Abstract
Tamoxifen had been the only available hormonal option for the systemic treatment for breast cancer from 1979–2000. Enormous efforts have led to the development of potent and selective third generation aromatase inhibitors including anastrozole, letrozole and exemestane. Due to their superior efficacy to tamoxifen, aromatase inhibitors are presently approved as first line agents for the treatment of advanced estrogen receptor (ER) positive breast cancer and adjuvant therapy in early ER positive early breast cancer in postmenopausal women. Selective ER Modulators (SERMS), tamoxifen and raloxifene are the only agents presently used in breast cancer prevention in high risk women and their use has increased substantially over the last decade. Third generations SERMS, lasofoxifene and bazedoxifene have shown significant reduction in bone loss compared to placebo in postmenopausal women and are currently approved in the European Union for the treatment of postmenopausal osteoporosis. This review outlines the current strategies employed in the use of endocrine therapy in the management and prevention of breast cancer.
Keywords: Selective estrogen receptor modulator, Tamoxifen, Aromatase Inhibitors, Estrogen Receptor
1. Introduction
The journey to determine the mechanism that lies behind the growth of breast cancer started more than 100 years ago. The first medical evidence was the suppression of estrogen levels through oophrectomy to cause regression of metastatic breast cancer[1]. Similar antitumor effects were observed following adrenelectomy and hypophysectomy in postmenopausal women with breast cancer [2]. This led to the evolution of endocrine therapies, with the principal goal of depriving tumor cells of estrogen to induce tumor regression. The story of the reinvention of tamoxifen to become the gold standard for the adjuvant treatment of breast cancer and the pioneering medicine for the reduction of breast cancer incidence in high risk women, has been told in detail elsewhere [3, 4]. The translational laboratory research work in the 1970’s [5]catalyzed the move from orphan drug for the adjuvant treatment and prevention of breast cancer resulting in tamoxifen becoming the standard of care for the long term adjuvant therapy of ER positive breast cancer and the extension of the lives of millions of women worldwide. Despite the clinical success of tamoxifen, development of drug resistance and endometrial cancer led to the requirement of alternative hormonal therapy to avoid these issues. The clinical efficacy of third generation non steroidal aromatase inhibitors (AIs), anastrozole and letrozole and steroidal AI, exemestane has been extensively studied in comparison to tamoxifen. Although AIs have shown some superiority to tamoxifen as first-line agents in the treatment of postmenopausal women with breast cancer [6–8]selective estrogen receptor modulators (SERMs) remain the mainstay of treatment in breast cancer prevention. In this review, we focus on current published data on the treatment strategies using hormonal therapy in the treatment and prevention of breast cancer.
2.1. Tamoxifen versus Aromatase inhibitors
2.1. Advanced Breast Cancer
A meta-analysis [9]of comparative studies of AIs with tamoxifen, in postmenopausal women with advanced breast cancer demonstrated a significant difference favoring AIs over tamoxifen as first line agents in overall response rate (ORR; OR, 1.56; 95% CI, 1.17–2.07; P = 0.002) and clinical benefit (CB; OR, 1.70; 95% CI, 1.24–2.33; P =0.0009). Although the overall survival (OS) was increased for the AIs arm compared to the tamoxifen arm, the differences observed were not statistically significant (OR, 1.95; 95% CI, 0.88–4.30; P = 0.10).
2.2. Adjuvant monotherapy
In estrogen receptor (ER) positive early breast cancer, 5 years of adjuvant tamoxifen significantly reduces breast cancer recurrence and mortality throughout the first 10 years and 15 years respectively[10]. Incorporation of AIs as adjuvant therapy in breast cancer has been extensively studied. Several randomized trials [11–13]have compared AIs to 5 years of tamoxifen as primary adjuvant treatment of postmenopausal women with early breast cancer. The results are summarized in Table 1. Although anastrozole and letrozole showed significant improvements for disease free survival (DFS) and time to distant recurrence (TTDR) and exemestane only improved TTDR, none of the AIs showed significant overall survival (OS). A meta-analysis of the ATAC and BIG trials [14]revealed that the AIs achieved a 2.9% absolute decrease in recurrence (9.6% for AI vs. 12.6% for tamoxifen; P < 0.00001) and a nonsignificant reduction in breast cancer mortality. In both studies, the incidence of bone fractures was observed more frequently in the AI arm but there were no significant difference in the risk of cardiovascular adverse events in both treatment groups. However, gynecological problems and thromboembolic events were more frequent with tamoxifen therapy. At 10 year follow up of the ATAC trial, the incidence of most cancers was similar between groups and continue to be increased with anastrozole for colorectal (66 vs. 44; OR 1·51, 1·01–2·27)) and lung cancer (51 vs. 34; OR 1·51, 95% CI 0·96–2·41)), and lower for endometrial cancer (6 vs. 24; OR 0·25, 95% CI 0·08–0·63), melanoma (8 vs. 19; 0·42, 0·16–1·00), and ovarian cancer (17 vs. 28). Although long term effects of AIs are not yet established, it is suggested that bisphosphonates be added to AIs regimens to prevent AI associated bone loss.
Table 1.
