Abstract
ANF-RGC is the prototype receptor membrane guanylate cyclase being both the receptor and the signal transducer of the most hypotensive hormones, ANF and BNP. It is a single trans-membrane protein. After binding these hormones at the extracellular domain, ANF-RGC at its intracellular domain signals the activation of the C-terminal catalytic module and accelerates the production of the second messenger, cyclic GMP, which controls blood pressure, cardiac vasculature, and fluid secretion. At present this is the sole transduction mechanism and the physiological function of ANF-RGC. Through comprehensive studies involving biochemistry, immunohistochemistry, and blood pressure measurements in mice with targeted gene deletions, the present study demonstrates a new signaling model of ANF-RGC that also controls blood pressure. In this model (1) ANF-RGC is not the transducer of ANF and BNP; (2) its extracellular domain is not used for signaling; and (3) the signal-flow is not downstream from the extracellular domain to the core catalytic domain. Instead, the signal is the intracellular Ca2+, which is translated at the site of its reception, at the core catalytic domain of ANF-RGC. A model for this Ca2+ signal transduction is diagrammed. It captures Ca2+ through its Ca2+ sensor myristoylated neurocalcin δ and up-regulates ANF-RGC activity with a K1/2 of 0.5 μM. The neurocalcin δ-modulated domain resides in the 849DIVGFTALSAESTPMQVV866 segment of ANF-RGC, which is a part of the core catalytic domain. Thereby, ANF-RGC is primed to receive, transmit and translate the Ca2+ signals into the generation of cyclic GMP at a rapid rate. The study defines a new paradigm of the membrane guanylate cyclase signaling, which is linked to the physiology of cardiac vasculature regulation and possibly also to fluid secretion.
Keywords: ANF-RGC, membrane guanylate cyclase, calcium, cyclic GMP, neurocalcinδ, blood pressure, adrenal gland
Introduction
The discovery of ANF-RGC (Atrial Natriuretic Factor Receptor Guanylate Cyclase), the first member of the membrane guanylate cycles family, was a landmark event in the field of cellular signaling1–9. It established a new field of membrane guanylate cyclases. With the inclusion of two other members, CNP-RGC, the receptor of C-type natriuretic peptide (CNP)10, 11 and STa-RGC, the receptor of heat stable enterotoxin, guanylin and uroguanylin12, 13, it demonstrated that the membrane guanylate cyclase is a surface receptor family. The sequential developments in the field disclosed three branches of the family: (1) the original, surface receptor; (2) the Ca2+-modulated, ROS-GC, with two members, ROS-GC1 and ROS-GC2; and (3) the odorant (uroguanylin) surface receptor and Ca2+-modulated with one member, ONE-GC8. ROS-GC primarily exists in the vision-linked sensory neurons. There it is a central component of phototransduction. It is also present in the olfactory bulb neurons but its physiological linkage with olfaction has not been established14. ONE-GC is the olfactory receptor of the odorant uroguanylin15, 16 and, indirectly, of atmospheric CO217, 18.
Common structural traits of the membrane guanylate cyclase family are that its members are single transmembrane-spanning proteins, composed of modular blocks8. Functionally, they are homodimeric. In each monomeric subunit, the transmembrane module divides the protein into two roughly equal portions, extracellular and intracellular. Their core catalytic domains are conserved, all residing in the intracellular region of their respective cyclases.
A striking topographical difference on the orientation of the core catalytic domain between the subfamilies of the surface receptor and the ROS-GC and ONE-GC exists. This is caused by the C-terminal extension (CTE) tails of ROS-GC and ONE-GC, which are absent in the surface receptor. Thus, in the ROS-GC and ONE-GC subfamilies the catalytic domain flows into CTE, which is not the case with the surface receptor subfamily.
The core catalytic domain of ROS-GC and ONE-GC is modulated differently than that of surface receptor subfamily members. ROS-GC and ONE-GC sense Ca2+ signals via their Ca2+ sensor domains, which reside in the intracellular region on the N- and C-terminal sides. There are four Ca2+ sensors of ROS-GC1: GCAP1, GCAP2, neurocalcin δ and S100B, each bound to its respective domain19. Importantly, the GCAP2 and S100B domains overlap and reside on CTE and the GCAP1-binding domain is located N-terminally to the catalytic domain. ONE-GC is modulated by three Ca2+ sensors: GCAP1, neurocalcin δ and hippocalcin20–22. Their targeted domains reside on the core catalytic domain of ONE-GC20–22. Intriguingly, GCAP1-modulated Ca2+ signal stimulates ONE-GC activity20, in contrast to ROS-GC1 where it inhibits the cyclase activity.
There is one prominent difference between the transduction mechanisms of ROS-GC and ONE-GC. While ROS-GC is solely modulated by the Ca2+ signals generated inside the sensory neurons, ONE-GC transduction mechanism is more complex. Being a uroguanylin receptor, it generates the uroguanylin signal at its extracellular domain and via its Ca2+ sensors amplifies the signal at its intracellular domain23. Additionally, ONE-GC is also modulated in a Ca2+-independent fashion. It transduces atmospheric CO2 signal via carbonic anhydrase enzyme17. The enzyme generates bicarbonate which, in turn, binds and stimulates the ONE-GC core catalytic domain, aa880-102818.
Noting these mechanistic complexities and similarities, these authors proposed a unified signaling theme of the ROS-GC and ONE-GC subfamilies where “Ca2+-sensors and ROS-GC are interlocked sensory transduction elements”19. The present study extends this theme to ANF-RGC. It discloses new ANF-RGC transduction mechanism. In this mechanism, the receptor domain where ANF and BNP bind is not involved in signaling. In fact, the extracellular domain, trans-membrane and the ATP-regulated domain are bypassed and the core catalytic domain is directly stimulated and generates cyclic GMP. The signal for the stimulation is Ca2+. In mice, the disabling of the Ca2+ signaling mechanism through genetic modification leads to hypertension. The findings define a new signal transduction model of the membrane guanylate cyclase family and link it with blood pressure regulation.
EXPERIMENTAL PROCEDURES
Expression in COS cells
COS cells maintained in DMEM medium supplemented with 10% fetal bovine serum and antibiotics were transfected with ANF-RGC cDNA using calcium phosphate co-precipitation technique24. 64 hr after transfection cells were washed with 50mM Tris-HCl pH 7.4/10 mM Mg2+ buffer, homogenized and the particulate fraction pelleted by centrifugation.
ANF-RGC soluble construct aa 788-1029
A full-length ANF-RGC cDNA in pcDNA3 expression vector was used for PCR amplification. The amplified fragment coding for ANF-RGC region aa 788-1029 was cloned into pFastBac vector (Bac-to-Bac Baculovirus expression system, Invitrogen system) yielding 6-His tag at N-terminus. The plasmid was sequenced to confirm its identity. Using DH10Bac cells the recombinant bacmid was generated and transfected into Sf-9 cells to produce recombinant baculoviruses. For protein expression, suspension of Sf-9 cells was infected at a rate of 6–10 MOI at a cell density of ~1×106 cells/ml. Cells were harvested 70–80 hrs after infection lysed and the protein was purified on a Ni-NTA column and through FPLC on Superdex 75 column.
Guanylate cyclase activity assay
The membrane fraction was incubated on ice-bath with or without neurocalcin δ in the assay system containing 10 mM theophylline, 15 mM phosphocreatine, 20 μg creatine kinase and 50 mM Tris-HCl, pH 7.5. Appropriate Ca2+ concentrations were adjusted with pre-calibrated Ca2+/EGTA solutions of a Ca2+ buffer kit (Molecular Probes/Invitrogen). The total assay volume was 25 μl. The reaction was initiated by addition of the substrate solution (4 mM MgCl2 and 1mM GTP, final concentration) and maintained by incubation at 37 °C for 10 min. The reaction was terminated by the addition of 225 μl of 50 mM sodium acetate buffer, pH 6.2 followed by heating on a boiling water bath for 3 min. The amount of cyclic GMP formed was determined by radioimmunoassay25.
Expression and purification of neurocalcin δ
Myristoylated neurocalcin δ was expressed and purified according to the protocol described previously26. Nonmyristoylated neurocalcin δ was expressed and purified following the same protocol except that the cells expressing neurocalcin δ were not co-transfected with N-myristoyltransferase and myristic acid was not added to the culture.
Antibodies
Antibodies against ANF-RGC and neurocalcin δ were raised in rabbits. Their specificities were described previously20, 26. The antibodies were affinity purified. PDE2A antibody was purchased from Santa Cruz Biotechnology, Inc., Santa Cruz, CA. Secondary antibodies conjugated to a fluorescent dye (DyLight 488 and DyLight 549) were purchased from Jackson ImmunoResearch Laboratories, Inc., West Grove, PA.
Protein quantification for determination of ANF-RGC catalytic efficiency
The antigen for ANF-RGC antibody (ANF-RGC fragment aa 486-661) was expressed and purified as described previously27. Its concentration was determined using purified bovine serum albumin standards. Aliquots of the antigen (10 - 0.1 ng) were diluted in a Laemmli SDS sample buffer and loaded next to 50 μg (total protein) of COS cells membranes expressing ANF-RGC. After electrophoresis in 7% SDS-polyacrylamide gel the proteins were transferred onto PVDF membrane. The membrane was blocked overnight in blocking solution (2% BSA in Tris-buffered saline containing Tween-20), immunostained with anti-ANF-RGC polyclonal antibody and developed using a Pierce/ThermoFisher Scientific SuperSignal reagent kit and goat anti rabbit peroxidase conjugates. The images were collected by exposing the membrane to X-ray Kodak film. The intensities of the signals were quantified from the calibration curve produced by the antigen standards.
