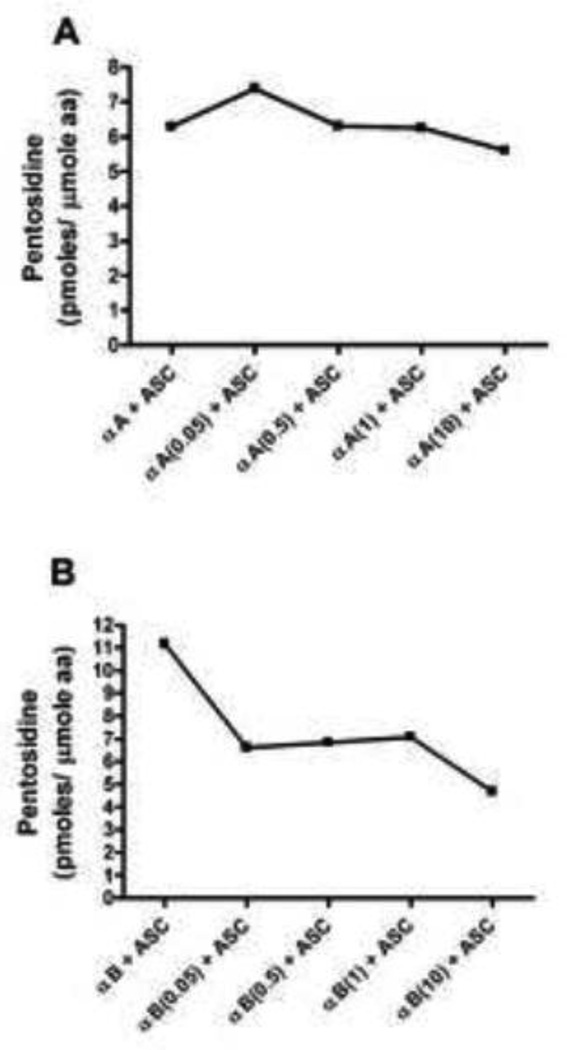
The effect of acetylation on pentosidine synthesis was then investigated. While pentosidine readily formed from ascorbate in both αA- and αB-crystallin (Fig. 6), it was not formed with MGO. Pentosidine synthesis from ascorbate was higher in αB-crystallin (Fig. 6B) compared to αA-crystallin (Fig. 6A). Lysine acetylation reduced pentosidine synthesis from ascorbate mostly in αB-crystallin. With highest acetylation, the inhibition was ~8% and ~50% in αA- and αB-crystallin, respectively. Together, these data suggest that prior acetylation of lysine residues inhibits AGE formation in α-crystallin. The previous observations that aspirin inhibits sugar-mediated crosslinking of lens proteins [40, 41] could be due to inhibition of crosslinking AGEs.
Fig. 6. Nε-acetylation inhibits pentosidine formation from ascorbate in α-crystallin.
Unacetylated and acetylated αA-crystallin (A) and αB-crystallin (B) was incubated with ascorbate for one week. Pentosidine was measured by fluorescence HPLC.