Third generation aromatase inhibitors versus tamoxifen as first line adjuvant therapy.
| TRIAL | ARM | MEDIAN FOLLOW-UP (MONTHS) |
n | DFS | TTDR |
|---|---|---|---|---|---|
| ATAC [11] | ANA vs.TAM | 120 | 6241 | HR 0.91, 95% CI 0.83–0.99 | HR 0·87, 95% CI 0·77–0·99 |
| p=0.04 | p=0.03 | ||||
| BIG [12] | LET vs. TAM | 76 | 4922 | HR 0.88, 95%CI 0.78–0.99 | HR 0.85, 95% CI 0.72–1.00 |
| p=0.03 | p=0.05 | ||||
| TEAM [13] | EXE vs. TAM | 33 | 9766 | HR 0.91, 95% CI 0.83–0.99 | HR 0.81, 95% CI 0.67–0.98 |
| p=0.12 | p<0.03 |
ANA, anastrozole; ATAC, Arimidex, Tamoxifen, Alone or in combination; BIG, Breast International Group; DFS, disease free survival; EXE, exemestane; LET, letrozole; LET, letrozole TAM, tamoxifen; TEAM, Tamoxifen, Exemestane Adjuvant Multicenter; TTDR, time to distant recurrence.
2.3. Sequential therapy
It is well known that despite an initial response to tamoxifen, disease progression can occur due to acquired resistance. Prevention of breast cancer recurrences and improvement of survival has been explored with the use of sequential therapy with AIs after 2–3 years of tamoxifen to a total of 5 years of endocrine therapy. Pooled analysis [14]of 4 trials [15–17]in which 2–3 years of tamoxifen is switched to either 2–3 years of AIs or tamoxifen revealed that AI therapy was associated with an absolute 3.1% (SE=0.6%) reduction in recurrence (5% for AI vs. 8.1% for tamoxifen;2P<0.00001) and an absolute 0.7%(SE=0.3%) decrease in breast cancer mortality(1.7% for AI vs. 2.4% for tamoxifen;2P=0.02) after approximately 5 years of hormonal therapy. Whereas breast cancer mortality was significantly reduced, none of the individual trials reported a significant overall survival. However, updated data from the Anastrozole-Nolvadex (ARNO)-95trial, showed significant reduction in the risk of recurrences (p=0.049) and improved overall survival (p=0.045) with sequential treatment with anastrozole compared to tamoxifen monotherapy[18].
Two studies compared primary AI monotherapy with sequential therapy including tamoxifen followed by an AI. In addition to assessment of letrozole monotherapy compared to tamoxifen, the BIG 1–98 trial [12]also evaluated sequential therapy of 2 years of letrozole followed by 3 years of tamoxifen or 2 years of tamoxifen followed by 3 years of letrozole. A median follow up of 71 months revealed that there was no significant difference in terms of DFS with either sequential therapy when compared with letrozole alone. The TEAM trial was initially designed to evaluate the clinical efficacy of exemestane compared to 5 years of tamoxifen as initial adjuvant endocrine therapy. The study design was changed, based on the results of the Intergroup Exemestane Study (IES) trial, to include the sequential use of exemestane after 2.5–3 years of tamoxifen treatment. Updated analysis from the TEAM trial [19]at 5.1 years follow up showed that there was no significant difference in DFS between exemestane alone and tamoxifen followed by exemestane.
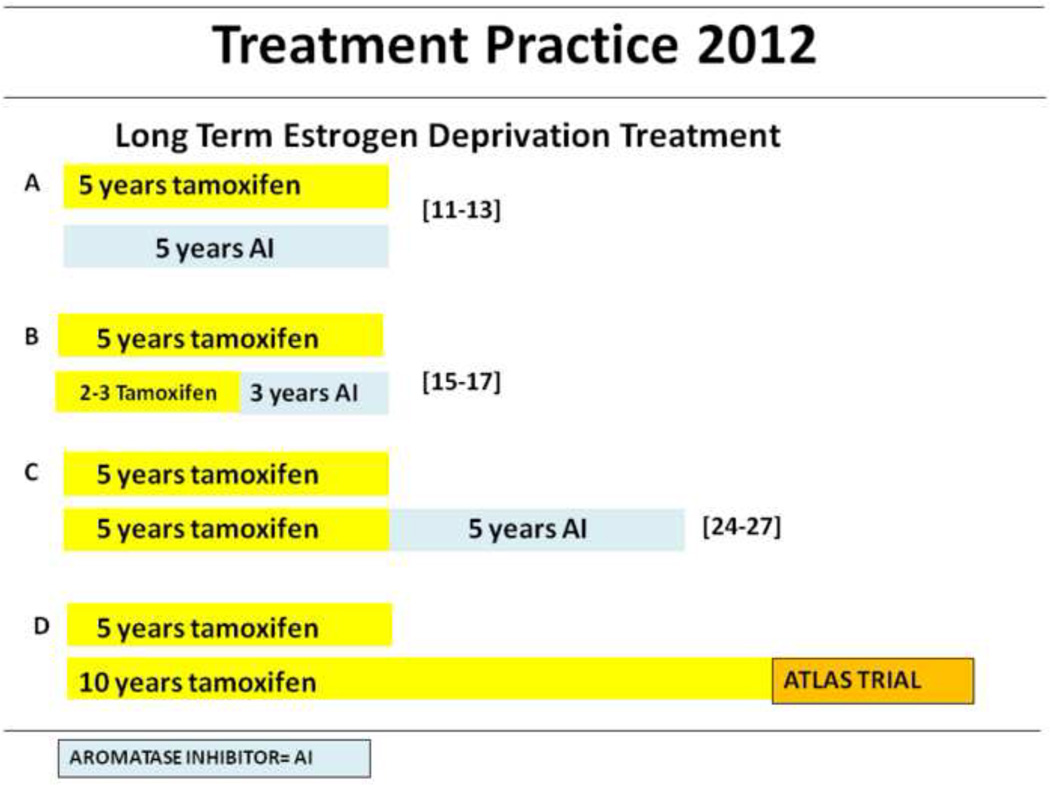
Therefore current recommendation in adjuvant endocrine treatment of ER positive breast cancer(Fig. 2.) is that postmenopausal women take AIs as a primary agent for 5 years or for 2 to 3 years after tamoxifen, while tamoxifen is recommended as a first line treatment for pre or perimenopausal women [20]. However which AI to use as either initial or sequential adjuvant therapy is yet to be determined. Studies [21]have shown that letrozole was more potent than anastrozole in the inhibition of aromatization and estrogen suppression in postmenopausal women with locally advanced and invasive ER positive breast cancer. But the superiority of letrozole was not observed in the head to head comparison of letrozole and anastrozole as second line agents in metastatic breast cancer [22]. The ACSOG trial [23]compared the clinical efficacy of all three AIs in the neoadjuvant treatment of locally advanced breast cancer. Preliminary results showed no significant difference in the clinical response rate. To date no meaningful clinical differences have been demonstrated between third generation AIs.