Immunohistochemistry
Mice were sacrificed by lethal injection of ketamine/xylazine (the protocol approved by the Salus University IUCAC) and perfused through the heart, first with a standard Tris-buffered saline (TBS) and then with freshly prepared 4% paraformaldehyde in TBS. The adrenal glands were removed and fixed for 1–4 hours in 4% paraformaldehyde with TBS at 4°C, cryoprotected in 30% sucrose overnight at 4°C and cut into 20 μm sections using Hacker-Bright OTF5000 microtome cryostat (HACKER Instruments and Industries Inc., Winnsboro, SC). The sections were washed with TBS, blocked in 10% normal donkey serum in TBS/0.5% Triton X-100 (TBST) for 1hr at room temperature, washed with TBST, incubated with respective antibody in blocking solution overnight at 4°C, washed with TBST, incubated with DyLight (488 or 549) conjugated donkey anti-rabbit (or as necessary donkey anti-goat) antibody (200:1) for 1 hr and again washed with TTBS. Images were acquired using an inverted Olympus IX81 microscope/FV1000 Spectral laser confocal system, and analyzed using Olympus FluoView FV10-ASW software. Digital images were processed using Adobe Photoshop software.
Genetically modified mice
Care of the experimental animals conformed to the protocols approved by the IACUC at Salus University and was in strict compliance with the NIH guidelines.
Neurocalcin δ+/− (NCδ+/−)
Two mouse genomic fragments of neurocalcin δ were amplified by PCR: first, from intron 14 to codon for V12 (exon 15); second, within intron 15. The genomic distance between these two fragments was ~1000 bp. They were cloned individually into the multiple cloning sites, separated by the PGK-neo cassette of the pPNT vector. The linear DNA consisting of both neurocalcin δ fragments separated by the PGK-neo cassette was released from the vector and electroporated into mouse ES cells. The clones with homologous recombination were injected into C57BL/6 blastocytes. Male chimera was bred to C57BL/6 females. NCδ+/− heterozygotes were obtained. The attempts (one-year) to breed homozygous NCδ−/− mice have to date been unsuccessful.
Membrane preparation
Adrenal glands were removed from the wild type (control) or the genetically modified mice. The tissues were powdered using pistol ant mortar under liquid nitrogen and homogenized in a buffer consisting of 250 mM sucrose, 10 mM Tris-HCl pH 7.4, 1 mM EGTA (or 10 μM Ca2+), containing a protease inhibitors cocktail, centrifuged at 1,000g and then at 100,000g to pellet the membrane fraction. This fraction was suspended in 50 mM Tris-HCl pH 7.4/10 mM MgCl2 buffer and used for guanylate cyclase activity assay.
Blood pressure measurements
Systolic blood pressures of neurocalcin δ gene-targeted mice (NCδ+/−) and their isogenic controls (wild type: NCδ+/+) were measured every day for one week by the noninvasive computerized tail-cuff method with CODA (Kent Scientific) according to the manufacturer’s protocol. An average blood pressure level of 10 sessions per day was calculated for analysis after 3 days of mice training. The mice were maintained on normal chow and drinking water available ad libitum.
Statistical analysis
The blood pressure data are expressed as mean ± SD. Differences between the two groups analyzed, NCδ+/+ (wt, control) and NCδ+/−, were compared using Student’s t test. A P value of <0.05 was considered significant.
RESULTS
ANF-RGC is a bimodal transduction switch
ANF-RGC is the receptor and the transducer of the signals generated by the peptide hormones ANF and BNP9. Binding of these hormones at the ANF-RGC extracellular domain signals activation of the C-terminal catalytic module and accelerates the production of their second messenger, cyclic GMP, which controls blood pressure, cardiac vasculature, and fluid secretion. The mechanism of ANF-RGC signal transduction is that ligand binding to its receptor domain triggers a chain of structural changes. These changes are carried through the trans-membrane to the intracellular domain where, by ATP-dependent changes within the ARM domain they are processed further. Ultimately, they are sensed by the core catalytic module, which translates them into the production of cyclic GMP9. This is the traditional and the only established ANF-RGC signal transduction mechanism.
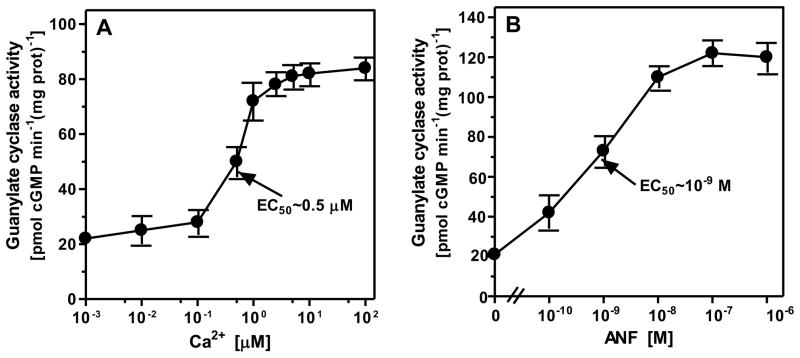
The myristoylated (myr) form of neurocalcin δ (NCδ) is a Ca2+-sensor protein of ROS-GC1 signaling19, 26. In the course of mapping its domain on ROS-GC1 to which it binds and transmits Ca2+ signal for its translation into the generation of cyclic GMP, these authors made a remarkable observation. This was that it binds directly to the core catalytic domain and, thereby, activates ROS-GC128. Protein database search indicated sequence conservation of the catalytic domain in the membrane guanylate cyclase family. Therefore, to test whether ANF-RGC is also linked with its Ca2+ signaling20, recombinant ANF-RGC expressed in COS cells was exposed to varying concentrations of Ca2+ in the presence of 2 μM of myr-NCδ. The results, presented in figure 1A, show that Ca2+ in a dose-dependent fashion with an EC50 of 0.5 μM stimulated ANF-RGC activity, Vmax occurring at ~ 1 μM Ca2+. Thus, Ca2+ via its sensor NCδ signals ANF-RGC activation. As expected, side-by-side experiment showed that ANF in the presence of ATP stimulated ANF-RGC with an EC50 of 1 nM (Fig. 1B). These results demonstrate that ANF-RGC is a bimodal signal transduction switch; one mode transducing the ANF signal and the other, the Ca2+ signal into the production of cyclic GMP.
Figure 1. ANF-RGC is responsive to ANF and Ca2+ signals.
COS cells were transfected with ANF-RGC cDNA. 60 hr after transfection the membrane fraction was prepared and assayed for guanylate cyclase activity in the presence of increasing concentrations of Ca2+ and 2 μM recombinant myristoylated neurocalcin δ (A) or indicated concentrations of ANF and 0.8 mM ATP. Ca2+ or EGTA were not added to the assay mixture. (B). The experiments were done in triplicate and repeated two times for reproducibility. The results presented are average ± SD from these experiments. The EC50 values were determined graphically.
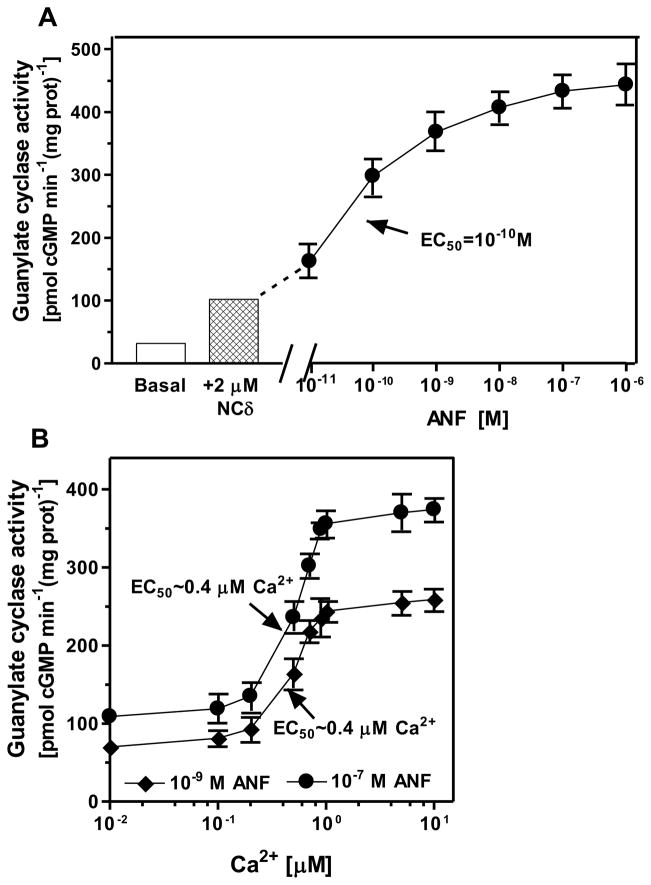
To determine the liaison between these two signaling modes, the membranes of COS cells expressing ANF-RGC were exposed first to 1 μM Ca2+ in the presence of 2 μM myr-NCδ and then to increasing concentrations, ranging from 10−11 M to 10−6 M, of ANF and constant 0.8 mM ATP. Myr-NCδ caused 3.2-fold stimulation from 31.6 to 102 pmol cyclic GMP min−1 (mg prot)−1 and ANF an additional 4.5-fold stimulation to 454 pmol cyclic GMP min−1 (mg prot)−1, amounting to a total of 7.7-fold stimulation of ANF-RGC activity (Fig. 2A). Thus, the results demonstrate that the NCδ and ANF effects are additive. To further substantiate this conclusion, membranes of COS cells expressing ANF-RGC were exposed to 2 μM NCδ, 10−9 or 10−7 M ANF, 0.8 mM ATP and increasing concentrations of Ca2+ and the cyclase activity was assessed. At low, ~10 nM Ca2+, the ANF-RGC activity was 70 ± 5 pmol cyclic GMP min−1(mg protein)−1 in the presence 10−9 M ANF and 109 ± 9 pmol cyclic GMP min−1(mg protein)−1 in the presence 10−7 M ANF (Fig. 2B). These activities are comparable to those achieved by ANF-RGC in the presence of only 10−9 or 10−7 M ANF (compare figure 1A) and consistent with the fact that Ca2+-free NCδ does not stimulate ANF-RGC (compare figure 1B). Thus, the cyclase activity at low Ca2+ concentration is regulated exclusively by ANF. Increasing concentration of Ca2+ in the assay mixture resulted in increased cyclase activity at both ANF concentrations. Because NCδ concentration was constant, 2 μM, these results indicate that increasing amount of Ca2+-bound NCδ in the assay mixture resulted in increased ANF-RGC activity. The half-maximal ANF-RGC activation was achieved at ~0.4 μM Ca2+ and maximal, at ~1 μM. Based on these results it is concluded that the two modes of ANF-RGC signaling, hormonal ANF and NCδ-mediated Ca2+, are processed by individual mechanisms. Because deletion of the extracellular domain of ANF-RGC has no influence on the NCδ-modulated Ca2+ signaling but is critical for the ANF signaling20, it is concluded that the two signaling modes are generated through different domains of ANF-RGC: ANF by the extracellular domain and Ca2+ by the intracellular domain.