3.6. Effect on the anti-apoptotic function of αA and αB-crystallin
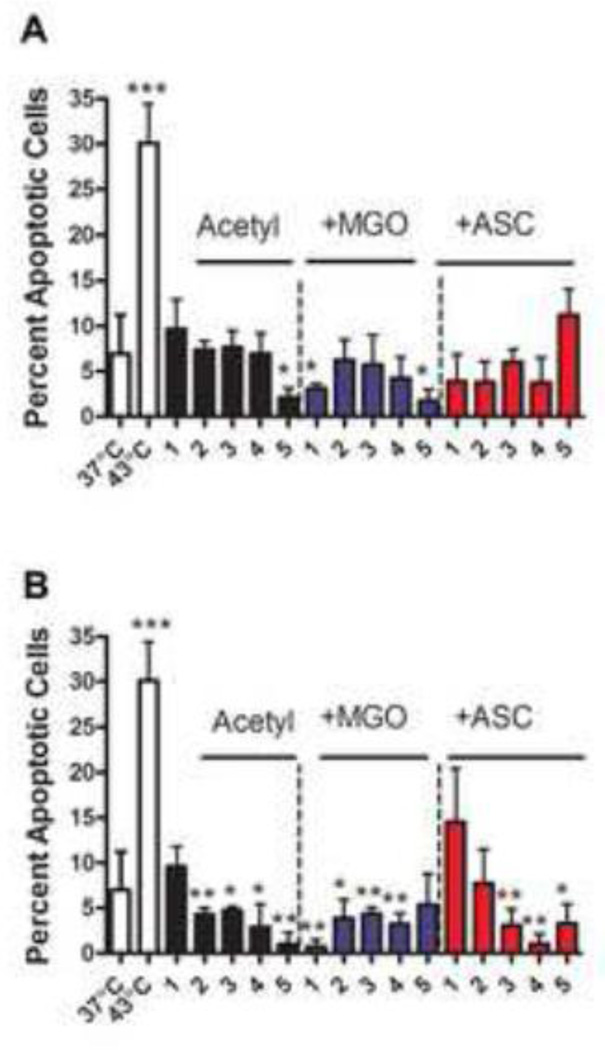
Next, we investigated the effects of acetylation and glycation on the anti-apoptotic function of αA and αB-crystallin in CHO cells upon heat stress. Native, acetylated, and acetylated + glycated αA- and αB-crystallin were transferred to CHO cells using a cationic lipid BioPORTER. Cells were stressed at 43°C for 1 hr to induce apoptosis. Thermal stress induced apoptosis in nearly 30% of cells (Fig. 7). Introduction of αA-crystallin reduced this apoptosis to ~10%. αA- and αB-crystallin acetylated at 10 fold molar excess Ac2O significantly inhibited apoptosis compared to the unmodified protein. The anti-apoptotic function of unacetylated and acetylated αA-crystallin was enhanced by glycation with MGO. The apoptotic cell number dropped to ~7% and ~3%, respectively, after glycation of unacetylated and acetylated αA-crystallin with MGO. The function of acetylated+MGO-glycated protein was slightly weaker than the MGO glycated protein (Fig. 7A), with the exception of protein acetylated with a 10 fold molar excess Ac2O. Glycation with ascorbate surprisingly improved the anti-apoptotic function of αA-crystallin, although at high levels of acetylation (10 fold molar excess Ac2O) there was a decrease in the anti-apoptotic function (12% when compared to 3% with ascorbate glycated protein).
Fig. 7. Effect of Nε-acetylation and glycation on the anti-apoptotic function of αA- and αB-crystallin.
α-Crystallin (unmodified, acetylated, and acetylated+glycated) (7.5 µg of protein) was treated with BioPORTER and added to the media of CHO cells when they reached 80% confluence. Cells were incubated at either 37°C or 43°C for 1 hr, followed by incubation at 37°C for 24 hrs. The percentage of apoptotic cells was determined by staining cells with Hoechst 3334. Panel A= αA-crystallin and panel B= αB-crystallin. In each panel, 1= unmodified protein and 2 to 5 = protein acetylated with 0.05, 0.5, 1.0, and 10 fold molar excess Ac2O or acetylated and glycated. Each bar represents the mean ± SD of three experiments. *p<0.05, **p<0.005 (compared to lane 1 in each case) and ***p<0.0005 (comparing cells at 43°C to cells at 37°C).
αB-Crystallin was as effective as αA-crystallin in inhibiting thermally induced apoptosis in CHO cells; the fraction of apoptotic cells decreased from ~30% to ~10% (Fig. 7B). Lysine acetylation enhanced this anti-apoptotic function; the percent of apoptotic cells dropped nearly to zero with αB-crystallin acetylated with 10 fold molar excess of Ac2O. Glycation by MGO further increased the anti-apoptotic function of unacetylated protein (percent apoptotic cells=2%), but did not significantly alter the anti-apoptotic function of acetylated αB-crystallin. However, protein acetylated with 10 fold molar excess Ac2O and glycated with MGO was less efficient than acetylated but not glycated protein. Glycation by ascorbate significantly reduced the anti-apoptotic function of αB-crystallin. However, prior lysine acetylation before glycation limited this loss in anti-apoptotic function. Together, these data suggest that glycation by MGO and lysine acetylation make both αA- and αB-crystallin better anti-apoptotic proteins, while glycation by ascorbate reduced the anti-apoptotic function of αB-crystallin without a significant affect on αA-crystallin.
