Abstract
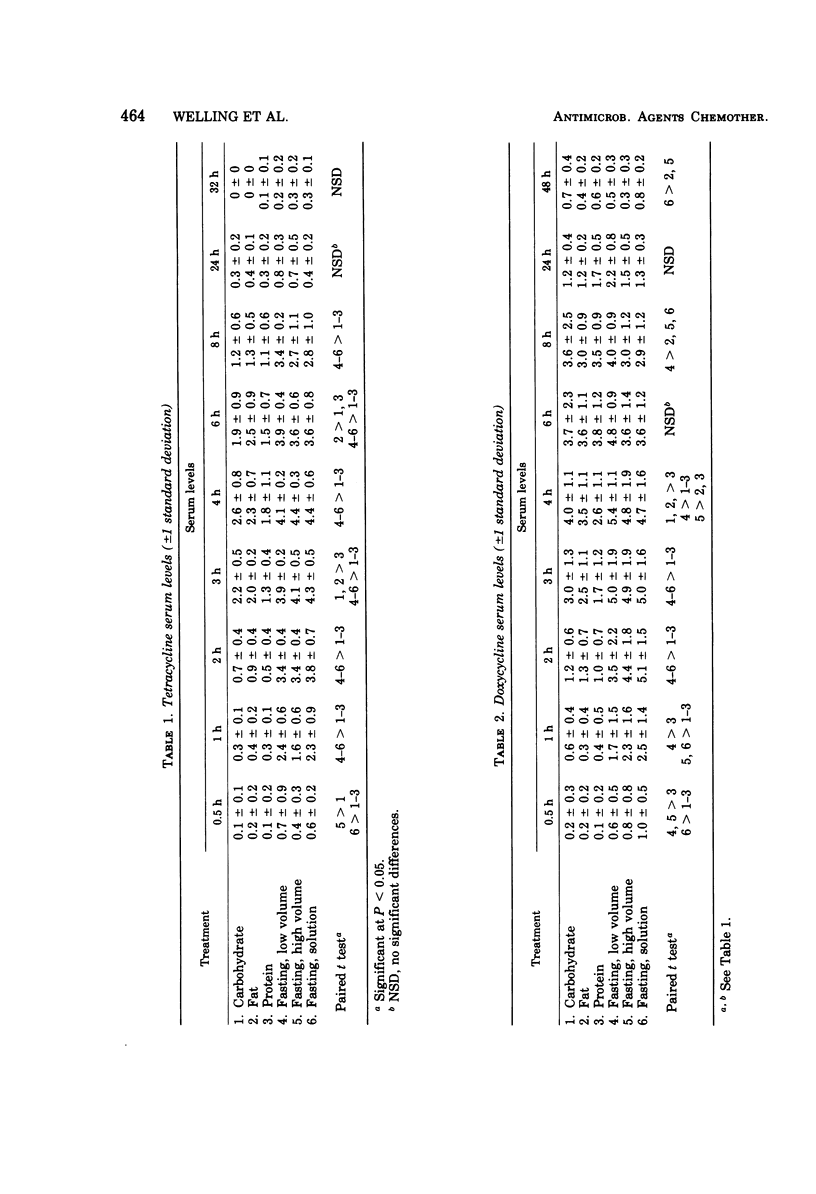
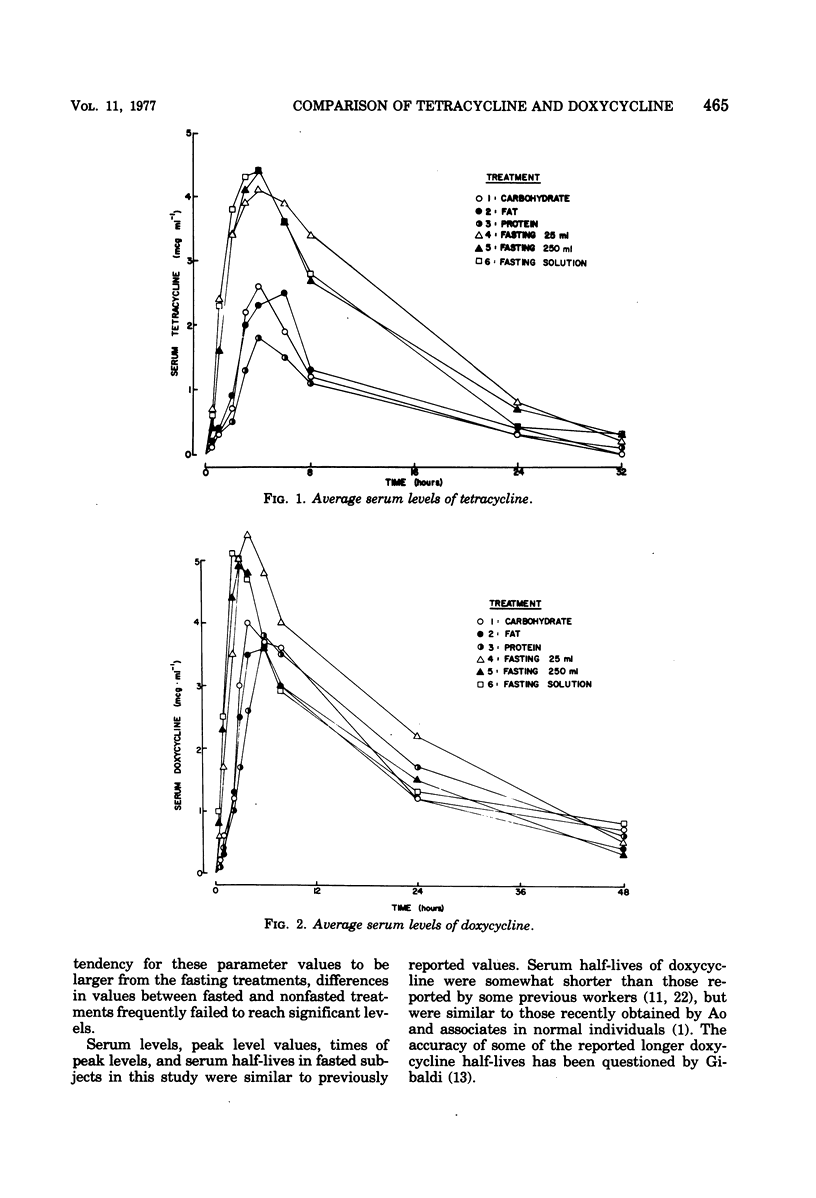
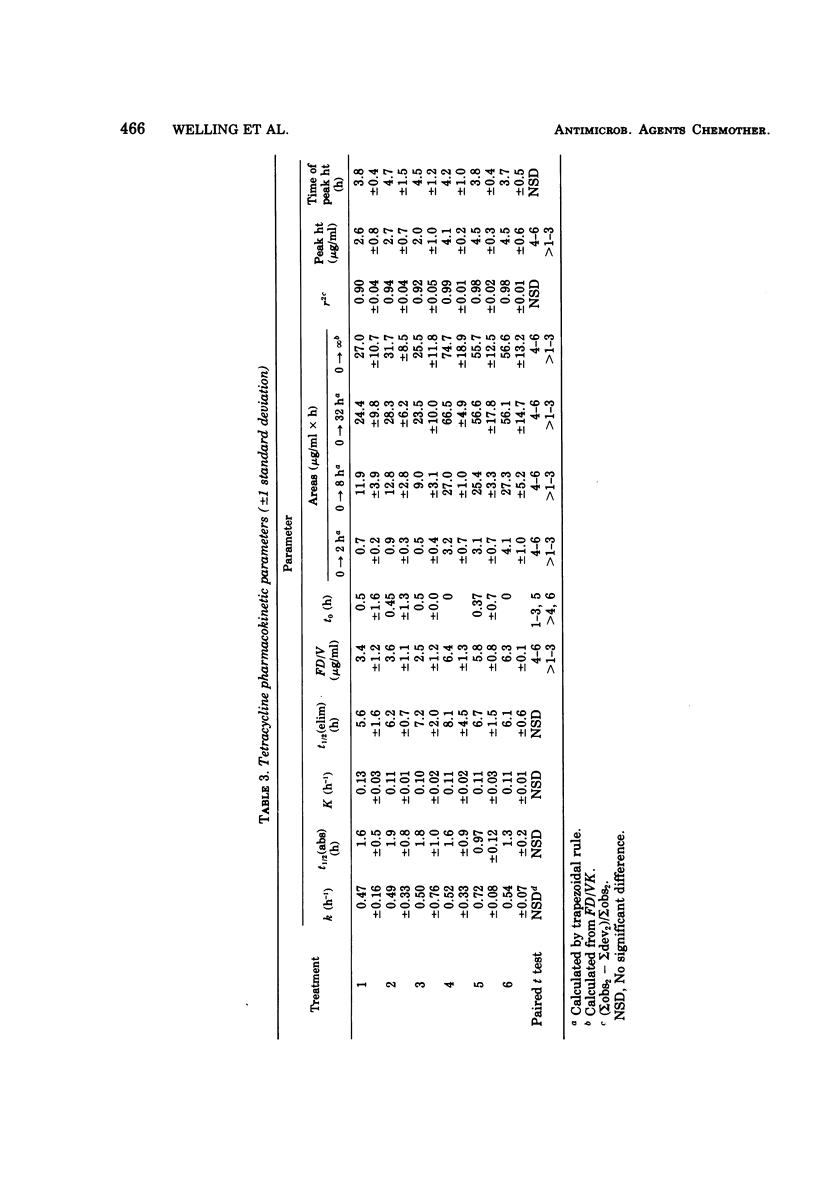
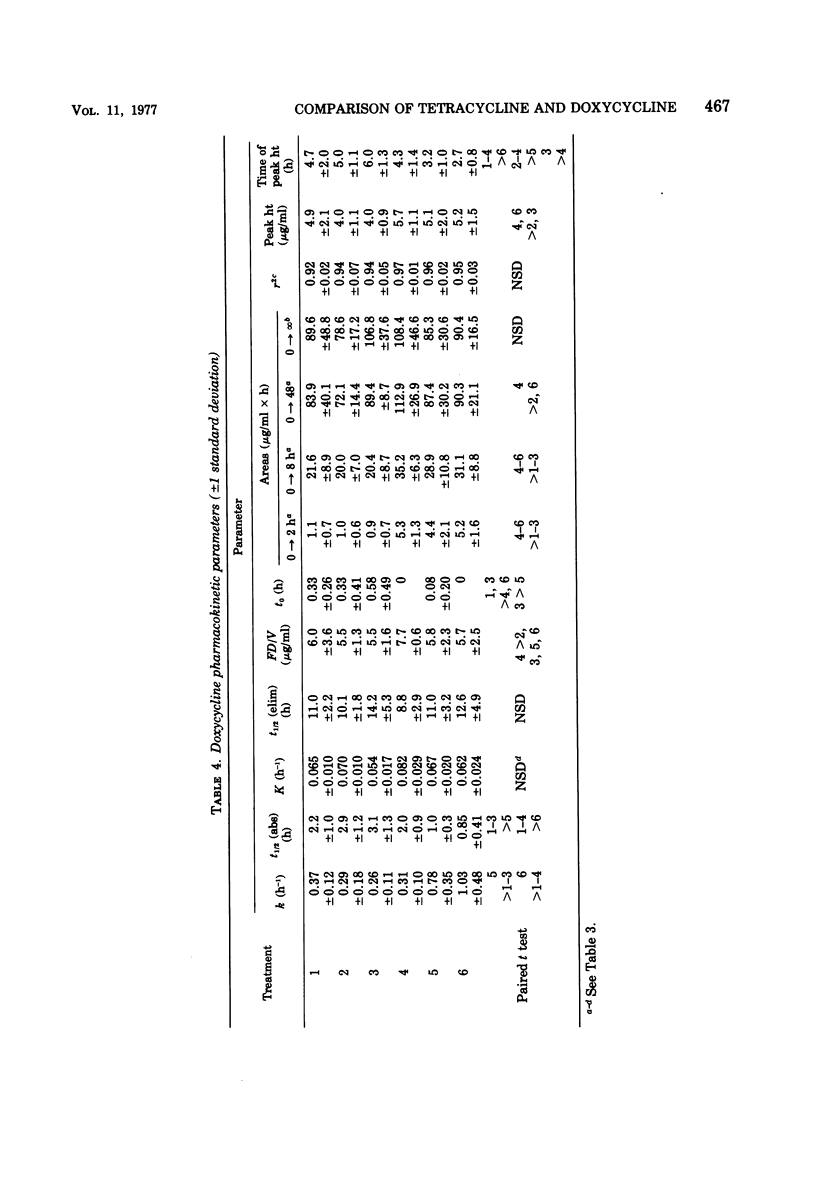
The influence of various test meals and fluid volumes on the relative bioavailability of commercial formulations of doxycycline hyclate and tetracycline hydrochloride was studied in healthy human volunteers. Serum levels of tetracycline were uniformly reduced by approximately 50% by all test meals, whereas serum levels of doxycycline were reduced by 20%. The reduction of tetracycline serum levels will likely be of clinical significance. The bioavailability of each drug was almost identical from an oral solution and from capsules in fasted subjects. The rate of doxycycline absorption was reduced when capsules were administered with a small volume of water, but the overall efficiency of absorption of both drugs was essentially independent of co-administered fluid volume. The use of 8-h serum data provides a reliable estimate of drug bioavailability for tetracycline and, to a lesser extent, for doxycycline.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ao N. K., Taneja O. P., Bhatia V. N., Aggarwal D. S. Serum concentration and urinary excretion of doxycline in normal subjects and patients with renal insufficiency. Chemotherapy. 1974;20(3):129–140. doi: 10.1159/000221802. [DOI] [PubMed] [Google Scholar]
