Abstract
Mechanisms of the transition from compensatory hypertrophy to heart failure are poorly understood and the roles of vascular endothelial growth factors (VEGFs) in this process have not been fully clarified. We determined the expression profile of VEGFs and relevant receptors during the progression of left ventricular hypertrophy (LVH). C57BL mice were exposed to transversal aortic constriction (TAC) and the outcome was studied at different time points (1 day, 2, 4, and 10 weeks). A clear compensatory phase (2 weeks after TAC) was seen with following heart failure (4 weeks after TAC). Interestingly, VEGF-C and VEGF-D as well as VEGF receptor-3 (VEGFR-3) were upregulated in the compensatory hypertrophy and VEGF-B was downregulated in the heart failure. After treatment with adeno-associated virus serotype 9 (AAV9)-VEGF-B186 gene therapy in the compensatory phase for 4 weeks the function of the heart was preserved due to angiogenesis, inhibition of apoptosis, and promotion of cardiomyocyte proliferation. Also, the genetic programming towards fetal gene expression, a known phenomenon in heart failure, was partly reversed in AAV9-VEGF-B186–treated mice. We conclude that VEGF-C and VEGF-D are associated with the compensatory LVH and that AAV9-VEGF-B186 gene transfer can rescue the function of the failing heart and postpone the transition towards heart failure.
Introduction
Left ventricular hypertrophy (LVH) is a major maladaptive response to chronic pressure overload and an important risk factor for hypertensive patients.1 It is an early step towards heart failure and increases the risk of subsequent cardiac morbidity and mortality.2 Initially, LVH is a response to a sustained increased work load where heart mass is increased to maintain circulatory function. Thus, LVH takes place in the early phase of cardiac stress and is accompanied by cardiac remodeling.3 Compensatory LVH is associated with a thickening of the myocardial wall and maintenance of contractility. When the pathological stress is prolonged, compensatory LVH is accompanied by interstitial fibrosis, contractile dysfunction, altered gene expression, changes in metabolism, and abnormalities in electrophysiology.2,4 This phase is followed by decompensated hypertrophy that is more prominently associated with a significant increase in the risk for sudden cardiac death or progression to heart failure in humans.5,6 Although the shift from the compensatory LVH to the pathological LVH is strongly associated with a disruption in the coordination between angiogenesis and cardiomyocyte growth,7,8,9 there is very little data available about the expression and role of vascular endothelial growth factors (VEGFs) in different phases of LVH.
VEGFs are among the most powerful regulators of vascular growth.10 The genes, VEGF-A, -B, -C, -D, -E, and placental growth factor share similar structures but differ in their physiological and biological properties largely due to their different interactions with three specific tyrosine kinase receptors: VEGF receptor (VEGFR)-1, VEGFR-2, and VEGFR-3.11 VEGF-A, a ligand of VEGFR-1 and -2, is known for its direct strong angiogenic effects.10,12 VEGF-B is a ligand of VEGFR-1 and NRP-1 and has recently been associated with cardiac angiogenesis13,14 as well as with specific effects on metabolism, survival, and apoptosis.15,16,17,18 Full-length VEGF-C and VEGF-D are ligands of VEGFR-3 and promote mostly lymphangiogenic effects whereas their processed forms are ligands of VEGFR-2 and increase angiogenesis and vascular permeability.19,20,21
Here, we have studied the progression of LVH and heart failure in an aortic constriction mouse model with echocardiography and related this process to the expression of VEGFs, their receptors, and relevant metabolic genes. Myocardial fibrosis, glycogen accumulation, angiogenesis, proliferation, and apoptosis were also studied, as well as the effects of adeno-associated virus serotype 9 (AAV9)-mediated gene transfer of VEGF-B186 on the progression of LVH and gene expression in LVH.
It was found that VEGF-C and VEGF-D were associated with the compensated LVH and that AAV9-VEGF-B186 gene transfer was able to rescue the function of the failing heart and postpone the development of heart failure.
Results
Progression of LVH
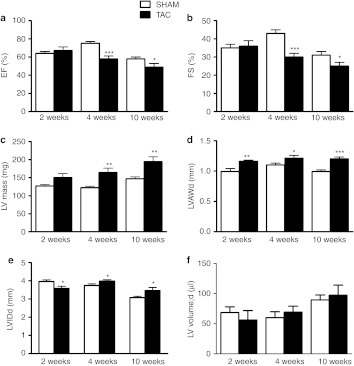
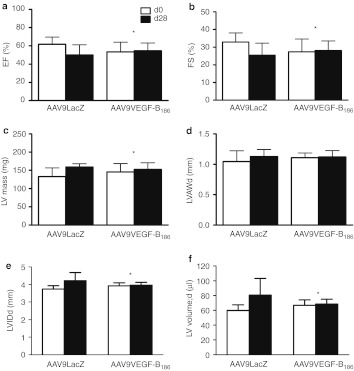
The progression of LVH was followed by echocardiography 2, 4, and 10 weeks after transversal aortic constriction (TAC) operation (Figure 1). Aortic constriction was confirmed by measuring a significant decrease in transverse aorta diameter which was later on (2 and 4 weeks after TAC operation) associated with an increase in the mRNA levels of so-called fetal genes, also known as hypertrophic markers, atrial natriuretic peptide (ANP) and skeletal α-actin (data not shown). Two weeks after TAC, compensatory hypertrophy was indicated by an elevated thickness of the anterior wall of the left ventricle (LVAW, Figure 1d) and decreased LV internal diameter (Figure 1e), while the systolic function measured by ejection fraction (EF, Figure 1a) and fractional shortening (FS, Figure 1b) were preserved. Decompensatory phase (4 and 10 weeks after TAC) was similarly associated with hypertrophic changes but in addition showed a decrease in EF (Figure 1a) and FS (Figure 1b) and an increase in LV mass (Figure 1c), LVAW (Figure 1d) as well as in LV internal diameter (Figure 1e). LV volume (Figure 1f) did not change significantly. Mortality rate was 18% of which 48% died within the first 24 hours after the TAC operation (n = 128).