Fig. 2.
Clinical guidelines in the adjuvant treatment of estrogen positive breast cancer in postmenopausal patients (A–C) or pre/postmenopausal patients (D).
A. Five years of Tamoxifen or aromatase inhibitors can be used as first line adjuvant hormonal therapy in pre or perimenopausal or postmenopausal women respectively. B. In postmenopausal women, sequential therapy with aromatase inhibitor after 2–3 years of tamoxifen is comparable alternative to AI monotherapy. C. Additional 5 years with AIs after 5 years of tamoxifen, have shown significant disease free survival. D. Investigation of extension of tamoxifen beyond 5 years is presently ongoing.
2.4. Extended therapy
The MA-17 [24]randomly assigned 5187 patients who have completed 5 years of tamoxifen to 5 years of letrozole or placebo to determine the risk of recurrence. The study was stopped early when the first interim analysis showed that letrozole significantly lowered recurrence rate at a median follow up of 2.4 years. As a result the study was unblinded and 66% of patients on placebo crossed over to the letrozole group. An updated intent to treat analysis [25]revealed that letrozole treatment achieved a 2.9% improvement in DFS at 4 years (HR 0.68 P= 0.0001). Similarly, ABCSG-6a [26]evaluated anastrozole for 3 years in comparison with placebo. Favorable results were obtained with anastrozole which resulted in a significant reduction in risk of recurrence (p= 0.031). Exemestane was also compared with placebo after tamoxifen adjuvant therapy by the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-33. Similar to the MA-17, the study was stopped prematurely and unblinded due to significant improvement in DFS [27]. However all 3 extended adjuvant trials showed no significant improvement in overall survival. Although 10 year follow up of patients who received 5 years of tamoxifen yielded beneficial effects compared with 2 years of tamoxifen [28]extension of adjuvant therapy with tamoxifen beyond 5 years is not yet recommended. Results are currently awaited from the Adjuvant Tamoxifen-Longer Against Shorter (ATLAS) and adjuvant Tamoxifen Treatment offer more (aTTOM) which should give more insight to extending tamoxifen beyond 5 years. Furthermore, no data is available for the use of AIs beyond 5 years, therefore the recommended limit on AIs is 5 years total across strategies [20].
3. The SERM concept and breast cancer prevention
As a result of a focused effort to decipher the pharmacology and toxicology of tamoxifen, conclusions were built one upon the other, in the same laboratory, to define the properties of a new drug group called the SERMs and to articulation a roadmap to apply that drug group to prevent multiple diseases in women health. The mention of “modulation” at an ER target site occurred with the examination of the structure function relationships of estrogenic triphenylethylene derivatives of tamoxifen at a prolactin gene target in vitro 29]. The estrogenic compounds could activate or suppress prolactin synthesis by altering the shape of the ER complex between the extremes of an “antiestrogenic” or an “estrogenic” conformation [30]. This idea of the molecular modulation of the receptor at a single target site was then expanded to consider the physiologic responses that occurred with nonsteriodal antiestrogen at multiple target sites in the body – simultaneously. A simultaneous series of translational studies focused on the uterus, breast (mammary gland) and bone together created the laboratory rationale for further clinical trials by the pharmaceutical industry [31–34]. It was clear in 1990 that the toxicological issues with tamoxifen e.g. endometrial cancer[34, 35]needed another approach. A roadmap was stated because few women would have a prevention of breast cancer even in high risk populations; all would be exposed to side effects: “We have obtained valuable clinical information about this group of drugs that can be applied in other disease states. Important clues have been garnered about the effects of tamoxifen on bone and lipids so it is possible that derivatives could find targeted applications to retard osteoporosis or atherosclerosis. The ubiquitous application of novel compounds to prevent diseases associated with the progressive changes after menopause may as a side effect, significantly retard the development of breast cancer. The target population would be post-menopausal women in general, thereby avoiding the requirement to select a high risk group to prevent breast cancer” [36].