- Barr W. H., Adir J., Garrettson L. Decrease of tetracycline absorption in man by sodium bicarbonate. Clin Pharmacol Ther. 1971 Sep-Oct;12(5):779–784. doi: 10.1002/cpt1971125779. [DOI] [PubMed] [Google Scholar]
- Barr W. H., Gerbracht L. M., Letcher K., Plaut M., Strahl N. Assessment of the biologic availability of tetracycline products in man. Clin Pharmacol Ther. 1972 Jan-Feb;13(1):97–108. doi: 10.1002/cpt197213197. [DOI] [PubMed] [Google Scholar]
- Blanchard P., Rudhardt M., Fabre J. Behaviour of doxycycline in the tissues. Chemotherapy. 1975;21 (Suppl 1):8–18. doi: 10.1159/000221886. [DOI] [PubMed] [Google Scholar]
- Brice G. W., Hammer H. F. Therapeutic nonequivalence of oxytetracycline capsules. JAMA. 1969 May 19;208(7):1189–1190. [PubMed] [Google Scholar]
- DEARBORN E. H., LITCHFIELD J. T., Jr, EISNER H. J., CORBETT J. J., DUNNETT C. W. The effects of various substances on the absorption of tetracycline in rats. Antibiotic Med Clin Ther (New York) 1957 Oct;4(10):627–641. [PubMed] [Google Scholar]
- Davis C. M., Vandersarl J. V., Kraus E. W. Tetracycline inequivalence: the importance of 96-hour testing. Am J Med Sci. 1973 Jan;265(1):69–74. doi: 10.1097/00000441-197301000-00007. [DOI] [PubMed] [Google Scholar]
