Abstract
Objective
To investigate the effect of acute hyperinsulinemia and the resulting decrease in plasma free fatty acid (FFA) concentrations on intramuscular TG synthesis.
Materials/Methods
U-13C16-palmitate was infused for 3 hours in anesthetized rabbits after overnight food deprivation. Arterial blood and leg muscle were sampled during the tracer infusion. Plasma samples were analyzed for free and TG-bound palmitate enrichments and concentrations. The enrichments and concentrations of palmitoyl-CoA and palmitoyl-carnitine as well as the enrichment of palmitate bound to TG were measured in muscle samples. Fractional synthetic rate (FSR) of intramuscular TG was calculated using the tracer incorporation method. The rabbits were divided into a control group and a hyperinsulinemic euglycemic clamp group.
Results
Insulin infusion decreased the rate of appearance of plasma free palmitate (2.00 ± 0.15 vs. 0.68 ± 0.20 µmol . kg−1 . min−1; p<.001), decreased plasma FFA concentration (327 ± 61 vs. 72 ± 25 nmol/mL; p<.01), decreased the total concentration of intramuscular fatty acyl-CoA plus fatty acyl-carnitine (12.1 ± 1.6 vs. 7.0 ± 0.7 nmol/g; p<.05), and decreased intramuscular TG FSR (0.48 ± 0.05 vs. 0.21 ± 0.06 %/h; p<.01) in comparison with the control group. Intramuscular TG FSR was correlated (p<.01) with both plasma FFA concentrations and intramuscular fatty acyl-CoA concentrations.
Conclusion
Fatty acid availability is a determinant of intramuscular TG synthesis. Insulin infusion decreases plasma and intramuscular fatty acid availability and thereby decreases TG synthesis.
Keywords: stable isotopes, fatty acyl-carnitine, fatty acyl-CoA
Introduction
Skeletal muscle accounts for 75–80% of whole body insulin-mediated glucose uptake and is therefore of central importance in the development of insulin resistance [1–3]. When insulin is ineffective in stimulating skeletal muscle glucose uptake hyperglycemia ensues. Thus, skeletal muscle insulin resistance is closely linked to hyperglycemia and type 2 diabetes [3]. A large body of evidence indicates that muscle insulin resistance is often associated with intramuscular (IM) accumulation of triglyceride (TG) and fatty acid intermediate metabolites [4–7]. IM TG content is inversely related to insulin sensitivity in almost all populations studied except in endurance-trained athletes [8, 9]. In order to understand the relationship between intracellular TG accumulation and the metabolic mechanisms underlying the development of insulin resistance it is therefore necessary to measure IM TG kinetics.
The amount of IMTG is determined by the balance between continuous synthesis and degradation. Fatty acid precursors for synthesis of IMTG come in large part from plasma FFA. In this regard, changes in plasma FFA levels are considered to be important in IM lipid dysregulation and insulin resistance [10]. Boden et al [11] reported that lowering of plasma FFA by acute hyperinsulinemia trended to decrease IM TG content, whereas increasing of plasma FFA increased IM TG content in healthy volunteers. Nevertheless, in that experiment the IM TG kinetics were not measured, so it is not known if the changes of IM TG content were due to the response of IM TG synthesis to changes in plasma FFA concentrations.
Isotopic techniques represent the most practical approach to measuring IM lipid kinetics. Several approaches have been published for measuring the fractional synthetic rate (FSR) of IM TG. The common problem of all published methods is measuring the IM precursor for TG synthesis, namely fatty acyl-coenzyme A (CoA). Because of the difficulty in measuring the fatty acyl-CoA, either plasma FFA or IM FFA have been used as surrogates of the precursor without necessary validations [12–16]. In a recent experiment we found that plasma free palmitate overestimated and that IM free palmitate underestimated the true precursor enrichment due to active lipid breakdown in vivo and during muscle sampling and processing [17]. IM PalCn enrichment was an acceptable surrogate for IM PalCoA enrichment, because it was less affected by IM lipid breakdown. This finding confirmed that PalCn enrichment can be used as the precursor enrichment for calculating IM TG FSR when direct measurement of IM PalCoA is problematic [18].
The goal of the present experiment was two-fold: to investigate the effect of acute hyperinsulinemia on IM TG synthesis, and to test the usefulness of IM PalCn enrichment as the precursor enrichment for IM TG synthesis. Because acute hyperinsulinemia (without changes in plasma FFA) has been reported to inhibit IM TG synthesis [19] and lowering of plasma FFA tended to decrease IM TG content [20], we anticipated that a combination of hyperinsulinemia and lowering of plasma FFA would inhibit IM TG synthesis.
Methods
Animals
Adult male New Zealand White rabbits (Myrtle’s Rabbitry; Thompson Station, TN), weighing ~4.5 kg, were used for this study. The rabbits were housed in individual cages and were given 150 g/day of unpurified diet (Lab Rabbit Chow 5326, Purina Mills; St. Louis, MO) for weight maintenance. This protocol complied with the Public Health Service Policy on Humane Care and Use of Laboratory Animals, incorporated in the Institute for Laboratory Animal Research Guide for Care and Use of Laboratory Animals, and was approved by the Animal Care and Use Committee of The University of Texas Medical Branch at Galveston.
The animals were studied after overnight food deprivation with free access to water. Surgery was performed to insert catheters into the carotid artery and jugular vein under general anesthesia [18]. The arterial line was used for drawing blood and monitoring arterial blood pressure and heart rate; the venous line was used for infusion of anesthetics and saline as well as insulin and glucose. An additional venous line was installed in a marginal ear vein by means of a Teflon-top needle (24 G 3/4 in Introcan® SafetyTM; B. Braum Medical Inc.; Bethlehem, PA), which was used exclusively for tracer administration. Tracheotomy was performed for placement of a tracheal tube, which was connected to a hood filled with oxygen-enriched room air.
Stable isotope tracer infusion
After surgery, we observed blood pressure, heart rate and rectal temperature for 20–30 minutes to ensure stable physiological conditions before the start of tracer infusion. In the insulin group, regular human insulin was infused at 2.5 mU . kg−1. min−1 after a priming dose calculated to raise plasma insulin to 150 µU/mL. Arterial blood was measured every 10 minutes to adjust the infusion rate of 25% glucose. When the infusion rate of glucose and plasma glucose concentrations were stable, which took 60–90 min, the tracer infusion was started.
