Abstract
Objectives
The objective of this study was to compare the accuracy of contrast-enhanced CT (CECT) and contrast-enhanced MRI (CEMRI) in the detection of perineural spread (PNS) of adenoid cystic carcinoma (ACC) in the oral and maxillofacial regions.
Methods
This study consisted of 13 ACCs from 13 patients, all of which were histopathologically diagnosed. Both CECT and CEMRI were performed in all patients before the treatment. The images of each patient were retrospectively evaluated for the detection of PNS. The definitions of PNS included abnormal density/signal intensity, contrast enhancement or widening of the pterygopalatine fossa, palatine foramen, incisive canal, mandibular foramen and mandibular canal, and enlargement or excessive contrast enhancement of a nerve.
Results
11 out of 13 cases were proven to exhibit PNS histopathologically. 8 of the 11 cases for which PNS was histopathologically proven exhibited PNS on MR images. Six of the eight cases for which PNS was exhibited on MR images also exhibited PNS on CT images. The sensitivity, specificity and accuracy for the detection of PNS were 55%, 100% and 62% on CT images and 73%, 100% and 77% on MR images, respectively. Although the accuracy of PNS on MR images was slightly superior to that on CT images, there were no statistically significant differences between the detection of PNS on CT images and on MR images.
Conclusions
CT and MR images are equally useful for the detection of PNS of ACC in the oral and maxillofacial regions.
Keywords: adenoid cystic carcinoma, perineural spread, contrast-enhanced computed tomography, contrast-enhanced magnetic resonance imaging
Introduction
Adenoid cystic carcinoma (ACC) is a rare malignant tumour of the major and minor salivary glands, accounting for 1% to 2% of all head and neck malignancies and approximately 10% of all salivary gland neoplasms.1 ACC is characterized by a high rate of late local failure and distant metastasis.2 60% to 70% of ACCs arise in the minor salivary glands, which may be localized in the palate, paranasal sinuses and nose, although they may also occur in the parotid or submandibular glands.3
In addition, ACC is the most common primary tumour to spread via the perineural mechanism.4 Perineural spread (PNS) of head and neck tumours most commonly occurs along the branches of the trigeminal and facial nerves.5-7 The incidence of PNS in patients with head and neck cancer dramatically alters the treatment and prognosis.8 However, some patients with radiologically or pathologically proven PNS have normal nerve function upon clinical examination.9 Many treatment failures are related to unrecognized PNS,10 so it is important to diagnose the presence of PNS precisely before treatment. Both CT and MRI can help detect PNS, but MRI is the modality of choice because of its superior soft-tissue contrast and the decreased amount of artefact disruption from dental hardware in the oral and maxillofacial regions.9 However, there have been few reports that investigate the CT and MRI features of PNS of ACC in the oral and maxillofacial regions. In particular, there have been few reports on both contrast-enhanced CT (CECT) and contrast-enhanced MRI (CEMRI) being performed on all patients with ACC in the oral and maxillofacial regions.
The aim of this study was to compare the accuracy of the CECT and CEMRI in the detection of PNS of ACC in the oral and maxillofacial regions using histopathology as a gold standard.
Materials and methods
We designed and implemented a retrospective case series study. Our institutional review board at Osaka University Dental Hospital, Japan, approved this retrospective study and written informed consent for enrolment was obtained from the patients.
The study sample was derived from the population of patients who presented to Osaka University Dental Hospital, and were histopathologically diagnosed with ACC between June 2000 and December 2007. Patients eligible for study inclusion had primary ACC in the oral and maxillofacial regions, underwent both CECT and CEMRI before the treatment and consented to study enrolment. Patients were excluded from the study if they had recurrent lesions, failed to undergo CECT or CEMRI before the treatment or refused study enrolment.
The predictor variable was the presence of PNS by the histopathological diagnosis. The outcome variable was the presence of PNS on CT or MR images. The other variables were age, sex and primary tumour location.
