Abstract
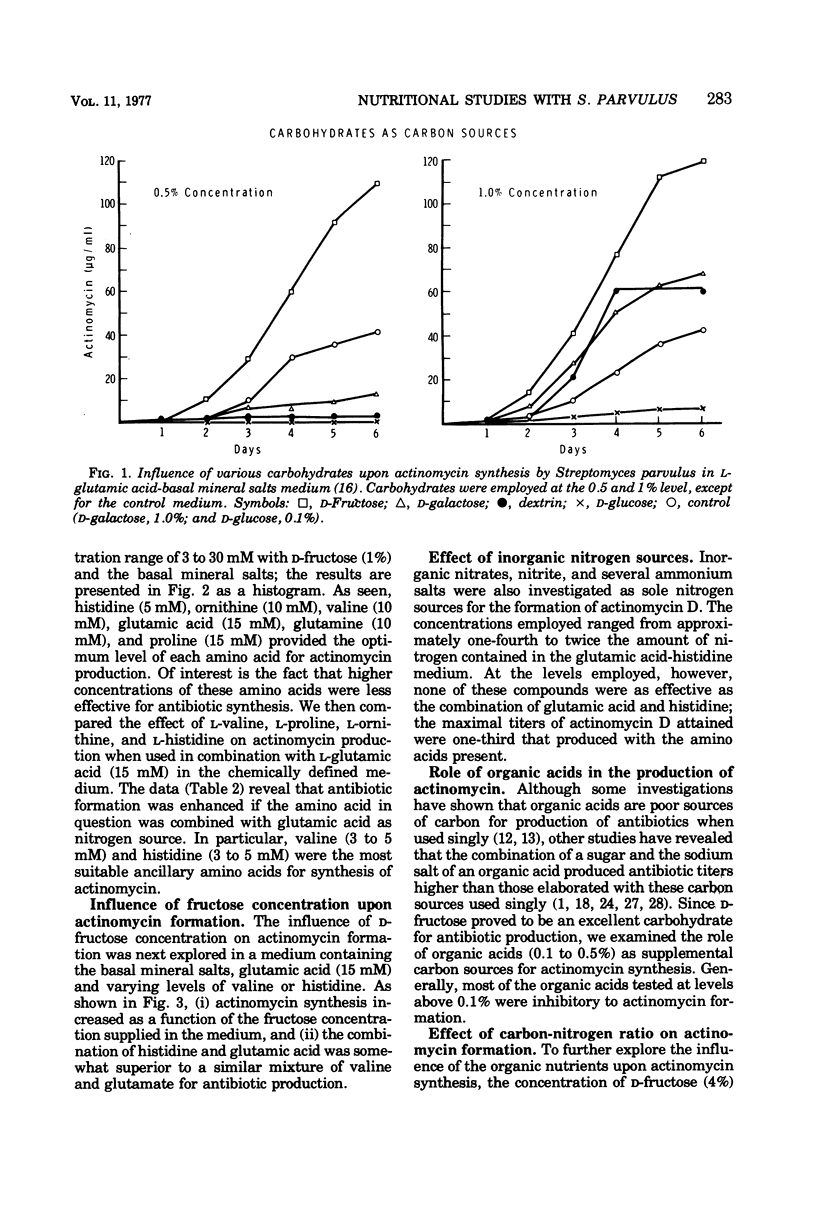
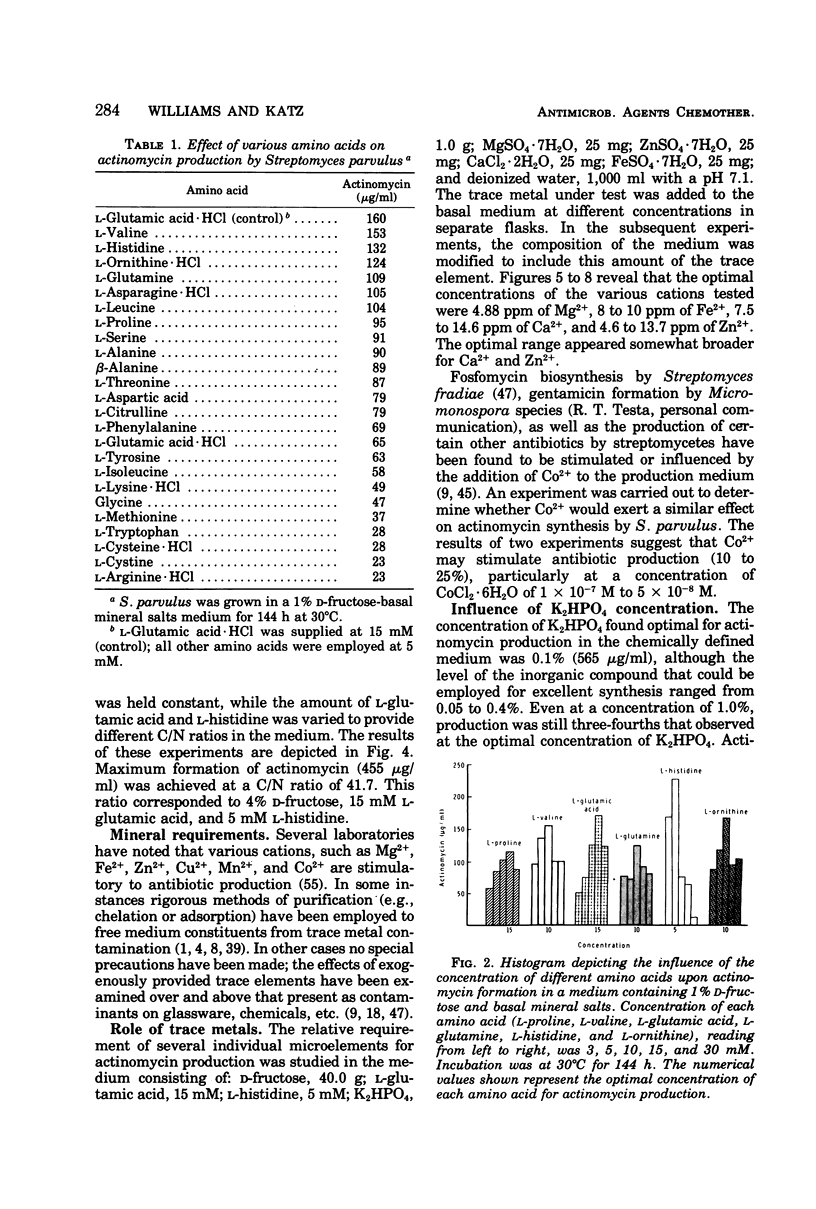
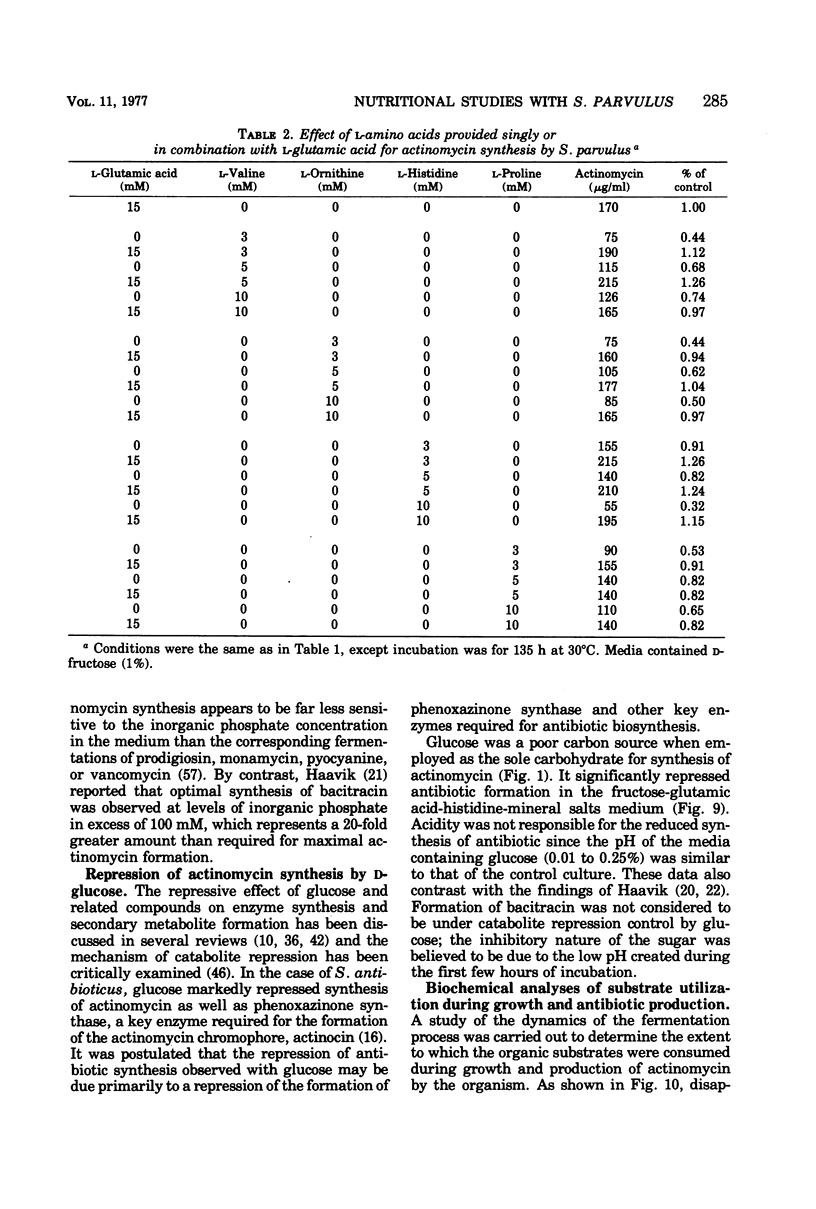
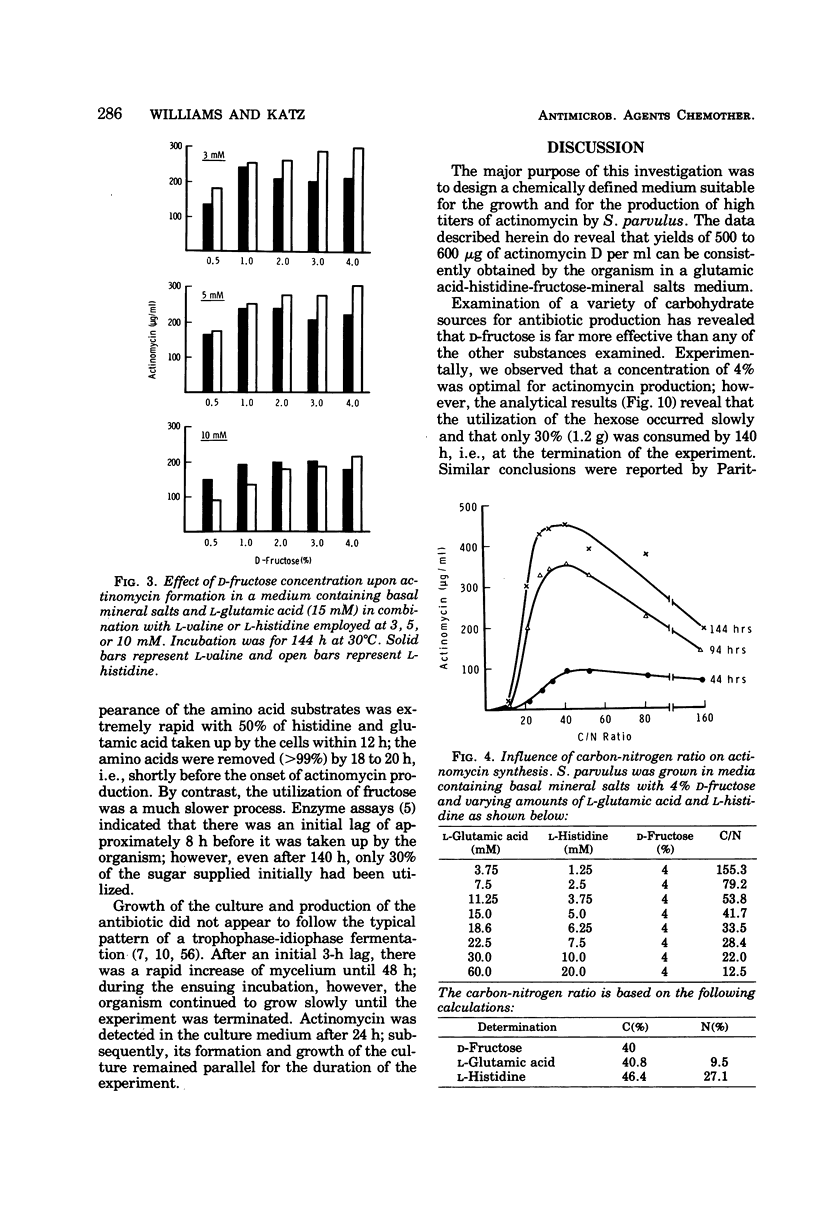
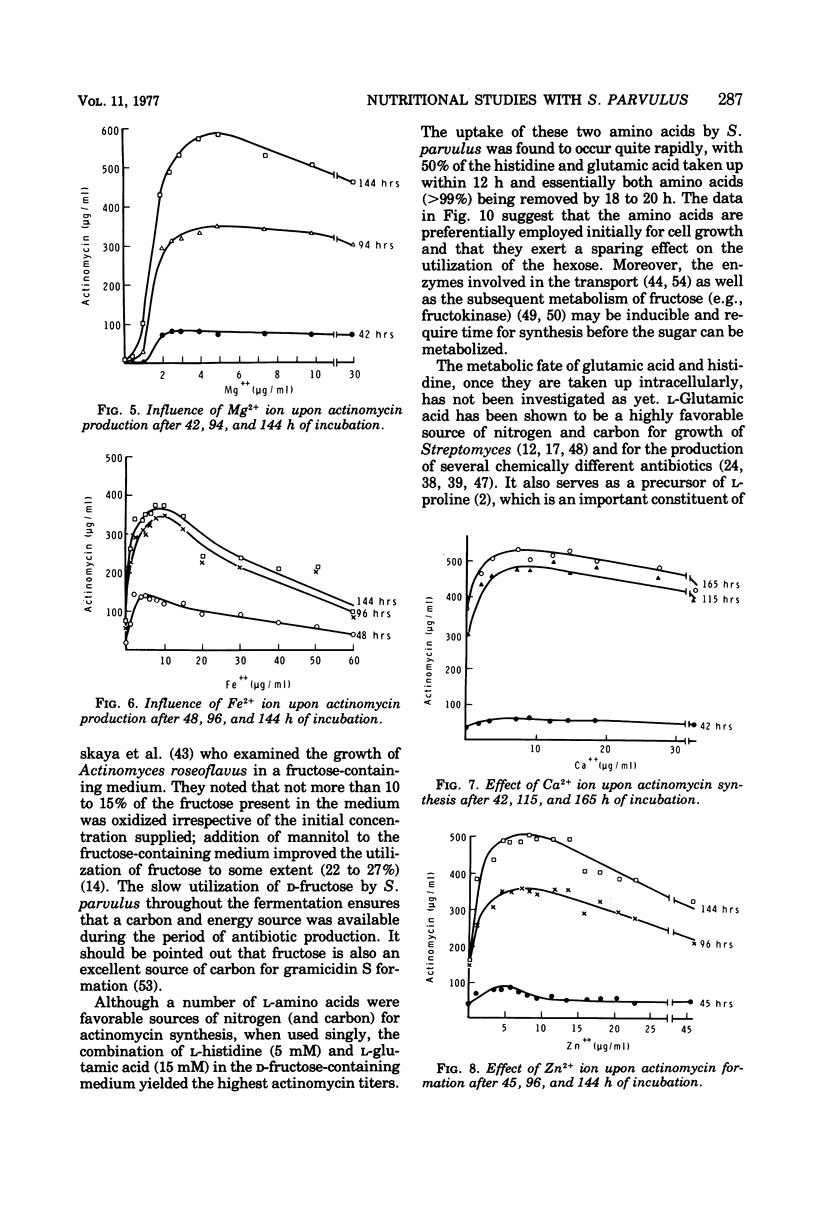
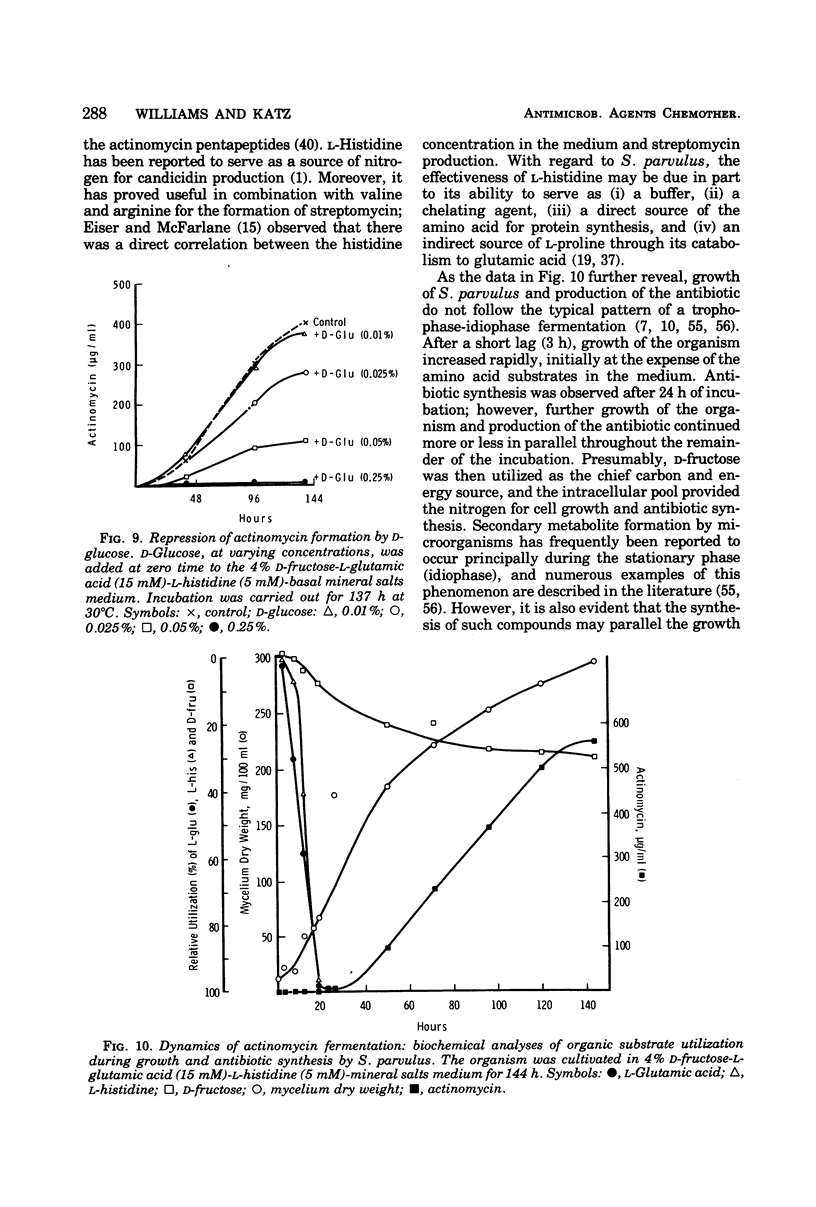
A chemically defined medium, consisting of d-fructose, l-glutamic acid, l-histidine, K2HPO4, MgSO4·7H2O, ZnSO4·7H2O, CaCl2·2H2O, FeSO4·7H2O, CoCl2·6H2O, and deionized water, was developed for synthesis of high yields (500 to 600 μg/ml) of actinomycin D by Streptomyces parvulus. Under these nutritional conditions, growth and actinomycin formation did not follow a typical trophophase-idiophase pattern. The amino acids appeared to have a sparing action on the utilization of d-fructose which was slowly and incompletely metabolized during mycelium development and antibiotic production. A significant repression of actinomycin synthesis by S. parvulus was observed when d-glucose (0.01 to 0.25%) was added to the culture medium. The repression was not due to a decline in the pH of the medium during glucose catabolism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKER R. F., LECHEVALIER H. Some nutritional requirements of Streptomyces griseus 3570 for growth and candicidin production. Appl Microbiol. 