Although tamoxifen is the pioneering SERM, raloxifene is the medicine that first exploited the ’roadmap” successfully starting in 1992 [37]. Scientists at Eli Lilly [38]confirmed the concept in animal models measuring bone density, uterine weights and circulating cholesterol and initiated the Multiple Outcomes of Raloxifene Evaluation (MORE) trial. Raloxifene would be the first SERM to be approved for two of the three properties of the “ideal SERM”: reduction in the incidence of fractures in osteoporosis and the reduction in the incidence of breast cancer [39–41]. Raloxifene does not reduce the risk of coronary heart disease [42]. It is however, perhaps pertinent to note that the original work on the prevention of rat mammary carcinogenesis [33] concluded that because the pharmacokinetics of tamoxifen were superior to raloxifene then raloxifene was unlikely to be superior clinically in breast chemoprevention. Initially, data demonstrated that raloxifene was extremely effective at preventing ER positive breast cancer in 90% of osteoporotic women [40]but in the Study of Tamoxifen and Raloxifene or STAR trial in healthy postmenopausal women tamoxifen and raloxifene were equivalent in producing a 50% decrease in breast cancer incidence[41]. However, the latter evaluations were during treatment with the SERMs. If an evaluation of breast cancer incidence occurs after the end of a 5 year treatment regimen tamoxifen is superior to raloxifene that is only 78% as effective as tamoxifen 3 years following stopping treatment [43]. The laboratory study was accurate. As a result, continuous treatment with raloxifene can be considered and is efficacious at maintaining an antitumor environment [44]. Most importantly, there is no increased risk of endometrial cancer with raloxifene this again demonstrating the veracity of the translational research. Due to its breast cancer and osteoporosis preventive effects, raloxifene is recommended to be the ideal treatment of choice in high risk postmenopausal women.
4. New generation SERMS
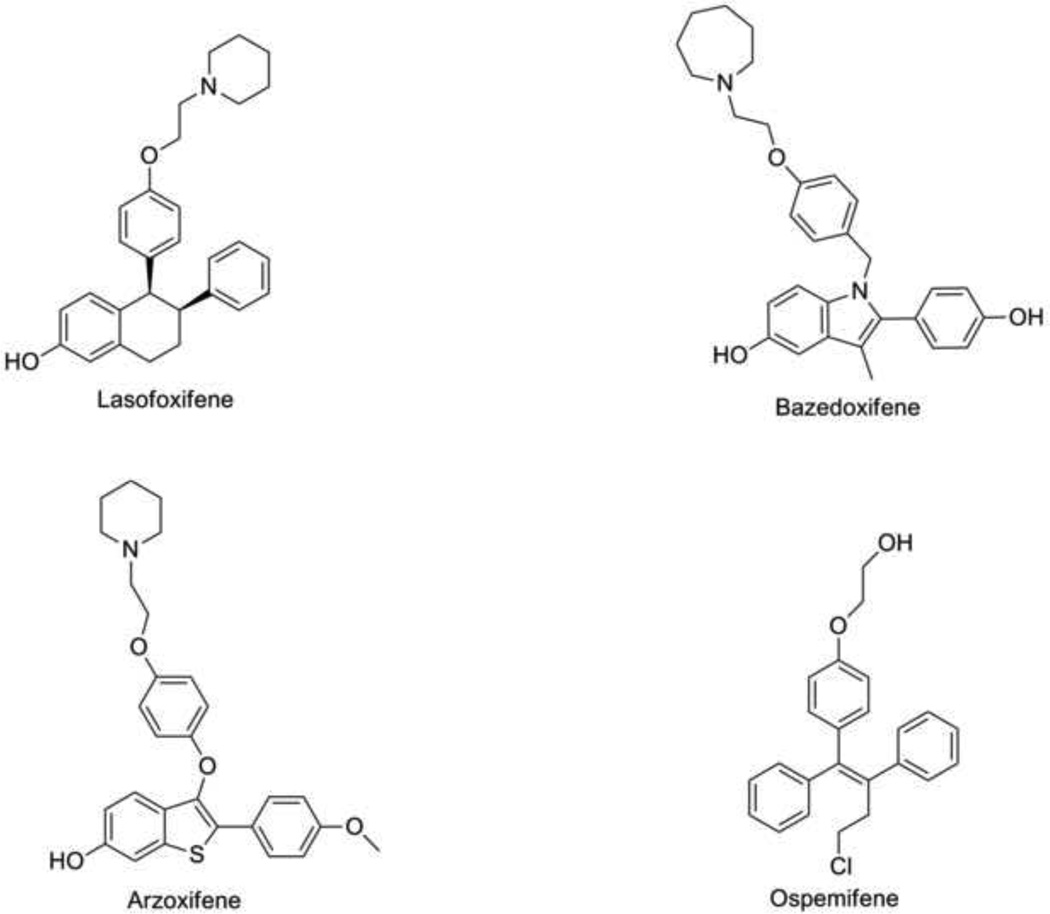
The development of third generation SERMs was based on preclinical studies which showed beneficial estrogenic effects on the bone without the detrimental stimulation on the endometrium or breast tissue [45, 46].Lasofoxifene, Bazedoxifene, Arzoxifene and Ospemifene (Fig. 3.) have been assessed in the treatment and prevention of osteoporosis as well as prevention of breast cancer. The Osteoporosis Prevention and Lipid Lowering (OPAL) and PEARL studies evaluated lasofoxifene, a third generation SERM in the treatment of osteoporosis. The OPAL study consists of two identical double-blind placebo-controlled studies assessing the vaginal and bone effects of lasofoxifene in nonosteoporotic women. Bone mineral density (BMD) was significantly reduced with an improvement in vaginal pH after 2 years of therapy [47–49]. The PEARL trial [50]is a randomized placebo controlled study involving 8556 postmenopausal women with low bone density. Five years treatment with 0.5mg of lasofoxifene induced a significant 79% reduction of all breast cancers as well as a statistically significant reduction of vertebral (42%) and non vertebral fractures (24%), major coronary events (32%) and stroke (36%) when compared to placebo [51]. The CORAL trial [52]compared the effects of lasofoxifene, raloxifene and placebo on BMD of postmenopausal women. Although lasofoxifene and raloxifene had a similar adverse effect profile, lasofoxifene significantly improved lumbar spine BMD (P≤ 0.05), and significantly reduced low-density lipoprotein cholesterol levels (P≤ 0.05) at 2 years of therapy compared to raloxifene and placebo. Lasofoxifene was approved for the treatment of osteoporosis in the European Union in March 2009; however it is still under review by the FDA in the United States. The medicine has not been marketed.