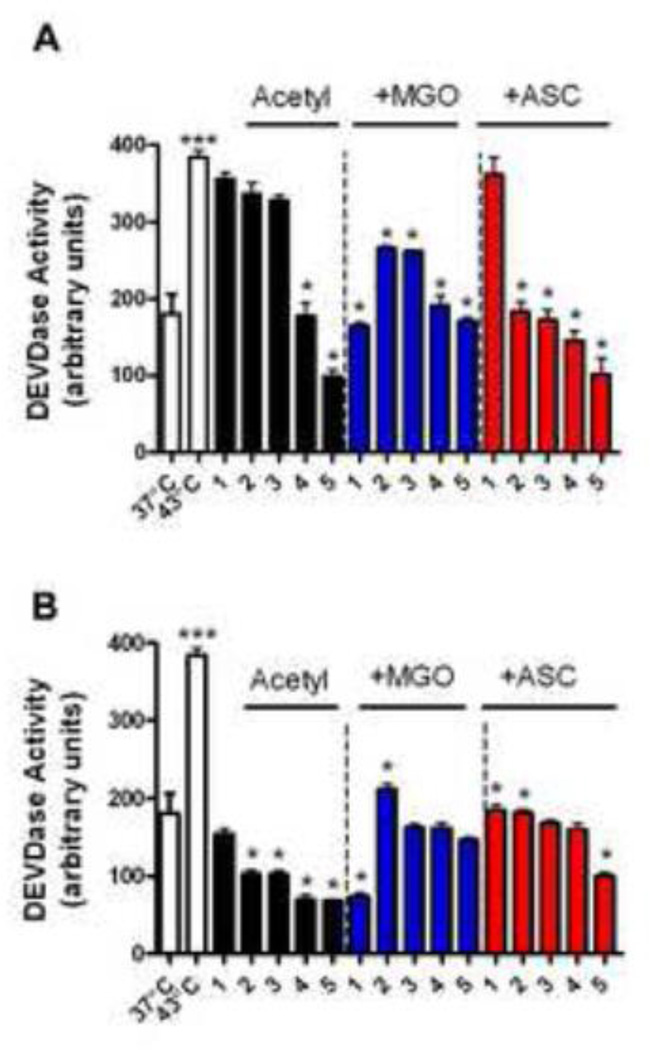
To determine if apoptosis occurred as a result of caspase-3 activation in the above experiment, we measured enzyme activity using DEVD as substrate. Caspase-3 activity increased by ~50% in thermally stressed CHO cells (Fig. 8), which was inhibited by ~5% and ~55%, respectively, by αA- and αB-crystallin. Lysine acetylation of both αA- and αB-crystallin provided better protection against caspase-3 activity, although the effect was more pronounced with αB-crystallin. Transfer of MGO-glycated protein further reduced caspase-3 activity by ~60% and ~65% in αA- and αB-crystallin. Light acetylation together with MGO glycation slightly lowered the ability of both αA- and αB-crystallin to inhibit caspase-3 activity. On the other hand, acetylation and ascorbate glycation increased the ability of αA-crystallin to inhibit caspase-3 activity compared to unacetylated αA-crystallin. A similar, but less apparent, increase was observed with αB-crystallin. These results imply that αB-crystallin and, to a somewhat lesser extent, αA-crystallin can block caspase-3 activity in thermally stressed CHO cells, and this function was retained in acetylated and acetylated+glycated proteins.
Fig. 8. Effect of Nε-acetylation and glycation of α-crystallin on caspase-3 activity in CHO cells.