1954 May;2(3):152–157. doi: 10.1128/am.2.3.152-157.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams E. Metabolism of proline and of hydroxyproline. Int Rev Connect Tissue Res. 1970;5:1–91. doi: 10.1016/b978-0-12-363705-5.50007-5. [DOI] [PubMed] [Google Scholar]
- Audhya T. K., Russell D. W. Enniatin production by Fusarium sambucinum: primary, secondary, and unitary metabolism. J Gen Microbiol. 1975 Feb;86(2):327–332. doi: 10.1099/00221287-86-2-327. [DOI] [PubMed] [Google Scholar]
- BU'LOCK J. D. Intermediary metabolism and antibiotic synthesis. Adv Appl Microbiol. 1961;3:293–342. doi: 10.1016/s0065-2164(08)70514-8. [DOI] [PubMed] [Google Scholar]
- Basak K., Majumdar S. K. Mineral nutrition of Streptomyces kanamyceticus for kanamycin formation. Antimicrob Agents Chemother. 1975 Oct;8(4):391–395. doi: 10.1128/aac.8.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanch H. W., Rogers P. L. Production of gramicidin S in batch and continuous culture. Biotechnol Bioeng. 1971 Nov;13(6):843–864. doi: 10.1002/bit.260130609. [DOI] [PubMed] [Google Scholar]