U-13C16-palmitate (99% enriched; Cambridge Isotope Laboratories), bound to 5% albumin, was infused continuously for 3 hours at the dose of ~0.1 µmol . kg−1. min−1 after a priming dose of 1.0 µmol/kg. The tracer was infused into the marginal ear vein using a Harvard Syringe pump (Harvard Apparatus; Boston, MA) set at 15 mL/h. Blood samples were taken from the carotid artery catheter at 0 (before the tracer infusion), 5, 30, 60, 90, 120, 150, and 180 minutes using tubes containing EDTA. After centrifugation plasma was separated and stored at −20 °C for later analysis. Muscle samples were taken from the adductor muscle of both legs at 0, 5, 60, 120, and 180 minutes. The adductor muscle was dissected to be free from visible facial and adipose tissues before excision with scissors. The muscle samples were washed in ice-cold saline or blotted with soft tissues very briefly, and were frozen in liquid nitrogen immediately using a metal net. After being submerged in liquid nitrogen for 1 minute, the frozen muscle samples were transferred to cryogenic tubes and stored at −80°C.
Mean arterial blood pressure, heart rate and rectal temperature were maintained stable by adjusting the infusion rates of anesthetics and physiological saline, and by using a heating blanket. These vital signs were recorded every 30 minutes during the 3-hour tracer infusion. At the end of the experiment, the rabbits were killed by intravenous injection of 5 mL saturated KCl under general anesthesia.
Sample analysis
Plasma FFA and TG were processed for measuring palmitate enrichment on a gas chromatograph-mass spectrometer (GC-MS, MSD system, Aligent) [18]. Heptadecanoic acid and triheptadecanoin were added to the plasma samples as internal standards. Ions were selectively monitored at mass-to-charge ratios of 270, 285 and 286 for palmitate. Ten fatty acids in plasma FFAs and TG were measured using a GC system with flame ionization detection (model 6890, Agilent); the 10 fatty acids were myristate (14:0), palmitate (16:0), palmitoleate [16:1(n-1)], stearate (18:0), oleate [C18:1(n-9)], linoleate [18:2(n-6)], linolenate [18:3(n-3)], arachidonic acid [(20:4(n-6)], timnodonic acid [20:5(n-30)], and decosahexenoic acid [22:6(n-3)].
Plasma insulin concentration was measured using an immunoassay kit (Mercodia AB; Sylveniusgatan, Sweden). Plasma glucose concentration was measured by an YSI glucose/lactate analyzer (2300 STAT; Yellow Spring, OH).
Five representative frozen muscle samples were thawed and were observed under a stereo microscope (10–40 ×, Konus microscopes). No overt adipose tissue contamination was found under the microscope. Therefore, frozen muscle samples were pulverized into powder with a mortar and pestle pre-chilled in liquid nitrogen. Heptadecanoyl-CoA (Sigma Chemical, St. Louis, MO) or d3-Palmitoyl-carnitine (PalCn) (CIL, Andover, MA) was added as an internal standard for calculating the concentration of Palmitoyl-CoA (PalCoA) or PalCn, respectively. Fatty acyl-CoA was extracted with KH2PO4 and 2-propanol, and fatty acyl-carnitine was extracted with KH2PO4 and acetronitrile/methanol from 40–50 mg tissue [21, 22]. The enrichments of PalCoA and PalCn along with percent composition of 7 fatty acyl-CoA and 7 fatty acyl-carnitine were measured on an Agilent 1100 series liquid chromatograph–1956B SL single quadrupole mass spectrometer (Agilent Technologies, Palo Alto, CA, USA) as previously described [21, 22]. The concentrations of PalCoA and PalCn were calculated from their ratios to the internal standards. The concentrations of fatty acyl-CoA and fatty acyl-carnitine were calculated from the concentration of PalCoA and PalCn divided by the percent of total fatty acids made up of palmitate. The total fatty acid concentration was taken to be the sum of the following fatty acids: myristate (14:0), palmitate (16:0), palmitoleate [16:1(n-1)], stearate (18:0), oleate [C18:1(n-9)], linoleate [18:2(n-6)], linolenate [18:3(n-3)].
Muscle TG and phospholipids (PL) were extracted from 30–50 mg of muscle powder overnight at 4°C in 1:2 (v/v) methanol: chloroform solution containing 0.05 mg/mL butylated hydroxytoluene. Internal standards added for quantification of lipids were triheptadecanoin for TG and L-a-phosphatidylcholine, diheptadecanoyl for PL. After extraction, the samples were centrifuged; the supernatant was dried under nitrogen gas. The samples were reconstituted with 50 µl chloroform for TLC (Partisil LK5D, Silica Gel 150 Å, Schleicher & Schuell, Maidstone, England) isolation of TG; the isolation was processed in a tank with a mixture of hexane: ethyl ether: acetic acid (70:30:1 in mL). The sample spots of PL were recovered from the TLC plate and re-extracted with methanol: chloroform solution by 30 minutes shaking on a shaker (Eberbach, Ann Arbor, MI). After centrifugation, the supernatant was loaded on TLC plates again; PL was isolated in a tank with chloroform: methanol: water (65:30:5 in mL) for 20–30 minutes and another tank with heptane: ethyl ether: glacial acid (80:20:2 in mL) for 45 minutes. The isolated PL fraction was recovered from the TLC plates. The isolated TG and PL were hydrolyzed in HNO3 to obtain FFA and glycerol. The FFA from IM TG and PL were derivatized to their methylester for measurement of palmitate enrichment on a gas chromatograph-combustion-isotopic ratio mass spectrometer (Finnigan, MAT, Bremen, Germany). The measured 13C enrichment was multiplied by 17/16 to convert to the enrichment of the U-13C16-palmitate, because there are 16 carbons in the total of 17 carbons in the molecule of palmitate methylester that have a chance to be labeled.
Calculations
The concentrations of palmitate in plasma or muscle lipids were calculated by the internal standard method [23]. Palmitate concentrations were divided by % palmitate in total fatty acids to obtain total fatty acid concentrations.