Fig. 3.
Chemical structures of third generation SERMS.
A 2 year randomized double-blind study [53]assessed the clinical efficacy of bazedoxifene compared with placebo. Raloxifene was added as a positive control. 10mg, 20 mg and 40 mg of bazedoxifene had superior advantage over placebo in the improvement of BMD at all skeletal sites (p<0.001). These effects were comparable to that obtained with 60mg raloxifene. Incidence of cardiovascular disease, thromboembolic events, endometrial abnormalities and breast cancer did not significantly differ between treatment groups. Silverman and Colleagues [54]reported that the incidence of new vertebral fractures was significantly lower in the bazedoxifene group compared to placebo (p< 0.05), while incidence of non vertebral fractures was not statistically different from the placebo group at 3 years. This trend was maintained on extension of the study for an additional 2 years [55]. A posthoc analysis of a subgroup of women at higher fracture risk showed that bazedoxifene induced a 50% and 44% reduction in nonvertebral fracture risk relative to placebo (p=0.02) and raloxifene (p=0.05). This effect by bazedoxifene in comparison to placebo was supported by a re-analysis using the fracture probability tool, FRAX [56]. Although incidence of breast cancer was lower in the bazedoxifene group, there were no significant differences noted in the incidence of breast or endometrial carcinoma as well as endometrial hyperplasia among treatment groups. Because of the favorable outcomes seen with bazedoxifene on the endometrium and bone, the (Selective Estrogen Menopause and Response to Therapy) SMART-1[57, 58]trial investigated the combination of bazedoxifene(BZA) and conjugated estrogens(CE) compared to placebo using endometrial hyperplasia and BMD as the primary endpoints. Although treatment with BZA/CE did not significantly reduce the incidence of endometrial hyperplasia over placebo at 2 years, BMD was increased significantly with BZA/CE at the lumbar spine and total hip. Perhaps the endometrial protective effects of BZA/CE may be seen with longer follow-up. This may alleviate the need for progestins in postmenopausal women with intact uterus on hormone replacement therapy. Bazedoxifene is currently approved for the treatment of osteoporosis in the European Union. Arzoxifene showed potential in the reduction of vertebral fractures but it was withdrawn from future clinical development based on nonvertebral efficacy [59]. QuatRx Pharmaceuticals is currently seeking FDA approval for the use of ospemifene in the treatment of vulvovaginal atrophy[60].
5. Conclusion
Tamoxifen continues to play a major role in the treatment and prevention of breast cancer. Parallel studies have shown that AIs are superior to tamoxifen in the management of metastatic breast cancer as well as an adjuvant agent in early breast cancer. Although most differences were statistically significant, however differences in overall survival was either non significant or was somewhat marginal. Clinical trials involving head to head comparison of AI are needed to determine the superiority (if any) in efficacy in tumor suppression. This will clarify the initial or sequential order in which these agents are used in clinical management. Tamoxifen and raloxifene are the only endocrine agents approved in the prevention of breast cancer in high risk women. Newer SERMs, lasofoxifene and bazedoxifene are well tolerated agents and could possibly act as an alternative in the prevention of postmenopausal osteoporosis. These SERMs have shown comparable efficacy to raloxifene. However clinical validation is needed to confirm beneficial effects in the reduction of the incidence of breast cancer, cardiovascular and thromboembolic events. Progress with the new SERMS is currently dependent upon the financial advantages of new agents over old SERMS now as generics (tamoxifen) or ending their patent life (raloxifene). So what about no estrogen at all for chemoprevention? Two trials were established to evaluate the efficacy of anastrozole (IBISII trial) and exemestane (MAP.3) with placebo in the prevention of breast cancer in high risk postmenopausal women. Recently MAP.3 has demonstrated the value of reducing breast cancer incidence by a reported low incidence of side effects[61]. Nevertheless, AIs are not currently recommended for breast cancer risk reduction outside of a clinical trial. No other drugs have shown greater efficacy than those currently approved for breast cancer treatment and prevention.
In summary, it is reasonable to note that much progress has been made in women’s health and a menu of medicines is now available and validated approaches are proven compared to none when all this started nearly 40 years ago [5].
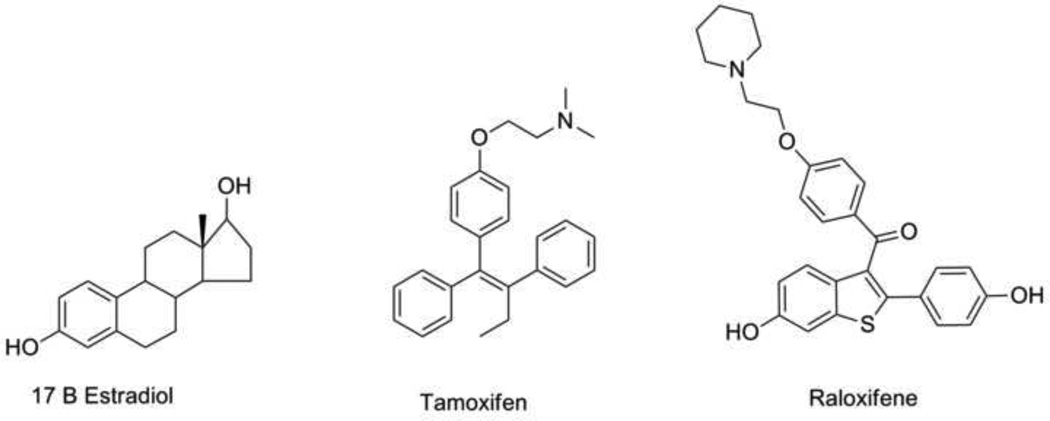
Fig. 1.
