Summary
Diverse sensory organs, including mammalian taste buds and insect chemosensory sensilla, show a striking compartmentalization of receptor cells. However, the functional impact of this organization remains unclear. Here we show that compartmentalized Drosophila olfactory receptor neurons (ORNs) communicate with each other directly. The sustained response of one ORN is inhibited by the transient activation of a neighboring ORN. Mechanistically, such lateral inhibition does not depend on synapses and is likely mediated by ephaptic coupling. Moreover, lateral inhibition in the periphery can modulate olfactory behavior. Together, the results show that integration of olfactory information can occur via lateral interactions between ORNs. Inhibition of a sustained response by a transient response may provide a means of encoding salience. Finally, a CO2-sensitive ORN in the malaria mosquito Anopheles can also be inhibited by excitation of an adjacent ORN, suggesting a broad occurrence of lateral inhibition in insects and possible applications in insect control.
Introduction
An intriguing feature of a number of sensory systems is the compartmentalization of their primary sensory cells. These cells are housed together in specialized structures such as the taste buds of vertebrates and the chemosensory sensilla of invertebrates. The compartmentalized primary sensory cells often respond to diverse stimuli. The functional consequence of such organization is unknown.
Olfactory receptor neurons (ORNs) are the primary units of odor perception1. ORNs are widely believed to function as autonomous units, each responding to odorants independent of other ORNs. In some organisms, such as insects, ORNs are compartmentalized into sensilla (Fig. 1a). An individual sensillum encapsulates the dendrites of neurons2–4. The neighboring ORNs exhibit differing spike amplitudes and odorant sensitivities5. In Drosophila melanogaster, each ORN is assigned a designation indicating the type of sensillum in which it is housed and its relative spike amplitude among the ORNs of the sensillum. Thus the ab3A neuron is located in antennal basiconic sensilla of type 3, and the “A” indicates that its spike amplitude is greater than that of the neighboring “B” neuron. In fruit flies, moths, and mosquitoes, ORNs are grouped in stereotyped combinations5–9.
Figure 1. Lateral inhibition of ORNs.
a, An olfactory sensillum that houses two ORNs, A and B. Inset: a single-unit recording. “A” has a larger spike amplitude than “B”. b, The two-odor paradigm. c, The ab3 sensillum, whose ORNs express the Or22a and Or85b receptors, which are sensitive to the indicated odorants. d, Top: a sustained stimulus of methyl hexanoate (m-hex, 10−7 dilution, long blue bar) elicits a response from ab3A (large spikes, ~37 spikes/s). A 500-ms pulse of 2-heptanone (2-hep, 10−4 dilution, orange bar) activates ab3B (small spikes). The response of ab3A is inhibited by the 2-hep stimulus. Right, averaged responses. Grey traces indicate responses when a pulse of diluent is delivered instead of 2-hep. Shaded areas represent S.E.M.. Inset: blue dots indicate ab3A spikes. Bottom: genetic ablation of ab3B prevented inhibition. e, In flies expressing ChR2* in ab3B (top), a 500-ms pulse of blue light (473 nm, ~10mW/mm2) excited ab3B, which inhibited the response of ab3A to m-hex (~32 spikes/s, 10−6). The more phasic inhibition is likely due to the kinetics of ChR2-dependent activation. Bottom: flies without ChR2*. f, Top: activation of ab3A by a pulse of m-hex (10−6) inhibited the response of ab3B to 2-hep (~38 spikes/s, 5×10−7). Bottom: genetic ablation of ab3A prevented inhibition. Inset: orange dots indicate ab3B spikes. Very large spikes represent the coincidence of A and B spikes. g, ChR2* expressed in ab3A. A pulse of blue light (~25mW/mm2) excited ab3A, inhibiting the response of ab3B to 2-hep (~35 spikes/s, 5×10−7). n=12 in d–g.
The functional significance of this widespread pattern of ORN organization is unknown. In Drosophila, neighboring ORNs do not have obvious functional relationships10, and they do not project to adjacent regions in the brain11. In certain sensilla of flies, moths, and beetles, the activation of neighboring ORNs elicits opposing behaviors6,8,9,12–16. There are theoretical predictions based on electrical circuit modeling that the transient activation of one ORN may interfere with the signaling of a neighboring ORN44, and there is precedent for olfactory stimuli that activate one neuron and inhibit its neighbor15,16, but in the absence of molecular genetic analysis it is difficult to determine whether such stimuli act uniquely on one ORN or directly on both. Similar examples can also be found in insect taste sensilla17–21, but in Drosophila some bitter compounds have in fact been shown to act directly both on a sugar neuron and on a bitter neuron, inhibiting one and exciting the other22.
Here we use the molecular genetics of Drosophila to examine the coding of pairs of odors by the ORNs of olfactory sensilla. We find that the prolonged activation of one ORN is inhibited by the transient excitation of its neighbor. This lateral inhibition is observed within diverse types of Drosophila sensilla, and the activation of a mosquito ORN laterally inhibits the response of a neighboring ORN to CO2, a key cue used by mosquitoes to find their human hosts. The communication between neurons does not require a synapse, and likely proceeds via ephaptic coupling. Finally, we find that this lateral inhibition at the periphery of the olfactory circuit can modulate olfactory behavior. Together our results indicate that ORNs do not signal cell-autonomously in all circumstances, but rather their responses can be regulated by the activity of their ORN neighbors in a sensillum.
Results
Activation of an ORN inhibits its neighbor
To analyze the relationship between two ORNs in a sensillum, we used a paradigm that allows us to deliver two odors, one for each neuron (Fig. 1b,c). One odorant, the “background odorant”, is provided continuously via an airstream and elicits the sustained firing of one ORN, the A neuron in most experiments. Superimposed upon this background stimulus, a short pulse of a second odorant is delivered to activate the other ORN, usually the B neuron. This paradigm of odor presentation is distinct from the single-odorant paradigm used commonly in many studies5,10,23, but it simulates a coding problem that the system encounters in its natural environment, for example when a fly receiving sustained olfactory input from a local source receives a superimposed, transient stimulus from a distant source delivered by a gust of wind.
When the ab3 sensillum is stimulated with a prolonged dose of methyl hexanoate (m-hex), the ab3A neuron responds with a sustained train of action potentials (large action potentials in Fig. 1d). When a pulse of 2-heptanone is superimposed on this background, not only does ab3B fire (small action potentials) but there is a dramatic reduction in the firing of ab3A (Fig. 1d).
This inhibitory effect could in principle be due to direct inhibition of Or22a, the receptor of ab3A, by 2-heptanone. However, ablation of ab3B by expression of the cell death gene reaper (rpr) completely abolished the inhibition of ab3A (Fig. 1d, bottom). This result suggests that the inhibition of the A neuron depends on the excitation of the B neuron.
