Abstract
The development of small interfering RNA (siRNA) for the treatment of human disorders has been often hampered by their low transfection efficiency in vivo. In order to overcome this major drawback, various in vivo siRNA transfection methods have been developed. However, their capacity to transfect immune or insulin-producing β-cells within the pancreas for the treatment of autoimmune diabetes remains undetermined. We found that lipid- or polyethylenimine-based delivery agents were efficient to address siRNA molecules within pancreas-associated antigen-presenting cells (APCs) (but not β-cells) and particularly a CD11b+ cell population comprising both CD11b+CD11cneg macrophages and CD11b+CD11c+ dendritic cells. However, the route of administration and the carrier composition greatly affected the transfection efficacy. Therapeutically, we showed that early (starting at 6-week-old) short-course treatment with lipid/Alox15-specific siRNA complex promoted long-term protection from type 1 diabetes (T1D) in wild-type (WT) nonobese diabetic (NOD) mice. Alox15 downregulation in pancreas-associated CD11b+ cells significantly upregulated a variety of costimulatory molecules and particularly the programmed death 1 ligand 1 (PD-L1) pathway involved in tolerance induction. Concomitantly, we found that regulatory T cells were increased in the pancreas of lipid/Alox15 siRNA-treated NOD mice. Collectively, our data provide new insights into the development of siRNA-based therapeutics for T1D.
Introduction
Type 1 diabetes (T1D) is a multifactorial autoimmune disease for which susceptibility is determined by genetic, environmental, and immunologic factors.1,2 Throughout the course of the disease, the body's immune system destroys its own insulin-producing pancreatic β-cells with a kinetic that can vary from one individual to another.3 T1D is usually diagnosed when ~80–90% of the initial β-cell mass has been destroyed leading to an elevation of the blood glucose level. To restore normoglycemia in T1D patients, long-term immune tolerance needs to be restored to stop β-cell death and provide a temporal window for β-cell regeneration or islet transplantation.4 From a therapeutic standpoint, a preventive strategy aiming at restoring tolerance before onset would be more effective and less invasive because it would preclude the need of β-cell replacement for maintaining normoglycemia. Since T1D is mostly afflicting children and young individuals, any preventive therapy would need to be associated with minimal side effects.
In vivo administration of antibody showed many successes in the prevention/treatment of graft rejection, cancer or autoimmune diseases. However, antibody-based therapies are often accompanied with systemic side effects since they are “active” components of the immune system and can mediate cytotoxic activities (antibody-dependent cell-mediated cytotoxicity, complement-dependent cytotoxicity or cytokines storm syndrome). RNA interference is a mechanism for RNA-guided regulation of gene expression in which double-stranded RNA of 21–25 base pairs inhibits the expression of genes with complementary nucleotide sequences using small interfering RNA (siRNA).5 Such molecules are already under clinical evaluation and do not lead to any major adverse events.6 Therefore, they have attracted a lot of attention and constitute a great hope for future targeted therapies which could avoid systemic side effects if they are being delivered site-specifically and without off-target silencing.7 Moreover, the use of siRNA sequences avoids the long and difficult process of monoclonal antibody production. Based on these characteristics, nonviral in vivo delivery of siRNA could be part of an immune intervention aiming at preventing autoimmune diabetes. Unfortunately, their efficacy in treating human diseases has been hampered by the low transfection efficiency of siRNA in vivo. In order to overcome this major drawback various lipid- or or polyethylenimine-based delivery agents have been developed to enhance the in vivo transfection of eukaryotic cells. However, their capacity to transfect immune or β-cells within the pancreas remains undetermined.
In this study, we hypothesized that siRNA/carrier complexes can be delivered within pancreas-associated immune cells to downregulate the expression of key proteins. Here, we provide a straightforward protocol to transiently knockdown gene expression in antigen-presenting cells (APCs) infiltrating the pancreas of prediabetic nonobese diabetic (NOD) mice. This approach allowed us to address whether both the CXCL10/CXCR3 pathway8,9 and the arachidonate 15-lipoxygenase (Alox15)10 expressed by pancreatic CD11b+ cells play a role during T1D pathogenesis in NOD mice. We showed that early short-course treatment with Alox15-specific but not CXCL10/CXCR3-specific siRNA/carrier complex promoted long-term protection from T1D in wild-type (WT) NOD mice.
Results
Efficacy of various carriers to deliver siRNA into pancreas-associated immune cells
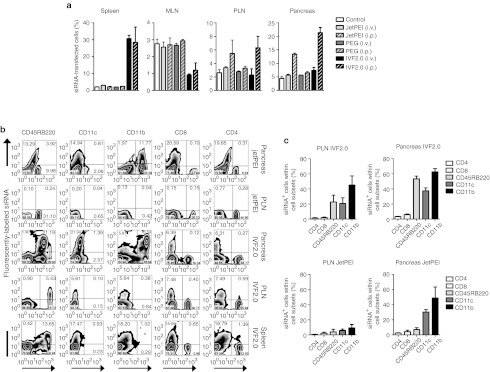
The use of siRNA-based therapeutics has drawn much attention because of their high specificity, high efficiency, and low toxicity.11 However, the effective in vivo delivery of siRNA remains a challenging task. In a first series of experiments, we studied the ability of two liposomes-based (PEG-liposomes (PEG) and invivofectamine2.0 (IVF2.0)) and one polyethylenimine-based (in vivo-JetPEI (JetPEI)) in vivo transfection reagents to deliver siRNA into β-cells or pancreas-associated immune cells. A solution of each carrier complexed with Alexa Fluor 647-labeled siRNA (5 mg/kg body weight) was injected either intravenously (i.v.) or intraperitoneally (i.p.) into prediabetic (10-week-old) NOD mice. The frequency of siRNA+ cells in total cells from the pancreas, pancreatic or mesenteric lymph nodes (PLN or MLN), and spleen of treated mice was evaluated ex vivo. As shown Figure 1a, only i.p. injections using JetPEI or IVF2.0 were able to transfect siRNA into pancreas-associated cells (13.4 ± 0.7 or 21.4 ± 3.3% respectively). Both i.v. and i.p. were effective in delivering IVF2.0-complexed siRNA into splenocytes (30.6 ± 3.5 and 28.4 ± 15.8% respectively). In contrast, cells derived from both PLN and MLN were poorly transfected by the different protocols tested.
Figure 1.
Efficacy of various liposome-based transfection reagents to deliver siRNA into pancreas-associated immune cells. Alexa Fluor 647-labeled siRNA (5 mg/kg of body weight) complexed with one of the three liposomes-based in vivo transfection reagents (in vivo-JetPEI (JetPEI), PEG-liposomes (PEG), and invivofectamine2.0 (IVF2.0)) was injected either intraperitoneally (i.p.) or intravenously (i.v.) into 9-week-old prediabetic NOD mice. (a) The spleen, pancreatic lymph nodes (PLN), mesenteric lymph nodes (MLN), and pancreas were collected 24 hours post-treatment and the frequency of total siRNA-transfected immune cells is given for each organ and transfection modality (n = 3 per group in three independent experiments). (b) After i.p. treatment with liposomes-complexed Alexa Fluor 647-labeled siRNA, single cell suspensions were obtained for each organ, stained with cell surface markers (CD4, CD8, CD45RB220, CD11c, and CD11b) and subsequently acquired by flow cytometry. (c) Pancreas- and PLN-derived immune cells were collected post-treatment with JetPEI- or IVF2.0-complexed Alexa Fluor 647-labeled siRNA. The frequency of siRNA+ cells within a specific immune cell subset is given as a mean ± SD (n = 3 per group in three independent experiments). NOD, nonobese diabetic; PEG, polyethylene glycol; siRNA, small interfering RNA.