- Elliott G. R., Armstrong M. F. Sodium bicarbonate and oral tetracycline. Clin Pharmacol Ther. 1972 May-Jun;13(3):459–459. [PubMed] [Google Scholar]
- Eneroth C. M., Lundberg C., Wretlind B. Antibiotic concentrations in maxillary sinus secretions and in the sinus mucosa. Chemotherapy. 1975;21 (Suppl 1):1–7. doi: 10.1159/000221885. [DOI] [PubMed] [Google Scholar]
- FINLAND M. [Antibiotic blood level enhancement]. Antibiotic Med Clin Ther (New York) 1958 Jun;5(6):359–363. [PubMed] [Google Scholar]
- Fabre J., Pitton J. S., Kunz J. P. Distribution and excretion of doxycycline in man. Chemotherapy. 1966;11(2):73–85. doi: 10.1159/000220439. [DOI] [PubMed] [Google Scholar]
- HIRSCH H. A., FINLAND M. Comparative activity of four tetracycline analogues against pathogenic bacteria in vitro. Am J Med Sci. 1960 Mar;239:288–294. doi: 10.1097/00000441-196003000-00004. [DOI] [PubMed] [Google Scholar]
- Kakemi K., Sezaki H., Ogata H., Nadai T. Absorption and excretion of drugs. XXXVI. Effect of Ca2+ on the absorption of tetracycline from the small intestine. (1). Chem Pharm Bull (Tokyo) 1968 Nov;16(11):2200–2205. doi: 10.1248/cpb.16.2200. [DOI] [PubMed] [Google Scholar]