To test further the possibility that activation of the ab3B neuron can inhibit the ab3A neuron, we expressed Channelrhodopsin2 (H134R-ChR2)24 in ab3B. As expected, blue light elicited an excitatory response in ab3B of these engineered flies (Fig. 1e). Activation of ab3B by light also inhibited the tonic firing of ab3A elicited by methyl hexanoate. Blue light had no effect on ab3A firing in control flies lacking H134R-ChR2 (Fig. 1e, bottom), indicating that it does not inhibit ab3A directly. The simplest interpretation of these results is that activation of ab3B inhibits the firing of ab3A.
We next asked whether activation of ab3A can inhibit ab3B. We first elevated ab3B activity by delivering 2-heptanone as the background odorant and then presented a pulse of methyl hexanoate to activate ab3A. Indeed, the pulse of methyl hexanoate inhibited the activity of ab3B (Fig. 1f, top). Genetic ablation of ab3A demonstrated that this inhibition depended on ab3A (Fig. 1f, bottom). Similarly, when H134R-ChR2 was expressed in ab3A, a blue-light stimulus activated ab3A and inhibited the tonic firing of ab3B (Fig. 1g).
Lateral inhibition in other sensilla
There are four morphological types of antennal sensilla: large basiconic sensilla, small basiconic sensilla, coeloconic sensilla, and trichoid sensilla1,25,26. ab3 is a large basiconic sensillum containing two ORNs. We analyzed four other sensilla, chosen for their morphological diversity and their functional specificities. Their ORNs express receptors that have been functionally characterized, and odorants have been identified that at certain concentrations selectively activate the receptor of only one ORN in each sensillum10,23.
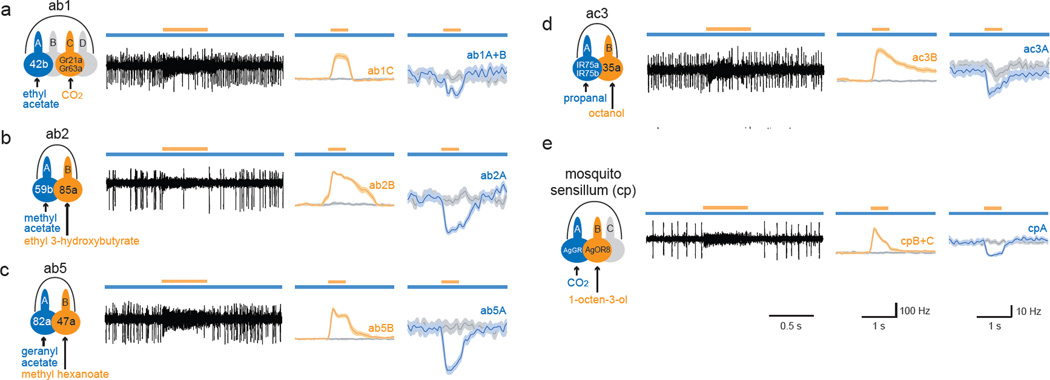
Lateral inhibition between ORNs was observed in all sensillar types examined: a large basiconic sensillum containing four ORNs (ab1), a large basiconic sensillum with two ORNs (ab2), a small basiconic sensillum (ab5), and a coeloconic sensillum (ac3). In each case, a short odorant pulse that activated one target ORN inhibited the tonic firing of a neighboring ORN (Fig. 2a–d). When the targeted ORN was ablated or nonfunctional, the short odorant pulse showed no inhibition of the neighboring ORN (Fig. S1). We note also that the pulsed odorant alone did not directly inhibit the spontaneous firing of the A neuron (Fig. S2). These results indicate that lateral inhibition is observed broadly in the Drosophila antenna.
Figure 2. Lateral inhibition in diverse sensilla.
Odorants at the tested concentrations activate only one ORN in each sensillum. a–d, Drosophila sensilla. Activation of the target ORN (orange) inhibited the response of the neighboring ORN (blue) to the background odorant. In a, ab1A and ab1B spikes could not be sorted reliably and were grouped. e, In the capitate-peg sensillum of Anopheles, activation of the cpB neuron by 1-octen-3-ol (10−4) inhibited the response of cpA to CO2. cpB and cpC spikes were combined. n=11~13. Odor dilutions and A neuron basal activities are in Table S2.
Lateral inhibition in a mosquito sensillum
ORNs are compartmentalized in sensilla in a wide variety of insects. We examined a sensillum of the malaria vector Anopheles gambiae that responds to CO27, a human volatile that attracts many mosquito species27. This sensillum contains an ORN, cpA, that responds to CO2, and a neighboring ORN, cpB, that is excited by 1-octen-3-ol7.
We used a prolonged CO2 stimulus to elicit a sustained response from cpA. When a short pulse of 1-octen-3-ol was superimposed, the cpB neuron was excited and cpA was robustly inhibited (Fig. 2e). We note that when 1-octen-3-ol was delivered in the absence of CO2, it did not inhibit the spontaneous firing of the CO2 -responsive cpA neuron directly (Fig. S2d), consistent with previous results7.
Taken together, our results show that lateral inhibition occurs in olfactory sensilla of multiple insect species, in sensilla of radically different morphology, and in sensilla containing two, three or four ORNs.