We next identified the immune cell subsets transfected by the carriers/siRNA complexes. APCs but not T cells were carrying fluorescently labeled siRNA after a single i.p. injection with either JetPEI- or IVF2.0-complexed siRNA (Figure 1b,c). Among the different cell subsets, CD11b+ cells were primarily transfected with the highest frequency of siRNA+ cells being observed in the pancreas when IVF2.0 was used (62.7 ± 7.4% siRNA+CD11b+ cells within the total CD11b+ cells). A vast majority of these CD11b+ cells were coexpressing the CD11c marker (Figure 1c and data not shown), consequently CD11c+CD11b+ dendritic cells within the pancreas are the major target for the carrier/siRNA complexes. However, pancreatic CD45RB220+ B cells were also highly transfected by siRNA molecules (53.4 ± 6.2% siRNA+CD45RB220+ cells within the total CD45RB220+ cells) when IVF2.0 but not JetPEI was used (Figure 1b,c). In addition, IVF2.0 was the only carrier to transfect siRNA into PLN-associated APCs with a frequency of siRNA+ cells within the different APC subsets between ~20 and 40% (Figure 1c); however, since APCs represent only a minor population in PLN (<15% of total cells, Figure 1b) the absolute number of siRNA+ cells in PLN remains low (Figure 1a). To conclude, the carrier composition and the route of administration are key to address siRNA within different immune cell subsets in various organs.
The presence of siRNA into pancreas-associated APCs is maintained
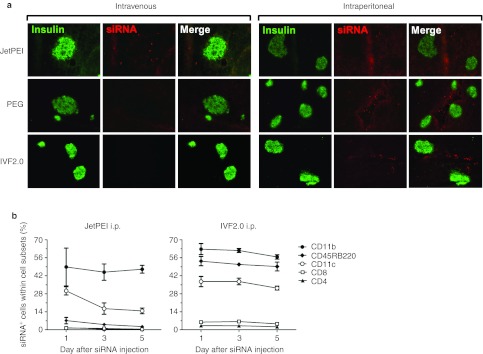
To evaluate whether insulin-producing β-cells were also transfected by carrier/siRNA complexes, pancreatic sections from prediabetic NOD mice treated with either JetPEI- or IVF2.0-complexed Dy547-siRNA were probed with an anti-insulin antibody (Figure 2a). None of the modalities examined resulted in a significant transfection of β-cells by Dy547-labeled siRNA therefore only APCs can be transfected by siRNA after i.p. administration of carrier/siRNA complexes. We next determined whether the presence of siRNA within pancreas-infiltrating immune cells was long lasting. After a single i.p. injection with Dy547-siRNA, our kinetic study revealed that a fluorescent signal was detected in APCs for up to 5 days (Figure 2b). It is worth mentioning that (i) the control-scrambled siRNA injected does not target any known sequences and (ii) APCs infiltrating the pancreas are not rapidly dividing, explaining why the signal remains relatively constant throughout the 5-day study period. Importantly, our results suggest that in vivo-delivered siRNA can be stably retained inside non-dividing cells for at least 5 days which is similar to the half-life observed for an antibody-based treatment.12
Figure 2.
In vivo delivery of liposome-complexed siRNA does not transfect pancreatic β-cells but survives in pancreas-associated immune cells. (a) Histological staining of pancreata from 8-week-old NOD mice. Pancreata were harvested 24 hours after intraperitoneal treatment with either JetPEI- or IVF2.0-complexed DY547-labeled scrambled control siRNA. Eight-µm tissue sections were cut and collected for immunohistochemistry. Sections were stained for insulin (green) and the DY547-labeled siRNA signal was collected in the Cy3 channel (red). (b) Ten-week-old NOD mice were subjected to a single intraperitoneal injection with control scrambled Dy547-labeled siRNA (100 µg) combined with either JetPEI or IVF2.0 reagents (day 0). The pancreata were collected at different time points after injection to evaluate the percentage of CD4+, CD8+, CD11c+, CD11b+, and CD45RB220+ cells carrying the fluorescent siRNA molecules. The data represent the mean ± SD, n = 4. NOD, nonobese diabetic; PEG, polyethylene glycol; siRNA, small interfering RNA.
Targeting the CXCL10/CXCR3 pathway with siRNA does not prevent T1D in WT NOD mice
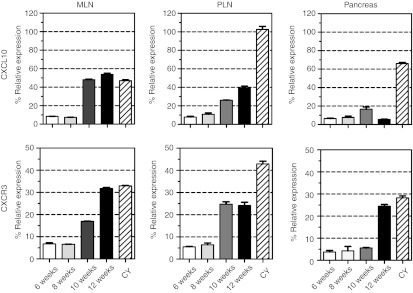
Next, we evaluated whether gene expression by pancreas-associated APCs could be effectively silenced by nonviral in vivo delivery of siRNA. The chemokine CXCL10 (also called IP-10) and its receptor CXCR3 were described, in several reports, to be involved in the recruitment of pathogenic T cells to the pancreas during pathogenesis of T1D.13 In humans, an elevation of CXCL10 was measured in the peripheral blood14 but also in the pancreas15 of T1D patients when compared with healthy subjects. Therefore, one can assume that the CXCL10/CXCR3 axis constitutes a potential target to prevent/cure T1D. To establish whether both CXCR3 and CXCL10 molecules could be potential targets for siRNA-based treatment, we investigated their expression levels in pancreas-infiltrating CD11b+ cells purified at various time points during T1D pathogenesis in the NOD mice. As shown Figure 3, both CXCL10 and CXCR3 genes were poorly expressed by intrapancreatic CD11b+ cells in WT NOD mice. However, the expression was significantly increased after 10 weeks of age in the PLN and MLN of NOD mice. Since both MLN and PLN are refractory to lipid- or polyethylenimine-based transfection (Figure 1), we decided to evaluate CXCR3 and CXCL10 gene expressions in the cyclophosphamide (CY)-induced model of T1D in NOD mice. In this accelerated model of T1D, CXCL10 relative expression by intrapancreatic CD11b+ cells was significantly augmented while the relative level of CXCR3 expression was approximately sixfold higher than in WT NOD mice at the same age (Figure 3). Concomitantly, both CXCR3 and CXCL10 relative expression levels were increased in PLN-derived CD11b+ cells. Altogether, these data suggest that the use of the CXCL10/CXCR3 pathway is amplified during the CY-accelerated pathogenesis of T1D in NOD mice (NOD-CY model). Accordingly, CXCL10 blockade using a neutralizing antibody suppressed T1D in NOD-CY mice.16
Figure 3.
Kinetics of CXCL10 and CXCR3 expressions by CD11b+ macrophages during T1D pathogenesis in NOD mice. Changes in CXCL10 and CXCR3 genes' expression were assessed by quantitative real-time PCR (qRT-PCR) in the pancreas, mesenteric, and pancreatic lymph nodes (MLN and PLN) collected either from wild-type NOD mice at different time points during T1D pathogenesis (6, 8, 10, and 12 weeks of age) or day 7 after intraperitoneal cyclophosphamide (CY; 250 mg/kg of body weight) injection in 8-week-old NOD mice. Results are presented as percentage compared with control. Data are representative of three independent experiments and represent the mean ± SD, n = 6. NOD, nonobese diabetic;T1D, type 1 diabetes.
Consequently, we decided to test the efficacy of CXCL10- and CXCR3-specific siRNA therapy to prevent T1D in both NOD-CY and WT NOD mice.