The rate of appearance (Ra) of plasma palmitate was calculated by tracer dilution [23]. The equation is
| (Eq. 1) |
where F is U-13C16-palmitate infusion rate in µmol . kg−1 . min−1 and E is plasma free palmitate enrichment at plateau.
FSRs of IM TG and PL were calculated by the tracer incorporation method, which is based on the precursor-product principle [23]. The general equation is
| (Eq. 2) |
where (Et2 − Et1) is the enrichment increment of palmitate bound to IM TG or PL from t1 to t2; EP(t2−t1) is the average precursor enrichment from t1 to t2. IM PalCn enrichment was used as the representative of PalCoA enrichment [17, 18].
We have previously reported that the proportionate contributions from plasma FFA, plasma TG, and IM lipids to IM precursor pool can be calculated from the enrichment ratio of IM palmitoyl-CoA to plasma free palmitate at the beginning of tracer infusion when plasma TG enrichment is zero and at the enrichment plateau of plasma TG-bound palmitate [18]; the equations are as follows.
| (Eq. 3) |
where ECoAt and EPalt are enrichments of IM PalCoA (represented by PalCn) and plasma free palmitate at time t, respectively. At the beginning of tracer infusion (i.e., t0) the enrichment of plasma TG-bound palmitate is zero, equation 3 calculates the % contribution exclusively from plasma FFA. When t is larger than zero, plasma TG-bound palmitate enrichment increases over time. When the enrichment of plasma TG-bound palmitate reaches a plateau, equation 3 calculates the total contribution from both plasma FFA and TG.
| (Eq. 4) |
where ECoAt0 and EPalt0 are enrichments of IM PalCoA (represented by PalCn) and Plasma free palmitate at time 0, lipid breakdown includes both plasma TG and IM lipids (mainly TG and PL).
Values are expressed as means ± SEM. Enrichment is expressed as mole % excess (MPE). Differences comparing two parameters were tested by 2-way t-test. A p value less than 0.05 was considered as statistically significant.
Results
The body weight of rabbits was 4.5 ± 0.1 kg in both groups (n = 5 in each group). During the tracer infusion the rabbits were maintained under stable physiological conditions and sufficient depth of anesthesia. The rectal temperature was 38.2 ± 0.1 in both group. Heart rate was faster (p<.05) in the insulin than in the control group (202 ± 11 vs. 154 ± 6 beats/min). Mean arterial pressure was not significantly different between the 2 groups (control: 65 ± 3 mmHg; insulin: 58 ± 3; p = .13).
The anesthesia and surgery caused a stress response as plasma glucose concentration increased to 307 ± 22 mg/dL in the control group. In the insulin group plasma glucose was clamped at 121 ± 9 mg/dL by exogenous glucose infusion at 5.9 ± 1.1 mg . kg−1 . min. Plasma insulin was 149 ± 9 µU/mL in the insulin group. In the control group plasma insulin concentrations were below the detectable level in some rabbits probably due to insensitivity of the insulin assay kit for rabbits.
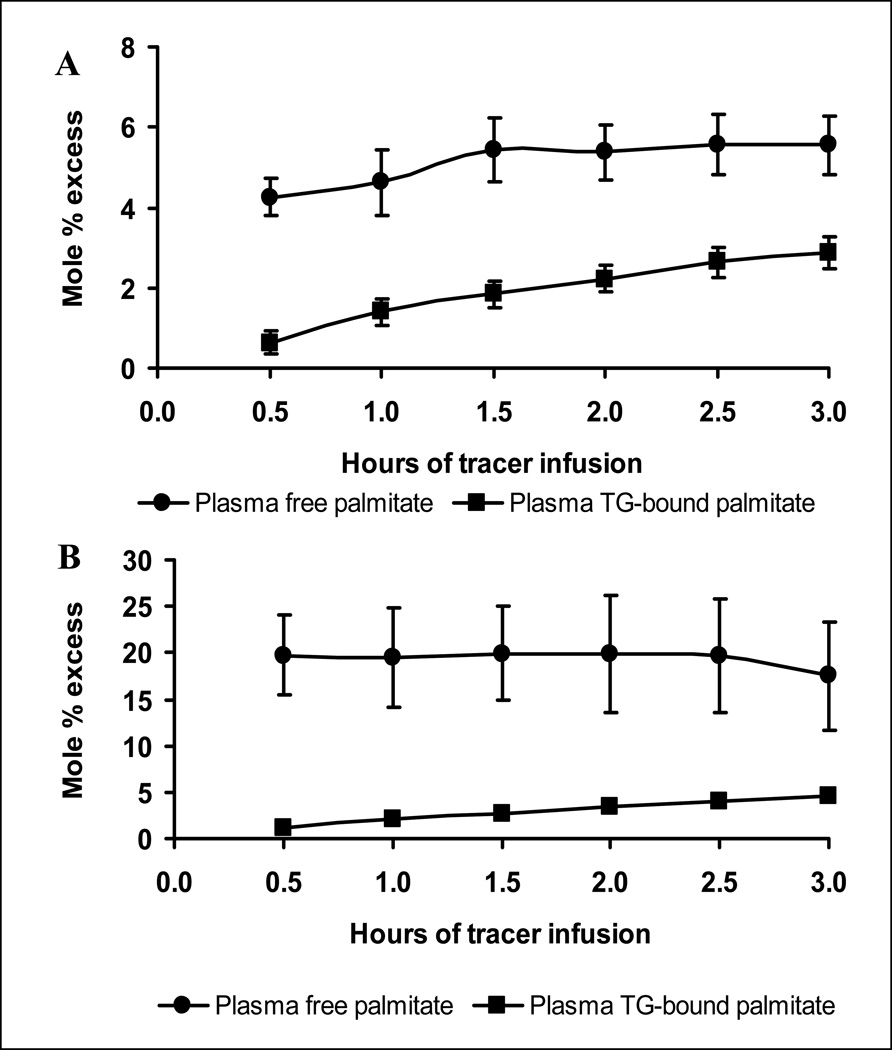
After the start of tracer infusion, plasma free palmitate enrichment increased rapidly and reached plateaus in both group (Fig. 1A and 1B). In contrast, plasma TG-bound palmitate enrichment increased gradually (Fig. 1A and 1B), reflecting the time delay for hepatocytes to take up plasma FFA and then secrete as VLDL-TG. The rate of plasma free palmitate appearance, which is a reflection of whole body lipolysis, was 2.00 ± 0.15 µmol . kg−1 . min in the control group, which was decreased (p<.001) to 0.68 ± 0.20 µmol . kg−1 . min in the insulin group. Insulin infusion decreased (p<.01) plasma FFA concentration in comparison with the control group; however, plasma TG concentrations were comparable (p=.75) between the 2 groups (Table 1).