Figure 2. The ANF and Ca2+ stimulatory effects on the activity of ANF-RGC are additive.
(A) Membranes of COS cells expressing ANF-RGC were preincubated with 1 μM Ca2+ and 2 μM recombinant myristoylated neurocalcin δ (NCδ). This was followed by incubation with indicated concentrations of ANF and 0.8 mM ATP and the guanylate cyclase activity was assayed as described in the Experimental Procedures. (B) Membranes of COS cells expressing ANF-RGC were incubated with 10−9 or 10−7 M ANF in the presence of 0.8 mM ATP, 2 μM NCδ and increasing concentrations of Ca2+. The guanylate cyclase activity was assessed. The experiments were repeated two times with different preparation of transfected cells. The results shown are from one experiment done in triplicate. The ANF EC50 values were determined graphically.
Myristoylated form of NCδ is necessary for effective Ca2+ signaling of ANF-RGC
NCδ belongs to the family of neuronal calcium sensor proteins (NCS). Characteristic feature of majority, but not all, of these proteins is that they are myristoylated at their N-termini and the myristoylation is important for their cellular functions.
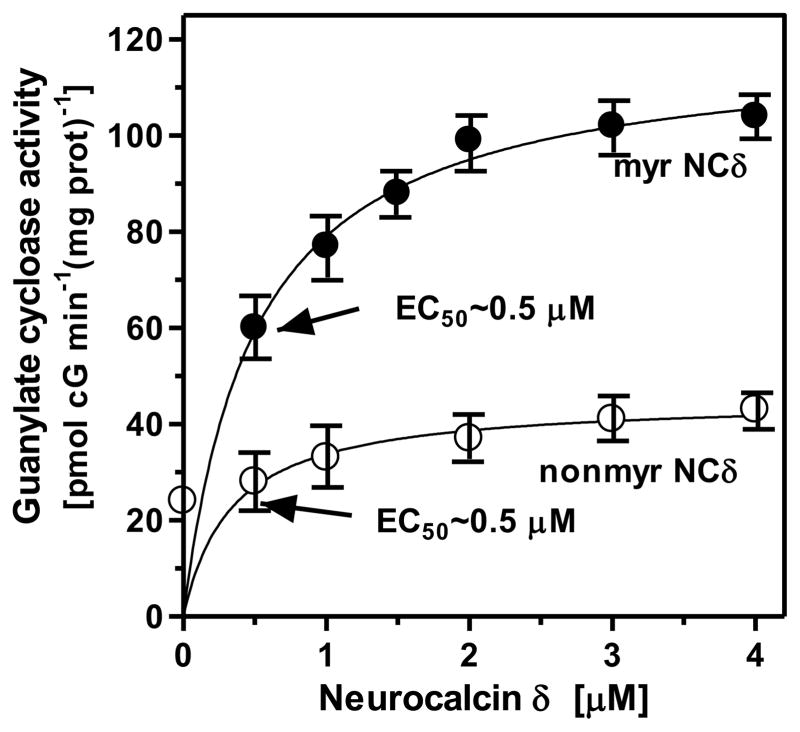
To determine if myristoylation is required for NCδ to transduce the Ca2+ signal for ANF-RGC activation, its myristoylated and nonmyristoylated forms were expressed, purified and individually incubated in the presence of 1 μM Ca2+ with membranes of COS cells expressing recombinant ANF-RGC. The results are shown in figure 3. The nonmyristoylated form caused only partial stimulation of ANF-RGC: from 24 to 43 pmol cyclic GMP min−1(mg prot)−1 (Fig. 3: open circles). In contrast, the myristoylated form robustly stimulated ANF-RGC: from 24 to 108 pmol cyclic GMP min−1(mg prot)−1 (Fig. 3: closed circles). The EC50 values of the both forms were identical, 0.5 μM, indicating their similar affinities for ANF-RGC. In the absence of Ca2+ [presence of 1 mM EGTA], both the myristoylated and nonmyristoylated forms caused no stimulation of ANF-RGC (not shown). The conclusion, therefore, is that the myristoylated form of NCδ is the transducer of Ca2+ signal in the activation of ANF-RGC.
Figure 3. Myristoylated neurocalcin δ effectively transmits the Ca2+ signal to ANF-RGC.
Membranes of COS cells expressing ANF-RGC were exposed to 1 μM Ca2+ and increasing concentrations of neurocalcin δ in its myristoylated (myr NCδ) or nonmyristoylated (nonmyr NCδ) form. The experiment was done in triplicate and repeated four times with separate membrane or neurocalcin d preparations. The results shown (mean ± SD) are from one experiment.
In addition to increasing the Vmax, myristoylation of NCδ influences other enzymatic properties of ANF-RGC. It lowers its KM for GTP from 0.86 mM to 0.37 mM and raises its enzymatic efficiency, kcat, from 6.5 ± 0.3 cyclic GMP sec−1 to 41.4 ± 0.5 cyclic GMP sec−1.
Core catalytic region of ANF-RGC, amino acids V851-V866, binds myristoylated NCδ
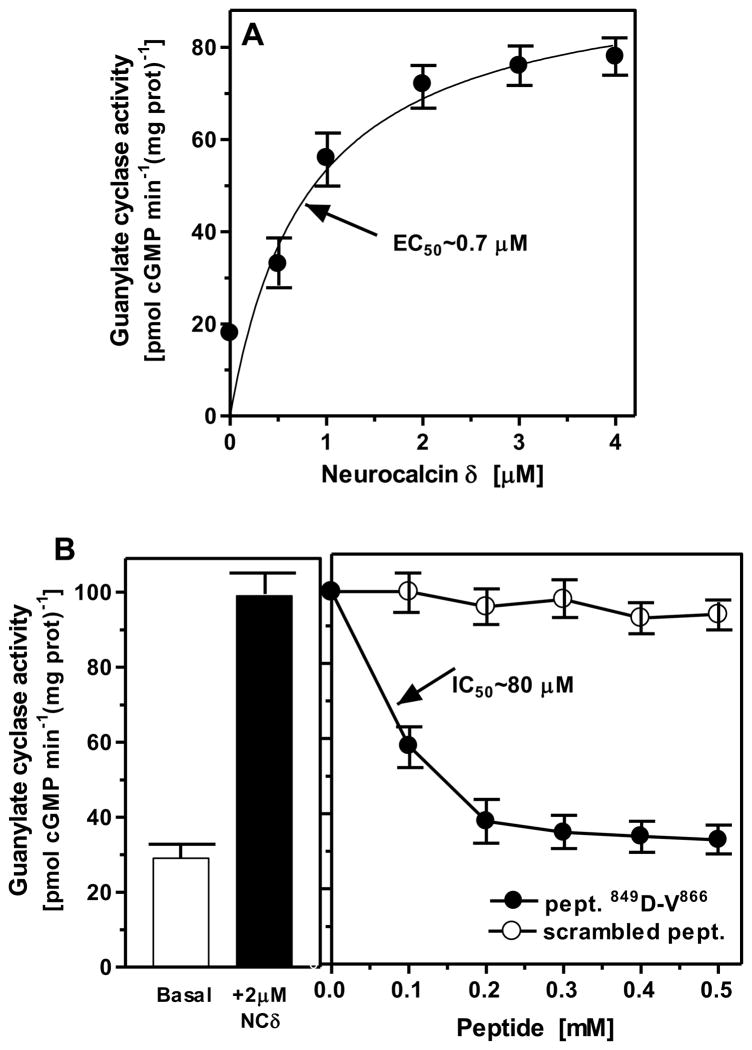
Guided by the information that the NCδ binding site on ROS-GC1, aa V892-D912, and on ONE-GC, aa M880-L921, resides on the core catalytic domain16, 28, and this domain is conserved in all members of the membrane guanylate cyclase family, it was tested whether the corresponding domain in ANF-RGC is the target site of NCδ. Two approaches were used. In first, the ANF-RGC fragment, aa 788-1029, encompassing the core catalytic domain, I820-G1029, was expressed as a soluble protein and purified to homogeneity. The expressed protein was biologically active and possessed intrinsic guanylate cyclase activity of 18 ± 4 pmol cyclic GMP min−1 mg prot−1. That the fragment is Ca2+ modulated via NCδ was verified by its dose-dependent response to varying concentrations of NCδ in the presence of 1 μM Ca2+. Myr-NCδ stimulated its guanylate cyclase activity in a dose-dependent fashion with an EC50 of 0.7 μM (Fig. 4A), the value comparable to 0.5 μM estimated for the full-length ANF-RGC. These results demonstrated that the NCδ signaling site resides in this fragment, which contains the core catalytic domain of ANF-RGC.
Figure 4.