Figure 1.
Progression of left ventricular hypertrophy. Myocardial function measured by (a) ejection fraction (EF) and (b) fractional shortening (FS) was preserved 2 weeks after TAC operation but significantly deteriorated 4 and 10 weeks after the operation. Compensatory hypertrophy was shown as (d) increased left ventricle anterior wall thickness during diastole (LVAWd) and (e) decreased LV internal diameter during diastole (LVIDd). In heart failure, 4 and 10 weeks after TAC operation, (c) LV mass (LV mass), (d) LVAWd, and (e) LVIDd were increased. (f) LV volume did not change significantly. Echocardiographic measurements were done on the day of killing. Results were obtained from parasternal short axis M-mode projections and each time point is compared with the SHAM-operated group of the same time point by Student's t-test. Data in different time points is from individual set of animals and is not therefore compared over time. Mean ± SD, n = 10/group, *P < 0.05, **P < 0.01, ***P < 0.001. TAC, transversal aortic constriction.
Chronic pressure overload effectively triggers endogenous myocardial angiogenesis and causes myocardial fibrosis
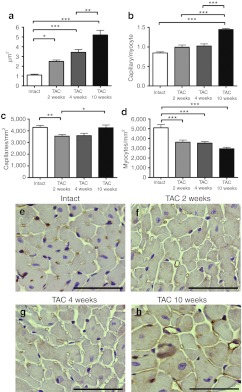
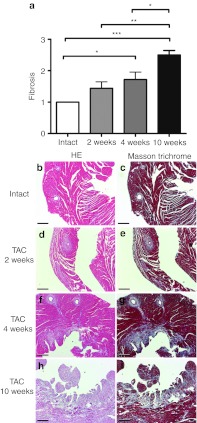
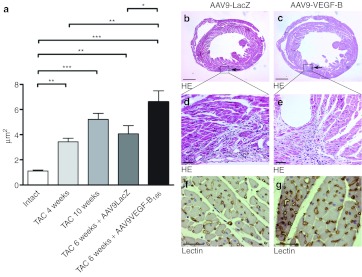
Chronic pressure overload triggered physiological angiogenesis in the myocardium indicated by increased mean capillary area (Figure 2a) and the number of capillaries per myocyte (Figure 2b). The number of capillaries per mm2 was decreased 2 weeks after TAC, and increased 10 weeks after TAC (Figure 2c). The progression of the hypertrophy was seen as a decrease in the amonut of myocytes per mm2 (Figure 2d). Representative pictures of the angiogenic response are shown in Figure 2e–h. Progression of hypertrophy was accompanied by increased myocardial fibrosis (Figure 3) while no difference was seen in glycogen accumulation, proliferation or apoptosis (data not shown). No differences were seen in the angiogenic or cardiomyocytes' response or in the amount of fibrosis between intact animals and sham-operated groups in different time points (Supplementary Figure S1).
Figure 2.
Angiogenic and cardiomyocyte responses to progressive left ventricular hypertrophy (LVH). (a,e–h) Progressive LVH triggered endogenous angiogenesis seen as enlarged capillaries. (b) Ten weeks after TAC operation, the number of capillaries per myocyte was significantly increased. Progressive LVH was seen as (c) decreased number of capillaries per mm2 2 weeks after the operation and (d) cardiomyocytes per mm2 in all time points. Quantification was done from five endothelium-stained microscopic fields from each animal at ×400 magnification. Mean ± SEM, n = 6/group (in a–d), statistical analyses with one-way analysis of variance and Bonferroni's multiple comparison test, *P < 0.05, **P < 0.01, ***P < 0.001. Representative pictures from endothelial stainings with Biotinylated Griffonia (Bandeiraea) Simplicifolia Lectin I, bars 50 µm (in e–h). TAC, transversal aortic constriction.
Figure 3.
Fibrosis in progressive left ventricular hypertrophy. (a–i) The amount of fibrosis increased progressively in TAC-operated animals while there was no fibrosis seen in intact animals. Quantification was done in a blinded fashion by three observers screening collagen-stained microscopic sections at ×12.5 magnification using the following grading criteria: 1, minor or no fibrosis; 2, moderate fibrosis; and 3, severe fibrosis. Mean ± SEM, statistical analyses with one-way analysis of variance and Bonferroni's multiple comparison test (n = 6/group), *P < 0.05, **P < 0.01, ***P < 0.001, (b,d,f,h) hematoxylin-eosin (HE) staining and (c,e,g,i) Masson trichrome staining for collagen, bars 100 µm. TAC, transversal aortic constriction.
The expression of VEGF-C, VEGF-D, and VEGFR-3 is increased in compensatory and VEGF-B expression is decreased in decompensatory phase of the LVH
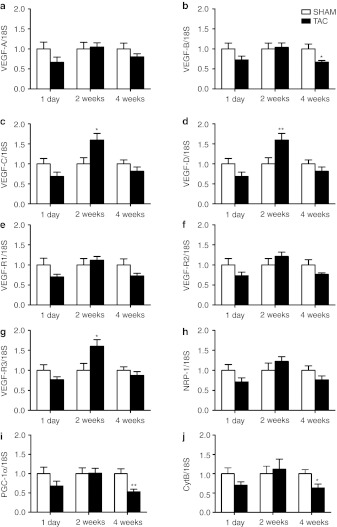
The amount of VEGFs mRNA in LV in all time points were as follows: VEGF-B > VEGF-A >> VEGF-C = VEGF-D (data not shown). No changes in the expression of VEGF-A, VEGF-B, VEGF-C, and VEGF-D was seen 1 day after TAC operation (Figure 4a–d). Interestingly, the expressions of VEGF-C and VEGF-D, growth factors previously unassociated with hypertrophic changes, were significantly increased at the compensatory hypertrophy, 2 weeks after TAC operation (Figure 4c,d) as was their primary receptor VEGFR-3 (Figure 4g). The expression of other receptors remained unchanged (Figure 4e,f,h). While the expression of VEGF-A mRNA remained unchanged and the expression of VEGF-C and VEGF-D mRNAs returned to basal level towards the later time point (4 weeks after TAC, Figure 4a,c,d), the expression of VEGF-B mRNA significantly decreased 4 weeks after TAC compared with SHAM-operated animals (Figure 4b).