Figure 1.
Isotopic enrichments of plasma free palmitate and plasma TG-bound palmitate. After the start of U-13C16-palmitate infusion, plasma free palmitate enrichment increased rapidly. Plasma TG-bound palmitate enrichment increased gradually, reflecting the time delay for hepatocytes to take up plasma FFA and then secrete as VLDL-TG. A, Control group; B, Insulin group.
Table 1.
Plasma FFA and TG concentrations
| Plasma FFA | Plasma TG | |||||
|---|---|---|---|---|---|---|
| Plasma free palmitate (nmol/ml) |
% palmitate | Plasma FFA (nmol/ml) |
Plasma TG- bound palmitate (nmol/ml) |
% palmitate | Plasma TG (nmol/ml) |
|
| Control | 120±16 | 38±3% | 327±61 | 163±34 | 44±5% | 139±37 |
| Insulin | 23±8 | 39±2% | 72±25 | 155±32 | 36±2 % | 163±36 |
| P | 0.001 | 0.80 | 0.006 | 0.87 | 0.26 | 0.86 |
Values are means ± SEM from 9 measurements in each rabbit. N = 5 in each group.
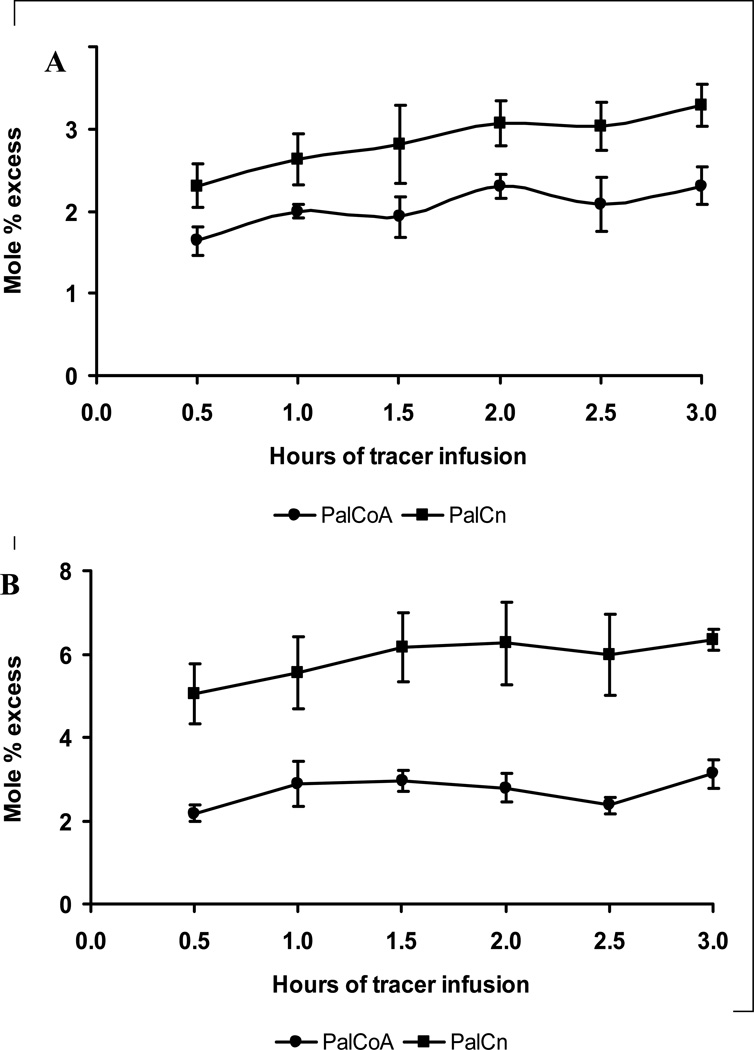
The enrichment of IM PalCoA was consistently lower than that of PalCn in both groups (Fig. 2). Because PalCn is a side-stream metabolite of PalCoA, the lower enrichment of PalCoA than PalCn was mostly likely due to tracee dilution (i.e., dilution of tracer by the tracee released from IM lipid breakdown) during sampling and processing, as the assays were conducted on biopsies that were not freeze-clamped [17, 18]. Therefore we used PalCn enrichment to represent PalCoA enrichment for FSR calculation. The FSR of IM TG was lower (p<.05) in the insulin group than in the control group (Table 3). In contrast, the FSRs of IM PL were close (p=.12) between the 2 groups.
Figure 2.
Isotopic enrichments of IM PalCoA and PalCn. The enrichment of PalCoA was consistently lower than that of PalCn. A, Control group; B, insulin group.
Table 3.
FSRs of IM TG and PL
| TG FSR in muscle | PL FSR in muscle | |||||
|---|---|---|---|---|---|---|
| Precursor MPE |
Increment of product MPE |
FSR (%/h) |
Precursor MPE |
Increment of product MPE |
FSR (%/h) |
|
| Control | 3.01±0.31 | 0.0425±0.0028 | 0.48±0.05 | 3.01±0.31 | 0.0680±0.0046 | 0.76±0.04 |
| Insulin | 6.50±0.69 | 0.0381±0.0052 | 0.21±0.06 | 6.50±0.69 | 0.1230±0.0140 | 0.64±0.06 |
| P value by t test |
0.0017 | 0.48 | 0.0055 | 0.0017 | 0.006 | 0.12 |
Values are means ± SEM. N = 5 in each group.
