Abstract
Background
Nanomaterials have unique properties compared to their bulk counterparts. For this reason, nanotechnology has attracted a great deal of attention from the scientific community. Metal oxide nanomaterials like ZnO and CuO have been used industrially for several purposes, including cosmetics, paints, plastics, and textiles. A common feature that these nanoparticles exhibit is their antimicrobial behavior against pathogenic bacteria. In this report, we demonstrate the antimicrobial activity of ZnO, CuO, and Fe2O3 nanoparticles against Gram-positive and Gram-negative bacteria.
Methods and results
Nanosized particles of three metal oxides (ZnO, CuO, and Fe2O3) were synthesized by a sol–gel combustion route and characterized by X-ray diffraction, Fourier-transform infrared spectroscopy, and transmission electron microscopy techniques. X-ray diffraction results confirmed the single-phase formation of all three nanomaterials. The particle sizes were observed to be 18, 22, and 28 nm for ZnO, CuO, and Fe2O3, respectively. We used these nanomaterials to evaluate their antibacterial activity against both Gram-negative (Escherichia coli and Pseudomonas aeruginosa) and Gram-positive (Staphylococcus aureus and Bacillus subtilis) bacteria.
Conclusion
Among the three metal oxide nanomaterials, ZnO showed greatest antimicrobial activity against both Gram-positive and Gram-negative bacteria used in this study. It was observed that ZnO nanoparticles have excellent bactericidal potential, while Fe2O3 nanoparticles exhibited the least bactericidal activity. The order of antibacterial activity was demonstrated to be the following: ZnO > CuO > Fe2O3.
Keywords: nanomaterial, ZnO, CuO, Fe2O3, antibacterial activity, metal oxides
Introduction
The emergence of infectious diseases in general poses a serious threat to public health worldwide, especially with the emergence of antibiotic-resistant bacterial strains. Generally, both Gram-positive and Gram-negative bacterial strains are thought to present a major public health problem. Over the years, antibiotics have been used to control infections resulting from both community and hospital environments.1–3 Current advances in the field of nanobiotechnology, particularly the ability to prepare metal oxide nanomaterials of specific size and shape, are likely to lead to the development of new antibacterial agents. The functional activities of nanoparticles are influenced largely by the particle size. Therefore, nanoparticles have received great attention due to their unique physical, chemical, and effective biological properties in various fields, including medicine. The properties of nanoparticles can easily be altered by reducing or changing their size, especially when the manipulations are done at the nanometer scale.4–7 Similarly, tailoring of materials at the atomic level in order to attain unique properties has been widely reported. Considering these unique properties, nanosized organic and inorganic particles are being generated for ultimate use in medical practices, such as metal oxides of zinc, copper, and iron in biomedical research.8,9 In addition, nanoparticles with smaller particle size have been reported to show good antimicrobial activity.10 Antimicrobial activity of nanoparticles has largely been studied with human pathogenic bacteria such as Escherichia coli11 and Staphylococcus aureus.12 Moreover, these microbes seem to be highly sensitive to ZnO and CuO nanoparticles.10,13 Bactericidal activity of such nanoparticles in part depends on (1) size, (2) stability, and (3) concentration in the growth medium. While growing in medium amended with nanoparticles, the bacterial population growth can be inhibited by specific nanoparticle interactions.7 In general, bacterial cell size is in the micrometer range, while its outer cellular membranes have pores in the nanometer range. Since nanoparticles can be smaller in size than bacterial pores, they will have a unique ability of crossing the cell membrane. There lies a strong challenge in preparing metal oxide nanomaterials stable enough to restrict bacterial growth significantly while in nutrient medium.
In comparison to published reports on physical and chemical properties, very limited information is available on the antimicrobial properties of metal oxide nanoparticles. Realizing the potential antimicrobial applications of metal oxide nanoparticles, we designed experiments to synthesize ZnO, CuO, and Fe2O3 nanoparticles using a gel-combustion method and subsequently tested their antibacterial activities against both Gram-positive (S. aureus and Bacillus subtilis) and Gram-negative (Pseudomonas aeruginosa and E. coli) bacterial strains. Furthermore, the antibacterial behavior of these metal oxide nanoparticles was compared.