A. Neurocalcin δ interacts with the catalytic domain of ANF-RGC. The ANF-RGC fragment aa 788-1029 was expressed in SF-9 cells and purified to homogeneity. The expressed protein was assayed guanylate cyclase activity in the presence of 1 μM Ca2+ and indicated concentrations of myristoylated neurocalcin δ. B. Site of neurocalcin δ interaction with ANF-RGC – functional interference. ANF-RGC expressed in COS cells was exposed first to 2 μM myristoylated neurocalcin δ and 1 μM Ca2+ than to increasing concentrations (up to 0.5 mM) of peptide covering ANF-RGC sequence aa D849-V866 or control scrambled peptide, which had the same as D849-V866 peptide amino acid composition but random sequence. The experiments were done in triplicate and repeated two times. The results presented are mean ± SD of these experiments. The IC50 values were determined graphically.
In the second approach, the NCδ binding site was precisely mapped through peptide competition analysis. Advantage was taken from the knowledge that the experimentally validated sites on ROS-GC1 and ONE-GC are located within the aa segments V837-L858 and V900-L921, respectively and the segments have, among themselves, 100% sequence conservation. The corresponding site on ANF-RGC, 849DIVGFTALSAESTPMQVVTLLMQ871, has 70% sequence conservation in comparison with ROS-GC1 and ONE-GC. An ANF-RGC sequence-specific peptide 849DIVGFTALSAESTPMQVV866 was synthesized and used in a functional interference experiment. A scrambled peptide VDASAIVMFVGLPSQTET was used as control in this experiment.
COS cell membranes expressing ANF-RGC were incubated with 2 μM myr-NCδ, increasing concentrations of the peptide and 1 μM Ca2+ (Fig. 4B). The peptide caused almost complete inhibition of the NCδ-stimulated ANF-RGC activity at 200 μM with an IC50 value of 80 μM. Under the same conditions the scrambled peptide did not exhibit any inhibitory effect. These results demonstrate that the ANF-RGC region 849DIVGFTALSAESTPMQVV866 mediates NCδ-dependent Ca2+ stimulation of ANF-RGC activity. This region is a part of the core catalytic domain and common to the corresponding sites in other membrane guanylate cyclases28, it has a secondary structure of helix-loop-helix and is acidic in nature with a pI of 3.37.
Interactive NCδ dimer with ANF-RGC dimer constitutes the functional Ca2+ signal transduction unit
The present concept based on the complementary biochemical and homology based modeling studies indicates that the secondary structure of the functional form of all membrane guanylate cyclases is homodimeric28–30. The contact points for their homo-dimeric formation reside (1) in their extracellular domain31, in the intracellular domains of (2) highly conserved dimerization domain32 and (3) core catalytic core domain28. The X-ray crystallographic studies have demonstrated that NCδ also exists as a dimer33. Thus the theoretical prediction was that the Ca2+-modulated functional unit is the interactive NCδ dimer and ANF-RGC dimer.
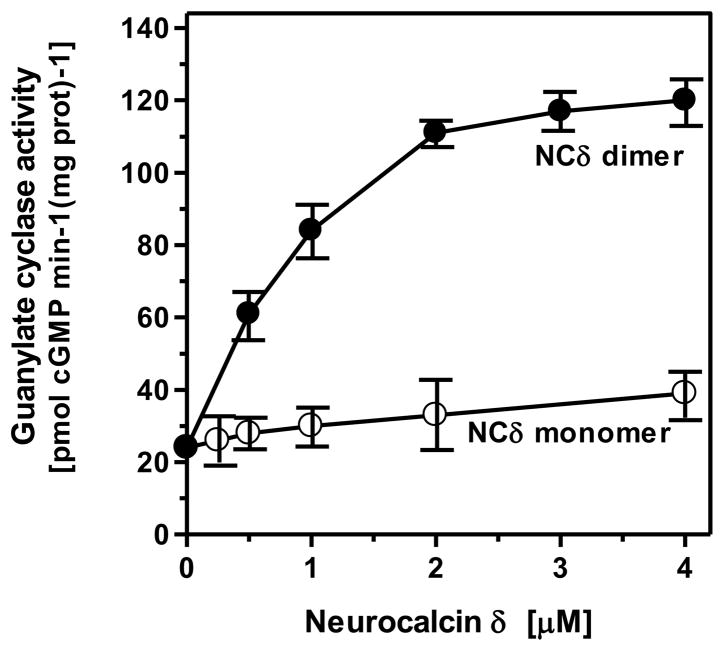
To assess this prediction experimentally, monomeric and dimeric forms of myristoylated NCδ were separated by FPLC and were used for reconstitution experiment with ANF-RGC. The dimeric form stimulated ANF-RGC 5-fold above the basal activity, from 24 to 120 pmol cGMP min−1 (mg prot)−1 (Fig. 5). Three separate experiments yielded calculated Hill coefficient values for the stimulation of ANF-RGC as 1.08 ± 0.21, 0.91 ± 0.29 and 0.9 ± 0.23. The stimulation by the monomeric form was only marginal, from 24 to 38 pmol cGMP min−1 (mg prot)−1 (Fig. 5). These results, thus, demonstrate and validate the prediction that the functional Ca2+ signal transduction unit is composed of one NCδ dimer and one ANF-RGC dimer.
Figure 5. Dimer is the functional entity of neurocalcin δ.
Myristoylated neurocalcin δ was expressed and purified by FPLC as described in “Experimental Procedures”. The monomeric and dimeric fractions were collected. These were individually used to assess their stimulatory effect on ANF-RGC activity expressed in COS cells. The experiment was done in triplicate and repeated four times with different neurocalcin δ preparations and ANF-RGC expressions. The results shown are from one experiment.
The functional Ca2+ signal transduction unit is present in the mouse adrenal gland
To grasp the physiological relevance of the above biochemical findings, these authors followed their preliminary study where they observed first through Western blot analysis and then through immunohistochemistry the co-presence of both elements of the functional transduction unit: NCδ and ANF-RGC in the glomerulosa cells of the mouse adrenal gland20.
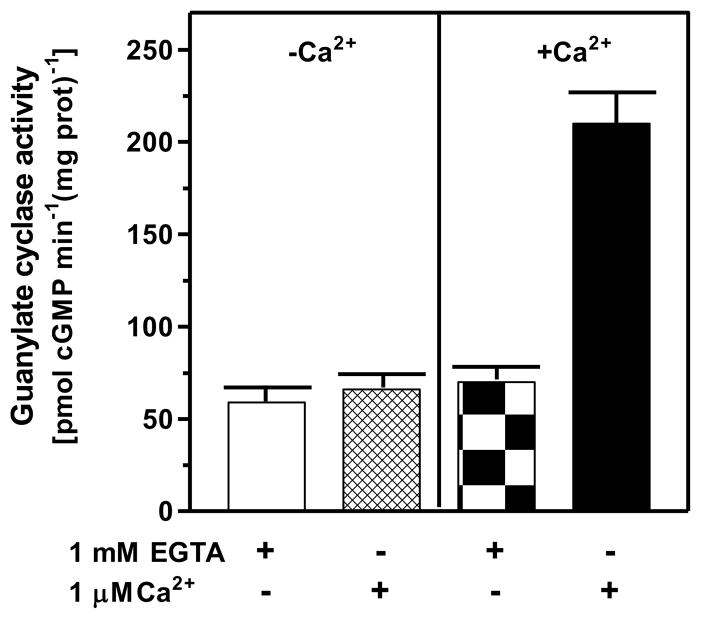
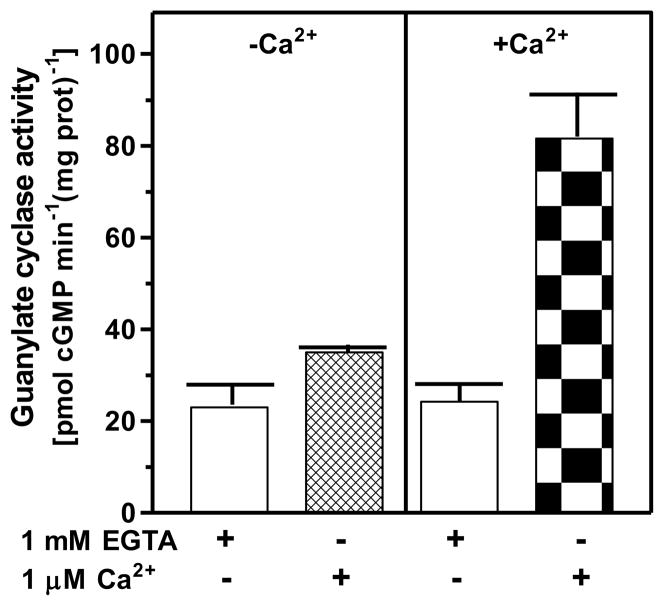
The first question was: does the mouse adrenal gland contain Ca2+-modulated membrane guanylate cyclase activity? The rational was founded on the earlier studies, which had shown that two vital endocrine glands containing functional ANF-RGC transduction system were adrenal and kidney7, 34, 35. Mouse adrenal gland was chosen for these studies. The gland was homogenized in the presence of 1 mM EGTA or 10 μM Ca2+ and the particulate fractions were prepared. Each preparation was analyzed for guanylate cyclase activity in the presence of 1 mM EGTA or 1 μM Ca2+. The membranes isolated in the absence of Ca2+ (1 mM EGTA) exhibited comparable cyclase activity regardless of the presence or absence of Ca2+ in the assay mixture. The activities were 59 ± 5 and 66 ± 5 pmol cyclic GMP min−1 (mg prot)−1 in the absence and presence of Ca2+, respectively (Fig. 6: panel “−Ca2+”). However, the membranes isolated in the presence of Ca2+ exhibited Ca2+-dependent activity; in the absence of Ca2+ in the assay mixture it was 70 ± 5 pmol cyclic GMP min−1 (mg prot)−1, the activity comparable to the activity in membranes isolated in the absence of Ca2+, but in its presence the activity was 210 ± 13 pmol cyclic GMP min−1 (mg prot)−1 (Fig. 6: panel “+Ca2+”). These results demonstrate that the adrenal gland houses functional Ca2+-dependent ANF-RGC signal transduction machinery.