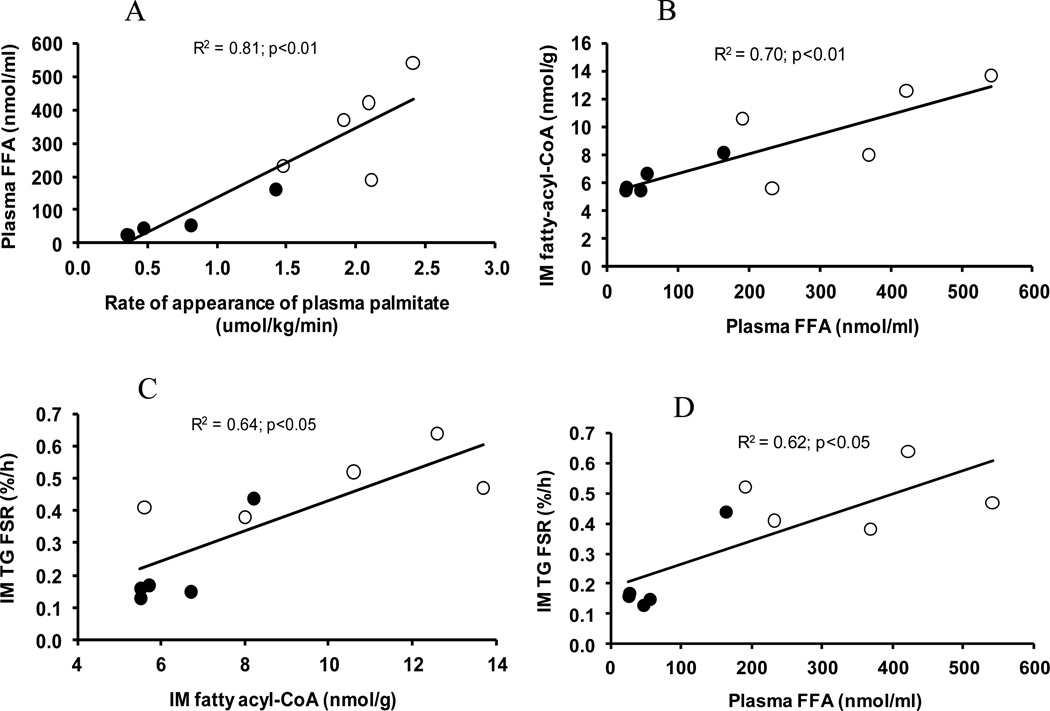
There were correlations (p<.01) between the rate of appearance of plasma free palmitate and plasma FFA concentration (Fig. 3A), between plasma FFA concentration and IM fatty acyl-CoA concentration (Fig. 3B), between IM fatty acyl-CoA concentration and TG FSR (Fig. 3C), and between plasma FFA concentration and IM TG FSR (Fig. 3D).
Figure 3.
Correlations between fatty acid availability and IM TG synthesis. A, Correlation between the rate of appearance of plasma palmitate and plasma FFA concentration. B, Correlation between plasma FFA concentration and IM fatty acyl-CoA concentration. C, Correlation between IM fatty acyl-CoA concentration and IM TG FSR. D, Correlation between plasma FFA concentration and IM TG FSR. Open circles represent control group, and closed circles, insulin group.
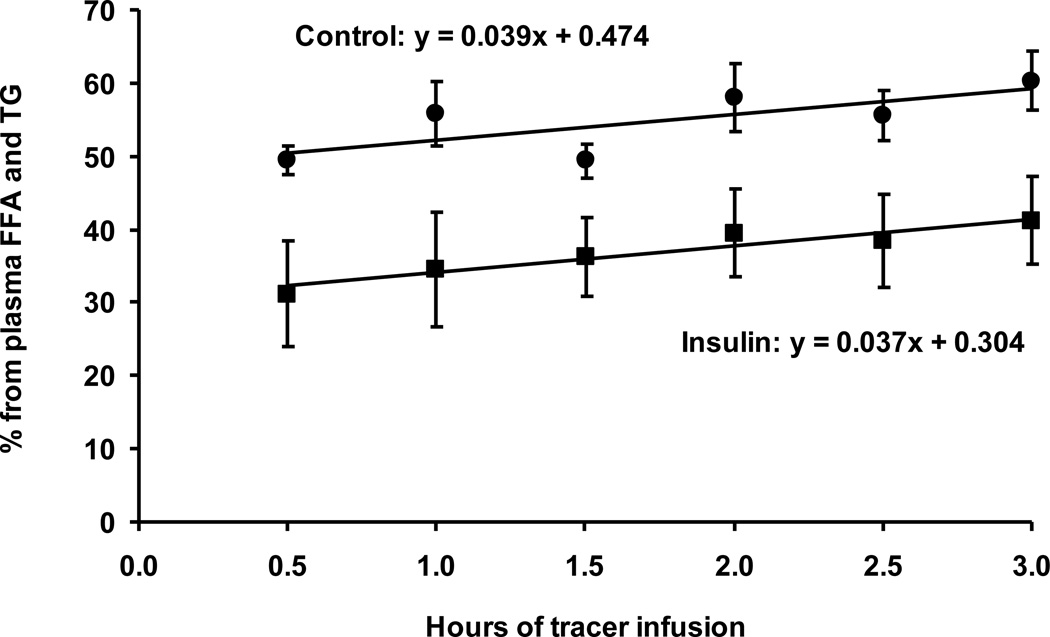
According to equation 3, the percentage contribution from plasma FFA to IM precursor can be calculated from plasma free palmitate enrichment and IM PalCn enrichment at time 0 when plasma TG-bound palmitate enrichment is zero. However, practically it is not possible to directly compare enrichment difference at time 0 because the tracer infusion was not started yet. So, we calculated the percentage contribution from all sampling points and extrapolated to time 0 using linear curve fitting (Fig. 4). The resultant percentage contributions from plasma FFA to IM precursor were 47 ± 4% and 30 ± 6% in the control and insulin groups, respectively (p<.05). The contributions from lipid breakdown were 53 ± 4% and 70 ± 6% in the control and insulin groups, respectively (p<.05). We also used the enrichment values at 0.5 hour as an approximation of 0 h. At 0.5 hour, plasma TG-bound palmitate enrichment was very low in relation to plasma free palmitate enrichment (Fig. 1), thus the contribution from plasma TG-bound palmitate enrichment to the precursor enrichment was minor, and equation 3 basically calculated the contribution from plasma free palmitate. The results showed that plasma FFA accounted for 50 ± 2% and 31 ± 7% of IM precursor in the control and insulin groups, respectively (p< .05). Using equation 4, lipid breakdown accounted for 50 ± 2% and 69 ± 7% in the control and insulin groups, respectively (p< .05). There were no significant differences between the percentage contributions calculated from the two approaches for the control (p=.4) and insulin (p=.6) groups. It is noteworthy that the calculated contribution from lipid breakdown included both IM lipid breakdown and plasma lipid breakdown via the action of lipoprotein lipase.