CT images were obtained with CT scanners (Light Speed QX/i, General Electric, Milwaukee, WI) at 120 kVp and 130–200 mA. On CT, the tumours were studied using the axial plane with the X-ray beam parallel to the occlusal plane. The field of view (FOV) was 25 × 25 cm, the matrix size was 512 × 512 and the slice thickness was 2.5–5 mm with no inter-slice spacing. A 100 ml dose of the contrast medium (iopamidol, Iopamiron 300, Bayer Yakuhin Ltd., Osaka, Japan; iohexol, Omnipaque 300, Daiichi Sankyo Co., Ltd., Tokyo, Japan; or iomeprol, Iomeron 300, Eisai Co., Ltd., Tokyo, Japan) was intravenously administered to all patients. Although the precise timing varied minimally from patient to patient, our typical scanning sequence consisted of an initial intravenous (IV) bolus injection of 70 ml at 0.6 ml s–1, followed by dynamic injection of 30 ml at 0.3 ml s–1 and simultaneous scanning.
MR images were obtained with a 1.5 T superconducting magnet scanner (Signa LX, General Electric) equipped with a head coil. On MRI, the tumours were studied using only the axial plane parallel to the occlusal plane. The scan protocol included T1 weighted (time of repetition (TR) 400–500 ms/time of echo (TE) 9–10 ms/number of excitations 1) spin-echo sequences with or without the chemical shift selective fat suppression (FS) technique and T2 weighted (TR 3500 ms/TE 98 ms/number of excitations 1) fast spin-echo sequences with the chemical shift selective FS technique. In all patients, the contrast medium (gadopentetate dimeglumine, Magnevist, Bayer Yakuhin Ltd.; gadodiamide, Omniscan, Daiichi Sankyo Co., Ltd., or gadoteridol, Prohance, Eisai Co., Ltd.) was intravenously administered and the T1 weighted spin-echo sequences using the FS technique were repeated before and after the contrast medium was administered. The typical scanning sequence consisted of IV bolus injection at approximately 0.2 ml kg–1 body weight, followed by a 1 min delay and scanning. The FOV was 25 × 25 cm, the matrix size was 512 × 256 and the slice thickness was 5 mm with 1 mm interslice spacing.
Both CT and MR images of each patient were retrospectively evaluated on the detection of PNS. On the basis of Ginsberg's criteria of PNS,11 the definitions of PNS included abnormal density/signal intensity, contrast enhancement or widening of the pterygopalatine fossa, palatine foramen, incisive canal, mandibular foramen and mandibular canal, and enlargement or excessive contrast enhancement of a nerve. These findings were independently evaluated on the CT and MR images by two radiologists who did not know the results of the histopathological examination and then consensus readings for interpretation discrepancies was performed.
For the statistical analysis, Fisher's exact probability test and Student's t-test were used to evaluate the presence of possible selection biases. Fisher's exact probability test was used to evaluate correlations between the presence of PNS by the histopathological diagnosis and the detectability of PNS on CT or MRI while the detectability of PNS on CT and MRI was compared with the McNemar test. p < 0.05 was considered to indicate a significant difference.
Results
21 patients corresponded to the inclusion criteria. Eight patients were excluded for the following reasons: three failed to undergo CT examination; two failed to undergo MRI examination; two had recurrent lesions; and one patient failed to inject the contrast medium in the MRI examination. All available data in the form of pathological specimens, image characteristics and clinical courses were reviewed and revealed that 13 patients had 13 ACC lesions (Table 1). The subjects included 5 males and 8 females, ranging from 27 years to 79 years of age (mean age 57.8 years). The site of origin of these tumours included five palates, three sublingual glands, two lips, one cheek, one retromolar and one angle of the mandible. Table 2 shows the summary of study variables. There were no possible selection biases (p > 0.05).
Table 1. Clinical summary and imaging findings of patients with perineural spread of adenoid cystic carcinoma in the oral and maxillofacial regions.