- Neuvonen P. J., Gothoni G., Hackman R., Björksten K. Interference of iron with the absorption of tetracyclines in man. Br Med J. 1970 Nov 28;4(5734):532–534. doi: 10.1136/bmj.4.5734.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuvonen P. J. Interactions with the absorption of tetracyclines. Drugs. 1976;11(1):45–54. doi: 10.2165/00003495-197611010-00004. [DOI] [PubMed] [Google Scholar]
- Neuvonen P. J., Turakka H. Inhibitory effect of various iron salts on the absorption of tetracycline in man. Eur J Clin Pharmacol. 1974 Aug 23;7(5):357–360. doi: 10.1007/BF00558206. [DOI] [PubMed] [Google Scholar]
- Steigbigel N. H., Reed C. W., Finland M. Absorption and excretion of five tetracycline analogues in normal young men. Am J Med Sci. 1968 May;255:296–312. doi: 10.1097/00000441-196805000-00005. [DOI] [PubMed] [Google Scholar]
- Wagner J. G., Northam J. I., Alway C. D., Carpenter O. S. Blood levels of drug at the equilibrium state after multiple dosing. Nature. 1965 Sep 18;207(5003):1301–1302. doi: 10.1038/2071301a0. [DOI] [PubMed] [Google Scholar]
- Welling P. G., Lyons L. L., Craig W. A., Trochta G. A. Influence of diet and fluid on bioavailability of theophylline. Clin Pharmacol Ther. 1975 Apr;17(4):475–480. doi: 10.1002/cpt1975174475. [DOI] [PubMed] [Google Scholar]
- Wilson D. M., Lever M., Brosnan E. A., Stillwell A. A simplified tetracycline assay. Clin Chim Acta. 1972 Jan;36(1):260–261. doi: 10.1016/0009-8981(72)90188-x. [DOI] [PubMed] [Google Scholar]