- CHESTERS C. G. C., ROLINSON G. N. Trace elements and streptomycin production. J Gen Microbiol. 1951 Aug;5(3):559–565. doi: 10.1099/00221287-5-3-559. [DOI] [PubMed] [Google Scholar]
- Claridge C. A., Rossomano V. Z., Buono N. S., Gourevitch A., Lein J. Influence of cobalt on fermentative methylation. Appl Microbiol. 1966 Mar;14(2):280–283. doi: 10.1128/am.14.2.280-283.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULMAGE H. T. The production of neomycin by Streptomyces fradiae in synthetic media. Appl Microbiol. 1953 Mar;1(2):103–106. doi: 10.1128/am.1.2.103-106.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulaney E. L. Observations on Streptomyces griseus: II. Nitrogen Sources for Growth and Streptomycin Production. J Bacteriol. 1948 Sep;56(3):305–313. [PMC free article] [PubMed] [Google Scholar]
- Eagon R. G., Phibbs P. V., Jr Kinetics of transport of glucose, fructose, and mannitol by Pseudomonas aeruginosa. Can J Biochem. 1971 Sep;49(9):1031–1041. doi: 10.1139/o71-151. [DOI] [PubMed] [Google Scholar]
- Gallo M., Katz E. Regulation of secondary metabolite biosynthesis: catabolite repression of phenoxazinone synthase and actinomycin formation by glucose. J Bacteriol. 1972 Feb;109(2):659–667. doi: 10.1128/jb.109.2.659-667.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENDLIN D. The nutritional requirements of a bacitracin-producing strain of Bacillus subtilis. Arch Biochem. 1949 Dec;24(2):435–446. [PubMed] [Google Scholar]