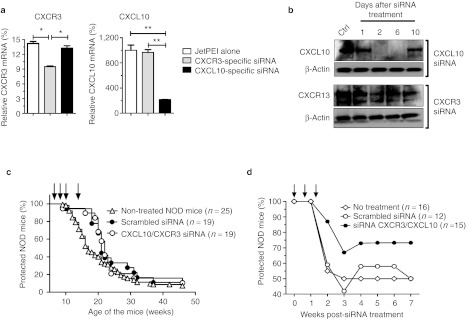
As shown in Supplementary Figure S1, various siRNA sequences targeting specifically the CXCR3 or CXCL10 mRNA were evaluated for their capacity to downregulate CXCR3 and CXCL10 at the protein level. Flow cytometry data using CXCL10- and CXCR3-expressing CHO cell lines revealed that CXCR3#2 and CXCL10#3 siRNA sequences were the most efficient with 56 and 46% inhibition of their targets, respectively and were used for subsequent experiments. We tested the efficacy of both siRNA sequences to silence CXCR3 and CXCL10 gene expression in pancreas-associated CD11b+ cells during the course of T1D in the NOD-CY model (day 9 after CY injection). By 48 hours after i.p. injection with either CXCR3#2 or CXCL10#3 siRNA complexed with JetPEI, we noted a significant and specific reduction in the CXCR3 or CXCL10 relative mRNA expression by intrapancreatic CD11b+ cells (Figure 4a). We also assessed CXCL10 and CXCR3 expressions at the protein level in NOD-CY–treated mice (Figure 4b) and evidenced that the amount of both proteins was significantly reduced in intrapancreatic CD11b+ cells between day 2 and 6 after siRNA delivery. In addition, while CXCL10 expression level was completely abolished from day 2 to 6, CXCR3 expression was only partially downregulated (Figure 4b).
Figure 4.
CXCL10- and CXCR3-specific siRNA treatment reduces T1D incidence in CY-induced diabetes but not wild-type NOD mice. (a) Quantitative analysis of transcript levels of CXCR3 and CXCL10 expressed by pancreas-associated macrophages, relative to housekeeping gene, determined by qRT-PCR after CXCL10- or CXCR3-specific siRNA treatment. Prediabetic NOD mice (9 weeks of age) were injected with cyclophosphamide (CY) and subsequently treated with JetPEI alone or complexed with CXCL10- or CXCR3-specific siRNA (day 9 after CY injection). CXCL10 and CXCR3 relative mRNA levels were measured on 48 hours post-siRNA treatment. Results are presented as percentage compared with control. Error bars indicate mean ± SD, n = 3 experiments; *P < 0.05; **P < 0.01. (b) Western blot analysis to assess the kinetics of CXCR3 and CXC10 downregulation. Total protein was extracted from pancreas-associated CD11b+ cells of NOD-CY–treated mice at 1, 2, 6, and 10 days after a single intraperitoneal injection of either CXCL10 or CXCR3 siRNA complexed with JetPEI on day 0. Fifty microgram per lane of total protein from untreated (Ctrl) and treated NOD mice was loaded. Staining of the indicated proteins on parallel blots is shown. Equal loading of tissue extracts was controlled by β-actin protein staining. Nonviral in vivo delivery of CXCR3/CXCL10 or scrambled control siRNA in (c) prediabetic wild-type or (d) CY-injected NOD mice. Data represents the cumulative diabetes incidence obtained from three independent experiments, n = 12–25 mice per group. Each single injection is shown with a black arrow on the graphs. Ctrl, control; mRNA, messenger RNA; NOD, nonobese diabetic; qRT-PCR, quantitative reverse transcription-PCR; siRNA, small interfering RNA;T1D, type 1 diabetes.
To evaluate the therapeutic efficacy of CXCR3/CXCL10 siRNA combination therapy, WT NOD or NOD-CY mice were dosed i.p. at 2.5 mg/kg of each siRNA in combination or 5.0 mg/kg of scrambled control siRNA each complexed with JetPEI. As expected, treatment of prediabetic WT NOD mice (at 8, 9, 10, and 13 weeks of age) did not significantly reduce diabetes incidence (Figure 4c), although a minimal but significant delay induced by JetPEI was observed as calculated by the Gehan-Breslow-Wilcoxon test (P = 0.0392). In the NOD-CY model (Figure 4d), T1D incidence was significantly reduced by ~50% when the mice were treated (on days 0, 5, and 10 after CY injection) with the CXCR3/CXCL10 combo siRNA as compared with control-scrambled siRNA (P = 0.0048) or non-treated mice (P = 0.0036).
Early downregulation of Alox15 by siRNA treatment prevents T1D in NOD mice
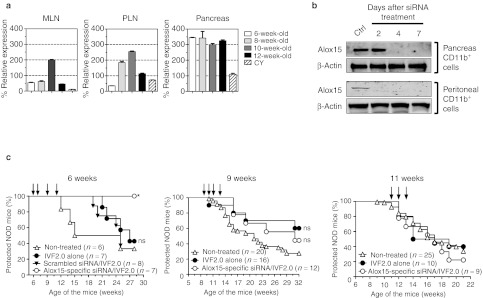
The arachidonate 12/15-lipoxygenase (or 12/15-LO), encoded by the Alox15 gene, is an enzyme expressed by myeloid cells (mostly macrophages) catalyzing the oxygenation of fatty acids to form lipid inflammatory mediators.10,17 By using Alox15-deficient NOD mice, McDuffie, M et al. 18 showed that Alox15 expression plays a major role in the pathogenesis of T1D. However, as pointed out,18,19 these data should also be interpreted with caution because the mechanisms underlying the altered phenotypes of the NOD-B6.129S2-Alox15tm1fun (Alox15-deficient NOD) mice may themselves be multigenic due to the congenic strategy used to generate Alox15-deficient NOD. To address this issue, we decided to use our siRNA-based strategy and downregulate Alox15 expression in macrophages at different time points during T1D pathogenesis. We first investigated the expression levels of Alox15 in CD11b+ cells purified from different organs during the course of T1D pathogenesis in WT NOD mice. As shown Figure 5a, CD11b+ cells retrieved from the pancreas of NOD mice throughout the disease course highly expressed Alox15. In contrast, Alox15 relative expression was lower in the lymph nodes and transiently peaked at 8–10 weeks of age.
Figure 5.
Early treatment with Alox15 siRNA (siAlox15) prevents T1D in NOD mice. (a) Changes in Alox15 gene expression were assessed by qRT-PCR in the pancreas, mesenteric, and pancreatic lymph nodes (MLN and PLN) collected either from wild-type NOD mice at different time points during T1D pathogenesis (6, 8, 10, and 12 weeks of age) or day 7 after intraperitoneal cyclophosphamide (CY; 250 mg/kg of body weight) injection in 8-week-old NOD mice. Results are presented as percentage compared with control. Data are representative of three independent experiments and represent the mean ± SD, n = 6. (b) Western blot analysis for treated NOD mice at different time points. Total protein was extracted from pancreas or peritoneal CD11b+ cells of NOD mice at 2, 4, and 7 days after a single intraperitoneal injection of siAlox15/IVF2.0 complex on day 0. Ten microgram per lane of total protein from untreated (Ctrl) and treated NOD mice was loaded. The intensity of both Alox15 and β-actin (loading control) signals is showed. (c) Diabetes incidence in NOD mice treated with IVF2.0 alone or complexed with Alox15-specific or scrambled siRNA. Short-course treatments started either at 6, 9 or 11 weeks of age and each single injection is shown with a black arrow on the graphs.*P = 0.0109 and ns, not significant. Ctrl, control; NOD, nonobese diabetic; qRT-PCR, quantitative reverse transcription-PCR; siRNA, small interfering RNA; T1D, type 1 diabetes.