Chemical Structures of SERMS currently used in breast cancer prevention. The structure of estradiol is included for comparison.
Acknowledgments
Funding Information. This work (VCJ) was supported by the Department of 1090 Defense Breast Program under Award number W81XWH-06-1-0590 Center of Excellence; subcontract under the SU2C (AACR) Grant number SU2C-AACR-DT0409; the Susan G Komen for the Cure Foundation under Award number SAC100009 and the Lombardi Comprehensive Cancer 1095 Center Support Grant (CCSG) Core Grant NIH P30 CA051008. The views and opinions of the author(s) do not reflect those of the US Army or the Department of Defense.
Footnotes
Competing Interest
The authors do not have any competing interest.
Contributor Information
Ifeyinwa Obiorah, Department of Oncology, Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, 3970 Reservoir Rd NW. Research Building, Suite E208, Washington, DC 20057, Tel: 202.687.4842, oi3@georgetown.edu
V. Craig Jordan, Scientific Director Lombardi Comprehensive Cancer Center, Vincent T. Lombardi Chair of Translational Cancer Research, Vice Chair of Department of Oncology, Professor of Oncology and Pharmacology, Georgetown University Medical Center, 3970 Reservoir Rd NW. Research Building, Suite E501, Washington, DC 20057, Tel: 202.687.2897, Fax: 202.687.6402, vcj2@georgetown.edu
REFERENCES
- 1.Beaston G. On the treatment of inoperable cases of carcinoma of the mamma. Suggestions for a new method of treatment with illustrative cases. Lancet. 1896;2:104–107. [PMC free article] [PubMed] [Google Scholar]
- 2.Jordan VC. A Century of Deciphering the Control Mechanisms of Sex Steroid Action in Breast and Prostate Cancer, The Origins of Targeted Therapy and Chemoprevention. Cancer res. 2009;69:1243–1254. doi: 10.1158/0008-5472.CAN-09-0029. [DOI] [PubMed] [Google Scholar]
- 3.Jordan VC. Tamoxifen (ICI46,474) as a targeted therapy to treat and prevent breast cancer. Br J Pharmacol. 2006;147:S269–S276. doi: 10.1038/sj.bjp.0706399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jordan VC. Tamoxifen, a most unlikely pioneering medicine. Nat Rev Drug Discov. 2003;2:205–213. doi: 10.1038/nrd1031. [DOI] [PubMed] [Google Scholar]
- 5.Jordan VC. Tamoxifen, catalyst for the change to targeted therapy. Eur J Cancer. 2008;44:30–38. doi: 10.1016/j.ejca.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonneterre J, Buzdar A, Nabholtz JM, et al. Anastrozole is superior to tamoxifen as first-line therapy in hormone receptor positive advanced breast carcinoma. Cancer. 2001;92:2247–2258. doi: 10.1002/1097-0142(20011101)92:9<2247::aid-cncr1570>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 7.Mouridsen H, Gershanovich M, Sun Y, et al. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women, analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol. 2003;21:2101–2109. doi: 10.1200/JCO.2003.04.194. [DOI] [PubMed] [Google Scholar]
- 8.Paridaens RJ, Dirix LY, Beex LV, et al. Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women, the European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:4883–4890. doi: 10.1200/JCO.2007.14.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu HB, Liu YJ, Li L. Aromatase inhibitor versus tamoxifen in postmenopausal woman with advanced breast cancer, a literature-based meta-analysis. Clin Breast Cancer. 2011;11:246–251. doi: 10.1016/j.clbc.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Early Breast Cancer Trialists' Collaborative Group E. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen, patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuzick J, Sestak I, Baum M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer-10-year analysis of the ATAC trial. Lancet Oncol. 2010;11:1135–1141. doi: 10.1016/S1470-2045(10)70257-6. [DOI] [PubMed] [Google Scholar]
- 12.Mouridsen H, Giobbie-Hurder A, Goldhirsch A, et al. Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med. 2009;361:766–776. doi: 10.1056/NEJMoa0810818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones S, Seynaeve C, Hasenburg A, et al. Results of the first planned analysis of the TEAM (tamoxifen exemestane adjuvant multinational) prospective randomized phase III trial in hormone sensitive postmenopausal early breast cancer. Cancer research. 2009;69(Suppl):15. [Google Scholar]
- 14.Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28:509–518. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 15.Boccardo F, Rubagotti A, Guglielmini P, et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer. Updated results of the Italian tamoxifen anastrozole (ITA) trial. Ann Oncol. 2006;17:vii10–vii14. doi: 10.1093/annonc/mdl941. [DOI] [PubMed] [Google Scholar]
- 16.Jakesz R, Jonat W, Gnant M, et al. Switching of postmenopausal women with endocrineresponsive early breast cancer to anastrozole after 2 years' adjuvant tamoxifen, combined results of ABCSG trial 8 and ARNO 95 trial. Lancet. 2005;366:455–462. doi: 10.1016/S0140-6736(05)67059-6. [DOI] [PubMed] [Google Scholar]