- Haavik H. I., Froyshov O. Function of peptide antibiotics in producer organisms. Nature. 1975 Mar 6;254(5495):79–82. doi: 10.1038/254079a0. [DOI] [PubMed] [Google Scholar]
- Haavik H. I. Studies on the formation of bacitracin by Bacillus licheniformis: effect of glucose. J Gen Microbiol. 1974 Apr;81(2):383–390. doi: 10.1099/00221287-81-2-383. [DOI] [PubMed] [Google Scholar]
- Haavik H. I. Studies on the formation of bacitracin by Bacillus licheniformis: effect of inorganic phosphate. J Gen Microbiol. 1974 Sep;84(1):226–230. doi: 10.1099/00221287-84-1-226. [DOI] [PubMed] [Google Scholar]
- Haavik H. I. Studies on the formation of bacitracin by Bacillus licheniformis: role of catabolite repression and organic acids. J Gen Microbiol. 1974 Oct;84(2):321–326. doi: 10.1099/00221287-84-2-321. [DOI] [PubMed] [Google Scholar]
- Hodgson B. Possible roles for antibiotics and other biologically active peptides at specific stages during sporulation of Bacillaceae. J Theor Biol. 1971 Jan;30(1):111–119. doi: 10.1016/0022-5193(71)90040-3. [DOI] [PubMed] [Google Scholar]