Next, we tested by quantitative real-time PCR the in vivo silencing activity of three different Alox15 siRNA sequences on pancreas-associated CD11b+ in NOD mice (Supplementary Figure S2). All three sequences led to a significant downregulation of Alox15 with ~50–80% inhibition when compared with control-scrambled siRNA treated or untreated NOD mice, respectively. The Alox15 siRNA#2 was used to assay its silencing capacity at the protein level. The Alox15 expression was almost entirely suppressed in peritoneal CD11b+ cells 2 days after i.p. treatment with IVF2.0/Alox15 siRNA#2 complex (Figure 5b). Pancreatic CD11b+ showed a delayed but sustained inhibition of the Alox15 protein (from day 4 to at least day 7 post-treatment).
Lastly, we investigated the ability of Alox15 siRNA to prevent T1D in NOD mice. As shown Figure 5c, the efficacy of IVF2.0/Alox15 siRNA treatment was highly dependent on the timing of administration of the lipid/siRNA complex. When treatment was initiated at 6 weeks of age, T1D was completely prevented until at least 28 weeks of age (Figure 5c, left panel). IVF2.0 alone or combined with scrambled siRNA delayed T1D onset but did not significantly change the final incidence (~60%) when compared with non-treated NOD mice. In striking contrast, if treatment is initiated later (9 or 11 weeks of age) Alox15 siRNA is not efficacious.
Phenotype of CD11b+ cells and frequency of regulatory T cells after Alox15-specific siRNA treatment
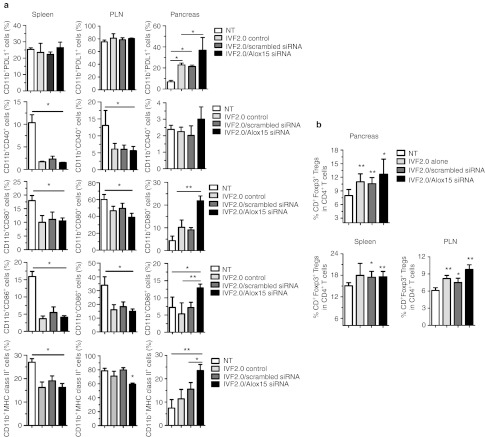
To understand why tolerance was induced when IVF2.0/Alox15 siRNA treatment was administered early during T1D pathogenesis, we analyzed the expression of key costimulatory molecules on CD11b+ cells upon therapy. The PD1-PDL1 pathway has been shown to control autoimmunity by inducing tolerance in many preclinical settings and animal models.20 In the pancreas, a significant augmentation of programmed death 1 ligand 1 (PDL1) expression by CD11b+ cells was observed after injection with IVF2.0 alone but Alox15 downregulation after IVF2.0/Alox15 siRNA treatment further increased this phenotype (Figure 6a). Simultaneously, the frequency of pancreatic CD11b+ cells coexpressing CD80, CD86, and major histocompatibility complex (MHC) class II was found significantly higher after IVF2.0/Alox15 siRNA treatment when compared with IVF2.0 alone or non-treated mice, while the percentage of CD11b+CD40+ cells remained unchanged (Figure 6a). In the PLN and spleen, PDL1 expression was not affected by the treatments. However, the frequency of CD11b+ cells expressing the other costimulatory molecules (CD80, CD86, CD40, and MHC class II) was decreased due to IVF2.0 lipid injection. These data show that the IVF2.0 transfection reagent, and possibly other lipids, have a direct effect on the phenotype of transfected cells such as CD11b+ cells which consequently may delay the onset of T1D (Figure 5c, left panel). Since regulatory T cells (Tregs) are major players in tolerance induction, we next measured the frequency of CD4+Foxp3+Tregs in various organs 2 weeks post-treatment (Figure 6b). Upon IVF2.0/Alox15 siRNA therapy, the frequency of CD4+Foxp3+Tregs was significantly higher in the spleen, PLN, and pancreas when compared with non-treated age-matched NOD mice. A lower but significant increase in the percentage of CD4+Foxp3+Tregs was also observed after IVF2.0 treatment alone in the PLN and pancreas. Altogether these data support the hypothesis that an early transient downregulation of Alox15 expression can prevent T1D through the promotion of anti-inflammatory CD11b+ cells and the expansion of Tregs.
Figure 6.
The phenotype of pancreas-associated macrophages and the frequency of regulatory T cells are modified by Alox15 siRNA/IVF2.0 treatment of prediabetic NOD mice. (a) The expression levels of various costimulatory molecules expressed by CD11b+ cells were assessed by flow cytometry in the spleen, pancreatic lymph nodes (PLN), and pancreas of IVF2.0 alone, Alox15-specific or scrambled siRNA/IVF2.0-treated NOD mice. The data represent the frequencies as a mean ± SD of three independent experiments, n = 6. (b) The frequency of CD4+Foxp3+ regulatory T cells (Tregs) was measured in the spleen, PLN, and pancreas of IVF2.0 alone or Alox15 siRNA/IVF2.0-treated NOD mice. Data are representative of two independent experiments and represent the mean ± SD, n = 6. *P < 0.05; **P < 0.01. MHC, major histocompatibility complex; NOD, nonobese diabetic; NT, non-treated; siRNA, small interfering RNA.
Discussion
Delivery to appropriate cells and tissues in vivo remains a major hurdle for harnessing the potent gene-silencing ability of siRNA molecules for treating human diseases. The aim of this study was to design a nonviral delivery method to address siRNA into pancreas-associated immune cells with the objective to investigate the role of key proteins during the pathogenesis of T1D and eventually prevent the disease onset. We studied the ability of three in vivo transfection reagents (carriers) to deliver siRNA complexes into β-cells or pancreas-associated lymphocytes. Here, we showed that two siRNA/carrier complexes were able to transfect APCs within the pancreas of prediabetic NOD mice but with differential cellular specificities and efficacies. The siRNA/carrier complexes preferentially targeted in the pancreas a CD11b+ cell population comprising both CD11b+CD11cneg macrophages and CD11b+CD11c+ dendritic cells. In the PLNs only one compound, namely IVF2.0, transfected siRNA into APCs. However, since PLN-derived APCs are mostly composed of CD45RB220+ B cells (Figure 1b) they constituted the main transfected population in the PLNs.
In addition, the route of administration greatly impacted the efficiency of the delivery agents to address the siRNA/carrier complex to pancreas-associated APCs. We observed that i.p. was more effective than i.v. injection to transfect intrapancreatic immune cells. The i.p. route of administration not only affected APCs in the pancreas but also led to an efficient transfection of peritoneal CD11b+ macrophages (data not shown). However, by using fluorescently labeled siRNA+CD11b+ cells purified from the peritoneal cavity of treated NOD mice and adoptively transferred i.p. into 9-week-old NOD mice, we demonstrated that pancreas-associated siRNA+CD11b+ cells did not result from the migration of these cells into the pancreas (data not shown). Then, i.p. treatment with siRNA/carrier complexes directly transfected APCs within the pancreas. We also showed that a single injection of siRNA/carrier complex can downregulate its target for at least 7 days within pancreatic CD11b+ cells (Figure 5b). This observation has important therapeutic implications for designing effective treatment schedules to obtain sustained downregulation of key protein involved in the pathogenesis of T1D without over dosing to optimize the efficacy/side effect ratio.