Materials and methods
Synthesis and characterization
In a typical synthesis procedure, metal nitrates of Zn, Cu, and Fe and citric acid were dissolved in distilled water with a molar ratio of 1:1. The solutions were stirred with a magnetic stirrer at 100°C. Stirring continues till the formation of gel for approximately 2 hours. As the gel was formed, it was allowed to burn at 200°C. A light fluffy mass was obtained as a result of combustion, which was further annealed at 400°C for 1 hour to obtain the respective crystalline metal oxide nanoparticles.14 The metal oxide nanoparticles thus obtained were characterized by X-ray diffraction (XRD), Fourier-transform infrared (FTIR) spectroscopy, and transmission electron microscopy (TEM). Crystallinity, structure, and crystallite size of nanoparticles were determined by XRD using a Rigaku (Tokyo, Japan) Miniflex X-ray diffractometer with Cu-Kα radiations (λ = 0.15406 nm) in the 2θ range from 20° to 80°. FTIR spectra of the samples were obtained using a PerkinElmer (Waltham, MA) FTIR spectrophotometer in the KBr matrix. TEM analysis was carried out using a 200 kV JEOL (Tokyo, Japan) transmission electron microscope.
Determination of antibacterial activity by well-diffusion method
Antimicrobial activities of the synthesized metal oxide nanoparticles were performed against both Gram-negative (E. coli and P. aeruginosa) and Gram-positive (B. subtilis and S. aureus) bacteria. The antibacterial activity was done by modified Kirby-Bauer disk diffusion method.15 In brief, the pure cultures of organisms were subcultured in Müller-Hinton broth at 35°C ± 2°C on a rotary shaker at 160 rpm. For bacterial growth, a lawn of culture was prepared by spreading the 100 μL fresh culture having 106 colony-forming units (CFU)/mL of each test organism on nutrient agar plates with the help of a sterile glass-rod spreader. Plates were left standing for 10 minutes to let the culture get absorbed. Then 8 mm wells were punched into the nutrient agar plates for testing nanomaterial antimicrobial activity. Wells were sealed with one drop of molten agar (0.8% agar) to prevent leakage of nanomaterials from the bottom of the wells. Using a micropipette, 100 μL (50 μg) of the sample of nanoparticle suspension was poured onto each of five wells on all plates. After overnight incubation at 35°C ± 2°C, the different levels of zone of inhibition were measured. Solvent blank was used as negative control. Antibiotic tetracycline was used as a positive control.
Determination of minimal bactericidal concentrations
Bacterial minimum bactericidal concentration (MBC) for metal oxide nanoparticles was determined by the broth-dilution method.16 In the present experiment, we used both Gram-positive and Gram-negative bacterial strains. Control experiments were also carried out in the presence of known standard antibiotics (tetracycline). A 10 mL nutrient broth medium amended with metal oxide nanomaterials (10–100 μg/mL) was prepared separately. Each set was inoculated aseptically with 100 μL of respective bacterial suspension (106 CFU/mL). The inoculated sets were incubated at 35°C ± 2°C for 24 hours. Viable bacterial colonies were counted and recorded by the naked eye determining the lowest concentration that locked bacteria growth, defining this as the MBC. The experiments were carried out in triplicate, and averages were reported.
Results
Morphological analysis
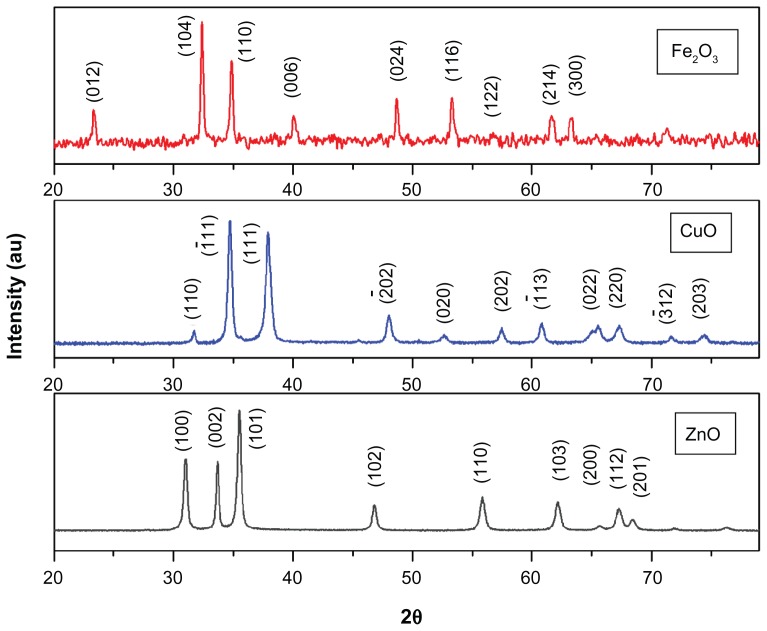
The typical XRD patterns of the ZnO, CuO, and Fe2O3 nanoparticles annealed at 400°C are shown in Figure 1. The peak positions of samples exhibit the hexagonal, monoclinic, and rhombohedral structures of ZnO, CuO, and Fe2O3, which were confirmed from the International Centre for Diffraction Data card numbers 80-0075, 80-1916, and 85-0987, respectively. Furthermore, no impurity peaks were observed in the XRD patterns, as all of the three metal oxides showed single-phase sample formation. The crystallite size was calculated using the Scherrer formula,
Figure 1.