- Hook D. J., Vining L. C. Biosynthetic precursors of etamycin, a peptidolactone antibiotic from Streptomyces griseoviridus. Can J Biochem. 1973 Dec;51(12):1630–1637. doi: 10.1139/o73-219. [DOI] [PubMed] [Google Scholar]
- KATZ E., GOSS W. A. Controlled biosynthesis of actinomycin with sarcosine. Biochem J. 1959 Nov;73:458–465. doi: 10.1042/bj0730458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ E., PIENTA P., SIVAK A. The role of nutrition in the synthesis of actinomycin. Appl Microbiol. 1958 Jul;6(4):236–241. doi: 10.1128/am.6.4.236-241.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ E., WALDRON C. R., MELONI M. L. Role of valine and isoleucine as regulators of actinomycin peptide formation by Streptomyces chrysomallus. J Bacteriol. 1961 Oct;82:600–608. doi: 10.1128/jb.82.4.600-608.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ E., WEISSBACH H. Incorporation of C14-labeled amino acids into actinomycin and protein by Streptomyces antibioticus. J Biol Chem. 1963 Feb;238:666–675. [PubMed] [Google Scholar]
- KATZ E., WISE M., WEISSBACH H. ACTINOMYCIN BIOSYNTHESIS. DIFFERENTIAL EFFECT OF CHLORAMPHENICOL ON PROTEIN AND PEPTIDE ANTIBIOTIC SYNTHESIS. J Biol Chem. 1965 Jul;240:3071–3078. [PubMed] [Google Scholar]