Inhibition is dose-dependent
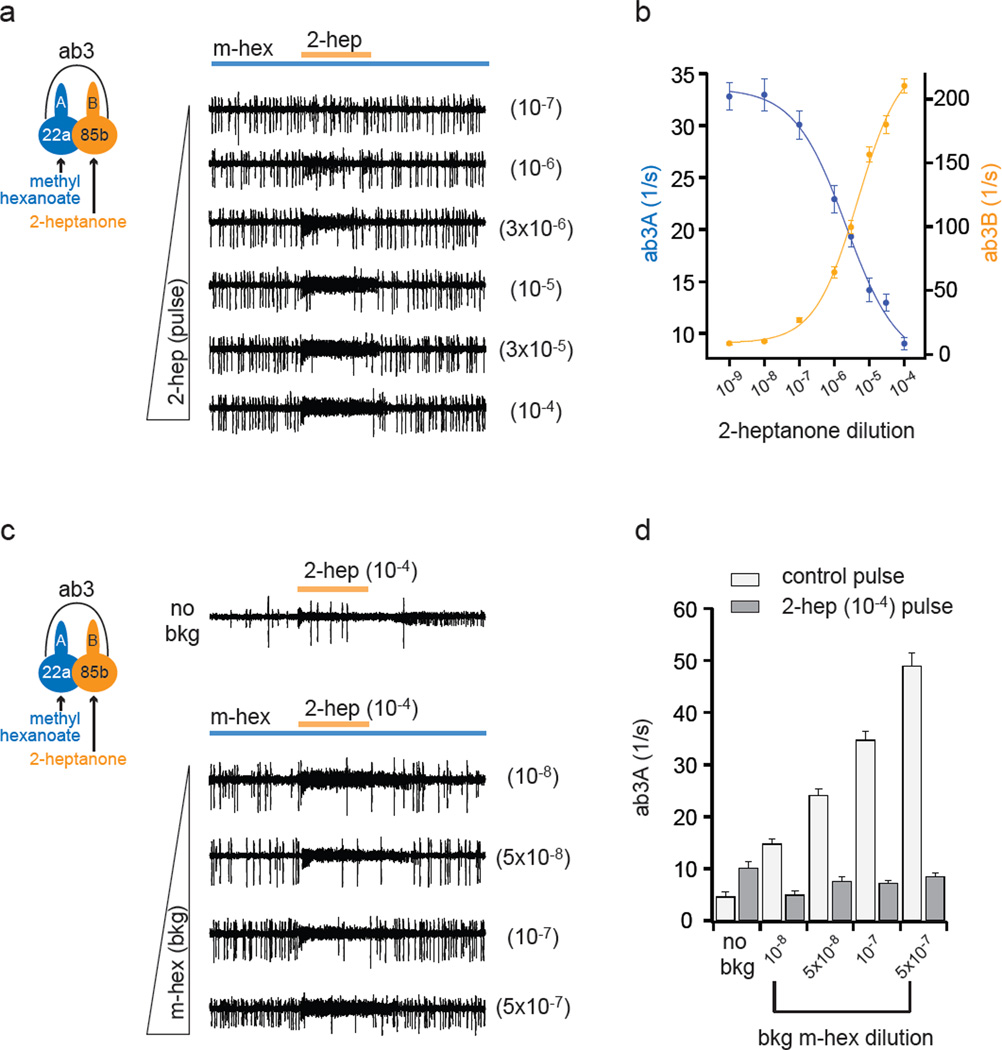
When ab3A was tonically excited with a constant concentration of methyl hexanoate, increasing doses of 2-heptanone produced increasing excitation of ab3B and increasing inhibition of ab3A (Fig. 3a,b). When the scales of the firing ranges are adjusted (Fig. 3b), the dose-response functions appear symmetric.
Figure 3. Lateral inhibition is dose-dependent.
a, Responses of ab3A and ab3B to a 500-ms pulse of 2-heptanone (orange) superimposed on a background odorant, methyl hexanoate (10−7 dilution; ~37 spikes/s). At these low concentrations, methyl hexanoate and 2-heptanone selectively activate ab3A and ab3B, respectively. 2-heptanone dilutions are at right. b, Activities of ab3A and ab3B during 2-heptanone pulses. Fit is with the Hill equation. n=12. c, Responses to a pulse of 2-heptanone (10−4) in presence of varying levels of methyl hexanoate, indicated at right. d, Responses of ab3A during 500-ms exposures to paraffin oil (control) or 2-heptanone with varying concentrations of background methyl hexanoate. n=12. In the absence of sustained stimulation of the A neuron (“no bkg”), strong activation of the B neuron elicited a small increase in the firing of A, which may represent passive depolarization of A resulting from close apposition of the neuronal membranes42,43. This effect appears to be overwhelmed by the passive hyperpolarization produced by ephaptic interactions (discussed below) when B is activated during sustained stimulation of A. Differences are significant in all conditions (p<0.002, paired t-test). n=12.
When the background odorant, methyl hexanoate, was delivered at increasing concentrations, the rate of ab3A tonic firing increased across a range of ~15 spikes/s to ~50 spikes/s (Fig. 3c,d, and Table S2). Inhibition by a strong ab3B stimulus was potent across all these concentrations; in all of these cases the rate of firing was reduced to approximately the same level. A genetic ablation experiment confirmed that these reductions depended on ab3B (Fig. S3). We note that 2-heptanone alone did not directly inhibit ab3A spontaneous activity (Fig. 3c,d, no bkg).
Transmission without a synapse
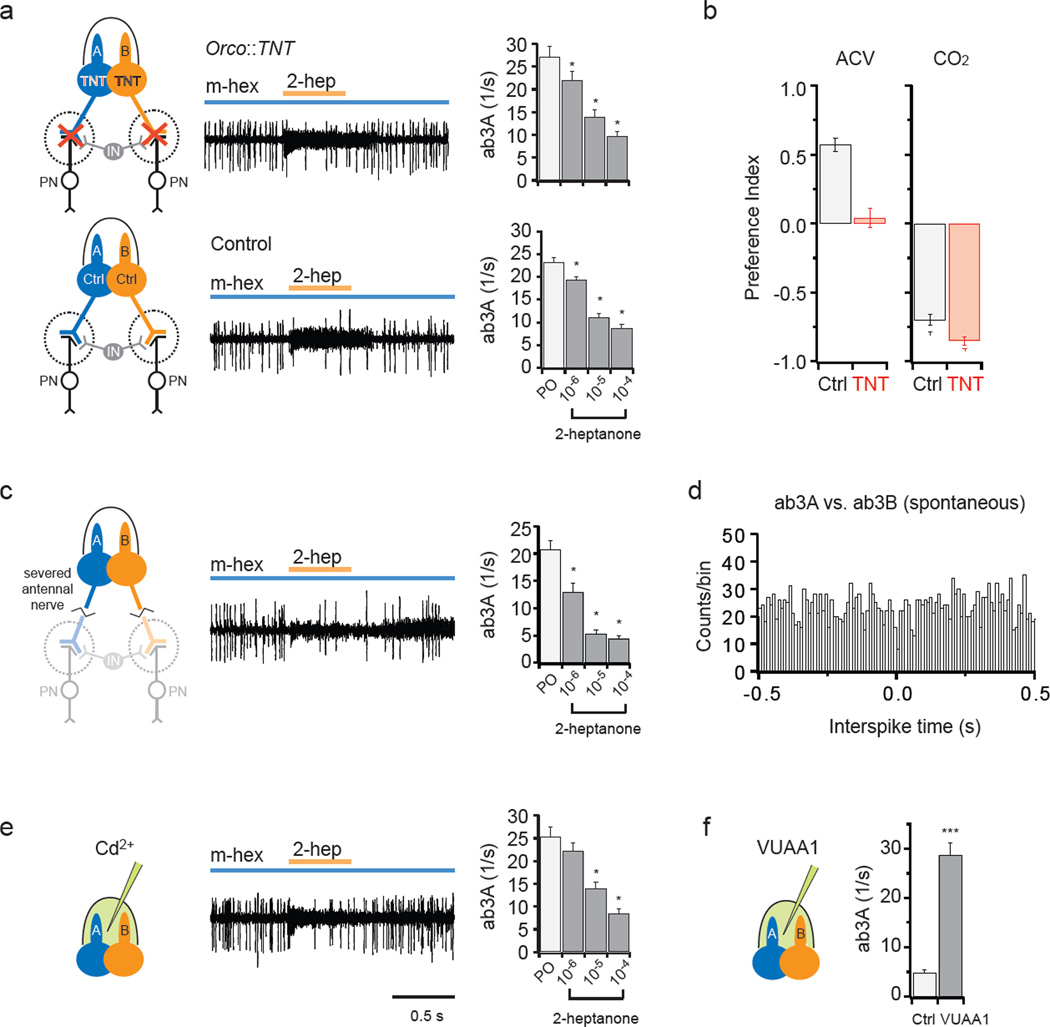
Next we asked whether the intrasensillar communication is mediated by synapses. First we used tetanus toxin (TNT)28 to block synaptic transmission. We expressed TNT in ORNs using the Orco promoter and the GAL4-UAS system, which is expected to drive expression in all basiconic ORNs29 except the CO2-sensitive ab1C neuron30,31. Activation of ab3B inhibited the tonic excitation of ab3A in these TNT-expressing flies (Fig. 4a, top). Moreover, the degree of inhibition was comparable to that in control flies (Fig. 4a, bottom). T-maze behavioral tests confirmed that synaptic transmission was blocked in the targeted ORNs (Fig. 4b).