In a therapeutic setting, the expression of two potential targets (CXCL10/CXCR3 pathway and Alox15) was downregulated by siRNA/carriers treatment for preventing T1D in NOD mice. Several reports described the CXCL10/CXCR3 pathway as involved in the recruitment of autoreactive T cells to the pancreas during pathogenesis of T1D.13,21,22,23 In humans, CXCL10 expression was increased in T1D patients when compared with healthy subjects.14,15 In WT NOD mice, our data clearly showed that silencing the CXCL10/CXCR3 pathways in pancreatic CD11b+ cells between 8 and 13 weeks of age do not delay or prevent T1D (Figure 4b). Two main reasons can account for this failure: (i) the expression levels of both CXCL10 and CXCR3 is relatively low in pancreatic CD11b+ cells without CY treatment (Figure 3) and (ii) other cell subsets express CXCL10 and CXCR3 during T1D pathogenesis. Indeed CXCL10 can be expressed by islet β-cells24 and acinar cells25 while CXCR3 has been observed on mouse and human regulatory T cells.26,27,28,29 In this case systemic blockade of CXCL10 and/or CXCR3 using blocking antibodies might be more efficacious as previously reported.30,31 Furthermore, the CXCR3 chemokine receptor is activated by three ligands: the CXCL9, CXCL10, and CXCL11chemokines.32 This redundancy might compensate the loss of CXCL10 signal during siRNA treatment and explain the absence of significant prevention from T1D in NOD mice. In addition, other chemokine/chemokine receptor pathways have been identified as playing a key role in T1D pathogenesis33,34 and could also compensate for the loss of CXCL10/CXCR3 activity in siRNA-treated mice.
Although transient silencing of Alox15 in CD11b+ cells provided complete prevention against T1D in NOD mice, the protection was only seen when the treatment was administered early in life. In contrast to what observed with Alox15-deficient NOD mice,18 our data pinpoint a critical time point (from 6 to 9 weeks of age) for targeting Alox15 expression in CD11b+ cells in order to significantly impact T1D pathogenesis (Figure 5c). Two non-mutually exclusive reasons might explain the lack of efficacy at later time points: (i) Other cell subsets and in particular the pancreatic β-cells can express the 12/15-LO protein in mice and in humans.35,36 In 2005, Chen M and colleagues37 described that one of the product generated by the enzymatic activity of 12/15-LO, the 12-hydroperoxyeicosatetraenoic acid (12-HPETE), is directly toxic to β-cells and human islets by markedly decreasing insulin secretory function and increasing β-cell death. (ii) Early Alox15 expression by pancreas-infiltrating CD11b+ cells triggers a pathogenic process leading to the migration of autoreactive effector T cells (Teff) into the pancreas. Consequently, once this process is in place the autoimmune pathogenesis can only be stopped by directly affecting the Teff compartment.
Mechanistically, our data highlighted for the first time that Alox15 downregulation in pancreas-associated CD11b+ cells can significantly upregulate a variety of costimulatory molecules and MHC class II (Figure 6a). In particular, PDL1 or CD274, involved in tolerance induction,20 was significantly increased. Some studies already described that PDL1+CD11b+ cells can play a major role in transplantation or autoimmune tolerance.38,39 In addition, PDL1 signaling on APCs was shown to be critical for the conversion of naive CD4+ T cells into adaptive CD4+Foxp3+Tregs.40 Collectively these data argue for a role of pancreatic Alox15-deficient CD11b+ cells, generated upon siRNA treatment, in the increase of pancreas-infiltrating CD4+Foxp3+Tregs (Figure 6b). It is worth mentioning that the frequency of PDL1+CD11b+ cells was not augmented in the spleen or PLN of treated NOD mice. Therefore, the inflammatory milieu found in the pancreas during the course of the disease seems to be required for upregulating PDL1 as previously suggested.39,41
A short-course treatment of prediabetic NOD mice with either in vivo-JetPEI or IVF2.0 complexed with scrambled siRNA significantly delayed T1D. The reasons behind this effect remains obscure but JetPEI is administered in a 5% glucose solution and recent studies showed that glucose and inflammation play a role in the control of islet vasculature and insulin content of β-cells in prediabetic NOD mice through increased production of vascular endothelial growth factor by pancreatic islets.42 Another study showed that IFN-α serum levels augmented in mice treated with in vivo-JetPEI.43 We confirmed that IFN-α but also other proinflammatory cytokines, such as IL-12, were rapidly upregulated in the serum of NOD mice after treatment with siRNA complexed with JetPEI (Supplementary Table S1). This upregulation was observed at 6 hours post-treatment but was completely lost at 24–48 hours (data not shown). Published data showed that upregulation of IFN-α or IL-12 in prediabetic NOD mice can delay and reduce T1D incidence.44,45 Although the role of these cytokines in T1D pathogenesis is still debated, it provides a rational for the modest antidiabetogenic activity observed in Figure 4c after in vivo-JetPEI treatment.
In contrast, IVF2.0 reagent demonstrated extremely low or nondetectable cytokine production upon in vivo administration in mice (Supplementary Table S1). Therefore, the delay in the onset of T1D showed after treatment with IVF2.0 alone (Figure 5c) might be at least partially due to changes in the expression of costimulatory molecules, (Figure 6) thanks to a direct signaling of IVF2.0 liposomes in transfected immune cells as suggested for other liposomes.46 Further mechanistic studies are warranted to shed new light on the activity of IVF2.0 liposomes on immune cells. In addition, other lipid-based siRNA delivery reagents such as DOTAP47 orTransIT-TKO48,49 have been shown to deliver siRNA to peritoneal macrophages when injected i.p. Even though their ability to target immune cells within the pancreas remains unknown, these reagents may have potential therapeutic efficacy and should be compared with JetPEI and IVF2.0 in future studies.
In summary, we have shown that both lipid-and polyethylenimine-based transfection reagents (IVF2.0 and JetPEI, respectively) provide a tool for targeted siRNA delivery to pancreas-associated macrophages in vivo. These findings overcome a critical barrier of in vivo delivery, significantly enhancing the prospect of siRNA-based therapeutics for autoimmune diabetes. Even though early downregulation of Alox15 prevents T1D pathogenesis in NOD mice, a combination of siRNAs to silence several inflammatory mediators may be necessary to enhance β-cell protection at a later time point in mice and maybe in humans.
Materials and Methods
Mice. NOD/LtJ female mice were purchased from the Jackson Laboratories (Bar Harbor, ME) and maintained in the La Jolla Institute for Allergy and Immunology under pathogen-free conditions and handled in accordance with protocols approved by the organization's animal care committee. Some 7–8-week-old female NOD mice received an i.p. injection of CY at 250 mg/kg body weight to accelerate the onset of T1D.
siRNA. In vivo ready (high-performance liquid chromatography purified) synthetic siRNA were purchased from Ambion/Life Technologies (Carlsbad, CA) and the most efficient sequence at knocking-down their specific targets in vitro were used in vivo. Scrambled control siRNA(sense 5′-AAAGUCGACCUUCAGUAAGGA-3′), Alox15-specific siRNA (sequence #2: sense 5′-GGCAAGUCAUGAAUCGGUAUU-3′), CXCL10- specific siRNA (sequence #3: sense 5′-GCCCACGUGUUGAGAUCA UUU-3′), CXCR3-specific siRNA (sequence #2: sense 5′-GGAUUUCAGCC UGAACUUUUU-3′).