Figure 4.
Relative mRNA expression of VEGFs, their receptors (VEGFRs, NRP-1) and mitochodrional genes (PGC-1α, CytB) in progressive LVH. (a) Relative expression of VEGF-A mRNA did not change 1 day, 2 or 4 weeks after TAC operation. (b) Relative expression of VEGF-B mRNA was decreased 4 weeks after TAC operation and (c,d,g) those of VEGF-C, VEGF-D, and VEGFR-3 mRNAs were increased 2 weeks after TAC operation. (e,f,h) The expressions of mRNA encoding VEGFR-1, VEGFR-2 or NRP-1 were not changed. (i,j) The expressions of PGC-1α and CytB mRNAs were decreased 4 weeks after TAC operation. mRNA expression of each gene was measured by RT-PCR and normalized to 18S ribosomal RNA as SHAM-operated group of each time point set to one. The relative expression of each gene in TAC-operated animals is compared with that in the SHAM group of the same time point by Student's t-test. Mean ± SEM, n = 6/group, *P < 0.05, **P < 0.01. CytB, cytochrome B; LVH, left ventricular hypertrophy; NRP-1, neuropilin-1; RT-PCR, reverse transcription-PCR; TAC, transversal aortic constriction; VEGFR, vascular endothelial growth factor receptor.
To evaluate the degree of metabolic remodeling, we measured the mRNA levels of mitochondria associated genes, PGC-1α and CytB, which were both significantly downregulated 4 weeks after TAC (Figure 4i,j).
VEGF-B gene therapy improves systolic function in heart failure
Since VEGF-B expression was decreased at the same time when systolic function of the heart was deteriorated between 2 and 4 weeks after TAC operation, we decided to treat the failing heart with VEGF-B186 delivered in AAV9 viral vector (1 × 1010 viral genomes in 10 µl) directly to the anterior wall of the LV. AAV9-LacZ (1 × 1010 viral genomes in 10 µl) was used as a control. The gene transfers were done 2 weeks after the TAC operation in the compensatory phase and animals were killed 4 weeks after the gene transfer. Human VEGF-B186 mRNA was detected in the LV 4 weeks after the gene transfer, as expected (data not shown).
Systolic function measured with EF and FS stayed at the normal level in VEGF-B186–treated animals compared with the LacZ controls (Figure 5a,b). Also, LV mass did not increase in VEGF-B186–treated group compared with LacZ group (Figure 5c) indicating a less severe hypertrophy. No differences were seen in the LVAW thickness during diastole (Figure 5d). VEGF-B186 gene therapy prevented the dilation of LV internal diameter (Figure 5e) as well as the increase in LV volume (Figure 5f), known features of heart failure, when compared with LacZ-treated controls.
Figure 5.
Progression of LVH after AAV9-VEGF-B186 gene transfer. AAV9-VEGF-B186 gene transfer (1 × 1010 viral genomes in 10 µl) sustained the systolic function ((a) EF and (b) FS) of TAC-operated mice when compared with AAV9-LacZ treated controls. (c) Left ventricle (LV) mass did not increase in VEGF-B186 treated group similarly than in LacZ control group (d) while LVAWd remained unchanged in both groups. (e) LVIDd and (f) LV volume stayed at the normal level in AAV9-VEGF-B186 treated animals, while both increased in AAV9-LacZ control animals. Echocardiographic measurements were done before the gene transfer and on the day of killing. The results were obtained from parasternal short axis M-mode projections. Mean ± SD, statistical analyses with repeated measurements two-way analysis of variance (n = 8/group), *P < 0.05. AAV, adeno-associated virus; EF, ejection fraction; FS, fractional shortening; LVAWd, left ventricle anterior wall thickness during diastole; LVH, left ventricular hypertrophy; LVIDd, left ventricle internal diameter during diastole; VEGF, vascular endothelial growth factor.
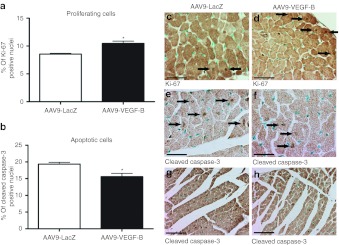
Gene therapy with hVEGF-B186 caused a minimal scaring in the anterior wall of the LV (Figure 6b–e) and apparently accelerated the angiogenic response in the myocardium indicated by the faster increase in mean capillary area compared with TAC control animals (Figure 6a,f,g). Cardiomyocyte proliferation, indicated by Ki-67 immunostaining, was more prevalent in VEGF-B186–treated group (Figure 7a,c,d), and there were less apoptotic cardiomyocytes (measured with cleaved caspase-3 immunostaining) when compared with LacZ-treated group (Figure 7b,e–h).
Figure 6.