- Katz E. Controlled biosynthesis of actinomycins. Cancer Chemother Rep. 1974 Jan-Feb;58(1):83–91. [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- MAJUMDAR M. K., MAJUMDAR S. K. EFFECTS OF MINERALS ON NEOMYCIN PRODUCTION BY STREPTOMYCES FRADIAE. Appl Microbiol. 1965 Mar;13:190–193. doi: 10.1128/am.13.2.190-193.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAJUMDAR S. K., BOSE S. K. Trace element requirements of Bacillus subtilis for mycobacillin formation. J Bacteriol. 1960 Apr;79:564–565. doi: 10.1128/jb.79.4.564-565.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meienhofer J., Atherton E. Structure-activity relationships in the actinomycins. Adv Appl Microbiol. 1973;16:203–300. [PubMed] [Google Scholar]
- Paritskaia N. S., Nikitina E. T., Kalakutskii L. V. Osobennosti rosta Actinomyces roseoflavus var. roseofungini na srede s fruktozoi. Mikrobiologiia. 1974 Jul-Aug;43(4):686–690. [PubMed] [Google Scholar]
- Phibbs P. V., Jr, Eagon R. G. Transport and phosphorylation of glucose, fructose, and mannitol by Pseudomonas aeruginosa. Arch Biochem Biophys. 1970 Jun;138(2):470–482. doi: 10.1016/0003-9861(70)90371-1. [DOI] [PubMed] [Google Scholar]