| Case |
Age (years)/sex |
Primary tumour location |
Left/Right |
Clinical symptoms |
PNS |
||
| CT | MRI | Histopathological diagnosis | |||||
| 1 | 29/F | Angle of mandible | L | Trismus | (+) | (+) | (+) |
| 2 | 27/F | Cheek | L | Paresthesia/trigeminal neuralgia | (+) | (+) | (+) |
| 3 | 67/M | Palate | R | Swelling | (+) | (+) | (+) |
| 4 | 62/M | Palate | R | Paresthesia/trigeminal neuralgia | (+) | (+) | (+) |
| 5 | 64/M | Palate | R | Swelling | (+) | (+) | (+) |
| 6 | 78/M | Palate | L | Contact pain | (+) | (+) | (+) |
| 7 | 37/F | Palate | L | Contact pain | (−) | (+) | (+) |
| 8 | 72/F | Retromolar | L | Paresthesia of tongue | (−) | (+) | (+) |
| 9 | 68/F | Sublingual gland | L | Paresthesia of tongue | (−) | (−) | (+) |
| 10 | 79/F | Sublingual gland | L | No symptom | (−) | (−) | (+) |
| 11 | 64/M | Lower lip | R | No symptom | (−) | (−) | (+) |
| 12 | 51/F | Sublingual gland | L | Swelling | (−) | (−) | (−) |
| 13 | 53/F | Upper lip | R | Contact pain | (−) | (−) | (−) |
(+), positive findings of PNS; (−), negative findings of PNS; F, female; L, left; M, male; PNS, perineural spread; R, right.
Table 2. Summary of study variables.
| PNS by the histopathological diagnosis |
|||
| Variable name (and type) | (+) | (−) | p-value |
| Sample size (n) | 11 (85%) | 2 (15%) | Not applicable |
| Sex: male (binary) | 5 (45%) | 0 (0%) | 0.22a |
| Primary tumour location (categorical) | 0.55a | ||
| Palate | 5 (45%) | 0 (0%) | |
| Sublingual gland | 2 (18%) | 1 (50%) | |
| Lip | 1 (9%) | 1 (50%) | |
| Cheek | 1 (9%) | 0 (0%) | |
| Retromolar | 1 (9%) | 0 (0%) | |
| Angle of mandible | 1 (9%) | 0 (0%) | |
| Age (continuous) | 58.8±18.8 | 52.0±1.41 | 0.63b |
(+), positive findings of PNS; (−), negative findings of PNS; PNS, perineural spread.
a Fisher’s exact probability test.
b Student's t-test.
11 of 13 cases were proven to exhibit PNS histopathologically. 8 of the 11 cases where the PNS was histopathologically proven exhibited PNS on MRI. Six of the eight cases where PNS was exhibited on MRI also exhibited PNS on CT images (Figures 1 and 2). In case 7, PNS within the pterygopalatine fossa was not detected on CT images, although it was detected on T1 weighted CEMRI with the FS technique (Figure 3). In case 8, PNS within the mandibular foramen and the mandibular canal was not detected on CT images, although it was detected on T1 weighted CEMRI, T2 weighted MR images with the FS technique and T1 weighted CEMRI with the FS technique (Figure 4).
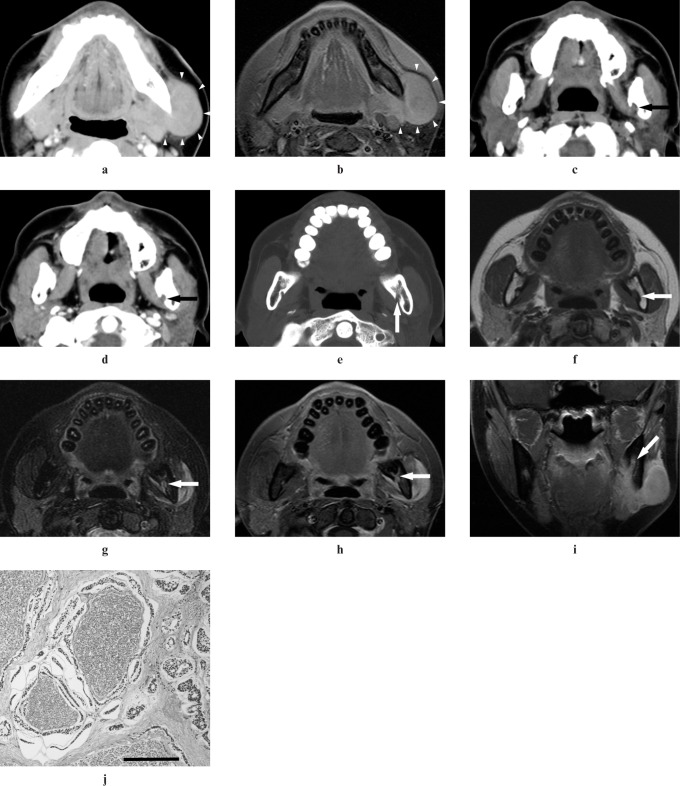
Figure 1.