The angiogenic response to AAV9-VEGF-B186 gene therapy. (a,f,g) AAV9-VEGF-B186 gene therapy significantly increased mean capillary area when compared with AAV9-LacZ group and the response was accelerated compared with the endogenous response of TAC-operated animals. Quantification was done from five endothelium-stained microscopic fields from each animal at ×400 magnification. (b–e) Gene therapy was done by direct ultrasound-guided injection and caused only a minor needle mark visible in hematoxylin-eosin (HE) staining. Mean ± SEM, statistical analyses with one-way analysis of variance and Bonferroni's multiple comparison test, n = 4/group, *P < 0.05, **P < 0.01, ***P < 0.001 (as shown in a), bars 1 mm (in b,c) and 50 µm (in d–g). HE staining (in b–e) and endothelium staining with Biotinylated Griffonia (Bandeiraea) Simplicifolia Lectin I (in f and g). AAV, adeno-associated virus; TAC, transversal aortic constriction; VEGF, vascular endothelial growth factor.
Figure 7.
The effect of AAV9-VEGF-B186 gene therapy on cell proliferation and apoptosis. AAV9-VEGF-B186 gene therapy (a,c,d) increased the number of proliferating cardiomyocytes and (b,e–h) decreased the number of apoptotic cardiomyocytes. Quantifications in a and b were done from five Ki-67 and cleaved caspase-3 microscopic fields, respectively, from each animal at ×400 magnification. Mean ± SEM, statistical analyses with Student's t-test, n = 4/group, *P < 0.05 (as shown in a,b), bars 25 µm (in c–f) and 50 µm (in g,h), Ki-67 staining for proliferating cells (c,d), and cleaved caspase-3 staining for apoptotic cells (e–h). AAV, adeno-associated virus; VEGF, vascular endothelial growth factor.
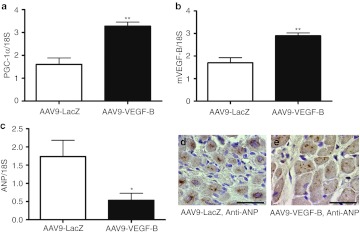
The expression of PGC-1α mRNA (Figure 8a) was increased and that of ANP mRNA (Figure 8c–e) was decreased after VEGF-B186 gene therapy when compared with LacZ-treated group. Also, the expression of endogenous VEGF-B (Figure 8b) mRNA was increased in VEGF-B186–treated group compared with LacZ group.
Figure 8.
Relative mRNA expression of PGC-1α, mVEGF-B, and ANP after AAV9-VEGF-B186 gene therapy. AAV9-VEGF-B gene therapy increased the relative expression levels of (a) PGC-1α and (b) mVEGF-B and decreased the relative expression of (c) ANP mRNA compared with LacZ control group. mRNA expression was measured by quantitative RT-PCR and normalized to 18S ribosomal RNA. Mean ± SEM, statistical analyses with Student's t-test, n = 4/group, *P < 0.05, **P < 0.01. (d,e) ANP staining, bars 25 µm. AAV, adeno-associated virus; ANP, atrial natriuretic peptide; VEGF, vascular endothelial growth factor.
Discussion
In the present study, we have identified the expression patterns of mouse VEGFs and their receptors in progressive LVH and treated the failing hearts with VEGF-B186 gene therapy to restore VEGF-B expression level. It was found that (i) in the compensatory phase of the LVH, VEGF-C, VEGF-D, and VEGFR-3 were significantly upregulated, (ii) when the LVH shifted towards heart failure, VEGF-B was downregulated, and (iii) by treating the animals with AAV9-mediated VEGF-B186 gene transfer in the compensatory phase we could overcome the defects in cardiac functional parameters, reduce metabolism-associated gene expression changes, and reverse the fetal gene expression pattern by lowering the expression of ANP, thus postponing the transition from LVH to heart failure.
In order to study the expression pattern of VEGFs and their receptors, we wanted to cause a chronic, long-term pressure overload recapitulating the different phases of pathological hypertrophy. Hypertrophy was seen in both phases as an increased thickness of the LVAW and an increase in the expression level of hypertrophic marker genes, ANP and skeletal α-actin. Compensatory hypertrophy was followed by heart failure within 4 weeks after TAC operation.
Angiogenesis is considered necessary to support ongoing hypertrophy and is the normal process enabling physiological hypertrophy during normal tissue growth.9,22 In our model of progressive LVH, a physiological angiogenic response was detected in hypertrophied myocardium. A physiological angiogenic response has also been described earlier.7,23 Angiogenesis is probably the reason for a slow progression from compensated to decompensated hypertrophy, since myocardium is able to compensate increased energy demands of the growing work load with dilatation of existing capillaries.
VEGF-B was the most abundant member of the VEGF family expressed in the adult myocardium as also described earlier.24 It has been previously reported that VEGF-A is upregulated 2 weeks after TAC operation8 but also that VEGF-A transcripts and protein levels remain at the control level 3, 7, and 12 weeks after aortic banding.25,26 We found VEGF-A levels to stay at the control level at all time points after TAC operation. In contrast to previous findings of upregulated VEGFR-1 levels 3 and 7 weeks after aortic banding,26 we found no changes in VEGFR-1 or -2 expression.
We show for the first time that in the compensatory phase of the LVH, VEGF-C, VEGF-D, and VEGFR-3 were significantly upregulated and that the expression returned back to control levels as the hypertrophy progresses towards heart failure. VEGF-C and VEGF-D have not so far been associated with cardiac hypertrophy and the activity of these growth factors during chronic pressure overload has remained unclear. In patient data regarding ischemic and dilated forms of cardiomyopathy, VEGF-C has been reported to be upregulated at mRNA and protein levels27 and both VEGF-C and VEGF-D have been shown to be upregulated after acute myocardial infarction in mice.28 A recent finding revealed an increase in the number of lymphatic vessels in human hypertrophic myocardium.29 In gene therapy studies, VEGF-C has been reported to prevent progression of myocardial ischemia by inducing collateral formation in a large animal model.30 However, the specific roles of VEGF-C and VEGF-D in compensatory hypertrophy need to be studied further.