Figure 6. The ANF-RGC activity in mouse adrenal gland is Ca2+-dependent.
Particulate fractions of mouse adrenal gland were isolated in the presence of 1 mM EGTA (panel “−Ca2+”) or 10 μM Ca2+ (panel “+Ca2+”) as described in the “Experimental Procedures” section. The membranes were assayed for guanylate cyclase activity in the presence of 1 mM EGTA or 1 μM Ca2+. The experiment was repeated three times with separate membrane preparations. The results are mean ± SD of these experiments.
The second question was: Is the Ca2+-dependent ANF-RGC signal transduction machinery expressed in the mouse adrenocortical glomerulosa cells NCδ-modulated? This problem was approached through immunocytochemistry. Because both antibodies at hand, against ANF-RGC and NCδ, were raised in rabbits, using them for co-immunostaining experiments was not possible. Hence, the goal was achieved indirectly. It has been established that the cyclic GMP-stimulated phosphodiesterase PDE2A is a specific marker for the glomerulosa cells36. Therefore, the co-presence of ANF-RGC and PDE2A was determined first, then, of NCδ and PDE2A, and from them, the co-presence of ANF-RGC, NCδ and PDE2A was assessed.
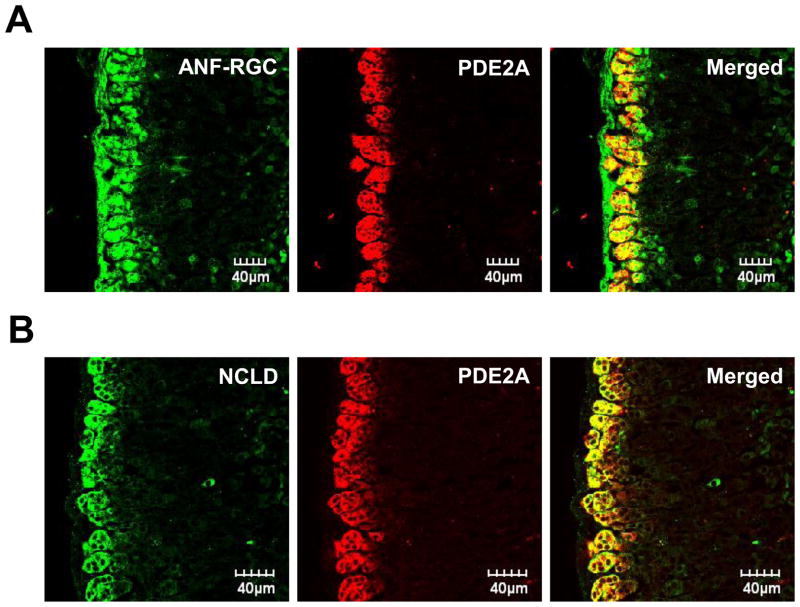
Figure 7A “ANF-RGC” shows that the glomerulosa cells in this section exhibit an intense signal (green) generated with the ANF-RGC antibody. Figure 7A “PDE2A” shows the same section with the red signal generated with the PDE2A antibody. The “merged” image of the two signals generating yellow fluorescence shows that the immunostaining of ANF-RGC and PDE2A overlap, demonstrating that these two proteins are co-present in the glomerulosa cells of the adrenal gland. Similarly, double staining with NCδ and PDE2A antibodies (Fig. 7B) demonstrates that NCδ and PDE2A are also co-present in these cells. It is, thus concluded that ANF-RGC and NCδ together with PDE2A are present in the same adrenocortical glomerulosa cells.
Figure 7. Neurocalcin δ is expressed in the same as ANF-RGC and PDE2A mouse adrenocortical zona glomerulosa cells.
Cryosections of the mouse adrenal gland were immuno-stained with ANF-RGC and PDE2A antibodies (A) or neurocalcin δ and PDE2A antibodies (B) as described in “Experimental Procedures”. The right-hand panels (“Merged”) present the composite images of ANF-RGC and PDE2 or neurocalcin δ and PDE2 staining and document that both ANF-RGC and neurocalcin δ are co-expressed with PDE2A.
NCδ is the Ca2+-sensor modulator of ANF-RGC in the adrenocortical glomerulosa cells
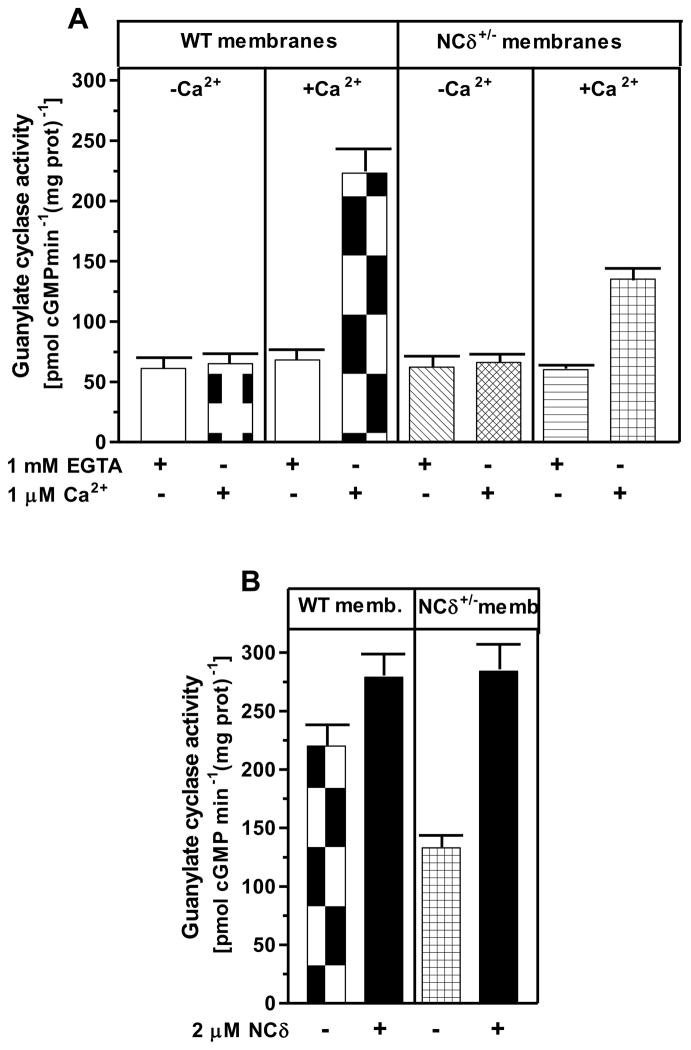
To demonstrate that the ANF-RGC Ca2+ signal transduction unit present in the mouse adrenocortical glomerulosa cells is functional, two independent approaches were used. First, a mouse model with deletion of one copy of the NCδ gene, NCδ+/−, was constructed. It was reasoned that if NCδ is indeed the Ca2+-sensor modulator of ANF-RGC, in the adrenal glands of these mice the NCδ-modulated Ca2+ signaling pathway should be half as active as in those of the wild type mice (wt; NCδ+/+). To test this prediction, the particulate fractions of the adrenal glands from wild type and NCδ+/− mice were prepared in the presence and absence of Ca2+ and the experiment was performed according to the protocol described earlier. Similar to the results presented in figure 6, the guanylate cyclase activity in membranes isolated in the absence of Ca2+ was about 65 ± 8 pmol cyclic GMP min−1 (mg prot)−1 for the wt and NCδ+/− mice and the activity was not affected by the presence or absence of Ca2+ in the assay mixture (Fig. 8A). However, the cyclase activity in membranes isolated in the presence of Ca2+ was strongly dependent on the mice genotype and Ca2+ in the assay mixture. When assessed in the absence of Ca2+, the activity was 70 ± 8 pmol cyclic GMP min−1 (mg prot)−1 for the wt and NCδ+/− mice but when assessed in the presence of 1 μM Ca2+ the activity was 223 ± 20 pmol cyclic GMP min−1 (mg prot)−1 for the wild type mice and 135 ± 10 pmol cyclic GMP min−1 (mg prot)−1 for the NCδ+/− mice. These results, as predicted, demonstrate that the Ca2+-dependent NCδ-modulated ANF-RGC signaling pathway in the mice with one copy of NCδ gene deleted (NCδ+/−) is functionally half as active as in the wild type mice. To further validate that the lowering of the Ca2+-dependent cyclase activity in the adrenal gland membranes of NCδ+/− mice is the exclusive consequence of lower NCδ expression, 2 μM exogenous NCδ was added to the NCδ+/+ and NCδ+/− membranes (isolated in the presence of Ca2+) and the cyclase activity was assessed in the presence of 1 μM Ca2+. The cyclase activity in the NCδ+/+ adrenal membranes increased only minimally, from 220 to 279 ± 21 pmol cyclic GMP min-1 (mg protein)-1 but in the NCδ+/− membranes, from 133 to 284 ± 24 (Fig. 8B). Thus, the activity achieved was practically the same for both types of membranes. These results demonstrate that addition of exogenous NCδ to the NCδ+/− adrenal gland membranes restores the guanylate cyclase activity and brings it to the level of activity in the NCδ+/+ membranes. The slight activity increase in the NCδ+/+ membranes can be explained by a partial loss of the native NCδ during the membrane preparation procedure.
Figure 8. Neurocalcin δ modulates ANF-RGC activity in mouse adrenal gland.
(A) Adrenal glands were removed from the wild type (WT) and neurocalcin δ+/− (NCδ+/−) mice and their particulate fractions were isolated in the presence of 1 mM EGTA (panels “−Ca2+” in “WT membranes” and “NCδ+/− membranes” sections of the figure) or 10 μM Ca2+ (panels “+Ca2+” in both sections of the figure). These were assayed for guanylate cyclase activity in the presence of 1 mM EGTA or 1 μM Ca2+. (B) 2 μM myr-NCδ was added to the adrenal gland membranes isolated in the presence of 10 μM Ca2+ from the wild type (“WT memb.”) or NCδ+/− (“NCδ+/− memb.”) mice and the guanylate cyclase activity was assessed in the presence of 1 μM Ca2+. The experiments were repeated two times with separate membrane preparations. The results are mean ± SD of these experiments.