- 17.Coombes RC, Kilburn LS, Snowdon CF, et al. Survival and safety of exemestane versus tamoxifen after 2–3 years' tamoxifen treatment (Intergroup Exemestane Study), a randomised controlled trial. Lancet. 2007;369:559–570. doi: 10.1016/S0140-6736(07)60200-1. [DOI] [PubMed] [Google Scholar]
- 18.Kaufmann M, Jonat W, Hilfrich J, et al. Improved overall survival in postmenopausal women with early breast cancer after anastrozole initiated after treatment with tamoxifen compared with continued tamoxifen, the ARNO 95 Study. J Clin Oncol. 2007;25:2664–2670. doi: 10.1200/JCO.2006.08.8054. [DOI] [PubMed] [Google Scholar]
- 19.van de Velde CJ, Rea D, Seynaeve C, et al. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM), a randomised phase 3 trial. Lancet. 2011;377:321–331. doi: 10.1016/S0140-6736(10)62312-4. [DOI] [PubMed] [Google Scholar]
- 20.Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline, update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geisler J, Helle H, Ekse D, et al. Letrozole is superior to anastrozole in suppressing breast cancer tissue and plasma estrogen levels. Clin Cancer Res. 2008;14:6330–6335. doi: 10.1158/1078-0432.CCR-07-5221. [DOI] [PubMed] [Google Scholar]
- 22.Rose C, Vtoraya O, Pluzanska A, et al. An open randomised trial of second-line endocrine therapy in advanced breast cancer, comparison of the aromatase inhibitors letrozole and anastrozole. Eur J Cancer. 2003;39:2318–2327. doi: 10.1016/s0959-8049(03)00630-0. [DOI] [PubMed] [Google Scholar]
- 23.Ellis MJ, Suman VJ, Hoog J, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer, clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype--ACOSOG Z1031. J Clin Oncol. 2011;29:2342–2349. doi: 10.1200/JCO.2010.31.6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793–1802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 25.Ingle JN, Tu D, Pater JL, et al. Intent-to-treat analysis of the placebo-controlled trial of letrozole for extended adjuvant therapy in early breast cancer, NCIC CTG MA. 17. Ann Oncol. 2008;19:877–882. doi: 10.1093/annonc/mdm566. [DOI] [PubMed] [Google Scholar]
- 26.Jakesz R, Greil R, Gnant M, et al. Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients, results from the randomized Austrian Breast and Colorectal Cancer Study Group Trial 6a. J Natl Cancer Inst. 2007;99:1845–1853. doi: 10.1093/jnci/djm246. [DOI] [PubMed] [Google Scholar]
- 27.Mamounas EP, Jeong JH, Wickerham DL, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen, intention-to-treat analysis of the National Surgical Adjuvant Breast And Bowel Project B-33 trial. J Clin Oncol. 2008;26:1965–1971. doi: 10.1200/JCO.2007.14.0228. [DOI] [PubMed] [Google Scholar]
- 28.Hackshaw A, Roughton M, Forsyth S, et al. Long-term benefits of 5 years of tamoxifen-10-year follow-up of a large randomized trial in women at least 50 years of age with early breast cancer. J Clin Oncol. 2011;29:1657–1663. doi: 10.1200/JCO.2010.32.2933. [DOI] [PubMed] [Google Scholar]
- 29.Jordan VC, Koch R, Mittal S, Schneider MR. Oestrogenic and antioestrogenic actions in a series of triphenylbut-1-enes, modulation of prolactin synthesis in vitro. Br J Pharmacol. 1986;87:217–223. doi: 10.1111/j.1476-5381.1986.tb10174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lieberman ME, Gorski J, Jordan VC. An estrogen receptor model to describe the regulation of prolactin synthesis by antiestrogens in vitro. J Biol Chem. 1983;258:4741–4745. [PubMed] [Google Scholar]
- 31.Jordan VC, Robinson SP. Species-specific pharmacology of antiestrogens, role of metabolism. Fed Proc. 1987;46:1870–1874. [PubMed] [Google Scholar]
- 32.Jordan VC, Phelps E, Lindgren JU. Effects of anti-estrogens on bone in castrated and intact female rats. Breast Cancer Res Treat. 1987;10:31–35. doi: 10.1007/BF01806132. [DOI] [PubMed] [Google Scholar]
- 33.Gottardis MM, Jordan VC. Antitumor actions of keoxifene and tamoxifen in the N-nitrosomethylurea- induced rat mammary carcinoma model. Cancer Res. 1987;47:4020–4024. [PubMed] [Google Scholar]
- 34.Gottardis MM, Robinson SP, Satyaswaroop PG, Jordan VC. Contrasting actions of tamoxifen on endometrial and breast tumor growth in the athymic mouse. Cancer Res. 1988;48:812–815. [PubMed] [Google Scholar]
- 35.Fornander T, Rutqvist LE, Cedermark B, et al. Adjuvant tamoxifen in early breast cancer, occurrence of new primary cancers. Lancet. 1989;1:117–120. doi: 10.1016/s0140-6736(89)91141-0. [DOI] [PubMed] [Google Scholar]
- 36.Lerner LJ, Jordan VC. Development of antiestrogens and their use in breast cancer, eighth Cain memorial award lecture. Cancer Res. 1990;50:4177–4189. [PubMed] [Google Scholar]
- 37.Lewis J, Jordan V. Comprehensive Medicinal Chemistry. In: Taylor JDT, editor. Case Histories, Raloxifene Edition. Oxford, UK: Elsevier Limited; 2006. pp. 103–121. [Google Scholar]