Strikingly, during the development of heart failure, 2 weeks onwards from TAC operation VEGF-B mRNA expression was significantly downregulated. Therefore, we hypothesized that we could improve the functional status of the heart by restoring VEGF-B expression. AAV9-mediated, direct gene transfer to LVAW improved the systolic function of the heart by expediting the angiogenic response of the myocardium, lowering the number of apoptotic cardiomyocytes, increasing cardiomyocyte proliferation, and decreasing the overall workload of the heart. We have previously shown that diastolic dysfunction in angiotensin-II–induced LVH could be prevented with adenoviral VEGF-B gene transfer in rats.16 In this work, the improvements in E/A ratio LV isovolumic relaxation time were suggested to be due to a combined effect of the increased proliferation of cardiomyocytes and an increase in capillary area. Others have also described cardiomyocyte proliferation and renewal in hypertrophy and after injury,31,32,33 even though cardiomyocytes have traditionally been regarded as permanently differentiated cells.
In the present study, we wanted to see a long-term gene expression driven by AAV9, which is reported to be cardiotropic.34 Long-term VEGF-B186 expression led, indeed, to a better systolic outcome. It has been previously reported that VEGF-B has an antiapoptotic effect,18,35 promotes cardiomyocyte proliferation36 and acts as a protective agent after myocardial infarction.13,17,35 Recently, Pepe and colleagues37 reported that AAV9-VEGF-B167 treatment delayed the progression of tachypacing-induced hypertrophy towards heart failure via a nonangiogenic cardioprotective effect by inhibiting apoptosis.
Metabolic disturbances are known to play a significant role in the transition from compensated to decompensated hypertrophy.38 It has been previously shown that in heart failure, PGC-1α, a factor associated with mitochondrial biogenesis, is downregulated.39,40 We also found that PGC-1α and CytB were downregulated at the onset of heart failure 4 weeks after TAC operation. This indicates metabolic remodeling associated with pathological hypertrophy. In AAV9-VEGF-B186–treated animals, PGC-1α levels were higher compared with control group indicating a reduction in metabolic remodeling compared to LacZ-treated controls. This could help heart to better meet its metabolic needs and therefore enhance the functional outcome. Also, we found ANP levels to be reduced in AAV9-VEGF-B186–treated group when compared with control group. ANP is an independent marker of myocardial workload41 and activity42 and since AAV9-VEGF-B186 gene transfer reduced ANP expression it could indicate that VEGF-B186 acts to reduce the overall cardiac workload after TAC. Interestingly, we saw that also the endogenous mouse VEGF-B level was upregulated in VEGF-B–treated animals. Knowing that VEGF-B increases fatty acid uptake via endothelium,15 this upregulation might increase the fatty acid utilization as an energy supply and therefore reduce the energy deprivation in the failing heart.
We conclude that VEGF-C and VEGF-D seem to be associated with the compensatory phase of LVH. Also, VEGF-B level is downregulated at the onset of heart failure and AAV9-mediated VEGF-B186 gene therapy improved the functional outcome of the hypertrophied heart by inducing angiogenesis, inhibiting apoptosis, promoting cardiomyocyte proliferation, and altering the expression of metabolism-associated genes in the heart. VEGF-B186 is a potential therapeutic target in LVH and for the prevention of heart failure.
Materials and Methods
Experimental animals. All animal procedures were approved by The National Animal Experiment Board of Finland and carried out in accordance with the guidelines of The Finnish Act on Animal Experimentation; 128 10–15 weeks old C57BL male mice (Harlan Laboratories, Indianapolis, IN) were used to perform TAC or sham operation. The animals were kept in standard housing conditions in The National Laboratory Animal Center of The University of Eastern Finland. Diet and water were provided ad libitum.
TAC. A pressure overload was induced by a modified TAC model.43 Briefly, mice were anesthetized with medetomidine (1 mg/kg, Domitor; Pfizer, New York, NY) and ketamine (75 mg/kg, Ketalar; Pfizer) subcutaneously and intubated with 0.75 mm polyethylene tube for ventilation (MicroVent; Harvard Apparatus, Holliston, MA). The thorax was opened by cutting the sternum from the midline and transverse aorta was visualized by blunt dissecting. A 7-0 silk suture was placed around the aorta between the brachiocephalicus and the arteria carotis communis sinistra and tied around a 25-G needle, which was subsequently removed. Sham-operated mice went through a similar surgical operation without the ligation. Postoperative analgesic (carprofen (Rimadyl) 5 mg/kg; Pfizer, buprenorphine (Temgesic) 0.05–1 mg/kg; RB Pharmaceuticals Limited, Berkshire, UK) and antisedatative (atipamezole hydrochloride (Antisedan) 0.1–1 mg/kg; Orion Oyj, Espoo, Finland) were given. Animals were killed 1 day, 2, 4 or 10 weeks after the TAC operation.
Echocardiography. Echocardiographic measurements were performed before the TAC operations and at 2, 4, and 10 weeks after the operations using Vevo770 Ultrasound System (VisualSonics, Toronto, Ontario, Canada). A high-frequency ultrasound probe (RMV-707B) operating at 30 MHz, with a focal depth of 12.7 mm was used. The animals were anesthetized with isoflurane (induction: 4.5% isoflurane, 450 ml air, maintenance: 2.0% isoflurane, 200 ml air; Baxter International, Deerfield, IL). EF, FS, LV mass, transverse aorta diameter, and LV diastolic wall thickness were determined from parasternal short axis M-mode measurements. EF was calculated by Vevo770 software (VisualSonics) by using the Teicholz formula.