In the second approach, Ca2+-dependent reconstitution of the transduction system was conducted using the heterologous system of COS cells. The cells were co-transfected with ANF-RGC and NCδ cDNAs. Their membranes were isolated in the presence and absence of Ca2+ and assayed for guanylate cyclase activity without and with Ca2+ in the assay mixture. The results are shown in figure 9. The cyclase activity remained at the basal level in the membranes isolated without Ca2+ but it increased 3.5-fold in membranes isolated and assayed in a buffer containing Ca2+. Membranes of COS cells transfected with ANF-RGC cDNA alone exhibited cyclase activity of 25 ± 4 pmol cyclic GMP min−1 (mg prot)−1 and the activity was unaffected by the presence or absence of Ca2+ during their preparation or activity assay. Because the only difference between the co-transfected cells and those transfected with ANF-RGC cDNA only was the expression of NCδ in the co-transfected cells, these results demonstrate that in living cells NCδ exhibits Ca2+-dependent association with the membranes and transmits the Ca2+ signal to ANF-RGC.
Figure 9. Ca2+-neurocalcin δ modulation of ANF-RGC activity is reconstituted in co-transfected COS cells.
COS cells were co-transfected with ANF-RGC and neurocalcin δ cDNAs. On the third day post-transfection their membranes were isolated and assayed for guanylate cyclase activity as described in legends to figures 8 and 10. The experiment was repeated two times. The results presented (mean ± SD) are from one experiment.
Together, the results of these two approaches establish that the Ca2+ modulation of ANF-RGC is not only an in vitro phenomenon but it also occurs in vivo both in mouse adrenal gland and in reconstituted system of transfected cells, and NCδ is the transducer of the Ca2+ signal.
NCδ-modulated Ca2+ signaling ANF-RGC transduction system modulates blood pressure
The primary role of ANF-RGC in the adrenal gland is to offset the renin-angiotensin system and inhibit aldosterone synthesis, thus, to lower blood pressure37–39. It does so by responding to the two most hypotensive peptide hormones, ANF and BNP, and producing their second messenger cyclic GMP. Cyclic GMP then elicits the natriuretic, diuretic, vasorelaxant, and anti-proliferative effects programmed by the ANF and BNP signals. The obvious question, therefore, was: Is the Ca2+-neurocalcin δ-ANF-RGC transduction system also involved in rgulation of blood pressure. To answer it, the systolic blood pressure of the NCδ+/− and of the isogenic control (wild type; NCδ+/+) was measured.
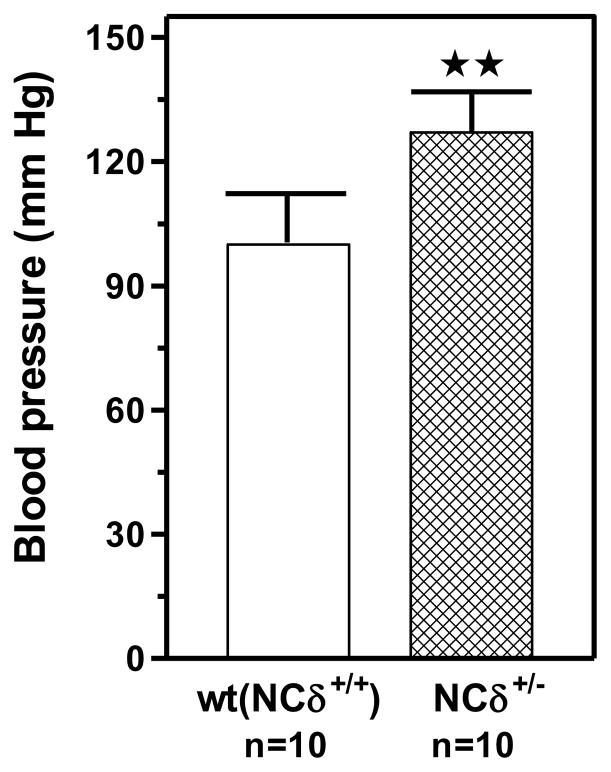
After three days of mock measuremnt sessions for training purposes, the systolic blood pressure was determined to be 92 ± 6 mm Hg for the wild type mice and 127 ± 9 mm Hg for the NCδ+/− mice (Fig. 10). The increase was statistically highly significant (P<0.005). These results clearly show that the deletion of one gene copy of neurocalcin δ leads to significant increase of blood pressure. Thus, it is concluded that the Ca2+-neurocalcin δ-ANF-RGC transduction system is a physiological modulator of the blood pressure.
Figure 10. Systolic blood pressure in NCδ gene-targeted mice.
The mice used were NCδ 2-copy (+/+) wild type allele (control) and NCδ 1-copy (+/−) gene-disrupted heterozygous allele. The mice were fed a normal-salt diet. Systolic blood pressure was measured every day for one week by the noninvasive computerized tail-cuff method. An average blood pressure level of 10 sessions a day was calculated for analysis after 3 days of training. Bars indicate means ± SD values for the representative genotypes. n describes the number of mice analyzed for each genotype. ** indicates that the P value was <0.005.
DISCUSSION
Coinciding with time periods of observations that the hormone-dependent guanylate cyclase which is independent of the soluble form exists in the mammalian cells25, 40, 41, the discovery of the ANF was announced42. ANF regulated sodium excretion, water balance and blood pressure43, 44. A hint that there is a link between ANF actions and the particulate form of guanylate cyclase emerged with the findings that ANF stimulated membrane guanylate cyclase activities in the rat tissues45, 46. The linkage was firmed with the findings that ANF-RGC is the receptor of ANF and is also its signal transducer1–8. This marked the beginning of the membrane guanylate cyclase field and also its entry into cardio-vasculature research.
More than 3-decades of research involving ANF and ANF-RGC gene deletions and biochemical studies8, 47 has now established that abnormalities in the ANF-GC transduction pathway cause many cardio-vascular complications, including hypertension and heart failure. The ANF/ANF-RGC gene-deleted mice possess salt-sensitive and salt-insensitive hypertension. These defects in cardio-vasculature occur because ANF-RGC does not properly transduce the ANF signal in the generation of cyclic GMP, the second messenger of ANF. Cyclic GMP is a major regulator of blood pressure. For these reasons, it is critical to understand the basic mechanism/s by which ANF-RGC signal transduction machinery operates. Many gaps remain, yet important strides have been made in this direction. The current, and (note) the only, model is that ANF-RGC signal transduction is only meant to process the hormonal signals of ANF and BNP, the signals generated in the domains at the extracellular region of ANF-RGC. These signals are then processed through ATP-dependent two-step activation process48. The model is: the ANF signal originates by the binding of one molecule of ANF to the extracellular domain of ANF-RGC. The binding modifies the juxtamembrane region where the disulfide 423C-C432 structural motif is a key element in this modification. The signal twists the trans-membrane domain, induces structural changes in the ARM domain, and prepares it for the ATP activation. Step 1: ATP binding to the ARM domain leads to a cascade of temporal and spatial changes. They involve (1) shift in ATP binding pocket position by 3–4 Å and rotation of its floor by 15°; G505 acts as a critical PIVOT for both the shift and the rotation; (2) movement by 2–7 Å but not the rotation of its β4 and β5 strands and its loop; and (3) movement of its αEF helix by 2–5 Å. This movement exposes its hydrophobic motif, 669WTAPELL675, which facilitates its direct (or indirect) interaction with the catalytic module resulting in its partial, about 50%, activation. Step 2: The six phosphorylation sites are brought from their buried to the exposed state. Through ATP and a hypothetical protein kinase they get phosphorylated, and the full activation (additional 50%) of ANF-RGC is achieved. Concomitantly, phosphorylation converts the ATP binding site from high to low affinity, ATP dissociates and ANF-RGC returns to its ground state. A conspicuous feature of this model is that the trajectory of the pathway originating at the extracellular domain to the core catalytic domain, the site of signal translation into the production of cyclic GMP, is downstream (Fig. 11).
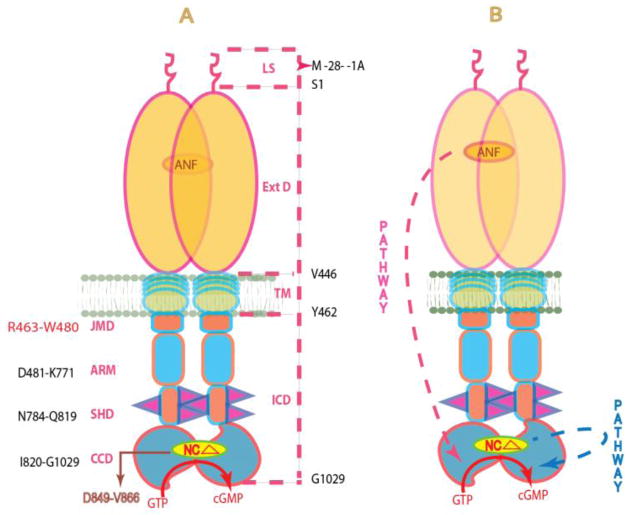
Figure 11.
(A) Schematic representation of the structural topography of ANF-RGC. ANF-RGC is a single transmembrane spanning homodimer protein. The dashed lines on the right show the defined boundaries of its segments: LS, leader sequence; ExtD, extracellular domain; TM, transmembrane domain; ICD, intracellular domain. The functional domains housed in ICD, their designated names and the amino acid residues constituting their boundaries are indicated at the left: JMD, juxtamembrane domain; ARM, the ATP regulated module; SHD-signaling helix domain; CCD-core catalytic domain. The site targeted by neurocalcin δ (NCΔ) (encircled) is located within CCD. (B) The signaling pathways of ANF and of NCδ are independent. The trajectory of the ANF pathway is shown in red dashed arrow. From its origin at the ExtD, it passes through the structural domains of TM, ARM and SHD in its course to CCD. In contrast, the trajectory of the NCδ pathway (shown in blue dashed arrow) is within the CCD. The CCD exists as an antiparallel homodimer.