Adenoid cystic carcinoma (ACC) of the left angle of mandible in a 29-year-old female (Case 1). (a,b) A well-defined-margin tumour in the left angle of mandible can be detected on CT and MR images (arrowheads). (c) A plain CT image shows obliteration of fat compared with the right side in the left mandibular foramen (arrow). (d) A contrast-enhanced CT image shows inhomogeneous enhancement in the left mandibular foramen (arrow). (e) A reformatted CT image with bone algorithm shows widening of the left mandibular foramen (arrow). (f) A T1 weighted MR image shows intermediate signal intensity the same as that of adjacent muscle in the left mandibular foramen (arrow). (g) A T2 weighted MR image with the fat suppression (FS) technique shows higher signal intensity than that of adjacent muscle in the left mandibular foramen (arrow). (h,i) Contrast-enhanced T1 weighted MR images with the FS technique show contrast enhancement of the left mandibular foramen (arrows). (j) A histopathologic section shows perineural spread of tumour cells along the inferior alveolar nerve and infiltration of cribriform-type ACC cells for the surrounding tissues (bar 200 μm)
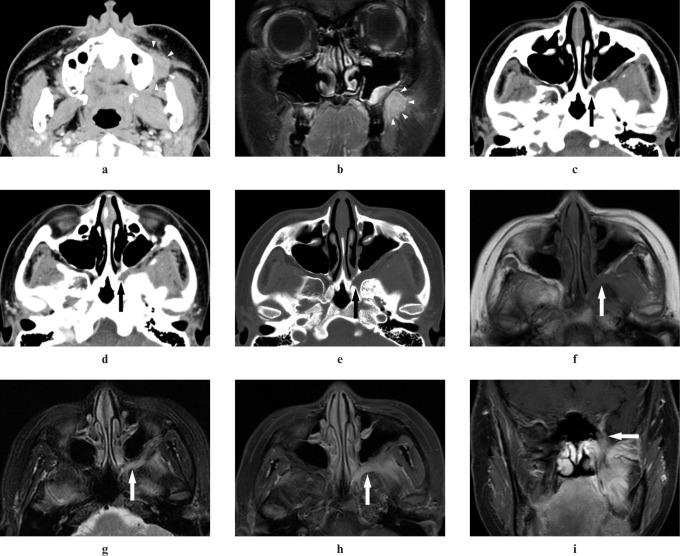
Figure 2.
Adenoid cystic carcinoma of the left cheek in a 27-year-old female (Case 2). (a,b) An ill-defined-margin tumour in the left cheek can be detected on CT and MR images (arrowheads). (c) A plain CT image shows obliteration of fat in the left pterygopalatine fossa (arrow). (d) A contrast-enhanced CT image shows inhomogeneous enhancement in the left pterygopalatine fossa (arrow). (e) A reformatted CT image with bone algorithm shows widening of the left pterygopalatine fossa (arrow). (f) A T1 weighted MR image shows intermediate signal intensity the same as that of adjacent muscle in the left pterygopalatine fossa (arrow). (g) A T2 weighted MR image with the fat suppression (FS) technique shows higher signal intensity than that of adjacent muscle in the left pterygopalatine fossa (arrow). (h,i) A contrast-enhanced T1 weighted MR image with the FS technique shows inhomogeneous enhancement of the left pterygopalatine fossa (arrows)
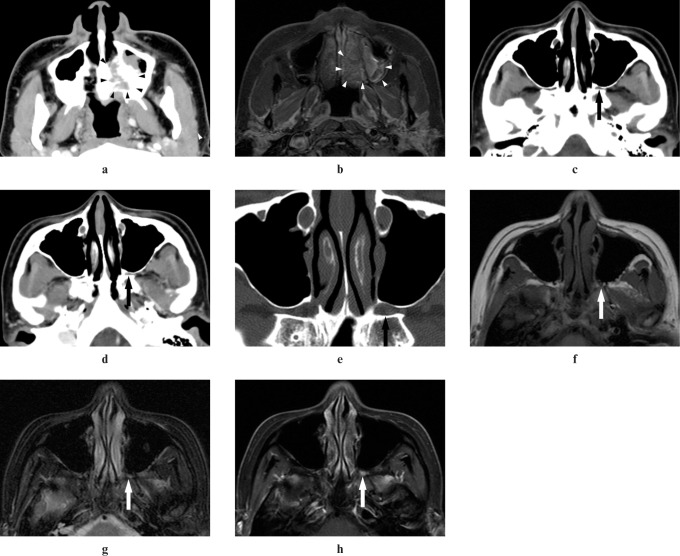
Figure 3.