Immunohistochemistry. Myocardial fibrosis (Masson trichrome, Accustain trichrome stains; Sigma-Aldrich, St Louis, MO), glycogen accumulation (Periodic acid Schiff's glycogen staining), proliferation (Ki-67, ab15580, dilution 1:200; Abcam, Cambridge, UK), apoptosis (Cleaved Caspase-3, Asp175, dilution 1:250; Cell Signaling Technology, Danvers, MA), angiogenesis (endothelium staining, Biotinylated Griffonia (Bandeiraea) Simplicifolia Lectin I, dilution 1:100; Vector Laboratories, Burlingame, CA), and ANP (N-20, SC-18811, dilution 1:100; Santa Cruz Biotechnologies, Santa Cruz, CA) were analyzed from 5 µm thick paraffin-embedded sections fixed with 4% paraformaldehyde in 7.5% sucrose for 4 hours. Capillary area, capillary/myocyte, capillary/mm2, and myocyte/mm2 ratios were analyzed from five microscopic fields of endothelium-stained sections at ×400 magnification within each animal as well as the number of proliferating and apoptotic cardiomyocytes from five microscopic fields of Ki-67 and cleaved caspase-3, respectively, stained sections within each animal at ×400 magnification. Glycogen accumulation was quantified from three microscopic fields of glycogen-stained sections within each animal at ×200 magnification. All quantifications were done in a blinded fashion by using AnalySIS software (Soft Imaging System, Muenster, Germany). The number of proliferating and apoptotic cardiomyocytes is presented as a mean percentage of all cardiomyocytes in a field. For proliferation, apoptosis and glycogen accumulation analyzes the microscopic pictures taken from the site of maximum staining in each section. Myocardial fibrosis was analyzed in a blinded fashion by three observers screening collagen-stained microscopic sections at ×12.5 magnification using the following grading criteria: 1, minor or no fibrosis; 2, moderate fibrosis; and 3, severe fibrosis. The result is shown as a mean of all observations.
Quantitative reverse transcription-PCR. Total RNA from cell cultures was isolated using the GenElute Mammalian Total RNA Miniprep Kit (Sigma-Aldrich). cDNA was synthesized using the First Strand cDNA Synthesis Kit (MBI Fermentas, Amherst, NY). Relative expression levels of mRNA encoding mVEGF-A (forward: 5′-GAT CCG CAG ACG TGT AAA TGT TC-3′, reverse: 5′-TTA ACT CAA GCT GCC TCG CC-3′), mVEGF-B (forward: 5′-CCA CTG GGC AAC ACC AAG TC-3', reverse: 5′-GCT GTG TTC TTC CAG GGA CAT C-3′), mVEGF-C (forward: 5′-TCA GCA AGA CGT TGT TTG AAA TTA C-3′, reverse: 5′-TGA TTG GCA AAA CTG ATT GTG ACT-3′), mVEGF-D (forward: 5′-TGG ACC AGT GAA GGA TTT TTC TTT-3′, reverse: 5′-TGC TCG GAT CTG TTG TTC AGA-3′), VEGFR-1 (forward: 5′-CTT TTC AAG GAC GGC TTT GC-3′, reverse: 5′-GCT CAT GAA TTT GAA AGC GTT TAC-3′), VEGFR-2 (forward: 5′-AAA ACT CTG GAA GAC AGG AAC AAA TT-3′, reverse: 5′-GCC ACA GAC TCC CTG CTT TTA-3′), VEGFR-3 (forward: 5′-TCT CCA ACT TCT TGC GTG TCA-3′, reverse: 5′-CGT TGC TCC GGA GAC TTC TC-3′), NRP-1 (forward: 5′-CTA TGA CCG GCT GGA GAT CTG-3′, reverse: 5′-GCC CAC AAT AAC GCC CAA T-3′), CytB (forward: 5′-CCA CTT CAT CTT ACC ATT TAT C-3′, reverse: 5′-TGA TCC TGT TTC GTG GAG GAA-3′), PGC-1α (forward: 5′-AGC GAC CAA TCG GAA ATC AT-3′, reverse: 5′-GCA AGT TTG CCT CAT TCT CTT CA-3′), ANP (forward: 5′-GAA AAG CAA ACT GAG GGC TCT G-3′, reverse: 5′-CCT ACC CCC GAA GCA GCT-3′), hVEGF-B (forward: 5′-GCC CAG GCC CCT GTC T-3′, reverse: 5′-ACA TCT ATC CAT GAC ACC ACT TTC C-3′), and 18S (forward: 5′-TGG TTG CAA AGC TGA AAC TTA AAG-3′, reverse: 5′-AGT CAA ATT AAG CCG CAG GC-3′) in LV were measured using quantitative reverse transcription-PCR with the ABI 7700 Sequence Detection System (Applied Biosystems, Foster City, CA) using SYBR Green chemistry (Applied Biosystems). The expression levels were normalized to 18S and the results are shown normalized to the expression of sham-operated control group at each time point or compared with the control gene therapy group.
Echocardiography-guided myocardial gene transfer. AAV9-hVEGF-B186 or AAV9-LacZ gene transfers were done as described earlier36 with a dose of 1 × 1010 viral genomes in 10 µl. Gene transfers were done 2 weeks after TAC operation and the animals were killed 6 weeks after the TAC operation. AAV9 vectors were produced in 293T cells using standard plasmid transfection methods and purified through sucrose-cushion ultracentrifugation and an anion-exchange column chromatography (Q-Sepharose; GE Healthcare, Waukesha, WI) followed by concentration through sucrose-cushion ultracentrifugation.44,45 The final virus pellets were resuspended with a solution which consists of 10 mmol/l Tris-HCl, pH 7.9, 1 mmol/l MgCl2, 3% sucrose and diluted further with sterile phosphate-bufferes saline to the final concentration. Virus titers were determined by measuring the genome copies by real-time quantitative PCR using virus genome DNAs prepared from the purified virus preparations.
Statistical analyses. Results are presented as mean ± SD or ± SEM, statistical significances were evaluated using Student's t-test, one-way analysis of variance or repeated measures two-way analysis of variance with Bonferroni's multiple comparison test used as a post-test. The used statistical analyses are specified in the figure legends. P < 0.05 was considered statistically significant. The following symbols are used in the figures: *P < 0.05, **P < 0.01, ***P < 0.001.