- ROMANO A. H., NICKERSON W. J. Utilization of amino acids as carbon sources by Streptomyces fradiae. J Bacteriol. 1958 Feb;75(2):161–166. doi: 10.1128/jb.75.2.161-166.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickenberg H. V. Cyclic AMP in prokaryotes. Annu Rev Microbiol. 1974;28(0):353–369. doi: 10.1146/annurev.mi.28.100174.002033. [DOI] [PubMed] [Google Scholar]
- Rogers T. O., Birnbaum J. Biosynthesis of fosfomycin by Streptomyces fradiae. Antimicrob Agents Chemother. 1974 Feb;5(2):121–132. doi: 10.1128/aac.5.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIVAK A., MELONI M. L., NOBILI F., KATZ E. Biosynthesis of the actinomycin chromophore. Studies with DL-[7alpha-14C] tryptophan and L-[Me-14C] methionine. Biochim Biophys Acta. 1962 Feb 26;57:283–289. doi: 10.1016/0006-3002(62)91121-6. [DOI] [PubMed] [Google Scholar]
- Sabater B., Sebastián J., Asensio C. Identification and properties of an inducible and highly specific fructokinase from Streptomyces violaceoruber. Biochim Biophys Acta. 1972 Oct 12;284(2):414–420. doi: 10.1016/0005-2744(72)90137-4. [DOI] [PubMed] [Google Scholar]