Adenoid cystic carcinoma of the left palate in a 37-year-old female (Case 7). (a,b) An ill-defined margin tumour in the left palate can be detected on CT and MR images (arrowheads). (c) A plain CT image does not show elevation of CT value in the left pterygopalatine fossa (arrow). (d) A contrast-enhanced CT image does not show contrast enhancement in the left pterygopalatine fossa (arrow). (e) A plain CT image with bone window does not show widening of the left pterygopalatine fossa (arrow). (f,g) T1 weighted and T2 weighted MR images with the fat suppression (FS) technique do not show abnormal signal intensity in the left pterygopalatine fossa (arrows). (h) However, a contrast-emhanced T1 weighted MR image with the FS technique shows abnormal enhancement of the left pterygopalatine fossa (arrow)
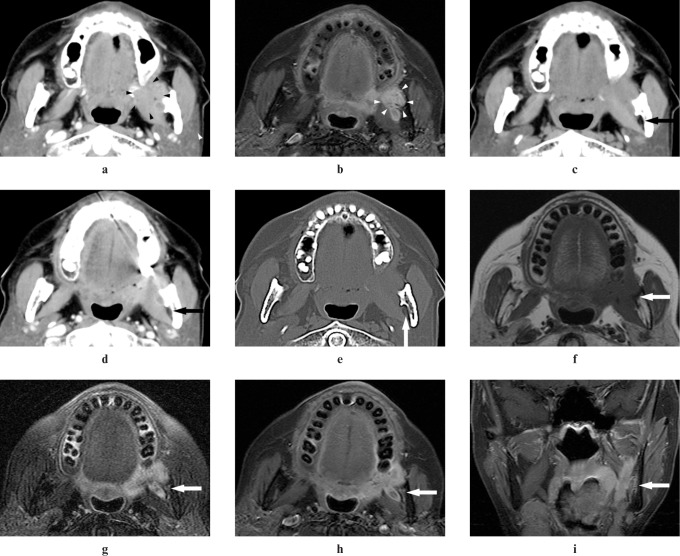
Figure 4.
Adenoid cystic carcinoma of the left retromolar in a 72-year-old female (Case 8). (a,b) An ill-defined margin tumour in the left retromolar can be detected on CT and MR images (arrowheads). (c) A plain CT image does not show elevation of CT value in the left mandibular foramen (arrow). (d) A contrast-enhanced CT image does not show inhomogeneous enhancement in the left mandibular foramen (arrow). (e) A reformatted CT image with bone algorithm does not show widening of the left mandibular foramen (arrow). (f) A T1 weighted MR image shows intermediate signal intensity the same as that of adjacent muscle in the left mandibular foramen and the left mandibular canal (arrow). (g) A T2 weighted MR image with the fat suppression (FS) technique shows higher signal intensity than that of adjacent muscle in the left mandibular foramen and the left mandibular canal and widening of the left mandibular foramen (arrow). (h,i) Contrast-enhanced T1 weighted MR images with the FS technique show contrast enhancement of the left mandibular foramen and the left mandibular canal and widening of the left mandibular foramen (arrows)
The sensitivity, specificity and accuracy of CT images for the detection of PNS were 55%, 100% and 62%, respectively (Table 3). The sensitivity, specificity and accuracy of MR images for the detection of PNS were 73%, 100% and 77%, respectively (Table 4). There were no statistically significant correlations between the presence of PNS by the histopathological diagnosis and the detectability of PNS on CT or MR images (p = 0.26 and 0.12, respectively). Although the accuracy of PNS on MR images was slightly superior to that on CT images, there were no statistically significant differences between the detectability of PNS on CT images and that on MR images (p = 0.48).