SUPPLEMENTARY MATERIAL Figure S1. Angiogenic and cardiomyocyte parameters of intact and SHAM-operated mice in different time points.
Acknowledgments
The study was done in Kuopio, Finland. This study was funded by Finnish Academy, University of Eastern Finland Spearhead Program, Sigrid Juselius Foundation, European Research Council Advanced grant, Finnish Foundation for Cardiovascular Research, Emil Aaltonen Foundation, and The Finnish Cultural Foundation's Northern Savo Fund. The authors like to acknowledge the staff at the Laboratory Animal Center of The University of Eastern Finland for the maintenance of the animals. Anniina Oksman (Charles River Discovery Services, Kuopio, Finland) is acknowledged for help regarding the animal work. The authors declared no conflict of interest.
Supplementary Material
Angiogenic and cardiomyocyte parameters of intact and SHAM-operated mice in different time points.
REFERENCES
- Katholi RE., and, Couri DM. Left ventricular hypertrophy: major risk factor in patients with hypertension: update and practical clinical applications. Int J Hypertens. 2011;2011:495349. doi: 10.4061/2011/495349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JJ., and, Chien KR. Signaling pathways for cardiac hypertrophy and failure. N Engl J Med. 1999;341:1276–1283. doi: 10.1056/NEJM199910213411706. [DOI] [PubMed] [Google Scholar]
- Rimbaud S, Sanchez H, Garnier A, Fortin D, Bigard X, Veksler V.et al. (2009Stimulus specific changes of energy metabolism in hypertrophied heart J Mol Cell Cardiol 46952–959. [DOI] [PubMed] [Google Scholar]
- Lorell BH., and, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102:470–479. doi: 10.1161/01.cir.102.4.470. [DOI] [PubMed] [Google Scholar]
- Levy D, Garrison RJ, Savage DD, Kannel WB., and, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- Frey N., and, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- Walsh K., and, Shiojima I. Cardiac growth and angiogenesis coordinated by intertissue interactions. J Clin Invest. 2007;117:3176–3179. doi: 10.1172/JCI34126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumiya Y, Shiojima I, Sato K, Sawyer DB, Colucci WS., and, Walsh K. Vascular endothelial growth factor blockade promotes the transition from compensatory cardiac hypertrophy to failure in response to pressure overload. Hypertension. 2006;47:887–893. doi: 10.1161/01.HYP.0000215207.54689.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R.et al. (2005Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure J Clin Invest 1152108–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylä-Herttuala S, Rissanen TT, Vajanto I., and, Hartikainen J. Vascular endothelial growth factors: biology and current status of clinical applications in cardiovascular medicine. J Am Coll Cardiol. 2007;49:1015–1026. doi: 10.1016/j.jacc.2006.09.053. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- Rissanen TT., and, Ylä-Herttuala S. Current status of cardiovascular gene therapy. Mol Ther. 2007;15:1233–1247. doi: 10.1038/sj.mt.6300175. [DOI] [PubMed] [Google Scholar]
- Lähteenvuo JE, Lähteenvuo MT, Kivelä A, Rosenlew C, Falkevall A, Klar J.et al. (2009Vascular endothelial growth factor-B induces myocardium-specific angiogenesis and arteriogenesis via vascular endothelial growth factor receptor-1- and neuropilin receptor-1-dependent mechanisms Circulation 119845–856. [DOI] [PubMed] [Google Scholar]
- Bry M, Kivelä R, Holopainen T, Anisimov A, Tammela T, Soronen J.et al. (2010Vascular endothelial growth factor-B acts as a coronary growth factor in transgenic rats without inducing angiogenesis, vascular leak, or inflammation Circulation 1221725–1733. [DOI] [PubMed] [Google Scholar]
- Hagberg CE, Falkevall A, Wang X, Larsson E, Huusko J, Nilsson I.et al. (2010Vascular endothelial growth factor B controls endothelial fatty acid uptake Nature 464917–921. [DOI] [PubMed] [Google Scholar]
- Serpi R, Tolonen AM, Huusko J, Rysä J, Tenhunen O, Ylä-Herttuala S.et al. (2011Vascular endothelial growth factor-B gene transfer prevents angiotensin II-induced diastolic dysfunction via proliferation and capillary dilatation in rats Cardiovasc Res 89204–213. [DOI] [PubMed] [Google Scholar]
- Li X, Tjwa M, Van Hove I, Enholm B, Neven E, Paavonen K.et al. (2008Reevaluation of the role of VEGF-B suggests a restricted role in the revascularization of the ischemic myocardium Arterioscler Thromb Vasc Biol 281614–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang F, Nagai N, Tang Z, Zhang S, Scotney P.et al. (2008VEGF-B inhibits apoptosis via VEGFR-1-mediated suppression of the expression of BH3-only protein genes in mice and rats J Clin Invest 118913–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achen MG, Jeltsch M, Kukk E, Mäkinen T, Vitali A, Wilks AF.et al. (1998Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4) Proc Natl Acad Sci USA 95548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E.et al. (1996A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases EMBO J 151751. [PMC free article] [PubMed] [Google Scholar]
- Rissanen TT, Markkanen JE, Gruchala M, Heikura T, Puranen A, Kettunen MI.et al. (2003VEGF-D is the strongest angiogenic and lymphangiogenic effector among VEGFs delivered into skeletal muscle via adenoviruses Circ Res 921098–1106. [DOI] [PubMed] [Google Scholar]