Figure 4. Lateral inhibition does not require synapses.
a, ab3 sensilla in flies expressing TNT in ORNs via the Orco promoter . Neurons were exposed to a 500-ms pulse of the ab3B odorant, 2-heptanone (orange, 10−4), superimposed on the background ab3A odorant, methyl hexanoate (blue, 10−7). IN: representative interneuron. Right: ab3A activity during a 500-ms exposure to paraffin oil (PO) or 2-heptanone in the presence of methyl hexanoate. Error bars= S.E.M. *, p<0.05, one-way repeated measures ANOVA, multiple comparison versus control group (PO) with Dunnett’s method (n=12). b, T-maze choice between water and 25% ACV or between air and 0.67% CO2. CO2 neurons do not express Or genes. (n=9). c, Severed antennae (n=7). d, Cross-correlation analysis of spontaneous spikes from an ab3 sensillum, showing intervals between ab3A spikes and ab3B spikes, binned in 10 ms increments. Each ab3B spike is used as a reference. Another ab3 sensillum gave similar results. e, Recordings made 15 min after introduction of Cd2+ (n=12). f, VUAA1 (1 mM) or vehicle (1% DMSO) was delivered via the recording electrode35. ab3A responses were recorded for 10 s. *** p<0.001, t-test (n=12).
Second, we performed single-unit recordings from isolated antennae, severed from the heads of flies. Activation of ab3B again inhibited the tonic excitation of ab3A (Fig. 4c), supporting the conclusion that lateral inhibition between neighboring ORNs occurs in the periphery without involvement of central synapses.
Third, we tested the possibility of axo-axonic synapses between ORNs with a cross-correlation analysis32. Analysis of ab3A and ab3B spontaneous spikes did not reveal coordinated spiking patterns and thus provided no evidence for axo-axonic synaptic interactions (Fig. 4d), similar to what has been found between homotypic ORNs in Drosophila33.
Finally, we used Cd2+ to block synaptic neurotransmission34. We included a high concentration of Cd2+ in the recording pipette so as to allow Cd2+ to diffuse into the sensillum lymph and block any peripheral dendro-dendritic synapses in sensilla of Orco-GAL4; UAS-TNT flies. We observed little if any effect on the inhibition of ab3A firing following ab3B excitation (Fig. 4e; compare with Fig. 4a). To verify the efficacy of our drug delivery method, we applied the Orco agonist VUAA135 via the recording pipette and observed elevated ORN spike activities as expected (Fig. 4f). Together, these results indicate that lateral inhibition does not depend on chemical synapses.
Intrasensillar communication could in principle be mediated via gap junctions; however, the activation of one ORN would then likely lead to the activation, rather than the inhibition, of its neighbor. Moreover, we found that nitric oxide signaling inhibitors had no effect on lateral inhibition (not shown). In summary, conventional forms of neuronal communication are unlikely to mediate lateral inhibition in a sensillum.
Lateral inhibition modulates behavior
To determine whether intra-sensillar neuronal inhibition can modulate olfactory behavior, we examined a pair of neighboring ORNs whose activation leads to opposing behavioral outputs (Fig. 5). ab1A mediates attraction to apple cider vinegar (ACV)13, whereas its neighbor ab1C mediates aversion to low concentrations of CO212,31,36,37. We confirmed that in a T-maze assay, when given a choice between CO2 and air alone, flies avoid CO2, whereas when faced with a choice between ACV and water, they are attracted to ACV (Fig. 5a, black bars).
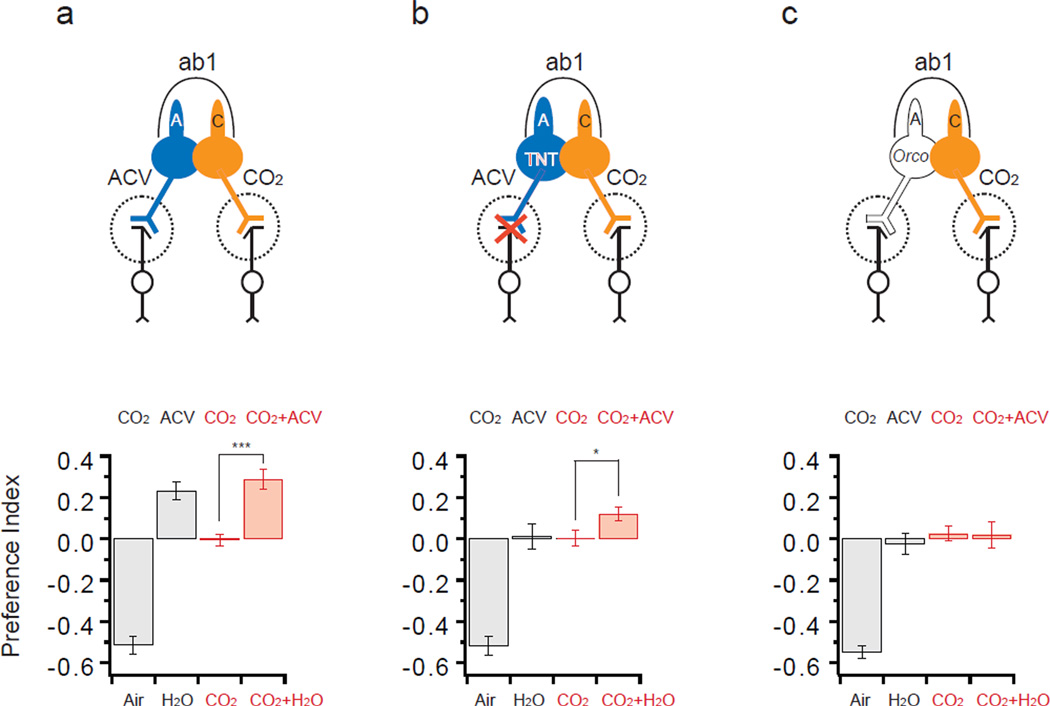
Figure 5. Lateral inhibition modulates behavior.