Conditions were the same as in Fig. 7. Caspase-3 activity was measured using Ac-DEVD-AFC substrate. In each panel 1= unacetylated and 2 to 5 = acetylated with 0.05, 0.5, 1.0, and 10 fold molar excess Ac2O. Panel A= αA-crystallin and panel B= αB-crystallin. Each bar represents the mean ± SD of three experiments. *p<0.05 (compared to lane 1 in each case) and ***p<0.0005 (comparing cells at 43°C to cells at 37°C).
4. Discussion
The purpose of our study was to determine the combined effect of acetylation and AGE formation on two important functions of α-crystallin, viz., chaperone and antiapoptosis. Previously, we had shown that acetylation occurs in human lens αA- and αB-crystallin, and enhances the chaperone function of αA-crystallin [25]. In this study we found that such acetylation also enhances the chaperone function of αB-crystallin. We analyzed acetylation status of αB-crystallin in lens water-soluble proteins from donors of age between 15 and 86 and found that acetylation of αB-crystallin occurs very early in age and remains throughout life. Previously we had shown that αA-crystallin is also acetylated at an early age in human lenses [25]. Together, these observations suggest that acetylation could be necessary for maintaining chaperone function of α-crystallin. With regard to lysine acetylation, it is not clear whether it is mediated by lysine acetyl transferase, or if it is formed from other post-translational modification, such as glycation as reported recently [42].
Glycation occurs in lens proteins throughout life, but is minimal in young lenses. Relatively high levels are found usually in aged and cataractous lenses [35]. This suggests that AGE formation is promoted by conditions in aged and cataractous lenses. One mechanism could be through oxidation of ascorbate due to oxidative stress or loss of GSH in aged lenses, which could lead to production of highly reactive sugars that can form AGEs [43–46]. The loss of GSH might also reduce the activity of GSH-dependent glyoxalase leading to decreased metabolism of MGO, and consequently, to formation of MGO-mediated AGEs [47]. Since lysine acetylation in both αA-and αB-crystallin occurs early in life, and remains throughout life, lysine acetylation might be an inhibitory mechanism against AGE formation, as lysine is a major site for AGE formation. This may be particularly important for ascorbate-mediated glycation, as lysine residues are primary targets in those reactions, unlike MGO-mediated glycation, in which arginine residues are the primary targets.
Our immunoprecipitation studies revealed that AGEs and acetylated lysine residues are together present in αA- and αB-crystallin modifications. This implies that these two types of modifications can occur concurrently in α-crystallin, and can change its function in aged and cataractous lenses. This observation led us to acetylate lysine residues followed by glycation with MGO or ascorbate, and to then determine the effects on two major functions of α-crystallin, chaperoning of destabilized proteins and inhibition of apoptosis.
We tested the effect of acetylation on glycation by two methods. In the first, we analyzed acetylated and glycated proteins by SDS-PAGE for protein crosslinking, as many AGEs are formed as crosslinking adducts in proteins [35]. Prior lysine acetylation decreased protein crosslinking by glycation by both MGO and ascorbate, suggesting broad inhibition in AGE synthesis. If this phenomenon were occurring in the lens, lysine acetylation at a young age could help lens proteins remain soluble by limiting AGE-mediated protein crosslinking. In the second method, specific AGEs were measured. We measured HI and argpyrimidine, which are arginine modifications, CML, which is a lysine modification, and pentosidine, which is a lysine-arginine crosslinking modification. Prior acetylation largely inhibited formation of these AGEs. The inhibition of AGE formation by lysine acetylation thus controls AGE accumulation in lens proteins during aging. These observations lead to an important question—how does acetylation of lysine residues inhibit HI and argpyrimidine formation in α-crystallin? In our previous study, we showed that acetylation leads to structural perturbations in α-crystallin [25]. It is possible that such structural changes could lead to masking of arginine residues, which otherwise would be available for HI and argpyrimidine modifications.