Figure 4.
Percentage contribution to IM precursor pool from plasma FFA and TG. This contribution increased over time in both the control and insulin groups due to the increasing enrichment of plasma TG-bound palmitate. The increases can be expressed by y = 0.039× + 0.474 and y = 0.037× + 0.0304 in the control and insulin groups, respectively.
Discussion
The major finding of this experiment is that acute hyperinsulinemia decreased the FSR of IM TG in comparison with the control group. In response to acute hyperinsulinemia plasma FFA concentration decreased (Table 1), so it is impossible to distinguish the effects of hyperinsulinemia from the response to a lowering of the plasma FFA levels. Nonetheless, the correlations of IM TG FSR with plasma FFA concentration and with IM fatty acyl-CoA concentration (Fig. 3C & 3D) indicate that fatty acid availability was a determinant of the rate of IM TG synthesis.
We found that the pool size of IM fatty acid metabolites is small relative to that of plasma FFA. In this experiment, the concentrations of PalCoA and PalCn were 2.47 ± 0.43 nmol/g and 0.47 ± 0.05 nmol/g in the control group, respectively (Table 2). If we assume that IM water accounts for 80% by weight, the concentration of PalCoA plus PalCn was 3.7 nmol/mL, a concentration gradient from plasma to IM pool of 32:1. This means a small change in FFA inflow from plasma to the IM precursor pool would significantly increase or decrease the PalCoA and PalCn pool, provided the synthetic and/or breakdown rate of IM TG does not react to compensate for the change of the plasma FFA inflow. Fatty acids and their intermediate metabolites are cytotoxic; their accumulation can impair the insulin signaling cascade [5–7]. On the other hand, a depletion of IM fatty acids and metabolites would also be detrimental to normal biological activities. Thus, the response of IM TG synthesis could be an important regulatory mechanism that reacts to changes in fatty acid inflow in order to maintain to some extent the homeostasis of the intracellular pools of fatty acids and their metabolites. In this context, partition of IM fatty acids to TG synthesis would prevent accumulation of potentially lipotoxic metabolites in the case of increased uptake of plasma FFA. This may explain why increased IM TG content has been reported to be associated with high insulin sensitivity in endurance trained athletes [8, 11]. The underlying mechanism responsible for the high insulin sensitivity in those athletes might involve enhanced capability of maintaining homeostasis of IM lipotoxic metabolites as a result of increased partitioning of fatty acids to TG synthesis despite a high rate of fatty uptake by muscle during and following exercise [24]. On the other hand, a low plasma FFA level decreases fatty acid inflow into the IM precursor pool, which is reasonably associated with a low TG synthesis rate. The reduction of IM TG synthesis in response to reduced fatty acid inflow protects the intracellular pool of fatty acids and their metabolites from depletion.
Table 2.
IM fatty acyl-CoA and fatty acyl-carnitine contents
| Fatty acyl-CoA | Fatty acyl-Cn | Total fatty acyl-CoA and fatty acyl-Cn (nmol/g) |
|||||
|---|---|---|---|---|---|---|---|
| PalCoA (nmol/g) |
% PalCoA |
Fatty acyl-CoA (nmol/g) |
PalCn (nmol/g) |
% PalCn |
Fatty acyl- Cn (nmol/g) |
||
| Control | 2.47±0.43 | 24±2% | 10.1±1.5 | 0.47±0.05 | 25±1% | 2.00±0.25 | 12.1±1.6 |
| Insulin | 1.12±0.07 | 18±0.5% | 6.3±1.2 | 0.15±0.03 | 25±1% | 0.66±0.14 | 7.0±0.7 |
| P | 0.01 | 0.007 | 0.04 | 0.0006 | 0.92 | 0.0016 | 0.018 |
Values are means ± SEM from 3 measurements in each rabbits. N = 5 in each group
The observation that a low plasma FFA concentration in the insulin group was correlated with a low IM TG synthetic rate is contrary to a previous finding that fatty acids inhibit IM TG synthesis in high fat-fed obese rats [25]. The discrepancy could be explained by the different responses of IM TG kinetics to fatty acid availability between obese and non-obese subjects. In obesity, chronic elevation of plasma FFA might disable the regulatory mechanisms for IM lipid homeostasis so that IM TG synthesis does not increase in response to a fatty acid load. Consequently, the resultant accumulation of IM lipotoxic metabolites induces insulin resistance.
The IM TG FSR in the control group (0.48 ± 0.05%/h; Table 3) was greater than what we reported previously in lean and obese rabbits (0.092 – 0.095%/h) in our previous experiment [18]. The difference in IM TG synthesis may reflect an adaptation to fasting because in this experiment the rabbits were studied after 16 hours food deprivation whereas in the previous experiment the rabbits were fasted for 24 hours before tracer infusion. With the prolonged food deprivation more IM fatty acids might be partitioned to the oxidative pathway than to TG synthesis. In contrast, IM PL FSRs in this experiment were comparable not only between the control and insulin group (0.76 ± 0.04%/h in control and 0.64 ± 0.06%/h in insulin; Table 3) but also between lean and obese rabbits in our previous experiment (0.76 ± 0.15%/h in lean and 0.70 ± 0.07 in obese rabbits; ref. 18). Phospholipids are essential components of cell membrane in skeletal muscle [26]. In adults, skeletal myofibers are terminal cells that do not proliferate [27]. Thus, it is not surprising that IM PL synthesis remains constant despite changes in TG synthesis in response to hormonal and nutritional interventions. In this context, a stable rate of IMPL synthesis provides a control of sorts for the assessment of changes in TG synthesis.