- Sabater B., Sebastián J., Asensio C. Identification and properties of an inducible mannokinase from Streptomyces violaceoruber. Biochim Biophys Acta. 1972 Oct 12;284(2):406–413. doi: 10.1016/0005-2744(72)90136-2. [DOI] [PubMed] [Google Scholar]
- Vicente M. The uptake of fructose by Pseudomonas putida. Arch Microbiol. 1975;102(2):163–166. doi: 10.1007/BF00428362. [DOI] [PubMed] [Google Scholar]
- Weinberg E. D. Secondary metabolism: raison d'être. Perspect Biol Med. 1971;14(4):565–577. doi: 10.1353/pbm.1971.0033. [DOI] [PubMed] [Google Scholar]
- Yajim T., Mason K. T., Kaltz E. Branched-chain amino acid substitutions in the biosynthesis of the antibiotic actinomycin. Antimicrob Agents Chemother. 1975 Jun;7(6):773–780. doi: 10.1128/aac.7.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima T., Grigg M. A., Katz E. Biosynthesis of antibiotic peptides with isoleucine stereoisomers. Arch Biochem Biophys. 1972 Aug;151(2):565–575. doi: 10.1016/0003-9861(72)90534-6. [DOI] [PubMed] [Google Scholar]
- Yajima T., Mason K., Katz E. Biogenetic origin of the D-isoleucine and N-methyl-L-alloisoleucine residues in the actinomycins. Antimicrob Agents Chemother. 1976 Feb;9(2):224–232. doi: 10.1128/aac.9.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]