X-ray diffraction spectra of ZnO, CuO, and Fe2O3 nanoparticles.
| (1) |
where λ is the wavelength of X-ray radiation and β is the full width at half maximum of the peaks at the diffracting angle θ. Crystallite sizes were calculated to be 18 nm, 22 nm, and 26.1 nm for ZnO, CuO, and Fe2O3 nanoparticles, respectively.
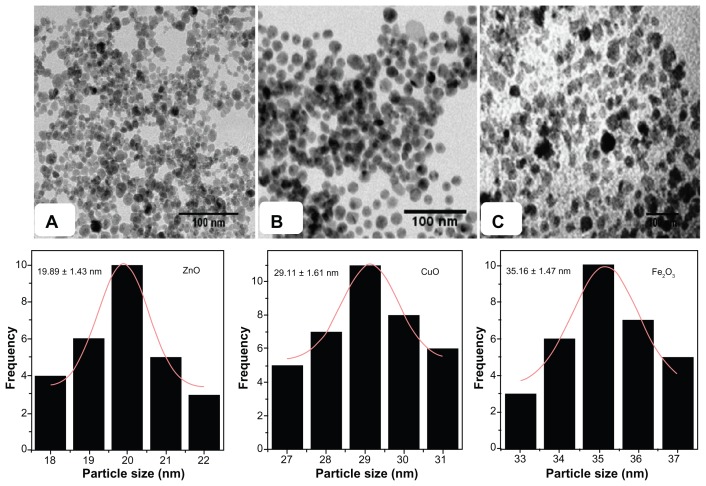
Figure 2 exhibits TEM images and histograms of particle-size distribution of ZnO, CuO, and Fe2O3 nanoparticles sintered at 400°C. Average particle sizes obtained from TEM images were found to be 19.89 ± 1.43 nm, 29.11 ± 1.61 nm, and 35.16 ± 1.47 nm for ZnO, CuO, and Fe2O3 nanoparticles, respectively. The average particle sizes determined by TEM images were very close to the crystallite size calculated from XRD results. Thus, the TEM results correlate well with XRD results.
Figure 2.
Transmission electron microscopy images of (A) ZnO, (B) CuO, and (C) Fe2O3 nanoparticles and histogram of particle-size distribution for different metal oxide nanoparticles.