siRNA/IVF2.0 complex preparation. Invivofectamine 2.0 (Invitrogen/Life technologies, Carlsbad, CA) was used according to the manufacturer's protocol. Stock siRNA solution (3 mg/ml) was diluted at 1.5 mg/ml with the complexation buffer (250 µl final) and subsequently combined with Invivofectamine 2.0 reagent (250 µl). The mixed complex was incubated at 50 °C for 30 minutes and dialyzed against PBS1X for 2 hours in a Spectra/Por Float-A-Lyzer G2 8-10KD (cat. no. G235031; Spectrum Medical Laboratories, Rancho Dominguez, CA). The siRNA concentration is adjusted with PBS1X at 0.25 mg/ml or 0.5 mg/ml for i.p. or i.v. injections, respectively. For the in vivo experiments, a total of 0.1 mg (~7.7 nmol or ~5 mg/kg) of siRNA was injected per NOD mouse.
siRNA/In vivo JET-PEI complex preparation. In vivo JET-PEI (Polyplus transfection, Illkirch, France) was used according to the manufacturer's protocol. Preparation of the siRNA/in vivo JET-PEI complex (N/P ratio 8) was performed as follows. The siRNA diluted in RNAse-free water (100 µl at 1 mg/ml) was mixed with 10% glucose (100 µl) to prepare solution A. In vivo JET-PEI (16 µl) in water (84 µl) was mixed with 10% glucose (100 µl) to prepare solution B. Solution A and B were combined, vortexed, and incubated for 15 minutes at room temperature. For the in vivo experiments, 100 µg of siRNA was injected per animal (~7.7 nmol or ~5 mg/kg).
siRNA/PEG-liposome complex preparation. PEG-liposome (AltogenBiosystems, Las Vegas, NV) was used according to the manufacturer's protocol. One hundred microgram of siRNA was diluted in 50 µl of PBS1X and combined with 40 µl of transfection Reagent. After 20 minutes incubation at room temperature, the transfection reagent enhancer (10 µl) was added (10 minutes at room temperature). PBS1X (100 µl) was added to reach a final volume of 200 µl. Each animal received 100 µg(~7.7 nmol or ~5 mg/kg) of siRNA/PEG-liposome solution (200 µl) i.v. or i.p.
Isolation of intrapancreatic immune cells. This protocol was previously described.50 Briefly, pancreata were cut into small pieces and digested for 30 minutes at 37 °C in 3 ml/pancreas of Hank's Balanced Salt Solution 1× with Ca2+ and Mg2+ complemented with collagenase P (2 mg/ml). The digestion was stopped by adding three volumes of RPMI-1640 buffer supplemented with 10% fetal calf serum (cRPMI). Large undigested tissue pieces were removed by filtration through a 500 µm mesh. After centrifugation (1,350 rpm, 5 minutes) the pellet was resuspended in a 40% Percoll solution (8 ml/pancreas) and transferred into a 15-ml conical tube. Immune cells were purified at the interphase of a 40/75% Percoll density gradient after centrifugation (2,200 rpm, 20 minutes at room temperature (RT)), rapidly washed in cRPMI and stored on ice until further analysis.
Antibodies and reagents for flow cytometry. We purchased the following antibodies from BD Biosciences (San Jose, CA): PE-conjugated CD4-specific (RM4-5), PerCPCy5.5-conjugated CD11c-specific (HL3), Alexa Fluor 488-conjugated CD8-specific (53-6.7), PE-Cy7-conjugated CD45RB220-specific (RA3-6B2), FITC-conjugated MHC class II-specific (10-3.6), PE-conjugated IFN-γ (XMG1.2), FITC-conjugated TNF-specific (MP6-XT22), and CD16/CD32-specific (FcBlock). We purchased the following antibodies from Biolegend (San Diego, CA): Pacific Blue- and PerCPCy5.5-conjugated CD11b-specific (M1/70), APC-conjugated CD183 (CXCR3)-specific (CXCR3-173), Pacific Blue-conjugated CD86-specific (GL-1). We purchased Alexa Fluor APC-conjugated streptavidin from Invitrogen/Life Technologies. We purchased the Foxp3 staining buffer set and the following antibodies from eBioscience (San Diego, CA): PE-Cy7-conjugated Foxp3-specific (FJK-16S), PE-Cy5-conjugated CD40-specific (1C10), APC-conjugated CD80-specific (16-10A1), PE-conjugated PDL1-specific (MIH5). Biotinylated anti-CXCL10 antibody (BAF466) was purchased from R&D systems (Minneapolis, MN).
Selection of siRNA against CXCR3, CXCL10 or Alox15. Mouse CXCL10- and CXCR3-expressing pVITRO2-mcs (Invivogen, San Diego, CA) plamids were synthesized by GenScript (Piscataway, NJ). Stable CHO cell lines expressing either mouse CXCL10 or CXCR3 were obtained by selection on hygromycin B. WT or CXCR3-expressing CHO cells were transfected with three single CXCR3 siRNA or a control-scrambled siRNA complexed with the lipofectamine 2000 (Invitrogen). The efficacy of each siRNA was assessed by measuring the surface expression of CXCR3 by flow cytometry using an APC-conjugated CXCR3-specific antibody (2 µg/ml, 20 minutes at 4 °C). WT or CXCL10-expressing CHO cells were transfected with four single CXCL10 siRNA or a control-scrambled siRNA complexed with the lipofectamine 2000. After fixation and permeabilization of the cells using the Cytofix/Cytoperm kit (BD Biosciences), the efficacy of each siRNA was assessed by measuring the intracellular expression of CXCL10 with a biotin-conjugated CXCL10-specific antibody (5 µg/ml at RT) and subsequent APC-labeled streptavidin staining (5 µg/ml, 20 minutes at 4 °C). A FACSCalibur cytometer (BD Biosciences) and CellQuest software (BD Biosciences) were used for flow cytometry, and data were analyzed using FlowJo 7.2.2 software.
Immunohistofluorescence. Snap-frozen sections of pancreas were fixed in 4% paraformaldehyde for 15 minutes at RT and washed three times in phosphate-buffered saline–3% bovine serum albumin (PBS-BSA). Subsequently insulin was detected by incubating a guinea pig anti-swine insulin (DakoCytomation, Glostrup, Denmark) at 3 µg/ml (1 hour 30 minutes, RT) and followed by incubation with Alexa Fluor 488-conjugated donkey anti-guinea-pig (Jackson ImmunoResearch, West Grove, PA) at 2 µg/ml (1 hour, RT). The DY547-labeled siRNA signal was collected in the Cy3 channel.
Quantitative real-time PCR. CD11b+ cells were purified by positive selection using CD11b+microbeads (#130-049-601; Miltenyi Biotec, Auburn, CA). mRNA was extracted from CD11b+ purified macrophages using the RNeasy kit (QIAGEN, Valencia, CA) and 2 µg of pure RNA was subjected to reverse transcription using the high capacity RNA-to-cDNA kit (cat. no. 4387406; Applied Biosystems, Carlsbad, CA). Quantitative real-time PCR was performed on 4 µl of cDNA with TaqMan fast universal PCR master mix (2×) (Applied Biosystems) and Roche LightCycler 480Real-Time PCR System (Roche Applied Science, Indianapolis, IN) according to the manufacturer's instructions. Amplification conditions were as follows: 45 cycles of denaturation at 95 °C for 10 seconds, annealing at 60 °C for 30 seconds, and extension at 72 °C for 1 second. TaqMan primers used were purchased from Applied Biosystems and were identified as follows: mouse Alox15 (assay ID: Mm00772337_m1), mouse CXCL10 (assay ID: Mm00445235_m1), mouse CXCR3 (assay ID: Mm00438259_m1), and mouse Gusb (assay ID: Mm00446953_m1). Relative CXCL10, CXCR3, and Alox15 quantification were determined by normalizing the expression for each gene to the Gusb gene following the 2−ΔΔCt method.