- Tirziu D, Chorianopoulos E, Moodie KL, Palac RT, Zhuang ZW, Tjwa M.et al. (2007Myocardial hypertrophy in the absence of external stimuli is induced by angiogenesis in mice J Clin Invest 1173188–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accornero F, van Berlo JH, Benard MJ, Lorenz JN, Carmeliet P., and, Molkentin JD. Placental growth factor regulates cardiac adaptation and hypertrophy through a paracrine mechanism. Circ Res. 2011;109:272–280. doi: 10.1161/CIRCRESAHA.111.240820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash AD, Baca M, Wright C., and, Scotney PD. The biology of vascular endothelial growth factor-B (VEGF-B) Pulm Pharmacol Ther. 2006;19:61–69. doi: 10.1016/j.pupt.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Hilfiker-Kleiner D, Hilfiker A, Kaminski K, Schaefer A, Park JK, Michel K.et al. (2005Lack of JunD promotes pressure overload-induced apoptosis, hypertrophic growth, and angiogenesis in the heart Circulation 1121470–1477. [DOI] [PubMed] [Google Scholar]
- Kaza E, Ablasser K, Poutias D, Griffiths ER, Saad FA, Hofstaetter JG.et al. (2011Up-regulation of soluble vascular endothelial growth factor receptor-1 prevents angiogenesis in hypertrophied myocardium Cardiovasc Res 89410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham D, Hofbauer R, Schäfer R, Blumer R, Paulus P, Miksovsky A.et al. (2000Selective downregulation of VEGF-A(165), VEGF-R(1), and decreased capillary density in patients with dilative but not ischemic cardiomyopathy Circ Res 87644–647. [DOI] [PubMed] [Google Scholar]
- Park JH, Yoon JY, Ko SM, Jin SA, Kim JH, Cho CH.et al. (2011Endothelial progenitor cell transplantation decreases lymphangiogenesis and adverse myocardial remodeling in a mouse model of acute myocardial infarction Exp Mol Med 43479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholová I, Dragneva G, Cermáková P, Laidinen S, Kaskenpää N, Hazes T.et al. (2011Lymphatic vasculature is increased in heart valves, ischaemic and inflamed hearts and in cholesterol-rich and calcified atherosclerotic lesions Eur J Clin Invest 41487–497. [DOI] [PubMed] [Google Scholar]
- Pätilä T, Ikonen T, Rutanen J, Ahonen A, Lommi J, Lappalainen K.et al. (2006Vascular endothelial growth factor C-induced collateral formation in a model of myocardial ischemia J Heart Lung Transplant 25206–213. [DOI] [PubMed] [Google Scholar]
- Boström P, Mann N, Wu J, Quintero PA, Plovie ER, Panáková D.et al. (2010C/EBPß controls exercise-induced cardiac growth and protects against pathological cardiac remodeling Cell 1431072–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD.et al. (2007Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury Nat Med 13970–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersell K, Arab S, Haring B., and, Kühn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- Kho C, Lee A, Jeong D, Oh JG, Chaanine AH, Kizana E.et al. (2011SUMO1-dependent modulation of SERCA2a in heart failure Nature 477601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentilin L, Puligadda U, Lionetti V, Zacchigna S, Collesi C, Pattarini L.et al. (2010Cardiomyocyte VEGFR-1 activation by VEGF-B induces compensatory hypertrophy and preserves cardiac function after myocardial infarction FASEB J 241467–1478. [DOI] [PubMed] [Google Scholar]
- Huusko J, Merentie M, Dijkstra MH, Ryhänen MM, Karvinen H, Rissanen TT.et al. (2010The effects of VEGF-R1 and VEGF-R2 ligands on angiogenic responses and left ventricular function in mice Cardiovasc Res 86122–130. [DOI] [PubMed] [Google Scholar]
- Pepe M, Mamdani M, Zentilin L, Csiszar A, Qanud K, Zacchigna S.et al. (2010Intramyocardial VEGF-B167 gene delivery delays the progression towards congestive failure in dogs with pacing-induced dilated cardiomyopathy Circ Res 1061893–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura-Clapier R, Garnier A., and, Veksler V. Energy metabolism in heart failure. J Physiol (Lond) 2004;555 Pt 1:1–13. doi: 10.1113/jphysiol.2003.055095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier A, Fortin D, Deloménie C, Momken I, Veksler V., and, Ventura-Clapier R. Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J Physiol (Lond) 2003;551 Pt 2:491–501. doi: 10.1113/jphysiol.2003.045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, Hsieh EJ, Liu Y, Chen T, Beyer RP, Chin MT.et al. (2012Mitochondrial proteome remodelling in pressure overload-induced heart failure: the role of mitochondrial oxidative stress Cardiovasc Res 9379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavi P, Laine M, Weckström M., and, Ruskoaho H. Cardiac mechanotransduction: from sensing to disease and treatment. Trends Pharmacol Sci. 2001;22:254–260. doi: 10.1016/s0165-6147(00)01679-5. [DOI] [PubMed] [Google Scholar]
- Ronkainen JJ, Vuolteenaho O., and, Tavi P. Calcium-calmodulin kinase II is the common factor in calcium-dependent cardiac expression and secretion of A- and B-type natriuretic peptides. Endocrinology. 2007;148:2815–2820. doi: 10.1210/en.2006-1676. [DOI] [PubMed] [Google Scholar]
- Rockman HA, Ross RS, Harris AN, Knowlton KU, Steinhelper ME, Field LJ.et al. (1991Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy Proc Natl Acad Sci USA 888277–8281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotukhin S, Potter M, Zolotukhin I, Sakai Y, Loiler S, Fraites TJ., Jret al. (2002Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors Methods 28158–167. [DOI] [PubMed] [Google Scholar]
- Gao G, Qu G, Burnham MS, Huang J, Chirmule N, Joshi B.et al. (2000Purification of recombinant adeno-associated virus vectors by column chromatography and its performance in vivo Hum Gene Ther 112079–2091. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Angiogenic and cardiomyocyte parameters of intact and SHAM-operated mice in different time points.