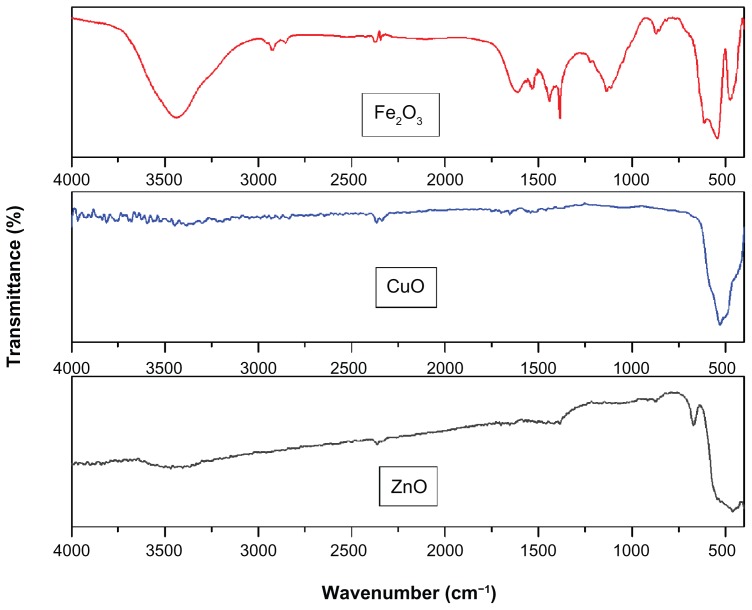
FTIR spectra were recorded in solid phase using the KBr pellet technique in the regions of 3500–400 cm−1. FTIR spectra of ZnO, CuO, and Fe2O3 nanoparticles are shown in Figure 3. FTIR spectra of all three metal oxide (ZnO, CuO, and Fe2O3) nanoparticles exhibited vibrations in the region 400–600 cm−1, which can be attributed to the vibrations of M−O (M = Zn, Cu, and Fe) which confirms the formation of ZnO, CuO and Fe2O3 nanoparticles. A weak band at around 2300 cm−1 may be attributed to the vibrations of atmospheric CO2. In the case of Fe2O3, the bands appearing at 1632 cm−1 can be attributed to the angular deformation of water δH−OH, while the band appearing at 3436 cm−1 can be assigned to the O−H stretching of water. The present findings agree well with the values reported in the available literature.17–21
Figure 3.
Fourier-transform infrared spectra of ZnO, CuO, and Fe2O3 nanoparticles.
Antimicrobial properties
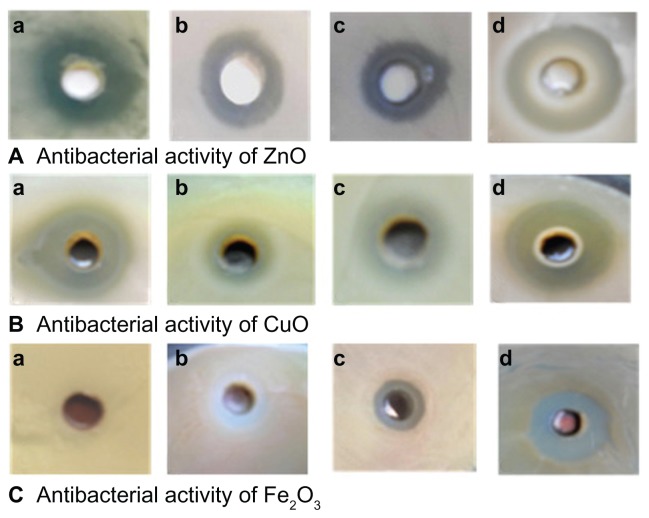
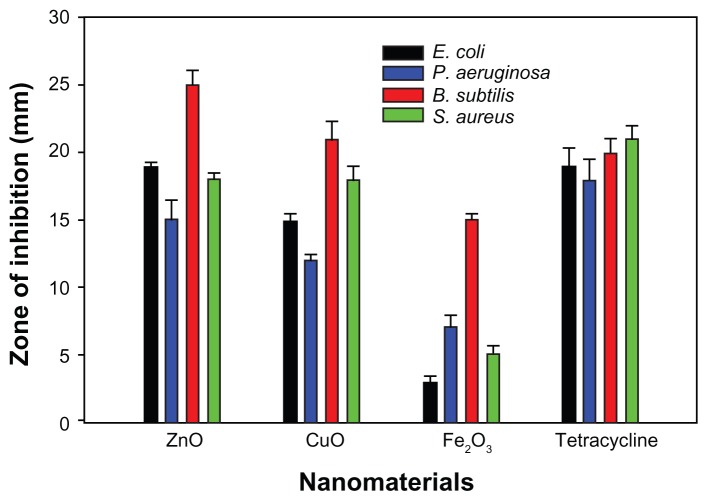
Antibacterial activity results revealed that ZnO and CuO nanoparticles acted as excellent antibacterial agents against both Gram-positive and Gram-negative bacteria when compared to Fe2O3 nanoparticles. It is clear from the XRD and TEM results that ZnO nanoparticles are smaller in size compared to CuO and Fe2O3. Figure 4 shows the zone of inhibition produced by different metal oxide nanoparticles against both Gram-positive and Gram-negative bacterial strains. ZnO (19.89 ± 1.43 nm) nanoparticles exhibited maximum (25 mm) bacterial growth inhibition against B. subtilis, in the form of zone-of-inhibition studies, where diffusion of nanoparticles on nutrient agar plates inhibits growth. In contrast, CuO and Fe2O3 showed zones of inhibition of 21 and 15 mm, respectively, against B. subtilis. In the case of E. coli maximum growth, inhibition zones were found to be the following; 19, 15, and 3 mm for ZnO, CuO, and Fe2O3, respectively (Figure 5). Similar patterns were observed in the case of P. aeruginosa and S. aureus, where the maximum zone of inhibition was exhibited by ZnO followed by CuO and Fe2O3. It appears that the antibacterial activity of the nanomaterials increased with increase in surface-to-volume ratio due to the decrease in size of nanoparticles.