A unique feature in this experiment was that IM PalCoA and PalCn were measured to estimate the precursor enrichment for TG and PL syntheses. It is known that fatty acyl-CoA is the true precursor for lipid synthesis [23]. We found that PalCoA enrichment was consistently lower than the corresponding PalCn enrichment as we reported previously [17, 18]. This enrichment difference is attributed to the tracee dilution from IM lipid breakdown as the use of freeze-clamp technique for in situ muscle sampling eliminated the enrichment difference between IM PalCoA and PalCn [17, 18]. However, because of severe tissue damage, the freeze-clamp technique is not suitable for multiple muscle biopsies. Thus, we have proposed the use of PalCn enrichment as a surrogate for precursor enrichment. In literature, IM free palmitate enrichment was often used as a surrogate of precursor enrichment for IM TG synthesis [13–16]. However, there have been no validations that IM free palmitate enrichment reflects PalCoA enrichment. In a recent experiment we found that IM free palmitate enrichment was much lower than PalCoA enrichment even when measured from the muscle samples taken with the freeze-clamp technique [17], indicating that IM free palmitate is more sensitive than PalCoA to the tracee dilution from lipid breakdown. PalCn is a side-stream metabolite of PalCoA and its enrichment is equilibrated with PalCoA enrichment, thus being a good surrogate of the precursor enrichment. The enrichment difference between IM PalCoA and PalCn was greater in the insulin than in the control group (Fig. 2). This was probably because in the insulin group IM PalCoA pool was smaller (Table 2) so that the PalCoA enrichment was diluted to a greater extent from lipid breakdown during sampling and processing
In the control group plasma FFA accounted for 47 ± 4% of IM precursor pool, and lipid breakdown accounted for the other 53 ± 4%. In response to insulin infusion the contribution from plasma FFA decreased to 30 ± 6% (p<.05 vs. control) and that from lipid breakdown increased to 70 ± 6% (p<.05 vs. control). The different contributions from plasma FFA to IM precursor are consistent with the corresponding plasma FFA concentrations in these two groups. Insulin infusion decreased the rate of appearance of plasma FFA thereby lowering plasma FFA concentrations. Consequently, less plasma FFA entered the IM pool, which might in turn be a signal for more fatty acid release from the IM lipid breakdown for the homeostasis of the IM precursor pool.
Gou et al recently reported that acute hyperinsulinemia inhibits intramyocellular triglyceride synthesis [19]. In their experiment, plasma concentrations of lipids, amino acids, and glucose were maintained constant by exogenous replacement. Their experimental design enabled the assessment of the insulin effect on IM TG synthesis. In contrast, the results from the present experiment reflected the more physiological circumstance of hyperinsulinemia with associated reduction in plasma FFA. Plasma amino acid concentrations were likely also reduced in our experiment by insulin [28]. We cannot exclude the possible effects of altered concentrations of plasma glucose and amino acids on IM TG synthesis in this experiment. For example, according to the theory of “glucose-fatty acid cycle”, elevated plasma FFA concentrations play a key role in the development of insulin resistance and hyperglycemia [29]. Thus, the hyperglycemia in the control group might be secondary to hyperlipidemia. In this regard, further experiments to investigate the independent and interactive effects of lipids, glucose and amino acids on IM TG kinetics would be useful in developing therapeutic strategies for reversing insulin resistance. Despite the complicated nature of IM TG metabolism, the correlations in Fig. 3 suggest the importance of fatty acid availability in the regulation of IM TG synthesis. This finding is consistent with the report that hyperinsulinemia and decreased FFA levels decreased IM TG content [20], presumably because of decreased IM TG FSR.
In summary, the isotopic method used in this experiment provides an improved approach to study the IM TG synthesis. The results support a link between plasma FFA availability and IM TG synthesis. Whereas the importance of plasma FFA availability in development of insulin resistance has been recognized for many years, we for the first time demonstrate the regulatory mechanisms of IM TG synthesis in response to plasma FFA concentrations. Partition of IM fatty acids to TG synthesis could be an important mechanism maintaining IM lipotoxic metabolites, which might explain the "athlete's paradox" in which endurance training results in increases intramyocellular lipids despite improved inslin sensitivity [8, 9].
Acknowledgements
The authors are grateful to Yun-xia Lin, Guy Jones, Guarang Jariwala, Cindy Locklin, and Christopher Danesi for technical assistance. We also thank the Animal Resource Center of University of Texas Medical Branch for professional care of rabbits.
Funding
This study was supported by Shriners grants 85600, 84090, 80500, 71008, and 84309, NIH grants DK 034817, RO1-GM56687, RO1-GM56687-11S1, P50-GM060338, and National Institute on Aging Special Emphasis Panel PEPPER CENTERS grant AG028718.
Abbreviations
- IM
intramuscular
- TG
triglyceride
- FFA
free fatty acid(s)
- CoA
coenzyme A
- PalCoA
palmitoyl-CoA
- PalCn
palmitoyl-carnitine
- PL
phospholipids(s)
- FSR
fractional synthetic rate(s)
- GC-MS
gas chromatograph-mass spectrometer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure: No conflict of interest exists with or among Drs.. X-j. Zhang, L. Wang, Z, D. Tuvdendorj, Z. Wu, N. A. Rodriguez, D.N. Herndon, R.R. Wolfe.
Author Contributions
Xia-jun Zhang: design and conduct of the study, data collection and analysis, data interpretation and manuscript writing.
Lijian Wang: conduct of the study, data collection and analysis, data interpretation and manuscript writing.
Demidmaa Tuvdendorj: conduct of the study, data collection and analysis, data interpretation and manuscript writing.
Zhanpin Wu: data collection and analysis, data interpretation and manuscript writing.
Noe A. Rodriguez: conduct of the study, data interpretation and manuscript writing.
David N. Herndon: design of the study, data interpretation and manuscript writing.
Robert R. Wolfe: design of the study, data collection and analysis, data interpretation and manuscript writing.