The ongoing studies by the present authors provide physiological support to this model (unpublished studies). The mice lacking the ANF-RGC gene encoded 669WTAPELL675 motif are hypertensive. The systolic blood pressure of the 669WTAPELL675-KO mice is 147 ± 4 Hg whereas for the isogenic wt-mice it is 99 ± 9 Hg.
The present comprehensive study defines a new paradigm of ANF-RGC signal transduction. Here, (1) ANF-RGC is the transducer of Ca2+ signal; (2) this pathway is independent of the ANF and BNP signal transduction, thus, does not utilize the extracellular domain of ANF-RGC; (3) the trajectory of the pathway is not downstream. It originates and acts directly on the core catalytic domain (Fig. 11), providing a novel mechanism of signal transduction in common with the sensory neuron-linked membrane guanylate cyclases: ROS-GC1 and ONE-GC; (4) Like the physiology of ANF and BNP signaling modes, the Ca2+ signaling mode is present in the glomerulosa cells of the adrenal cortex; and importantly, like those modes, regulates blood pressure in mice. The absence of those modes leads to hypertension.
There are two functional and independent structural compartments of ANF-RGC. One compartment processes the signals of ANF and BNP and the other, of intracellularly generated Ca2+ (Fig. 11). The transduction mechanisms of these two types of signals are fundamentally different yet both modulate blood pressure and the absence of either of them causes hypertension in the genetically targeted mice.
Ca2+ Signal Transduction Model
Based on facts provided by this study, a preliminary stepwise Ca2+ signal transduction is envisioned. Ground state: Dimer form of myr-NCδ is bound to a dimer of ANF-RGC. It constitutes the Ca2+ sensor element of ANF-RGC and it binds to the 849DIVGFTALSAESTPMQVV866 domain of ANF-RGC. The presence of the Ca2+ sensor element keeps the basic enzymatic efficiency of ANF-RGC at the threshold level in the production of cyclic GMP from GTP. Activation and activated state. A rise of Ca2+ to the semimicromolar range is sensed by NCδ; Ca2+ with K1/2 of 0.5 μM binds myr-NCδ, which undergoes a Ca2+-dependent configurational changes. These, in turn, cause a structural change in the NCδ binding domain of ANF-RGC, which is also a part of the core catalytic domain. This increases the catalytic efficiency of ANF-RGC by more than 6-fold: kcat, from 6.5 to 41 cyclic GMP sec−1 and results in the accelerated production of cyclic GMP.
This model opens up important avenues of future cardio-vascular research. It is envisioned that in the adrenal glomerulosa cells, cyclic GMP formed through the Ca2+-dependent mechanism, acts as a second messenger, offsets, at least partially, the rennin-angiotensin system, inhibits aldosterone synthesis and lowers the blood pressure. Significantly, the 0.4–0.5 μM Ca2+ concentration that, through NCδ, causes half-maximal activation of ANF-RGC (Fig. 1A and 2B) is within the range of cytoplasmic Ca2+ concentration in glomerulosa cells synthesizing aldosterone49. Whether, in these, the operation of the ANF-RGC-Ca2+-NCδ system precedes the operation of the ANF/ANF-RGC system or is concomitant with it remains to be determined.
The model may also aid in explaining the contractile and relaxation properties of vascular smooth muscle cells (VSMC). The magic signaling molecule in these cells is Ca2+. Volumes of documented evidence indicate that in VSMC the pulsated rise of Ca2+ causes their contraction and its fall, relaxation50–52. It is also well established that in these cells cyclic GMP-generated through ANF-RGC transduction system causes relaxation53. The present study demonstrates, however, that Ca2+ directly through ANF-RGC can generate cyclic GMP and cause relaxation of VSMC. Thus, the intracellular rise of Ca2+ in VSMC can cause both their contraction and relaxation. In accordance with the presently proposed Ca2+-relaxation related hypothesis, the present study demonstrates that deletion of the Ca2+ sensor, NCδ, from the ANF-RGC transduction system causes hypertension in the mice.
Ca2+-NCδ-modulated ANF-RGC transduction pathway is a new regulator of mouse blood pressure. The task for future research is to identify the source of Ca2+ that turns on the Ca2+ sensor element of the ANF-RGC transduction machinery.
Acknowledgments
Founding Statement: The study was supported by National Heart, Blood and Lung Institute grants HL084584 and S82701 and by the Pennsylvania Lions Sight Conservation and Eye Research Foundation
The authors acknowledge Guanylate Cyclase Innovative Biotechnologies (GCIB) for the support of neurocalcin δ+/− mice.
Abbreviations
- ANF
atrial natriuretic factor
- BNP
B-type natriuretic peptide
- CNP
C-type natriuretic peptide
- ANF-RGC
atrial natriuretic factor receptor guanylate cyclase
- ARM
ATP regulatory module
- EGTA
ethylene glycol tetraacetic acid
- GCAP
guanylate cyclase activating protein
- ONE-GC
olfactory neuroepithelial guanylate cyclase
- ROS-GC
rod outer segment guanylate cyclase
References
- 1.Paul AK. Doctoral thesis. University of Tennessee; 1986. Particulate guanylate cyclase from adrenocortical carcinoma 494. Purification, biochemical and immunological characterization. [Google Scholar]
- 2.Paul AK, Marala RB, Jaiswal RK, Sharma RK. Coexistence of guanylate cyclase and atrial natriuretic factor receptor in a 180-kD protein. Science. 1987;235:1224–1226. doi: 10.1126/science.2881352. [DOI] [PubMed] [Google Scholar]
- 3.Kuno T, Andresen JW, Kamisaki Y, Waldman SA, Chang LY, Saheki S, Leitman DC, Nakane M, Murad F. Co-purification of an atrial natriuretic factor receptor and particulate guanylate cyclase from rat lung. J Biol Chem. 1986;261:5817–5823. [PubMed] [Google Scholar]
- 4.Meloche S, McNicoll N, Liu B, Ong H, De Lean A. Atrial natriuretic factor R1 receptor from bovine adrenal zona glomerulosa: purification, characterization, and modulation by amiloride. Biochemistry. 1988;27:8151–8158. doi: 10.1021/bi00421a025. [DOI] [PubMed] [Google Scholar]
- 5.Takayanagi R, Inagami T, Snajdar RM, Imada T, Tamura M, Misono KS. Two distinct forms of receptors for atrial natriuretic factor in bovine adrenocortical cells. Purification, ligand binding, and peptide mapping. J Biol Chem. 1987;262:12104–12113. [PubMed] [Google Scholar]
- 6.Chinkers M, Garbers DL, Chang MS, Lowe DG, Chin HM, Goeddel DV, Schulz S. A membrane form of guanylate cyclase is an atrial natriuretic peptide receptor. Nature. 1989;338:78–83. doi: 10.1038/338078a0. [DOI] [PubMed] [Google Scholar]
- 7.Duda T, Goraczniak RM, Sharma RK. Site-directed mutational analysis of a membrane guanylate cyclase cDNA reveals the atrial natriuretic factor signaling site. Proc Natl Acad Sci USA. 1991;88:7882–7886. doi: 10.1073/pnas.88.17.7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma RK. Membrane guanylate cyclase is a beautiful signal transduction machine: overview. Mol Cell Biochem. 2010;334:3–36. doi: 10.1007/s11010-009-0336-6. [DOI] [PubMed] [Google Scholar]
- 9.Duda T. Atrial natriuretic factor-receptor guanylate cyclase signal transduction mechanism. Mol Cell Biochem. 2010;334:37–51. doi: 10.1007/s11010-009-0335-7. [DOI] [PubMed] [Google Scholar]
- 10.Schulz S, Singh S, Bellet RA, Singh G, Tubb DJ, Chin H, Garbers DL. The primary structure of a plasma membrane guanylate cyclase demonstrates diversity within this new receptor family. Cell. 1989;58:1155–1162. doi: 10.1016/0092-8674(89)90513-8. [DOI] [PubMed] [Google Scholar]
- 11.Duda T, Goraczniak RM, Sitaramayya A, Sharma RK. Cloning and expression of an ATP-regulated human retina C-type natriuretic factor receptor guanylate cyclase. Biochemistry. 1993;32:1391–1395. doi: 10.1021/bi00057a001. [DOI] [PubMed] [Google Scholar]
- 12.de Sauvage FJ, Camerato TR, Goeddel DV. Primary structure and functional expression of the human receptor for Escherichia coli heat-stable enterotoxin. J Biol Chem. 1991;266:17912–17918. [PubMed] [Google Scholar]
- 13.Currie MG, Fok KF, Kato J, Moore RJ, Hamra FK, Duffin KL, Smith CE. Guanylin: an endogenous activator of intestinal guanylate cyclase. Proc Natl Acad Sci USA. 1992;89:947–951. doi: 10.1073/pnas.89.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duda T, Venkataraman V, Krishnan A, Nagele RG, Sharma RK. Negatively calcium-modulated membrane guanylate cyclase signaling system in the rat olfactory bulb. Biochemistry. 2001;40:4654–4662. doi: 10.1021/bi0027985. [DOI] [PubMed] [Google Scholar]
- 15.Leinders-Zufall T, Cockerham RE, Michalakis S, Biel M, Garbers DL, Reed RR, Zufall F, Munger SD. Contribution of the receptor guanylyl cyclase GC-D to chemosensory function in the olfactory epithelium. Proc Natl Acad Sci USA. 2007;104:14507–14512. doi: 10.1073/pnas.0704965104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duda T, Sharma RK. ONE-GC membrane guanylate cyclase, a trimodal odorant signal transducer. Biochem Biophys Res Commun. 2008;367:440–445. doi: 10.1016/j.bbrc.2007.12.153. [DOI] [PubMed] [Google Scholar]