Figure 4.
Zone of inhibition produced by different metal oxide nanoparticles against both Gram-positive and Gram-negative bacterial strains. Antibacterial activity of (A) ZnO; (B) CuO; and (C) Fe2O3 of bacterial strains (a), Escherichia coli, (b) Staphylococcus aureus, (c) Pseudomonas aeruginosa, and (d) Bacillus subtilis.
Figure 5.
Bar graphs showing zone of inhibition introduced by different metal oxides against various microorganisms.
Discussion
We demonstrated that the order of antibacterial activities of nanomaterials was ZnO (19.89 ± 1.43 nm) > CuO (29.11 ± 1.61 nm) > Fe2O3 (35.16 ± 1.47 nm), which indicates the size of the nanoparticles might also play a role in the antibacterial activity of each sample. Similar activity observations have been made for nanoparticles composed of a single metal oxide.6,7,10 However, it should also be noticed that Gram-negative bacterial strains of E. coli and P. aeruginosa had inhibition-zone sizes that were 24% and 16% lower than Gram-positive bacterial strains of B. subtilis and S. aureus in the case of ZnO nanoparticles. And in the case of CuO nanoparticles, same Gram-negative bacterial strains of E. coli and P. aeruginosa had zone sizes that were 28% and 33% lower than Gram-positive B. subtilis and S. aureus bacterial strains, respectively. This observation could also be indicative of higher Gram-negative strain resistance/tolerance against such nanomaterials over Gram-positive bacterial strains. Our finding is in agreement with Premanathan et al, who reported that the ZnO nanoparticle effect is more pronounced against Gram-positive bacterial strains than Gram-negative bacterial strains.22
Furthermore, previous studies have shown that the smaller the ZnO particle size, the greater the efficacy in inhibiting the growth of bacteria, involving both the production of reactive oxygen species and the accumulation of nanoparticles.7,10,23 However, nanoparticles of ZnO were previously reported to act both as bactericidal agents24 and bacteriostatic agents,25 perhaps thereby limiting their biomedical use.
In another experiment, we analyzed the MBC of different metal oxide nanoparticles against both Gram-negative and Gram-positive bacterial strains (Table 1). ZnO nanoparticles were also found to be most bactericidal compared to Fe2O3 and CuO nanoparticles. In our MBC study of ZnO nanoparticles, ZnO was 72%, 80%, 88%, and 84% more effective than Fe2O3, while 28%, 31%, 27%, 50%, and 40% more bactericidal than CuO against against E. coli, S. aureus, P. aeruginosa, and B. subtilis, respectively. The bactericidal pattern of our synthesized nanomaterials against both Gram-negative and Gram-positive bacterial strains was again ZnO > CuO > Fe2O3. Our results are supported by Baek and An26 and Wang et al,27 who reported that ZnO was the most toxic nanomaterial among ten other nanomaterials. As previously observed with zone-of-inhibition studies, ZnO nanoparticles had 11% and 12% more bactericidal activity against Gram-positive S. aureus and B. subtilis than Gram-negative E. coli and P. aeruginosa, respectively. CuO nanoparticles were 12% and 21% more active against Gram-positive S. aureus and B. subtilis than Gram-negative E. coli and P. aeruginosa, respectively. Overall, our observations are that Gram-positive bacterial strains are more sensitive in comparison to Gram-negative strains against the nanomaterials tested.