As we reported previously [48–50], both MGO-glycation and acetylation improved the chaperone function of αA-crystallin. The observation in this study that prior acetylation further improves MGO-mediated effects on the chaperone function of αA-crystallin points to an additive effect of these two modifications. Further, lysine acetylation partially prevented the loss of chaperone function by ascorbate-glycation in both αA- and αB-crystallin. This is not surprising because ascorbate glycation mainly occurs on lysine residues, and therefore, prior lysine acetylation could have prevented glycation by ascorbate, and hence, the loss of chaperone function. In agreement with our previous observation [51], MGO-glycation did not improve the chaperone function of αB-crystallin. Why the MGO-mediated improvement in chaperone function is specific to αA-crystallin is not known and needs further investigation.
We also investigated the effect of acetylation and glycation on the other important function of α-crystallin, its ability to prevent apoptosis in cells experiencing external stresses. In this study we used thermal stress in CHO cells to interrogate the effects. CHO cells provided a clean background for the study as they lack α-crystallin. Previous studies, including our own, have shown that α-crystallin inhibits apoptosis by binding to procaspase-3 and Bax, inhibiting cytochrome-c release, activating PI3 kinase, activating PDK-1 and AKT, and inhibiting PTEN [9–12]. The transfer of both αA- and αB-crystallin resulted in a significant inhibition of apoptosis. This appears to be due to inhibition of caspase-3 activity. The increase in anti-apoptotic property by acetylation in both proteins suggests that acetylation of α-crystallin within lens epithelial cells would enhance protection offered by α-crystallin against metabolic and environmental stresses. The fact that glycation by either MGO or ascorbate did not drastically change this property indicates the anti-apoptotic property is resistant to these post-translational modifications. An interesting observation is that, while the chaperone function of αB-crystallin was not improved by MGO-glycation, its anti-apoptotic property was significantly improved. This suggests that there is disconnect between the chaperone and anti-apoptotic functions in αB-crystallin, unlike in αA-crystallin [9].
In summary, the findings in this study suggest that the lysine acetylation that occurs early in life in α-crystallin helps the protein retain its function in thwarting ill effects from ascorbate-glycation during aging.
Supplementary Material
Highlights.
Lysine acetylation inhibits advanced glycation end product formation in α-crystallin
Lysine acetylation inhibits the loss of chaperone function by ascorbate glycation in α-crystallin
Lysine acetylation further improves the chaperone function of methylglyoxal-modified α-crystallin
Lysine acetylation improves the anti-apoptotic function of α-crystallin
Acknowledgments
This study was supported from NIH grants R01EY-09912 (RHN), P30EY-11373 (the Visual Sciences Research Centre of CWRU); Research to Prevent Blindness (CWRU), NY; and Ohio Lions Eye Research Foundation (RHN). Research in TOI’s laboratory was supported by FY 2011 Grant-in-Aid for Scientific Research for Young Scientists, FY2012 Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science, and by FY 2011 and FY 2012 Adaptable and Seamless Technology Transfer Program through Target-driven R&D, and Independent Administrative Corporation, Japan Science and Technology Agency.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–485. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Andley UP. The lens epithelium: focus on the expression and function of the alphacrystallin chaperones. Int J Biochem Cell Biol. 2008;40:317–323. doi: 10.1016/j.biocel.2007.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srinivasan AN, Nagineni CN, Bhat SP. alpha A-crystallin is expressed in non-ocular tissues. J Biol Chem. 1992;267:23337–23341. [PubMed] [Google Scholar]