Activation of ab1A mediates attraction to ACV; activation of ab1C mediates aversion to CO2. Two of the four ORNs in ab1 are depicted. Preference indices of control (a), Orco-GAL4; UAS-TNT (b), and Orco (c) are shown (mean ± S.E.M.). The ab1A neurons of the TNT-expressing flies respond to ACV but are expected not to transmit information to post-synaptic neurons, whereas ab1A neurons in Orco are expected not to respond to ACV. In each T-maze assay, ~50 flies were allowed two min to choose. In single-odor experiments (black bars) the test arm contained either CO2 or ACV. *, p<0.05; ***, p <0.001, t-test (n=16). CO2 was 0.13%; ACV was 100%, pH 7.5. In physiological recordings from Orco flies, ACV did not inhibit the spontaneous firing of the CO2 neuron.
We then tested whether the two behavioral pathways interact. When both arms of the T-maze contained CO2 , the flies showed no preference (Fig. 5a). When ACV was added to one of the CO2-containing arms, the flies preferred that arm. The preference for the arm containing both CO2 and ACV could have two sources: the attraction to its ACV that is mediated by ab1A, and a reduction in the avoidance of its CO2 that is mediated by ab1C.
To evaluate the contributions of these sources, we used Orco-GAL4;UAS-TNT, which blocks synaptic transmission from ab1A but not ab1C. Consistent with the expected specificity of this block, these flies did not respond to ACV but avoided CO2 (Fig. 5b, black bars). We note that in these flies, physiological recordings confirmed that ab1A neurons respond to ACV (not shown). When given a choice between two arms, one with CO2 and one with CO2 and ACV, these flies preferred the arm with ACV (Fig. 5b). Since synaptic transmission from ab1A neurons is blocked and the flies have no attraction to ACV, the simplest interpretation of these results is that activation of ab1A attenuated the response of ab1C to CO2 via lateral inhibition: the reduced CO2 response decreased the avoidance of the arm containing CO2 and ACV relative to the arm containing CO2 alone, and this decreased avoidance is seen as an attraction to the arm containing CO2 and ACV.
If this interpretation is correct, and the preference for the arm containing CO2 and ACV depends on the activation of ab1A, then the preference should be abolished in Orco mutants, which lack a co-receptor required for the response of ab1A but not ab1C. Consistent with this prediction, Orco mutants showed no preference between the arm containing CO2 and the arm containing CO2 and ACV (Fig. 5c). We note that ACV does not inhibit ab1C directly (Fig. S4). Taken together, these results provide evidence that lateral inhibition within a sensillum can modulate behavior.
Discussion
Integration of olfactory information has long been known to occur in the CNS, and has more recently been shown to occur in individual ORNs38. We have demonstrated that integration also occurs at a third level, the sensillum, via lateral inhibition between ORNs responding to different components of a mixture. The sensillum thus acts as a processing unit in olfactory computation.
Lateral inhibition of a prolonged signal by a transient signal may provide a neural representation of the salience of an odor that has recently reached the fly39. Sustained responses were inhibited more strongly by stronger transient pulses. This graded pattern of lateral inhibition may give rise to a potent form of contrast enhancement in which the output of a sensillum is dominated by a pulse of a strong odor. Graded lateral inhibition may provide a peripheral mechanism for evaluating countervailing signals and allowing one to prevail. We note that in Drosophila, an ORN that responds to a pheromone40,41 is the only ORN that does not have a neighbor, as if to ensure that its sustained response is not inhibited by a pulse of any other odorant.
Our finding that lateral inhibition does not require synapses is consistent with anatomical data. Electron microscopy in Drosophila has not revealed synaptic structures or gap junctions between ORNs housed in the same sensillum2,3. Rather, as detailed below, the physiological features of olfactory sensilla suggest another mechanism of lateral information flow: ephaptic transmission, which refers to non-synaptic communication between adjacent neurons through an extracellular electrical field42,43. The ability of either neuron in a two-neuron sensillum to inhibit the other, as well as the grossly similar temporal dynamics of activation and lateral inhibition (Fig. S5), are consistent with ephaptic transmission.
In insect olfactory sensilla, a substantial electrical potential exists between two isolated compartments: the sensillum lymph, which bathes the dendrites, and the hemolymph, which surrounds the somata (Fig. 1a, Fig. S6). This “transepithelial” potential serves as the primary driving force for odorant-induced transduction currents of the ORNs44,45. Elaboration of an established electrical circuit model44,45 based on these physiological features predicts that strong activation of one ORN will hyperpolarize the soma of a co-compartmentalized ORN (Fig. S6), resulting in a reduced firing rate. This prediction is consistent with the results of our molecular genetic analysis and with our interpretation that lateral inhibition is due to ephaptic interactions.
The model further predicts that the magnitude of the hyperpolarization of the neighboring neuron, and hence its reduction in firing rate, is reflected by the change in the transepithelial potential (VA) (Fig. S6), measured experimentally as a local field potential (LFP) (Fig. S7a). Although strong activation of an ORN can influence the LFP in a neighboring sensillum46, we found that the magnitude of the LFP change in nearby unstimulated sensilla is small (Fig. S7). Consistent with this observation, lateral inhibition does not spread among homotypic sensilla that are in close proximity to one another (Fig. S8). These results further support the conclusion that the lateral inhibition is due to local electrical interactions between neighboring ORNs within a sensillum.
The two-odor paradigm used in this analysis, in which a transient odor is superimposed upon a sustained odor, differs from the classic one-odor paradigm in which a transient pulse of a single odor is delivered. A priori one might expect to observe ephaptic effects in the one-odor paradigm if one ORN were excited sufficiently strongly, but the effects may be expected to be less pronounced than in the two-odor paradigm. ORN spike frequency is determined not only by the somatic transmembrane potential Vm, but also by its rate of change, dVm/dt46. According to the model, transient activation of ORN2 reduces the depolarizing current of ORN1 (Fig. S6). In the two-odor paradigm, activation of ORN2 has a dramatic effect on the value of dVm1/dt, which changes from 0 to a negative value (dVm1/dt <<0; Fig. S6). By contrast, in the one-odor paradigm, the activation of ORN2 has a more subtle effect on dVm1/dt when the sensillum is stimulated with an odor that activates both neurons: dVm1/dt is positive either in the presence or absence of ORN2 activation, only somewhat less positive when ORN2 is activated. The more subtle influence of ORN2 activation on dVm1/dt in the one-odor paradigm may explain why in the one-odor paradigm, the excitatory responses of an ORN containing an ectopically expressed receptor were strikingly similar to those of the ORN that endogenously expresses the same receptor23, despite major differences in the response profiles of their neighbors.
We note finally that our results suggest the possibility of a new approach to insect control: the inhibition of key insect ORNs by activation of their neighbors with odorants.