Table 1.
Minimum bactericidal concentration (MBC) of different metal oxide nanoparticles against Gram-negative and Gram-positive bacteria
| Nanoparticles | MBC values (μg/mL) | |||
|---|---|---|---|---|
|
|
||||
| Escherichia coli | Pseudomonas aeruginosa | Staphylococcus aureus | Baccillus subtilis | |
| ZnO | 18 ± 0.5 | 14 ± 0.6 | 16 ± 0.2 | 12 ± 0.5 |
| CuO | 25 ± 0.3 | 28 ± 0.5 | 22 ± 0.5 | 20 ± 0.7 |
| Fe2O3 | 65 ± 1.5 | 120 ± 2.3 | 80 ± 1.5 | 78 ± 1.4 |
| Tetracycline* | 30 ± 0.4 | 30 ± 1.2 | 28 ± 1.2 | 25 ± 1.5 |
Notes: Values are mean of three replicates;
standard antibiotic.
Thus, in this report, ZnO nanoparticles have shown the best antibacterial behavior compared to CuO and Fe2O3 nanoparticles. However, it is important to identify the key physicochemical properties of nanometal oxides that govern antibacterial activity and cytotoxicity to mammalian cells, as has been previously initiated. It is essential to assess the contribution of the size, shape, morphology, and electronic properties on cytotoxicity if these particles are to have wide biomedical applications.28,29 Our expectation is first to see such nanomaterials applied as surface disinfectants, as their stability would allow long-term storage and prolonged activity.
Conclusion
The nanosized particles of pure ZnO, CuO, and Fe2O3 were synthesized by the sol–gel combustion method. XRD and TEM results showed that ZnO nanoparticles were smallest (18 nm) in size compared to CuO (22 nm) and Fe2O3 (26 nm). Furthermore, the antibacterial activity of all the three synthesized nanomaterials was compared and varied considerably. Antimicrobial activity increased with increase in surface-to-volume ratio due to a decrease in particle size of nanoparticles. Here, ZnO nanoparticles showed excellent bactericidal potential, while iron oxide nanoparticles had the least bactericidal activity. Our results indicate that nanomaterials were most effective against Gram-positive bacterial strains compared to Gram-negative bacterial strains.
Acknowledgments
Mr Arham S Ahmed and Mr M Oves are thankful to CSIR, New Delhi, for providing financial support in the form of SRF. Dr Adnan Memic would like to thank the Strategic Technologies Program of King Abdulaziz City for Science and Technology, grant number 10-NAN1081-3, for their partial support and funding of this project.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- Lowy F. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- Komolafe OO. Antibiotic resistance in bacteria – an emerging public health problem. Malawi Med J. 2003;15:63–67. doi: 10.4314/mmj.v15i2.10780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkey PM. The growing burden of antimicrobial resistance. J Antimicrob Chemother. 2008;62(Suppl 1):i1–i9. doi: 10.1093/jac/dkn241. [DOI] [PubMed] [Google Scholar]
- Lewis K, Klibanov AM. Surpassing nature: rational design of sterile-surface materials. Trends Biotechnol. 2005;23:343–348. doi: 10.1016/j.tibtech.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Rosi NL, Mirkin CA. Nanostructures in biodiagnostics. Chem Rev. 2005;105:1547–1562. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]
- Azam A, Ahmed AS, Oves M, Khan MS, Memic A. Size-dependent antimicrobial properties of CuO nanoparticles against Gram-positive and -negative bacterial strains. Int J Nanomedicine. 2012;7:3527–3535. doi: 10.2147/IJN.S29020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghupati KR, Koodali RT, Manna AC. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir. 2011;27:4020–4028. doi: 10.1021/la104825u. [DOI] [PubMed] [Google Scholar]
- Mahapatra O, Bhagat M, Gopalakrishnan C, Arunachalam KD. Ultrafine dispersed CuO nanoparticles and their antibacterial activity. J Exp Nanosci. 2008;3:185–193. [Google Scholar]
- Tran N, Mir A, Mallik D, Sinha A, Nayar S, Webster TJ. Bactericidal effects of iron oxide nanoparticles on Staphylococcus aureus. Int J Nanomedicine. 2010;5:277–283. doi: 10.2147/ijn.s9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N, Ray B, Ranjit KT, Manna AC. Antibacterial activity of ZnO nanoparticles suspensions on a broad spectrum of microorganisms. FEMS Microbiol Lett. 2008;279:71–76. doi: 10.1111/j.1574-6968.2007.01012.x. [DOI] [PubMed] [Google Scholar]