- 5.Bhat SP, Nagineni CN. alpha B subunit of lens-specific protein alpha-crystallin is present in other ocular and non-ocular tissues. Biochemical and biophysical research communications. 1989;158:319–325. doi: 10.1016/s0006-291x(89)80215-3. [DOI] [PubMed] [Google Scholar]
- 6.Dubin RA, Wawrousek EF, Piatigorsky J. Expression of the murine alpha B-crystallin gene is not restricted to the lens. Molecular and cellular biology. 1989;9:1083–1091. doi: 10.1128/mcb.9.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horwitz J. Alpha-crystallin. Experimental eye research. 2003;76:145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- 8.Andley UP. Crystallins in the eye: Function and pathology. Progress in retinal and eye research. 2007;26:78–98. doi: 10.1016/j.preteyeres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Pasupuleti N, Matsuyama S, Voss O, Doseff AI, Song K, Danielpour D, Nagaraj RH. The anti-apoptotic function of human alphaA-crystallin is directly related to its chaperone activity. Cell Death Dis. 2010;1:e31. doi: 10.1038/cddis.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andley UP. Effects of alpha-crystallin on lens cell function and cataract pathology. Curr Mol Med. 2009;9:887–892. doi: 10.2174/156652409789105598. [DOI] [PubMed] [Google Scholar]
- 11.Mao YW, Liu JP, Xiang H, Li DW. Human alphaA- and alphaB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 2004;11:512–526. doi: 10.1038/sj.cdd.4401384. [DOI] [PubMed] [Google Scholar]
- 12.Liu JP, Schlosser R, Ma WY, Dong Z, Feng H, Lui L, Huang XQ, Liu Y, Li DW. Human alphaA- and alphaB-crystallins prevent UVA-induced apoptosis through regulation of PKCalpha, RAF/MEK/ERK and AKT signaling pathways. Exp Eye Res. 2004;79:393–403. [PubMed] [Google Scholar]
- 13.Sharma KK, Santhoshkumar P. Lens aging: effects of crystallins. Biochim Biophys Acta. 2009;1790:1095–1108. doi: 10.1016/j.bbagen.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrington V, Srivastava OP, Kirk M. Proteomic analysis of water insoluble proteins from normal and cataractous human lenses. Mol Vis. 2007;13:1680–1694. [PubMed] [Google Scholar]
- 15.Wilmarth PA, Tanner S, Dasari S, Nagalla SR, Riviere MA, Bafna V, Pevzner PA, David LL. Age-related changes in human crystallins determined from comparative analysis of post-translational modifications in young and aged lens: does deamidation contribute to crystallin insolubility? J Proteome Res. 2006;5:2554–2566. doi: 10.1021/pr050473a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hains PG, Truscott RJ. Post-translational modifications in the nuclear region of young, aged, and cataract human lenses. J Proteome Res. 2007;6:3935–3943. doi: 10.1021/pr070138h. [DOI] [PubMed] [Google Scholar]
- 17.Nagaraj RH, Sell DR, Prabhakaram M, Ortwerth BJ, Monnier VM. High correlation between pentosidine protein crosslinks and pigmentation implicates ascorbate oxidation in human lens senescence and cataractogenesis. Proc Natl Acad Sci U S A. 1991;88:10257–10261. doi: 10.1073/pnas.88.22.10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagaraj RH, Shipanova IN, Faust FM. Protein cross-linking by the Maillard reaction. Isolation, characterization, and in vivo detection of a lysine-lysine cross-link derived from methylglyoxal. J Biol Chem. 1996;271:19338–19345. doi: 10.1074/jbc.271.32.19338. [DOI] [PubMed] [Google Scholar]
- 19.Hasan A, Smith JB, Qin W, Smith DL. The reaction of bovine lens alpha Acrystallin with aspirin. Exp Eye Res. 1993;57:29–35. doi: 10.1006/exer.1993.1095. [DOI] [PubMed] [Google Scholar]
- 20.Pal JK, Bera SK, Ghosh SK. Acetylation of alpha-crystallin with N-acetylimidazole and its influence upon the native aggregate and subunit reassembly. Curr Eye Res. 1999;19:358–367. doi: 10.1076/ceyr.19.4.358.5299. [DOI] [PubMed] [Google Scholar]