References
- 1.DeFronzo RA, Jacot E, Jequier E, et al. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 2.Shulman GI, Rothman DL, Jue T, et al. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopey. N Eng J Med. 1990;322:223–228. doi: 10.1056/NEJM199001253220403. [DOI] [PubMed] [Google Scholar]
- 3.Cahova M, Vavrinkova H, Kazdova L. Glucose-fatty acid interaction in skeletal muscle and adipose tissue in insulin resistance. Physiol Res. 2007;56:1–15. doi: 10.33549/physiolres.930882. [DOI] [PubMed] [Google Scholar]
- 4.Pan DA, Lillioja S, Kriketos AD, et al. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 1997;46:983–988. doi: 10.2337/diab.46.6.983. [DOI] [PubMed] [Google Scholar]
- 5.Cooney GJ, Thompson AL, Furler SM, et al. Muscle long-chain acyl-CoA esters and insulin resistance. Ann N.Y. Acad Sci. 2002;967:196–207. doi: 10.1111/j.1749-6632.2002.tb04276.x. [DOI] [PubMed] [Google Scholar]
- 6.Boden G. Ceramide: a contributor to insulin resistance or an innocent bystander? Diabetologia. 2008;51:1095–1096. doi: 10.1007/s00125-008-1015-y. [DOI] [PubMed] [Google Scholar]
- 7.Timmers S, Schrauwen P, de Vogel J. Muscular diacylglycerol metabolism and insulin resistance. Physiology & Behavior. 2008;94:242–251. doi: 10.1016/j.physbeh.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid induced insulin resistance. J Clin Invest. 2007;117:1690–1698. doi: 10.1172/JCI30566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodpaster BH, He J, Watkins S, et al. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Meatb. 2001;86:5755–5761. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- 10.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]
- 11.Boden G, Lebed B, Schatz M, et al. Effects of acute changes of plasma free fatty acids on intramylcellular fat content and insulin resistance in healthy subjects. Diabetes. 2002;50:1612–1617. doi: 10.2337/diabetes.50.7.1612. [DOI] [PubMed] [Google Scholar]
- 12.Bergman BC, Perreault L, Hunderdosse DM, et al. Increased intramuscular lipid synthesis and low saturation relate to insulin sensitivity in endurance-trained athletes. J Apply Physiol. 2010;108:1134–1141. doi: 10.1152/japplphysiol.00684.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergman BC, Perreault L, Hunerdosse DM, et al. Intramuscular lipid metabolism in the insulin resistance of smoking. Diabetes. 2009;58:2220–2227. doi: 10.2337/db09-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perreault L, Bergman BC, Hunerdosse DM, et al. Altered intramuscular lipid metabolism relates to diminished insulin action in men, but not women, in progression to diabetes. Obesity. 2010;18:2093–2100. doi: 10.1038/oby.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo Z, Jensen MD. Intramuscular fatty acid metabolism evaluated with stable isotopic tracers. J Appl Physiol. 1998;84:1674–1679. doi: 10.1152/jappl.1998.84.5.1674. [DOI] [PubMed] [Google Scholar]
- 16.Sacchetti M, Saltin B, Olsen DB, et al. High triacylglycerol turnover rate in human skeletal muscle. J Physiol. 2004;561.3:883–891. doi: 10.1113/jphysiol.2004.075135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X-j, Rodriguez NA, Wang L, et al. Measurement of precursor enrichment for calculating intramuscular triglyceride fractional synthetic rate. J Lipid Res. 2012;53:119–125. doi: 10.1194/jlr.M019901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X-J, Chinkes DL, Wu Z, et al. The synthetic rate of muscle triglyceride but not phospholipids is increased in obese rabbits in comparison with lean rabbits. Metabolism. 2009;58:1649–1656. doi: 10.1016/j.metabol.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Guo Z, Zhou L, Jensen MD. Acute hyperinsulinemia inhibits intramyocellular triglyceride synthesis in high-fat-fed obese rats. J Lipid Res. 2006;47:2640–2646. doi: 10.1194/jlr.M600116-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Boden G, Lebed B, Schatz M, et al. Effects of acute changes of plasma free fatty acids on intramylcellular fat content and insulin resistance in healthy subjects. Diabetes. 2002;50:1612–1617. doi: 10.2337/diabetes.50.7.1612. [DOI] [PubMed] [Google Scholar]
- 21.Sun D, Cree MG, Wolfe RR. Quantification of the concentration and 13C tracer enrichment of long-chain fatty acyl-coenzyme A in muscle by liquid chromatography/mass spectrometry. Analytic Biochem. 2006;349:87–95. doi: 10.1016/j.ab.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Sun D, Cree MG, Zhang X-J, et al. Measurement of stable isotopic enrichment and concentration of long-chain fatty acyl-carnitines in tissue by HPLC-MS. J Lipid Res. 2006;47:431–439. doi: 10.1194/jlr.D500026-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Wolfe RR, Chinkes DL. Isotopic Tracers in Metabolic Research: Principle and practice of kinetic analysis. 2nd ed. New Jersey: Wiley-Liss; 2004. pp. 325–360. [Google Scholar]
- 24.Romijn JA, Coyle EF, Sidossis L, et al. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol. 1993;265:E380–E391. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- 25.Guo Z, Zhou L. Fatty acids inhibit intramyocellular triglyceride synthesis and turnover acutely in high fat-fed obese rats. Horm Metab Res. 2006;38:721–726. doi: 10.1055/s-2006-955093. [DOI] [PubMed] [Google Scholar]
- 26.Price ER, Guglielmo CG. The effect of muscle phospholipid fatty acid composition on exercise performance: a direct test in the migratory white-throated sparrow. Am J Physiol. 2009;297:R775–R782. doi: 10.1152/ajpregu.00150.2009. [DOI] [PubMed] [Google Scholar]
- 27.Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: The stem cell that come in from the cold. J Histochem Cytochem. 2006;54:1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X-J, Chinkes DL, Irtun O, et al. Anabolic action of insulin on skin wound protein is augmented by exogenous amino acids. Am J Physiol. 2002;282:E1308–E1315. doi: 10.1152/ajpendo.00361.2001. [DOI] [PubMed] [Google Scholar]
- 29.Randle PJ, Garland PB, Hales CN, et al. The glucose-fatty acid cycle: its role in insulin sensitivity and metabolic disturbances of diabetes mellitus. Lancet. 1963;i:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]