- Yoon KY, Byeon JH, Park JH, Hwang J. Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci Total Environ. 2007;373:572–575. doi: 10.1016/j.scitotenv.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Ruparelia JP, Chatterjee AK, Duttagupta SP, Mukherji S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008;4:707–716. doi: 10.1016/j.actbio.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Heinlaan M, Ivask A, Blinova I, Dubourguier HC, Kahru A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere. 2008;71:1308–1316. doi: 10.1016/j.chemosphere.2007.11.047. [DOI] [PubMed] [Google Scholar]
- Jundale DM, Pawar SG, Patil SL, Chougule MA, Godse PR, Patil VB. Effect of annealing on structure, morphology and optoelectronic properties of nanocrystalline CuO thin films. AIP Conf Proc. 2011;1391:573–575. [Google Scholar]
- Bauer AW, Kirby WMM, Sherris JC, Truck M. Antibiotic susceptibility testing by standardized single disk method. Am J Clin Pathol. 1966;45:493–496. [PubMed] [Google Scholar]
- Wikler MA. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard. 5th ed. Wayne, PA: National Committee for Clinical Laboratory Standards (NCCLS); 2000. pp. M7–M5. [Google Scholar]
- Arshad M, Azam A, Ahmed AS, Mollah S, Naqvi AH. Effect of Co substitution on the structural and optical properties of ZnO nanoparticles synthesized by sol–gel route. J Alloys Compd. 2011;509:8378–8381. [Google Scholar]
- Jagminas A, Kuzmarskyte J, Niaura G. Electrochemical formation and characterization of copper oxygenous compounds in alumina template from ethanolamine solutions. Appl Surf Sci. 2002;201:129–137. [Google Scholar]
- Jagminas A, Niaura G, Kuzmarskyte J, Butkiene R. Surface-enhanced Raman scattering effect for copper oxygenous compounds array within the alumina template pores synthesized by ac deposition from Cu(II) acetate solution. Appl Surf Sci. 2004;225:302–308. [Google Scholar]
- Zhang YC, Tang JY, Wang GL, Zhang M, Hu XY. Facile synthesis of submicron Cu2O and CuO crystallites from a solid metallorganic molecular precursor. J Cryst Growth. 2006;294:278–282. [Google Scholar]
- Ansari SA, Azam A, Naqvi AH. Structural and morphological study of Fe2O3 nanoparticles. Asian J Res Chem. 2011;4:1638–1642. [Google Scholar]
- Premanathan M, Karthikeyan K, Jeyasubramanian K, Manivannan G. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomedicine. 2011;7:184–192. doi: 10.1016/j.nano.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Salah N, Habib SS, Khan ZH, et al. High-energy ball milling technique for ZnO nanoparticles as antibacterial material. Int J Nanomedicine. 2011;6:863–869. doi: 10.2147/IJN.S18267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, He Y, Irvin PL, Jin T, Shi X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl Environ Microbiol. 2011;77:2325–2331. doi: 10.1128/AEM.02149-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajjar P, Pettee B, Britt DW, Huang W, Johnson WP, Anderson AJ. Antimicrobial activities of commercial nanoparticles against an environmental soil microbe Pseudomonas putida KT2440. J Biol Eng. 2009;3:9. doi: 10.1186/1754-1611-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek Y, An Y. Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Sci Total Environ. 2011;409:1603–1608. doi: 10.1016/j.scitotenv.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Wang Z, Lee Y, Wu B, et al. Anti-microbial activities of aerosolized transition metal oxide nanoparticles. Chemosphere. 2010;80:525–529. doi: 10.1016/j.chemosphere.2010.04.047. [DOI] [PubMed] [Google Scholar]
- Xu M, Fujita D, Kajiwara S, et al. Contribution of physicochemical characteristics of nano-oxides to cytotoxicity. Biomaterials. 2010;31:8022–8031. doi: 10.1016/j.biomaterials.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Brayner R. The toxicological impact of nanoparticles. Nano Today. 2008;3:48–55. [Google Scholar]