Table 3. Comparison of the perineural spread (PNS) in the histopathological diagnosis with PNS on CT.
| PNS on CT |
||||
| (+) | (−) | Total | ||
| PNS in the histopathological diagnosis | (+) | 6 | 5 | 11 |
| (−) | 0 | 2 | 2 | |
| Total | 6 | 7 | 13 | |
(+), positive findings of PNS; (−), negative findings of PNS; PNS, perineural spread; Sensitivity 55%, specificity 100%, accuracy 62%.
p = 0.26 (Fisher's exact probability test).
Table 4. Comparison of the perineural spread (PNS) in the histopathological diagnosis with PNS on MRI.
| PNS on MRI |
||||
| (+) | (−) | Total | ||
| PNS in the histopathological diagnosis | (+) | 8 | 3 | 11 |
| (−) | 0 | 2 | 2 | |
| Total | 8 | 5 | 13 | |
(+), positive findings of PNS; (−), negative findings of PNS; PNS, perineural spread; Sensitivity: 73%, specificity: 100%, accuracy: 77%.
p = 0.12 (Fisher's exact probability test).
All five palate cases were proven to exhibit PNS histopathologically and also exhibited PNS on MR images. Only 4 of 11 cases where PNS was histopathologically proven showed paraesthesia clinically. Only three of eight true positive cases on MR images showed paraesthesia clinically.
Discussion
The aim of this study was to compare the accuracy of the CECT and CEMRI in the detection of PNS of ACC in the oral and maxillofacial regions using histopathology as a gold standard.
The results of this study confirm that CT and MR images are equally useful for the detection of PNS of ACC in the oral and maxillofacial regions. Although the accuracy of PNS on MR images was slightly superior to that on CT images, there were no statistically significant differences between the detectability of PNS on CT images and that on MR images.
The reported incidence of PNS in patients with ACC is widely variable and ranges from 20% to 80%.12 Almost any nerve can serve as a pathway for the spread of malignant disease around the extracranial head and neck. The pathway of PNS is predictable with knowledge of the pertinent anatomy.9 The maxillary nerve can carry a tumour from the palate, maxilla, midface, nose and nasopharynx.7,13 Tumours of the lip, chin and oral floor can follow the inferior alveolar nerve and tumours of the tongue can extend along the lingual nerve.14
There is some controversy on the mechanism of PNS.3 Some researchers believe that the tumour spreads along the lymphatic system associated with the nerve.13 Others think that the nerve simply represents the line of least resistance.15 Although the pathogenesis of PNS is poorly understood, several recent reports have shown that neural cell adhesion molecules may have a role in the pathogenesis of PNS of malignant tumours, including ACC and squamous cell carcinoma.4
As tumour cells accumulate along the nerve, they may cause foraminal enlargement and, eventually, foraminal destruction.6,13,14 This is well observed on cross-sectional CT images.13 However, foraminal enlargement is a delayed finding because the normal nerve is small in relation to the foramen, allowing considerable tumour growth to occur without foraminal change.8,16 Obliteration of fat planes at the foraminal opening is a common indicator of PNS.6,13,14 Fat is normally seen in the pterygopalatine fossa and mandibular canal, so loss of this fat should lead the radiologist to suspect PNS.13,14
MR images were found to have a higher sensitivity and specificity than CT in detecting PNS with ACC of the parotid gland, paranasal sinuses and nasopharynx.4 The results of this study also suggested that MR was slightly superior to CT in the detection of PNS of ACC in the oral and maxillofacial regions. In two of our cases (cases 7 and 8), PNS was not detected on CT images although it was detected on MR images. In particular, in case 7, PNS within the pterygopalatine fossa was only detected on T1 weighted CEMRI with the FS technique. In previous reports, T1 weighted CEMRI showed enhancement of the tumour infiltrating the nerve, which could not be imaged with CT.8,17 Moreover, enhancement of the tumour infiltrating the nerve was best observed on T1 weighted MR images with the FS technique because the enhancing nerve might be obscured by adjacent high-signal-intensity fat or a chemical shift artefact on routine T1 weighted MR images without the FS technique.7,8 Enhancement of the tumour infiltrating the nerve may depend on extensive destruction of the barrier functions of the intraneural microvascular circulation and the perineurium.18 In this study, gadolinium T1 weighted CEMRI with the FS technique were recommended for the detection of PNS of ACC in the oral and maxillofacial regions.