Western blotting. Total proteins were extracted from purified CD11b+ cells using the M-PER mammalian protein extraction reagent (Pierce, Rockford, IL). Protein concentrations were measured using a BCA Protein Assay Reagent kit (Pierce) and 50 µg (for CXCR3/CXCL10) or 10 µg (for Alox15) subjected to 10% SDS-PAGE separation. Samples are transferred onto a nitrocellulose membrane by semi-dry electrophoresis subsequently blocked using 5% non-fat dry milk in PBS1X. The presence of CXCL10 or CXCR3 were detected using a goat anti-mouse CXCL10 (AF-466-NA; R&D Systems) or CXCR3 (clone C-20, sc-6226; Santa Cruz Biotechnology, Santa Cruz, CA) polyclonal antibodies and revealed with a horseradish peroxidase (HRP)-conjugated donkey anti-goat antibody (sc-2033; Santa Cruz Biotechnology). Alox15 protein was detected using a rabbit polyclonal anti-Alox15 antibody (clone D01P; Abnova, Taipei, Taiwan) and revealed by a HRP-conjugated donkey anti-rabbit IgG (product number 31458; Thermo Scientific, Rockford, IL). We used β-actin as a loading control detected with HRP-conjugated β-actin antibody (Santa Cruz Biotechnology; sc-47778 HRP).
Statistical analysis. Data analysis was performed using GraphPad Prism 4.00 (GraphPad Software, La Jolla, CA). Survival curves were computed using the log-rank method. For some analyses, the Gehan-Breslow-Wilcoxon method was used when more weight to deaths at early time points was required. For other in vivo data the significance was evaluated using a two-way analysis of variance test. Statistical significance for other data was measured using an unpaired two-tailed Mann–Whitney U-test. *P < 0.05; **P < 0.01.
SUPPLEMENTARY MATERIAL Figure S1. Selection of CXCL10- and CXCR3-specific siRNA. Figure S2. Selection of Alox15-specific siRNA. Table S1. Serum proinflammatory cytokines.
Acknowledgments
The authors thank SinaFaton (La Jolla Institute for Allergy and Immunology, La Jolla, CA) for her technical help. We are also indebted to Xavier de Mollerat (Invitrogen/Life Technologies, Carlsbad, CA) for helpful discussions and recommendations on IVF2.0 technology. This work was successively supported by a pilot grant from the association “L'Aide aux JeunesDiabétiques” (to D.B.), the National Institutes of Health grant U19AI050864-10 (to D.B), and an Invitrogen/Life Technologies collaborative grant (to D.B.). The authors declared no conflict of interest.
Supplementary Material
Selection of CXCL10- and CXCR3-specific siRNA.
Selection of Alox15-specific siRNA.
Serum proinflammatory cytokines.
REFERENCES
- Bresson D., and, von Herrath M. Mechanisms underlying type 1 diabetes. Drug Discov Today Dis Mech. 2004;1:321–327. [Google Scholar]
- van Belle TL, Coppieters KT., and, von Herrath MG.2011Type 1 diabetes: etiology, immunology, and therapeutic strategies Physiol Rev 9179–118.doi: 10.1152/physrev.00003.2010). [DOI] [PubMed] [Google Scholar]
- von Herrath M, Sanda S., and, Herold K. Type 1 diabetes as a relapsing-remitting disease. Nat Rev Immunol. 2007;7:988–994. doi: 10.1038/nri2192. [DOI] [PubMed] [Google Scholar]
- von Herrath M, Rottembourg D., and, Bresson D. Progress in the development of immune-based therapies for type 1 diabetes mellitus. BioDrugs. 2006;20:341–350. doi: 10.2165/00063030-200620060-00004. [DOI] [PubMed] [Google Scholar]
- Kim DH., and, Rossi JJ. Strategies for silencing human disease using RNA interference. Nat Rev Genet. 2007;8:173–184. doi: 10.1038/nrg2006. [DOI] [PubMed] [Google Scholar]
- Davidson BL., and, McCray PB., Jr Current prospects for RNA interference-based therapies. Nat Rev Genet. 2011;12:329–340. doi: 10.1038/nrg2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas A., and, Vlassov AV. RNAi: a novel antisense technology and its therapeutic potential. Med Sci Monit. 2006;12:RA67–RA74. [PubMed] [Google Scholar]
- Lacotte S, Brun S, Muller S., and, Dumortier H. CXCR3, inflammation, and autoimmune diseases. Ann N Y Acad Sci. 2009;1173:310–317. doi: 10.1111/j.1749-6632.2009.04813.x. [DOI] [PubMed] [Google Scholar]
- Lee EY, Lee ZH., and, Song YW. CXCL10 and autoimmune diseases. Autoimmun Rev. 2009;8:379–383. doi: 10.1016/j.autrev.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Kühn H., and, O'Donnell VB. Inflammation and immune regulation by 12/15-lipoxygenases. Prog Lipid Res. 2006;45:334–356. doi: 10.1016/j.plipres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Gao Y, Liu XL., and, Li XR. Research progress on siRNA delivery with nonviral carriers. Int J Nanomedicine. 2011;6:1017–1025. doi: 10.2147/IJN.S17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresson D, Togher L, Rodrigo E, Chen Y, Bluestone JA, Herold KC.et al. (2006Anti-CD3 and nasal proinsulin combination therapy enhances remission from recent-onset autoimmune diabetes by inducing Tregs J Clin Invest 1161371–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotondi M, Chiovato L, Romagnani S, Serio M., and, Romagnani P. Role of chemokines in endocrine autoimmune diseases. Endocr Rev. 2007;28:492–520. doi: 10.1210/er.2006-0044. [DOI] [PubMed] [Google Scholar]
- Nicoletti F, Conget I, Di Mauro M, Di Marco R, Mazzarino MC, Bendtzen K.et al. (2002Serum concentrations of the interferon-gamma-inducible chemokine IP-10/CXCL10 are augmented in both newly diagnosed Type I diabetes mellitus patients and subjects at risk of developing the disease Diabetologia 451107–1110. [DOI] [PubMed] [Google Scholar]
- Roep BO, Kleijwegt FS, van Halteren AG, Bonato V, Boggi U, Vendrame F.et al. (2010Islet inflammation and CXCL10 in recent-onset type 1 diabetes Clin Exp Immunol 159338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto J, Yoneyama H, Shimada A, Shigihara T, Yamada S, Oikawa Y.et al. (2004CXC chemokine ligand 10 neutralization suppresses the occurrence of diabetes in nonobese diabetic mice through enhanced beta cell proliferation without affecting insulitis J Immunol 1737017–7024. [DOI] [PubMed] [Google Scholar]
- Dobrian AD, Lieb DC, Cole BK, Taylor-Fishwick DA, Chakrabarti SK., and, Nadler JL. Functional and pathological roles of the 12- and 15-lipoxygenases. Prog Lipid Res. 2011;50:115–131. doi: 10.1016/j.plipres.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuffie M, Maybee NA, Keller SR, Stevens BK, Garmey JC, Morris MA.et al. (2008Nonobese diabetic (NOD) mice congenic for a targeted deletion of 12/15-lipoxygenase are protected from autoimmune diabetes Diabetes 57199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP., and, Leibel RL. An apparent role for Alox15 in the pathogenesis of diabetes in the NOD mouse: parsing the supporting genetic data. Diabetes. 2008;57:1–2. doi: 10.2337/db07-1592. [DOI] [PubMed] [Google Scholar]
- Francisco LM, Sage PT., and, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinic MM, Juedes AE, Bresson D, Homann D, Skak K, Huber C.et al. (2007Minimal impact of a de novo-expressed beta-cell autoantigen on spontaneous diabetes development in NOD mice Diabetes 561059–1068. [DOI] [PubMed] [Google Scholar]