- 17.Sun L, Wang H, Hu J, Han J, Matsunami H, Luo M. Guanylyl cyclase-D in the olfactory CO2 neurons is activated by bicarbonate. Proc Natl Acad Sci USA. 2009;106:2041–2046. doi: 10.1073/pnas.0812220106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duda T, Sharma RK. Distinct ONE-GC transduction modes and motifs of the odorants: Uroguanylin and CO(2) Biochem Biophys Res Commun. 2010;391:1379–1384. doi: 10.1016/j.bbrc.2009.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma RK, Duda T. Ca(2+)-sensors and ROS-GC: interlocked sensory transduction elements: a review. Front Mol Neurosci. 2012;5:42. doi: 10.3389/fnmol.2012.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duda T, Pertzev A, Koch KW, Sharma RK. Antithetical modes of and the Ca(2+) sensors targeting in ANF-RGC and ROS-GC1 membrane guanylate cyclases. Front Mol Neurosci. 2012;5:44. doi: 10.3389/fnmol.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duda T, Fik-Rymarkiewicz E, Venkataraman V, Krishnan A, Sharma RK. Calcium-modulated ciliary membrane guanylate cyclase transduction machinery: constitution and operational principles. Mol Cell Biochem. 2004;267:107–122. doi: 10.1023/b:mcbi.0000049372.33965.4f. [DOI] [PubMed] [Google Scholar]
- 22.Krishnan A, Duda T, Pertzev A, Kobayashi M, Takamatsu K, Sharma RK. Hippocalcin, new Ca(2+) sensor of a ROS-GC subfamily member, ONE-GC, membrane guanylate cyclase transduction system. Mol Cell Biochem. 2009;325:1–14. doi: 10.1007/s11010-008-0015-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duda T, Sharma RK. Ca2+-modulated ONE-GC odorant signal transduction. FEBS Lett. 2009;583:1327–1330. doi: 10.1016/j.febslet.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook MJ, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1989. [Google Scholar]
- 25.Nambi P, Aiyar NV, Sharma RK. Adrenocorticotropin-dependent particulate guanylate cyclase in rat adrenal and adrenocortical carcinoma: comparison of its properties with soluble guanylate cyclase and its relationship with ACTH-induced steroidogenesis. Arch Biochem Biophys. 1982;217:638–646. doi: 10.1016/0003-9861(82)90545-8. [DOI] [PubMed] [Google Scholar]
- 26.Krishnan A, Venkataraman V, Fik-Rymarkiewicz E, Duda T, Sharma RK. Structural, biochemical, and functional characterization of the calcium sensor neurocalcin delta in the inner retinal neurons and its linkage with the rod outer segment membrane guanylate cyclase transduction system. Biochemistry. 2004;43:2708–2723. doi: 10.1021/bi035631v. [DOI] [PubMed] [Google Scholar]
- 27.Burczynska B, Duda T, Sharma RK. ATP signaling site in the ARM domain of atrial natriuretic factor receptor guanylate cyclase. Mol Cell Biochem. 2007;301:93–107. doi: 10.1007/s11010-006-9400-7. [DOI] [PubMed] [Google Scholar]
- 28.Venkataraman V, Duda T, Ravichandran S, Sharma RK. Neurocalcin delta modulation of ROS-GC1, a new model of Ca(2+) signaling. Biochemistry. 2008;47:6590–6601. doi: 10.1021/bi800394s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rondeau JJ, McNicoll N, Gagnon J, Bouchard N, Ong H, De Léan A. Stoichiometry of the atrial natriuretic factor-R1 receptor complex in the bovine zona glomerulosa. Biochemistry. 1995;34:2130–2136. doi: 10.1021/bi00007a005. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Ruohom AE, Rao VD, Hurley JH. Catalytic mechanism of the adenylyl and guanylyl cyclases: modeling and mutational analysis. Proc Natl Acad Sci USA. 1997;94:13414–13419. doi: 10.1073/pnas.94.25.13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Misono KS, Philo JS, Arakawa T, Ogata CM, Qiu Y, Ogawa H, Young HS. Structure, signaling mechanism and regulation of the natriuretic peptide receptor guanylate cyclase. FEBS J. 2011;278:1818–1829. doi: 10.1111/j.1742-4658.2011.08083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson EM, Chinkers M. Identification of sequences mediating guanylyl cyclase dimerization. Biochemistry. 1995;34:4696–4701. doi: 10.1021/bi00014a025. [DOI] [PubMed] [Google Scholar]
- 33.Vijay-Kumar S, Kumar VD. Crystal structure of recombinant bovine neurocalcin. Nat Struct Biol. 1999;6:80–88. doi: 10.1038/4956. [DOI] [PubMed] [Google Scholar]
- 34.Sharma RK, Marala RB, Duda T. Purification and characterization of the 180-kDa membrane guanylate cyclase containing atrial natriuretic factor receptor from rat adrenal gland and its regulation by protein kinase C. Steroids. 1989;53:437–460. doi: 10.1016/0039-128x(89)90024-x. [DOI] [PubMed] [Google Scholar]
- 35.Ballermann BJ, Marala RB, Sharma RK. Characterization and regulation by protein kinase C of renal glomerular atrial natriuretic peptide receptor-coupled guanylate cyclase. Biochem Biophys Res Commun. 1988;157:755–761. doi: 10.1016/s0006-291x(88)80314-0. [DOI] [PubMed] [Google Scholar]
- 36.Stephenson DT, Coskran TM, Wilhelms MB, Adamowicz WO, O’Donnell MM, Muravnick KB, Menniti FS, Kleiman RJ, Morton D. Immunohistochemical localization of phosphodiesterase 2A in multiple mammalian species. J Histochem Cytochem. 2009;57:933–949. doi: 10.1369/jhc.2009.953471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aoki H, Richmond M, Izumo S, Sadoshima J. Specific role of the extracellular signal-regulated kinase pathway in angiotensin II-induced cardiac hypertrophy in vitro. Biochem J. 2000;347:275–284. [PMC free article] [PubMed] [Google Scholar]
- 38.Burnett JC, Jr, Granger JP, Opgenorth TJ. Effect of synthetic atrial natriuretic factor on renal function and rennin release. Am J Physiol Renal Fluid Electrolyte Physiol. 1984;247:F863–866. doi: 10.1152/ajprenal.1984.247.5.F863. [DOI] [PubMed] [Google Scholar]
- 39.Shi S-J, Nguyen HT, Sharma GD, Navar G, Pandey KN. Genetic disruption of atrial natriuretic peptide receptor-A alters rennin and angiotensinII levels. Am J Physiol Renal Physiol. 2001;281:F665–673. doi: 10.1152/ajprenal.2001.281.4.F665. [DOI] [PubMed] [Google Scholar]
- 40.Nambi P, Sharma RK. Adrenocorticotropic hormone-responsive guanylate cyclase in the particulate fraction of rat adrenal glands. Endocrinology. 1981;108:2025–2027. doi: 10.1210/endo-108-5-2025. [DOI] [PubMed] [Google Scholar]
- 41.Sharma RK. Biochemical Control in Adrenocortical Carcinoma. In: Hollander VP, editor. Hormonally Responsive Tumors. Academic Press; New York: 1985. pp. 185–213. [Google Scholar]
- 42.de Bold AJ. Atrial natriuretic factor of the rat heart. Studies on isolation and properties. Proc Soc Exp Biol Med. 1982;170:133–138. doi: 10.3181/00379727-170-41408. [DOI] [PubMed] [Google Scholar]
- 43.Cantin M, Genest J. The heart, an endocrine gland. Ann Endocrinol (Paris) 1985;46:219–228. [PubMed] [Google Scholar]
- 44.Atlas SA, Laragh JH. Atrial natriuretic peptide: a new factor in hormonal control of blood pressure and electrolyte homeostasis. Annu Rev Med. 1986;37:397–414. doi: 10.1146/annurev.me.37.020186.002145. [DOI] [PubMed] [Google Scholar]
- 45.Waldman SA, Rapoport RM, Murad F. Atrial natriuretic factor selectively activates particulate guanylate cyclase and elevates cyclic GMP in rat tissues. J Biol Chem. 1984;259:14332–14334. [PubMed] [Google Scholar]
- 46.Hamet P, Tremblay J, Pang SC, Garcia R, Thibault G, Gutkowska J, Cantin M, Genest J. Effect of native and synthetic atrial natriuretic factor on cyclic GMP. Biochem Biophys Res Commun. 1984;123:515–527. doi: 10.1016/0006-291x(84)90260-2. [DOI] [PubMed] [Google Scholar]
- 47.Pandey KN. Guanylyl cyclase/atrial natriuretic peptide receptor-A: role in the pathophysiology of cardiovascular regulation. Can J Physiol Pharmacol. 2011;89:557–573. doi: 10.1139/y11-054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duda T, Yadav P, Sharma RK. Allosteric modification, the primary ATP activation mechanism of atrial natriuretic factor receptor guanylate cyclase. Biochemistry. 2011;50:1213–1225. doi: 10.1021/bi1018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quinn SJ, Williams GH, Tillotson DL. Calcium oscillations in single adrenal glomerulosa cells stimulated by angiotensin II. Proc Natl Acad Sci USA. 1988;85:5754–5758. doi: 10.1073/pnas.85.15.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamm KE, Stull JT. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol. 1985;25:593–562. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- 51.Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- 52.Somlyo AP, Himpens B. Cell calcium and its regulation in smooth muscle. FASEB J. 1989;3:2266–2276. doi: 10.1096/fasebj.3.11.2506092. [DOI] [PubMed] [Google Scholar]
- 53.Martel G, Hamet P, Tremblay J. Central role of guanylyl cyclase in natriuretic peptide signaling in hypertension and metabolic syndrome. Mol Cell Biochem. 2010;334:53–65. doi: 10.1007/s11010-009-0326-8. [DOI] [PubMed] [Google Scholar]