Methods Summary
Fly antennal preparations and single-unit recordings were performed essentially as described23, except for the isolated antennal preparation in which the stabilized antenna was severed from the head using the broken tip of a tapered glass microcapillary tube. Recordings were performed on adult female flies 5–7 day post-eclosion, except that flies 24–36 hours post-eclosion were used in UAS-TNT experiments because TNT-expressing ORNs began to lose spike activities in older flies. Table S1 lists fly genotypes for all experiments. Female Anopheles gambiae mosquitoes were used ~4 days post-eclosion. Extracellular recordings from the capitate-peg sensilla on the maxillary palp were performed as described7,47. AC signals (300–2000 Hz) were recorded, except for local field potential recordings where DC signals (low-pass filtered at 2 kHz) were recorded. ORN spikes were detected and sorted based on spike amplitude using routines in Igor Pro 6.01 and binned at 50-ms intervals.
For optogenetic experiments, flies were reared in constant darkness on fly food supplemented with ~100 µM all trans-retinal36. Recordings were performed on adult females 7 days post-eclosion using an established optics setup48. For pharmacological experiments, chemicals were delivered inside the sensillum via the recording glass electrode.
T-maze behavioral tests were performed essentially as described12. For experiments shown in Fig. 4b, flies were given one minute to choose between the two arms; air vs. CO2 (0.67% ) or H2O vs. ACV (25%). For experiments shown in Fig. 5, four experimental conditions were used: 1) air vs. CO2 (0.13%); 2) H2O vs. ACV (100%, pH 7.5); 3) CO2 (0.13%) vs. CO2 (0.13%); 4) CO2 (0.13%)+H2O vs. CO2 (0.13%)+ACV (100%, pH 7.5). Preference index was calculated as the fraction of the flies entering the test arm minus the fraction of the flies entering the control arm.
Materials and Methods
Drosophila stocks
Recordings were performed on adult female flies 5d post-eclosion, except that 7d flies were used in UAS-rpr experiments, and flies 24–36 hours post-eclosion were used in UAS-TNT experiments because TNT-expressing ORNs began to lose spike activities in older flies. Flies were reared at 25°C in an incubator with a 12-hr light-dark cycle. The following fly stocks were used: (1) UAS-rpr49, (2) w1118 and PBac[WH]Or35af02057 (3) UAS-TNT28, (4) UAS-H134R-ChR224, (5) Or-GAL4 lines (Bloomington stock center), (6) Gr21a-GAL430. Table S1 lists genotypes for all experiments.
Mosquitoes
Female Anopheles gambiae mosquitoes were used ~4 days post-eclosion. Extracellular recordings from the capitate-peg sensilla on the maxillary palp were performed as described7,47.
Electrophysiology and data analysis
For the standard antennal preparation, a fly was wedged into the narrow end of a truncated plastic pipette tip to expose the antenna, which was subsequently stabilized between a tapered glass microcapillary tube and a coverslip covered with double-sided type. For the isolated antennal preparation, a standard antennal preparation was made first and the stabilized antenna was gently severed from the head using the broken tip of a tapered glass microcapillary tube. Extracellular single-unit recordings were performed essentially as described23. Briefly, electrical activity of the ORNs was recorded extracellularly by placing a sharp electrode filled with Ringer solution23 into a sensillum and the reference electrode filled with the same Ringer solution was placed in the eye (standard antennal preparation) or in the first antennal segment (severed antennal preparation). No more than four sensilla from the same antenna were recorded in the standard preparation, and no more than two sensilla from the same antenna were recorded in the severed preparation. For each sensillum, one trial of each odorant concentration was presented. AC signals (300–2000 Hz) were recorded on an Iso-DAM amplifier (World Precision Instruments), except for local field potential recordings where DC signals (low-pass filtered at 2 kHz) were recorded and digitized at 5 kHz with Axoscope 10.2 (Molecular Devices). ORN spikes were detected and sorted based on spike amplitude using routines in Igor Pro 6.01 (Wavemetrics). Peri-stimulus time histograms (PSTHs) were obtained by averaging spike activities in 50-ms bins and smoothed using a binomial algorithm (Igor Pro 6.01, Wavemetrics).
Odor stimuli
Odorants were diluted in paraffin oil (v/v). For short odor pulses, odor stimuli (50 µl applied to a filter disc) were delivered from a Pasteur pipette via a pulse of air (200 ml/min) into the main air stream (2000 ml/min) as described previously23. In Fig. 2a, stimulation with CO2 was by filling the Pasteur pipette with pure CO2, which was subsequently puffed into the main air stream. Based on published dose-response relationships of ab1A to CO230,50, the concentration of CO2 was estimated to be ~1% (mean ab1C response shown in Fig. 2a: 163 Hz). Background odor stimuli were delivered from a 125-ml flask containing 3 ml of odor dilutions (or 25 ml of carbonated water for background CO2) directly downstream of the main air stream (2000 ml/min).
Optogenetic stimulation
Flies expressing H134R-ChR2 in targeted ORNs and control flies (UAS-H134R-ChR2; +) were reared in constant darkness on fly food supplemented with ~100 µM all trans-retinal (Sigma) as described36. Recordings were performed on adult females 7 days post-eclosion using an established optics setup48. Briefly, a light stimulus was generated via a blue laser (MBL-III-473/30 mW, Opto Engine LLC) and delivered by an optical fiber (200-µm core diameter, BFH22–200, Thorlabs). The tip of the optical fiber was positioned above the antenna. Light pulses (500-ms duration) were controlled by an isolated pulse stimulator (Model 2100, A-M Systems). Light output at the tip of the optical fiber was measured with an optical power meter (Model 1916-C, Newport).
Cross-correlation analysis
The basal spike activity was investigated using 30 sweeps of 10-s duration. Action potentials of the ab3A and ab3B neuron were identified based on size and their triphasic (ab3A neuron) or more biphasic (ab3B neuron) shape using Origin software (OriginLab Corporation). Spike times of ab3A and ab3B neurons of individual sweeps were cross-correlated using Matlab software (MathWorks). Interspike times were accumulated across all recorded sweeps and binned in 10-ms intervals. Such an analysis can reveal coordinated spiking patterns and was used to identify axo-axonic synapses between neighboring scorpion ORNs32.
Pharmacology
Drugs were prepared as concentrated stock solutions and diluted in Ringer solution prior to experiments. Chemicals were delivered inside the sensillum via the recording glass electrode. Recordings were performed in flies expressing TNT in the ORNs ~15 min after drug introduction, except for the experiments with VUAA1, where recordings were performed within minutes after electrode insertion. The electrode stayed inside the sensillum throughout the 15-min period. VUAA1 (Chemical Diversity Research Institute, Joint Stock Company, Russia) was used at 1 mM (stock: 100 mM in DMSO). CdCl2 (Aldrich) was used at 1 mM (stock: 100 mM in Ringer solution).