Mukherji et al19 evaluated the accuracy of CT in determining PNS in oral cavity and tongue base malignant tumours and reported the sensitivity and specificity as 88% and 83%, respectively. In contrast, there was low sensitivity (55%) in the present study. Hanna et al4 reported that the sensitivity and specificity of MRI in detecting PNS of ACC in the skull base were 100% and 85%, respectively. Nemzek et al18 concluded that the sensitivity of MR images for the detection of PNS of head and neck tumours was 95%; however, the sensitivity for mapping the entire extent of PNS fell to 63%. Eisen et al10 evaluated the accuracy of MRI in determining PNS in skull base tumours and reported 50% specificity and 59% overall accuracy. In this study, the sensitivity of MR images for the detection of PNS was 73%. The results of these studies suggested that micro-PNS could not be identified on CT or MR images, even if PNS was histopathologically proven.
All five palate cases demonstrated PNS on MR images. Carcinomas of hard- or soft-palate origin are known to spread perineurally along the palatine branches of the maxillary nerve.20 An abnormal attenuation within the pterygopalatine fossa on soft-tissue CT windows or abnormal enhancement on T1 weighted CEMRI with the FS technique is strong evidence of PNS.7,8,11,13,21 It was considered that upper invasion of a palatal malignant tumour was easily demonstrated because of contiguity to the palatal bone and its thin thickness, so PNS occurred more often than in other head and neck regions.
Catalano et al6 reported that 30% to 45% of cases with PNS were initially asymptomatic. When paraesthesia was recognized, clinical evidence of PNS was often non-specific and might include hypoesthesia, burning or stinging pain, trigeminal neuralgia, facial palsy or intracranial symptoms.5,6,8 In this study, only 4 of 11 cases (36%) for which PNS was histopathologically proven showed paraesthesia clinically.
In case 9, PNS was not detected on CT and MR images in spite of the clinical demonstration of paraesthesia. This result suggested that PNS cannot be completely identified on CT or MR images, even if paresthesia is clinically demonstrated.
Our study had several potential limitations. First, this study consisted of only 13 cases. The power analysis (p < 0.05 and power of 0.8) suggested that 39 cases on CT examinations and 20 cases on MRI examinations would be needed for significant correlations between the presence of PNS by the histopathological diagnosis and the detection of PNS on CT or MR images. Further studies are needed to give clear recommendations. Second, several different contrast mediums were intravenously administered to each patient in CT or MRI examinations. The compositions of these contrast mediums were different so the effects of contrast enhancement might vary slightly among them. However, the apparent differences among the contrast mediums were not shown in this study.
In conclusion, CT and MR images were found to be equally useful for the detection of PNS of ACC in the oral and maxillofacial regions. Although the accuracy of PNS on MR images was slightly superior to that on CT images, there were no statistically significant differences between the detection of PNS on CT images and on MR images. Further studies are needed to give clear recommendations.
Acknowledgments
This study was presented at the 17th International Congress of Dentomaxillofacial Radiology, 28 June to 2 July 2009, Amsterdam, the Netherlands.