- 21.Lin PP, Barry RC, Smith DL, Smith JB. In vivo acetylation identified at lysine 70 of human lens alphaA-crystallin. Protein Sci. 1998;7:1451–1457. doi: 10.1002/pro.5560070622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lapko VN, Smith DL, Smith JB. In vivo carbamylation and acetylation of watersoluble human lens alphaB-crystallin lysine 92. Protein Sci. 2001;10:1130–1136. doi: 10.1110/ps.40901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi Q, Yan H, Li MY, Harding JJ. Effect of a combination of carnosine and aspirin eye drops on streptozotocin -- induced diabetic cataract in rats. Molecular vision. 2009;15:2129–2138. [PMC free article] [PubMed] [Google Scholar]
- 24.Hasan A, Smith JB, Qin W, Smith DL. The reaction of bovine lens alpha Acrystallin with aspirin. Experimental eye research. 1993;57:29–35. doi: 10.1006/exer.1993.1095. [DOI] [PubMed] [Google Scholar]
- 25.Nagaraj RH, Nahomi RB, Shanthakumar S, Linetsky M, Padmanabha S, Pasupuleti N, Wang B, Santhoshkumar P, Panda AK, Biswas A. Acetylation of alphaA-crystallin in the human lens: effects on structure and chaperone function. Biochim Biophys Acta. 2012;1822:120–129. doi: 10.1016/j.bbadis.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed N. Advanced glycation endproducts--role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67:3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Beswick HT, Harding JJ. Conformational changes induced in lens alpha- and gammacrystallins by modification with glucose 6-phosphate. Implications for cataract. Biochem J. 1987;246:761–769. doi: 10.1042/bj2460761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed MU, Brinkmann Frye E, Degenhardt TP, Thorpe SR, Baynes JW. Nepsilon-( carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem J. 1997;324(Pt 2):565–570. doi: 10.1042/bj3240565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hains PG, Truscott RJ. Proteomic analysis of the oxidation of cysteine residues in human age-related nuclear cataract lenses. Biochim Biophys Acta. 2008;1784:1959–1964. doi: 10.1016/j.bbapap.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Derham BK, Harding JJ. Effects of modifications of alpha-crystallin on its chaperone and other properties. Biochem J. 2002;364:711–717. doi: 10.1042/BJ20011512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar MS, Reddy PY, Kumar PA, Surolia I, Reddy GB. Effect of dicarbonylinduced browning on alpha-crystallin chaperone-like activity: physiological significance and caveats of in vitro aggregation assays. Biochem J. 2004;379:273–282. doi: 10.1042/BJ20031633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ortwerth BJ, Feather MS, Olesen PR. The precipitation and cross-linking of lens crystallins by ascorbic acid. Exp Eye Res. 1988;47:155–168. doi: 10.1016/0014-4835(88)90032-2. [DOI] [PubMed] [Google Scholar]
- 33.Fan X, Reneker LW, Obrenovich ME, Strauch C, Cheng R, Jarvis SM, Ortwerth BJ, Monnier VM. Vitamin C mediates chemical aging of lens crystallins by the Maillard reaction in a humanized mouse model. Proc Natl Acad Sci U S A. 2006;103:16912–16917. doi: 10.1073/pnas.0605101103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan X, Monnier VM. Inhibition of crystallin ascorbylation by nucleophilic compounds in the hSVCT2 mouse model of lenticular aging. Invest Ophthalmol Vis Sci. 2008;49:4945–4952. doi: 10.1167/iovs.08-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagaraj RH, Linetsky M, Stitt AW. The pathogenic role of Maillard reaction in the aging eye. Amino Acids. 2012;42:1205–1220. doi: 10.1007/s00726-010-0778-x. [DOI] [PubMed] [Google Scholar]
- 36.Pirie A. Color and solubility of the proteins of human cataracts. Investigative ophthalmology. 1968;7:634–650. [PubMed] [Google Scholar]
- 37.Puttaiah S, Biswas A, Staniszewska M, Nagaraj RH. Methylglyoxal inhibits glycation-mediated loss in chaperone function and synthesis of pentosidine in alphacrystallin. Exp Eye Res. 2007;84:914–921. doi: 10.1016/j.exer.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 38.Grandhee SK, Monnier VM. Mechanism of formation of the Maillard protein crosslink pentosidine. Glucose, fructose, and ascorbate as pentosidine precursors. The Journal of biological chemistry. 1991;266:11649–11653. [PubMed] [Google Scholar]