T-maze assay
Flies were collected within ~8 hours post-eclosion without using CO2 anesthesia. Flies were tested 24–32 hours post-eclosion after ~24 hr starvation. For starvation, flies were gently tapped into empty vials with moistened foam plugs and kept at 25°C in an incubator.
Behavioral tests were performed as described previously12 at room temperature in a dark room. About 40–60 flies were transferred by an aspirator into a 15-ml centrifuge tube (Corning 430791), which was subsequently connected to the sliding chamber (elevator) of the T-maze apparatus. Flies were gently tapped into the elevator, which was then lowered to the opening where the test arm and the control arm were connected. A 16-inch 15-W fluorescent bulb was placed horizontally behind the test and control arms, and the light was on only for the duration of the assay. Phototaxis drew flies out of the elevator. Flies were given one minute to choose between the two arms, after which the elevator was partially lifted to block any further choices. Preference index was calculated as the fraction of the flies entering the test arm minus the fraction of the flies entering the control arm. The total number of flies used in calculation of the preference index included flies in both arms and in the elevator.
For the experiment shown in Fig. 4b, 10 µl of apple cider vinegar solution or 10 µl of water was added to a Whatman filter disc (1/2 inch diameter) that was positioned around the 1.5-ml mark of the 15-ml centrifuge tube. Twenty-five percent apple cider vinegar was used because it attracted flies in a T-maze assay without triggering the acid-mediated avoidance pathway37. Ten minutes of equilibrium time was allowed before the tubes were connected to the T-maze apparatus immediately before the assay. For the experiment in Fig. 4b using CO2, 0.1 ml of pure CO2 (UN1013, Airgas) was injected into the tube immediately before the assay. The positions of the test and control tubes were alternated for each trial. New groups of flies and new tubes were used for each test. The air inside the 15-ml tube was equilibrated with the air in the room for at least 4 hours before use.
For experiments shown in Fig. 5 to address the behavioral relevance of lateral inhibition, we used four experimental conditions: 1) air vs. CO2; 2) H2O vs. ACV; 3) CO2 vs. CO2; 4) CO2+H2O vs. CO2+ACV. Thirty microliters of neutralized apple cider vinegar (100%, pH 7.5) or water was added to a Whatman filter disc that was positioned horizontally via permanent double-sided tape (Scotch, 3M) around the 10-ml mark of the centrifuge tube. When CO2 was used, 0.1 ml of 20% CO2 was injected into the tube(s) (near the 5-ml mark) immediately before the assay. When CO2 was used in both arms, the CO2 was injected, the two tubes were connected to the T-maze apparatus, and then the apparatus was inverted gently ~10 times and allowed to equilibrate for an additional minute to ensure that CO2 was distributed evenly between the two arms. The elevator was then lowered to release the flies. The positions of the test and control tubes were alternated for each trial.
Supplementary Material
Acknowledgment
We thank J. Cardin for help establishing the optogenetics system, Y. Zhao and E. Fikrig for providing mosquitoes, A. Tzingounis and G. Lowe for suggestions, Z. Berman and P. Graham for technical assistance, T. Koh for suggestions and for sharing reagents, G. Thomas, F. Marion-Poll, R. Wyman and D. McCormick for comments on the manuscript. This work was funded by NIH grants to J.R.C. and by a grant from the Foundation for the NIH through the Grand Challenges in Global Health Initiative (GCGH No. 121); an NRSA postdoctoral fellowship to K.M. (NIH F32DC011242); and an NIH grant to J.R. (NIH DC009613).
Footnotes
Author Contributions
C.-Y.S. designed, performed the experiments and analyzed the data, except for coeloconic sensillum recordings and cross-correlation analysis, which were performed by K.M. and J.R., respectively. C.-Y.S., K.M. and J.R.C. wrote the manuscript. All authors contributed to the interpretation of the study.
References
- 1.Su CY, Menuz K, Carlson JR. Olfactory perception: receptors, cells, and circuits. Cell. 2009;139:45–59. doi: 10.1016/j.cell.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shanbhag SR, Muller B, Steinbrecht RA. Atlas of olfactory organs of Drosophila melanogaster 1. Types, external organization, innervation and distribution of olfactory sensilla. Int J Insect Morphol Embryol. 1999:377–397. [Google Scholar]
- 3.Shanbhag SR, Muller B, Steinbrecht RA. Atlas of olfactory organs of Drosophila melanogaster 2. Internal organization and cellular architecture of olfactory sensilla. Arthropod Struct Dev. 2000;29:211–229. doi: 10.1016/s1467-8039(00)00028-1. [DOI] [PubMed] [Google Scholar]
- 4.Keil TA. Reconstruction and morphometry of silkmoth olfactory hairs: A comparative study of sensilla trichodea on the antennae of male. Antheraea polyphemus and Antheraea pernyi (Insecta, Lepidoptera) Zoomorphology. 1984;104:147–156. [Google Scholar]
- 5.de Bruyne M, Foster K, Carlson JR. Odor coding in the Drosophila antenna. Neuron. 2001;30:537–552. doi: 10.1016/s0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 6.Kaissling KE. Peripheral mechanisms of pheromone reception in moths. Chem Senses. 1996;21:257–268. doi: 10.1093/chemse/21.2.257. [DOI] [PubMed] [Google Scholar]
- 7.Lu T, et al. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr Biol. 2007;17:1533–1544. doi: 10.1016/j.cub.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akers RP, O'Connell RJ. The contribution of olfactory receptor neurons to the perception of pheromone component ratios in male redbanded leafroller moths. J Comp Physiol A. 1988;163:641–650. doi: 10.1007/BF00603848. [DOI] [PubMed] [Google Scholar]
- 9.Takanashi T, et al. Unusual response characteristics of pheromone-specific olfactory receptor neurons in the Asian corn borer moth, Ostrinia furnacalis. J Exp Biol. 2006;209:4946–4956. doi: 10.1242/jeb.02587. [DOI] [PubMed] [Google Scholar]
- 10.Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 11.Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15:1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 12.Suh GS, et al. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- 13.Semmelhack JL, Wang JW. Select Drosophila glomeruli mediate innate olfactory attraction and aversion. Nature. 2009;459:218–223. doi: 10.1038/nature07983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berg BG, Mustaparta H. The significance of major pheromone components and interspecific signals as expressed by receptor neurons in the oriental tobacco budworm moth, Helicoverpa assulta. J Comp Physiol A. 1995:683–694. [Google Scholar]