- 38.Black LJ, Sato M, Rowley ER, et al. Raloxifene (LY139481 HCI) prevents bone loss and reduces serum cholesterol without causing uterine hypertrophy in ovariectomized rats. J Clin Invest. 1994;93:63–69. doi: 10.1172/JCI116985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene, results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637–645. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 40.Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women, results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189–2197. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- 41.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes, the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 42.Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125–137. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- 43.Vogel VG, Costantino JP, Wickerham DL, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial, Preventing Breast Cancer. Cancer Prevention Research. 2010;3:696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martino S, Cauley JA, Barrett-Connor E, et al. Continuing outcomes relevant to Evista, breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751–1761. doi: 10.1093/jnci/djh319. [DOI] [PubMed] [Google Scholar]
- 45.Komm BS, Lyttle CR. Developing a SERM, stringent preclinical selection criteria leading to an acceptable candidate (WAY-140424) for clinical evaluation. Ann N Y Acad Sci. 2001;949:317–326. doi: 10.1111/j.1749-6632.2001.tb04039.x. [DOI] [PubMed] [Google Scholar]
- 46.Ke HZ, Paralkar VM, Grasser WA, et al. Effects of CP-336,156, a new, nonsteroidal estrogen agonist/antagonist, on bone, serum cholesterol, uterus and body composition in rat models. Endocrinology. 1998;139:2068–2076. doi: 10.1210/endo.139.4.5902. [DOI] [PubMed] [Google Scholar]
- 47.McClung M, Siris E, Cummings S. Lasofoxifene increased BMD of the spine and hip and decreased bone turnover markers in postmenopausal women with low or normal BMD. J Bone Min Res. 2005;20:F429. [Google Scholar]
- 48.Davidson M, Moffett A, Welty F, et al. Extraskeletal effects of lasofoxifene on postmenopausal women. J Bone Min Res. 2005;20:S173. [Google Scholar]
- 49.Peterson GM, Naunton M, Tichelaar LK, Gennari L. Lasofoxifene, selective estrogen receptor modulator for the prevention and treatment of postmenopausal osteoporosis. Ann Pharmacother. 2011;45:499–509. doi: 10.1345/aph.1P604. [DOI] [PubMed] [Google Scholar]
- 50.Cummings SR, Ensrud K, Delmas PD, et al. Lasofoxifene in Postmenopausal Women with Osteoporosis. N Engl J Med. 2010;362:686–696. doi: 10.1056/NEJMoa0808692. [DOI] [PubMed] [Google Scholar]
- 51.LaCroix AZ, Powles T, Osborne CK, et al. Breast cancer incidence in the randomized PEARL trial of lasofoxifene in postmenopausal osteoporotic women. J Natl Cancer Inst. 2010;102:1706–1715. doi: 10.1093/jnci/djq415. [DOI] [PubMed] [Google Scholar]
- 52.McClung MR, Siris E, Cummings S, et al. Prevention of bone loss in postmenopausal women treated with lasofoxifene compared with raloxifene. Menopause. 2006;13:377–386. doi: 10.1097/01.gme.0000188736.69617.4f. [DOI] [PubMed] [Google Scholar]
- 53.Miller PD, Chines AA, Christiansen C, et al. Effects of bazedoxifene on BMD and bone turnover in postmenopausal women-2-yr results of a randomized, double-blind, placebo- and active-controlled study. J Bone Miner Res. 2008;23:525–535. doi: 10.1359/jbmr.071206. [DOI] [PubMed] [Google Scholar]
- 54.Silverman SL, Christiansen C, Genant HK, et al. Efficacy of bazedoxifene in reducing new vertebral fracture risk in postmenopausal women with osteoporosis, results from a 3-year, randomized, placebo- and active-controlled clinical trial. J Bone Miner Res. 2008;23:1923–1934. doi: 10.1359/jbmr.080710. [DOI] [PubMed] [Google Scholar]
- 55.Silverman SL, Chines AA, Kendler DL, et al. Sustained efficacy and safety of bazedoxifene in preventing fractures in postmenopausal women with osteoporosis, results of a 5-year, randomized, placebo-controlled study. Osteoporos Int Epub. 2011 Jul 21; doi: 10.1007/s00198-011-1691-1. [DOI] [PubMed] [Google Scholar]
- 56.Kanis JA, Johansson H, Oden A, McCloskey EV. Bazedoxifene reduces vertebral and clinical fractures in postmenopausal women at high risk assessed with FRAX. Bone. 2009;44:1049–1054. doi: 10.1016/j.bone.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 57.Pickar JH, Yeh IT, Bachmann G, Speroff L. Endometrial effects of a tissue selective estrogen complex containing bazedoxifene/conjugated estrogens as a menopausal therapy. Fertil Steril. 2009;92:1018–1024. doi: 10.1016/j.fertnstert.2009.05.094. [DOI] [PubMed] [Google Scholar]
- 58.Lindsay R, Gallagher JC, Kagan R, et al. Efficacy of tissue-selective estrogen complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril. 2009;92:1045–1052. doi: 10.1016/j.fertnstert.2009.02.093. [DOI] [PubMed] [Google Scholar]
- 59.Silverman SL. New selective estrogen receptor modulators (SERMs) in development. Curr Osteoporos Rep. 2010;8:151–153. doi: 10.1007/s11914-010-0025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bachmann GA, Komi JO Group aTOS. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women, results from a pivotal phase 3 study. Menopause. 2010;17:480–486. doi: 10.1097/gme.0b013e3181c1ac01. [DOI] [PubMed] [Google Scholar]
- 61.Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for Breast-Cancer Prevention in Postmenopausal Women. N Engl J Med. 2011;364:2381–2391. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]