References
- 1.Kim KH, Sung MW, Chung PS, Rhee CS, Park CI, Kim WH. Adenoid cystic carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg 1994;120:721–726 [DOI] [PubMed] [Google Scholar]
- 2.Iseli TA, Karnell LH, Graham SM, Funk GF, Buatti JM, Gupta AK, et al. Role of radiotherapy in adenoid cystic carcinoma of the head and neck. J Laryngol Otol 2009;123:1137–1144 [DOI] [PubMed] [Google Scholar]
- 3.Spiro RH, Huvos AG, Strong EW. Adenoid cystic carcinoma of salivary origin. A clinicopathologic study of 242 cases. Am J Surg 1974;128:512–520 [DOI] [PubMed] [Google Scholar]
- 4.Hanna E, Vural E, Prokopakis E, Carrau R, Snyderman C, Weissman J. The sensitivity and specificity of high-resolution imaging in evaluating perineural spread of adenoid cystic carcinoma to the skull base. Arch Otolaryngol Head Neck Surg 2007;133:541–545 [DOI] [PubMed] [Google Scholar]
- 5.Arcas A, Bescos S, Raspall G, Capellades J. Perineural spread of epidermoid carcinoma in the infraorbital nerve: case report. J Oral Maxillofac Surg 1996;54:520–522 [DOI] [PubMed] [Google Scholar]
- 6.Catalano PJ, Sen C, Biller HF. Cranial neuropathy secondary to perineural spread of cutaneous malignancies. Am J Otol 1995;16:772–777 [PubMed] [Google Scholar]
- 7.Parker GD, Harnsberger HR. Clinico-radiologic issues in perineural tumour spread of malignant diseases of the extracranial head and neck. Radiographics 1991;11:383–399 [DOI] [PubMed] [Google Scholar]
- 8.Laine FJ, Braun IF, Jensen ME, Nadel L, Som PM. Perineural tumour extension through the foramen ovale: evaluation with MR imaging. Radiology 1990;174:65–71 [DOI] [PubMed] [Google Scholar]
- 9.Caldemeyer KS, Mathews VP, Righi PD, Smith RR. Imaging features and clinical significance of perineural spread or extension of head and neck tumours. Radiographics 1998;18:97–110 [DOI] [PubMed] [Google Scholar]
- 10.Eisen MD, Yousem DM, Montone KT, Kotapka MJ, Bigelow DC, Bilker WB, et al. Use of preoperative MR to predict dural, perineural, and venous invasion of skull base tumours. AJNR Am J Neuroradiol 1996;17:1937–1945 [PMC free article] [PubMed] [Google Scholar]
- 11.Ginsberg LE, Demonte F. Imaging of perineural tumour spread from palatal carcinoma. AJNR Am J Neuroradiol 1998;19:1417–1422 [PMC free article] [PubMed] [Google Scholar]
- 12.Hutcheson JA, Vural E, Korourian S, Hanna E. Neural cell adhesion molecule expression in adenoid cystic carcinoma of the head and neck. Laryngoscope 2000;110:946–948 [DOI] [PubMed] [Google Scholar]
- 13.Curtin HD, Williams R, Johnson J. CT of perineural tumour extension: pterygopalatine fossa. AJNR Am J Neuroradiol 1985;144:163–169, free full text: http://www.ajronline.org/content/144/1/163.long [DOI] [PubMed] [Google Scholar]
- 14.Matzko J, Becker DG, Phillips CD. Obliteration of fat planes by perineural spread of squamous cell carcinoma along the inferior alveolar nerve. AJNR Am J Neuroradiol 1994;15:1843–1845 [PMC free article] [PubMed] [Google Scholar]
- 15.Larson DL, Rodin AE, Roberts DK, O'Steen WK, Rapperport AS, Lewis SR. Perineural lymphatics: myth or fact. Am J Surg 1966;112:488–492 [DOI] [PubMed] [Google Scholar]
- 16.Woodruff WW, Yeates AE, Mclendon RE. Perineural tumour extension to the cavernous sinus from superficial facial carcinoma: CT manifestations. Radiology 1986;161:395–399 [DOI] [PubMed] [Google Scholar]
- 17.Hudgins P. Contrast enhancement in head and neck imaging. Neuroimaging Clin N Am 1994;4:101–115 [PubMed] [Google Scholar]
- 18.Nemzek WR, Hecht S, Gandour-Edwards R, Donald P, McKennan K. Perineural spread of head and neck tumours: how accurate is MR imaging? AJNR Am J Neuroradiol 1998;19:701–706 [PMC free article] [PubMed] [Google Scholar]
- 19.Mukherji SK, Weeks SM, Castillo M, Yankaskas BC, Krishnan LA, Schiro S. Squamous cell carcinomas that arise in the oral cavity and tongue base: can CT help predict perineural or vascular invasion? Radiology 1996;198:157–162 [DOI] [PubMed] [Google Scholar]
- 20.Laccourreye O, Bely N, Halimi P, Guimaraes R, Brasnu D. Cavernous sinus involvement from recurrent adenoid cystic carcinoma. Ann Otol Rhinol Laryngol 1994;103:822–825 [DOI] [PubMed] [Google Scholar]
- 21.Barakos JA, Dillon WP, Chew WM. Orbit, skull base and pharynx: contrast-enhanced fat suppression MR imaging. Radiology 1991;179:191–198 [DOI] [PubMed] [Google Scholar]