- van Halteren AG, Kardol MJ, Mulder A., and, Roep BO. Homing of human autoreactive T cells into pancreatic tissue of NOD-scid mice. Diabetologia. 2005;48:75–82. doi: 10.1007/s00125-004-1613-2. [DOI] [PubMed] [Google Scholar]
- Szanya V, Ermann J, Taylor C, Holness C., and, Fathman CG. The subpopulation of CD4+CD25+ splenocytes that delays adoptive transfer of diabetes expresses L-selectin and high levels of CCR7. J Immunol. 2002;169:2461–2465. doi: 10.4049/jimmunol.169.5.2461. [DOI] [PubMed] [Google Scholar]
- Sarween N, Chodos A, Raykundalia C, Khan M, Abbas AK., and, Walker LS. CD4+CD25+ cells controlling a pathogenic CD4 response inhibit cytokine differentiation, CXCR-3 expression, and tissue invasion. J Immunol. 2004;173:2942–2951. doi: 10.4049/jimmunol.173.5.2942. [DOI] [PubMed] [Google Scholar]
- Sarkar SA, Lee CE, Victorino F, Nguyen TT, Walters JA, Burrack A.et al. (2012Expression and regulation of chemokines in murine and human type 1 diabetes Diabetes 61436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerning A, Koss K, Datta D, Boneschansker L, Jones CN, Wong IY.et al. (2011Subsets of human CD4(+) regulatory T cells express the peripheral homing receptor CXCR3 Eur J Immunol 412291–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukena SN, Velaga S, Geffers R, Grosse J, Baron U, Buchholz S.et al. (2011Human regulatory T cells in allogeneic stem cell transplantation Blood 118e82–e92. [DOI] [PubMed] [Google Scholar]
- Eksteen B, Miles A, Curbishley SM, Tselepis C, Grant AJ, Walker LS.et al. (2006Epithelial inflammation is associated with CCL28 production and the recruitment of regulatory T cells expressing CCR10 J Immunol 177593–603. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Inoue A, Kohno M, Lei J, Miyazaki T, Yoshie O.et al. (2008Therapeutic effect of CXCR3-expressing regulatory T cells on liver, lung and intestinal damages in a murine acute GVHD model Gene Ther 15171–182. [DOI] [PubMed] [Google Scholar]
- Oikawa Y, Shimada A, Yamada Y, Okubo Y, Katsuki T, Shigihara T.et al. (2010CXC chemokine ligand 10 DNA vaccination plus Complete Freund's Adjuvant reverses hyperglycemia in non-obese diabetic mice Rev Diabet Stud 7209–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigihara T, Shimada A, Oikawa Y, Yoneyama H, Kanazawa Y, Okubo Y.et al. (2005CXCL10 DNA vaccination prevents spontaneous diabetes through enhanced beta cell proliferation in NOD mice J Immunol 1758401–8408. [DOI] [PubMed] [Google Scholar]
- Groom JR., and, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. 2011;89:207–215. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboumrad E, Madec AM., and, Thivolet C. The CXCR4/CXCL12 (SDF-1) signalling pathway protects non-obese diabetic mouse from autoimmune diabetes. Clin Exp Immunol. 2007;148:432–439. doi: 10.1111/j.1365-2249.2007.03370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson MA., and, Wilson SB. Fatal attraction: chemokines and type 1 diabetes. J Clin Invest. 2002;110:1611–1613. doi: 10.1172/JCI17311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XS, Sheller JR, Johnson EN., and, Funk CD. Role of leukotrienes revealed by targeted disruption of the 5-lipoxygenase gene. Nature. 1994;372:179–182. doi: 10.1038/372179a0. [DOI] [PubMed] [Google Scholar]
- Chen W, Bluestone JA., and, Herold KC. Achieving antigen-specific tolerance in diabetes: regulating specifically. Int Rev Immunol. 2005;24:287–305. doi: 10.1080/08830180500379671. [DOI] [PubMed] [Google Scholar]
- Chen M, Yang ZD, Smith KM, Carter JD., and, Nadler JL. Activation of 12-lipoxygenase in proinflammatory cytokine-mediated beta cell toxicity. Diabetologia. 2005;48:486–495. doi: 10.1007/s00125-005-1673-y. [DOI] [PubMed] [Google Scholar]
- Garcia MR, Ledgerwood L, Yang Y, Xu J, Lal G, Burrell B.et al. (2010Monocytic suppressive cells mediate cardiovascular transplantation tolerance in mice J Clin Invest 1202486–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Zhao Z, Ventura E, Gran B, Shindler KS., and, Rostami A. The PD-1/PD-L pathway is up-regulated during IL-12-induced suppression of EAE mediated by IFN-gamma. J Neuroimmunol. 2007;185:75–86. doi: 10.1016/j.jneuroim.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH., and, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci USA. 2008;105:9331–9336. doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke P., and, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci USA. 2003;100:5336–5341. doi: 10.1073/pnas.0931259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav EM, Baquero MT, Opare-Addo LW, Akirav M, Galvan E, Kushner JA.et al. (2011Glucose and inflammation control islet vascular density and beta-cell function in NOD mice: control of islet vasculature and vascular endothelial growth factor by glucose Diabetes 60876–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba S, Nagahara S, Makita N, Tarumi Y, Ishimoto T, Matsuo S.et al. (2012Atelocollagen-mediated systemic delivery prevents immunostimulatory adverse effects of siRNA in mammals Mol Ther 20356–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong FS., and, Wen L. IFN-alpha can both protect against and promote the development of type 1 diabetes. Ann N Y Acad Sci. 2008;1150:187–189. doi: 10.1196/annals.1447.031. [DOI] [PubMed] [Google Scholar]
- Zhang J, Huang Z, Sun R, Tian Z., and, Wei H.2012IFN-gamma induced by IL-12 administration prevents diabetes by inhibiting pathogenic IL-17 production in NOD mice J Autoimmun 3820–28.doi: 10.1016/j.jaut.2011.11.017). [DOI] [PubMed] [Google Scholar]
- Lonez C, Vandenbranden M., and, Ruysschaert JM. Cationic liposomal lipids: from gene carriers to cell signaling. Prog Lipid Res. 2008;47:340–347. doi: 10.1016/j.plipres.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Sioud M., and, Sørensen DR. Cationic liposome-mediated delivery of siRNAs in adult mice. Biochem Biophys Res Commun. 2003;312:1220–1225. doi: 10.1016/j.bbrc.2003.11.057. [DOI] [PubMed] [Google Scholar]
- Lundberg P, Welander PV, Edwards CK., 3rd, , van Rooijen N., and, Cantin E. Tumor necrosis factor (TNF) protects resistant C57BL/6 mice against herpes simplex virus-induced encephalitis independently of signaling via TNF receptor 1 or 2. J Virol. 2007;81:1451–1460. doi: 10.1128/JVI.02243-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg P, Yang HJ, Jung SJ, Behlke MA, Rose SD., and, Cantin EM. Protection against TNFa-dependent liver toxicity by intraperitoneal liposome delivered DsiRNA targeting TNFa in vivo. J Control Release. 2012;160:194–199. doi: 10.1016/j.jconrel.2011.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamchak AA, Manenkova Y, Leconet W, Zheng Y, Chan JR, Stokes CL.et al. (2012Preexisting autoantibodies predict efficacy of oral insulin to cure autoimmune diabetes in combination with anti-CD3 Diabetes 611490–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Selection of CXCL10- and CXCR3-specific siRNA.
Selection of Alox15-specific siRNA.
Serum proinflammatory cytokines.