- 39.Nagaraj RH, Biswas A, Miller A, Oya-Ito T, Bhat M. The other side of the Maillard reaction. Ann N Y Acad Sci. 2008;1126:107–112. doi: 10.1196/annals.1433.045. [DOI] [PubMed] [Google Scholar]
- 40.Swamy MS, Abraham EC. Inhibition of lens crystallin glycation and high molecular weight aggregate formation by aspirin in vitro and in vivo. Investigative ophthalmology & visual science. 1989;30:1120–1126. [PubMed] [Google Scholar]
- 41.Stevens A. The effectiveness of putative anti-cataract agents in the prevention of protein glycation. Journal of the American Optometric Association. 1995;66:744–749. [PubMed] [Google Scholar]
- 42.Henning C, Smuda M, Girndt M, Ulrich C, Glomb MA. Molecular basis of maillard amide-advanced glycation end product (AGE) formation in vivo. The Journal of biological chemistry. 2011;286:44350–44356. doi: 10.1074/jbc.M111.282442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagaraj RH, Sell DR, Prabhakaram M, Ortwerth BJ, Monnier VM. High correlation between pentosidine protein crosslinks and pigmentation implicates ascorbate oxidation in human lens senescence and cataractogenesis. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:10257–10261. doi: 10.1073/pnas.88.22.10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linetsky M, Shipova E, Cheng R, Ortwerth BJ. Glycation by ascorbic acid oxidation products leads to the aggregation of lens proteins. Biochimica et biophysica acta. 2008;1782:22–34. doi: 10.1016/j.bbadis.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fan X, Reneker LW, Obrenovich ME, Strauch C, Cheng R, Jarvis SM, Ortwerth BJ, Monnier VM. Vitamin C mediates chemical aging of lens crystallins by the Maillard reaction in a humanized mouse model. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:16912–16917. doi: 10.1073/pnas.0605101103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng R, Lin B, Lee KW, Ortwerth BJ. Similarity of the yellow chromophores isolated from human cataracts with those from ascorbic acid-modified calf lens proteins: evidence for ascorbic acid glycation during cataract formation. Biochimica et biophysica acta. 2001;1537:14–26. doi: 10.1016/s0925-4439(01)00051-5. [DOI] [PubMed] [Google Scholar]
- 47.Mailankot M, Padmanabha S, Pasupuleti N, Major D, Howell S, Nagaraj RH. Glyoxalase I activity and immunoreactivity in the aging human lens. Biogerontology. 2009;10:711–720. doi: 10.1007/s10522-009-9218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagaraj RH, Oya-Ito T, Padayatti PS, Kumar R, Mehta S, West K, Levison B, Sun J, Crabb JW, Padival AK. Enhancement of chaperone function of alphacrystallin by methylglyoxal modification. Biochemistry. 2003;42:10746–10755. doi: 10.1021/bi034541n. [DOI] [PubMed] [Google Scholar]
- 49.Biswas A, Miller A, Oya-Ito T, Santhoshkumar P, Bhat M, Nagaraj RH. Effect of site-directed mutagenesis of methylglyoxal-modifiable arginine residues on the structure and chaperone function of human alphaA-crystallin. Biochemistry. 2006;45:4569–4577. doi: 10.1021/bi052574s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagaraj RH, Nahomi RB, Shanthakumar S, Linetsky M, Padmanabha S, Pasupuleti N, Wang B, Santhoshkumar P, Panda AK, Biswas A. Acetylation of alphaA-crystallin in the human lens: effects on structure and chaperone function. Biochimica et biophysica acta. 2012;1822:120–129. doi: 10.1016/j.bbadis.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagaraj RH, Panda AK, Shanthakumar S, Santhoshkumar P, Pasupuleti N, Wang B, Biswas A. Hydroimidazolone modification of the conserved Arg12 in small heat shock proteins: studies on the structure and chaperone function using mutant mimics. PloS one. 2012;7:e30257. doi: 10.1371/journal.pone.0030257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.