- 15.Nikonov AA, Leal WS. Peripheral coding of sex pheromone and a behavioral antagonist in the Japanese beetle Popillia japonica. J Chem Ecol. 2002;28:1075–1089. doi: 10.1023/a:1015274104626. [DOI] [PubMed] [Google Scholar]
- 16.O'Connell RR. Responses to pheromone blends in insect olfactory receptor neurons. J Comp Physiol A. 1985:747–761. [Google Scholar]
- 17.Schoonhoven LM, Van Loon JJA. An inventory of taste in caterpillars: each species its own key. Acta Zoologica Academiae Scientiarum Hungaricae. 2002;48(Suppl. 1):215–263. [Google Scholar]
- 18.Mitchell BK. Interactions of alkaloids with galeal chemosensory cells of Colorado potato beetle. Journal of Chemical Ecology. 1987;13:2009–2022. doi: 10.1007/BF01041728. [DOI] [PubMed] [Google Scholar]
- 19.Jorgensen K, Almaas TJ, Marion-Poll F, Mustaparta H. Electrophysiological characterization of responses from gustatory receptor neurons of sensilla chaetica in the moth Heliothis virescens. Chem Senses. 2007;32:863–879. doi: 10.1093/chemse/bjm057. [DOI] [PubMed] [Google Scholar]
- 20.de Brito Sanchez MG, Giurfa M, de Paula Mota TR, Gauthier M. Electrophysiological and behavioural characterization of gustatory responses to antennal 'bitter' taste in honeybees. Eur J Neurosci. 2005;22:3161–3170. doi: 10.1111/j.1460-9568.2005.04516.x. [DOI] [PubMed] [Google Scholar]
- 21.Dethier VG, Bowdan E. The effect of alkaloids on sugar receptors and the feeding behaviour of the blowfly. Physiological Entomology. 1989;14:127–136. [Google Scholar]
- 22.Meunier N, Marion-Poll F, Rospars JP, Tanimura T. Peripheral coding of bitter taste in Drosophila. J Neurobiol. 2003;56:139–152. doi: 10.1002/neu.10235. [DOI] [PubMed] [Google Scholar]
- 23.Hallem EA, Ho MG, Carlson JR. The molecular basis of odor coding in the Drosophila antenna. Cell. 2004;117:965–979. doi: 10.1016/j.cell.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Pulver SR, Pashkovski SL, Hornstein NJ, Garrity PA, Griffith LC. Temporal dynamics of neuronal activation by Channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. J Neurophysiol. 2009;101:3075–3088. doi: 10.1152/jn.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwon Y, et al. Drosophila TRPA1 channel is required to avoid the naturally occurring insect repellent citronellal. Curr Biol. 2010;20:1672–1678. doi: 10.1016/j.cub.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takken W, Knols BG. Odor-mediated behavior of Afrotropical malaria mosquitoes. Annu Rev Entomol. 1999;44:131–157. doi: 10.1146/annurev.ento.44.1.131. [DOI] [PubMed] [Google Scholar]
- 28.Keller A, Sweeney ST, Zars T, O'Kane CJ, Heisenberg M. Targeted expression of tetanus neurotoxin interferes with behavioral responses to sensory input in Drosophila. J Neurobiol. 2002;50:221–233. doi: 10.1002/neu.10029. [DOI] [PubMed] [Google Scholar]
- 29.Larsson MC, et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 30.Kwon JY, Dahanukar A, Weiss LA, Carlson JR. The molecular basis of CO2 reception in Drosophila. Proc Natl Acad Sci U S A. 2007;104:3574–3578. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- 32.Gaffin DD. Electrophysiological analysis of synaptic interactions within peg sensilla of scorpion pectines. Microsc Res Tech. 2002;58:325–334. doi: 10.1002/jemt.10140. [DOI] [PubMed] [Google Scholar]
- 33.Kazama H, Wilson RI. Origins of correlated activity in an olfactory circuit. Nat Neurosci. 2009;12:1136–1144. doi: 10.1038/nn.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yaksi E, Wilson RI. Electrical coupling between olfactory glomeruli. Neuron. 2010;67:1034–1047. doi: 10.1016/j.neuron.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones PL, Pask GM, Rinker DC, Zwiebel LJ. Functional agonism of insect odorant receptor ion channels. Proc Natl Acad Sci U S A. 2011;108:8821–8825. doi: 10.1073/pnas.1102425108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suh GS, et al. Light activation of an innate olfactory avoidance response in Drosophila. Curr Biol. 2007;17:905–908. doi: 10.1016/j.cub.2007.04.046. [DOI] [PubMed] [Google Scholar]
- 37.Ai M, et al. Acid sensing by the Drosophila olfactory system. Nature. 2010;468:691–695. doi: 10.1038/nature09537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su CY, Martelli C, Emonet T, Carlson JR. Temporal coding of odor mixtures in an olfactory receptor neuron. Proc Natl Acad Sci U S A. 2011;108:5075–5080. doi: 10.1073/pnas.1100369108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin JP, et al. The neurobiology of insect olfaction: sensory processing in a comparative context. Prog Neurobiol. 2011;95:427–447. doi: 10.1016/j.pneurobio.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 40.van der Goes van Naters W, Carlson JR. Receptors and neurons for fly odors in Drosophila. Curr Biol. 2007;17:606–612. doi: 10.1016/j.cub.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurtovic A, Widmer A, Dickson BJA. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- 42.Jefferys JG. Nonsynaptic modulation of neuronal activity in the brain: electric currents and extracellular ions. Physiol Rev. 1995;75:689–723. doi: 10.1152/physrev.1995.75.4.689. [DOI] [PubMed] [Google Scholar]
- 43.Faber DS, Korn H. Electrical field effects: their relevance in central neural networks. Physiol Rev. 1989;69:821–863. doi: 10.1152/physrev.1989.69.3.821. [DOI] [PubMed] [Google Scholar]
- 44.Vermeulen A, Rospars JP. Why are insect olfactory receptor neurons grouped into sensilla? The teachings of a model investigating the effects of the electrical interaction between neurons on the transepithelial potential and the neuronal transmembrane potential. Eur Biophys J. 2004;33:633–643. doi: 10.1007/s00249-004-0405-4. [DOI] [PubMed] [Google Scholar]
- 45.Kaissling KE. Chemo-electrical transduction in insect olfactory receptors. Annu Rev Neurosci. 1986;9:121–145. doi: 10.1146/annurev.ne.09.030186.001005. [DOI] [PubMed] [Google Scholar]
- 46.Nagel KI, Wilson RI. Biophysical mechanisms underlying olfactory receptor neuron dynamics. Nat Neurosci. 2011;14:208–216. doi: 10.1038/nn.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pellegrino M, Nakagawa T, Vosshall LB. Single sensillum recordings in the insects Drosophila melanogaster and Anopheles gambiae. J Vis Exp. 2010:1–5. doi: 10.3791/1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cardin JA, et al. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat Protoc. 2010;5:247–254. doi: 10.1038/nprot.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoo SJ, et al. Hid, Rpr and Grim negatively regulate DIAP1 levels through distinct mechanisms. Nat Cell Biol. 2002;4:416–424. doi: 10.1038/ncb793. [DOI] [PubMed] [Google Scholar]
- 50.Yao CA, Carlson JR. Role of G-proteins in odor-sensing and CO2-sensing neurons in Drosophila. J Neurosci. 2010;30:4562–4572. doi: 10.1523/JNEUROSCI.6357-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.