Antiretroviral therapy medication errors are common among hospitalized patients with human immunodeficiency virus (HIV) infection (29% of admissions on hospital day 1) but are quickly corrected (7% of admissions on hospital day 2). Compared with those admitted to the HIV/AIDS service, patients admitted to surgical services were at increased risk of errors.
Abstract
Background. Antiretroviral therapy (ART) medication errors can lead to drug resistance, treatment failure, and death. Prior research suggests that ART medication errors are on the rise in US hospitals. This analysis provides a current estimate of inpatient antiretroviral prescribing errors.
Methods. Retrospective review of medication orders during the first 48 hours of hospitalization for patients with human immunodeficiency virus (HIV) infection admitted to the Johns Hopkins Hospital between 1 January and 31 December 2009. Errors were classified as (1) incomplete regimen, (2) incorrect dosage, (3) incorrect schedule, and (4) nonrecommended drug-drug combinations. Multivariable regression was used to identify factors associated with errors.
Results. A total of 702 admissions occurred in 2009. Of these, 380 had ART medications prescribed on the first day and 308 on the second day of hospitalization. A total of 145 ART medication errors in 110 admissions were identified on the first day (29%), and 22 errors were identified in 21 admissions on the second day (7%). The most common errors were incomplete regimen and incorrect dosage or schedule. Protease inhibitors accounted for the majority of dosing and scheduling errors (71%–73%). Compared with patients admitted to the HIV/AIDS service, those admitted to surgical services were at increased risk of errors (adjusted odds ratio, 3.10; 95% confidence interval, 1.18–8.18).
Conclusions. ART medication errors are common among hospitalized HIV-infected patients on the first day of admission, but most are corrected within 48 hours. Interventions are needed to safeguard patients and prevent serious complications of ART medication errors especially during the first 24 hours of hospitalization.
Inpatient medication errors and adverse drug events are common, costly, and may lead to significant patient injury and death [1–9]. Persons living with human immunodeficiency virus (HIV) (PLWH) are at increased risk of medication errors given their complex medication regimens, multiple comorbid conditions, and interactions with inpatient providers who lack experience with antiretroviral therapy (ART) [10–12]. ART medication errors may have serious long-term consequences, including the development of drug resistance, treatment failure, or death [13].
Prior research suggests that ART medication errors are on the rise. In 1998, ART medication errors were detected in 12% of admissions, but by 2004–2007, the error rate had increased to 21%–26% of admissions [12, 14–16]. Since these studies were conducted, significant advances in ART and health information technology have occurred. Efavirenz, ritonavir-boosted atazanavir, ritonavir-boosted darunavir, and raltegravir are highly effective at decreasing HIV viral replication and have favorable side effect profiles and simple dosing schedules; thus, in combination with tenofovir and emtricitabine, these drugs are preferentially recommended for treatment naive patients by the Department of Health and Human Services Panel on Antiretroviral Guidelines for Adults and Adolescents [17]. Moreover, an increasing number of hospitals are using computerized provider order entry (CPOE) and clinical support tools, which have been touted as mechanisms for decreasing medication errors and improving patient safety [2, 18, 19]. However, recent data indicate that these technologies may facilitate certain types of medication errors [20–23].
The effect of improvements in HIV therapy and introduction of new technologies on ART medication errors is yet to be determined. As such, the goal of this analysis was to provide a current estimate of antiretroviral prescribing errors in the hospital setting, to evaluate the duration of ART medication errors, and to identify patient and hospital risk factors for these errors.
METHODS
Study Population and Setting
Since 1989, the Johns Hopkins HIV Clinical Cohort (JHHCC) has offered enrollment to all patients who initiate longitudinal care at the Johns Hopkins Hospital HIV Clinic. All patients are approached to enroll in the cohort, and <1% of patients refuse [24]. As described elsewhere, technicians abstract comprehensive demographic, laboratory, medication, and health services utilization data from medical records and health system databases [24]. Maintenance of the database and use of its contents for analysis of patient outcomes are approved by the Institutional Review Board of the Johns Hopkins University School of Medicine. All adult HIV-infected patients (≥18 years old) enrolled in the JHHCC and with ≥1 hospitalization at the Johns Hopkins Hospital between 1 January and 31 December 2009 were eligible for inclusion in this study.
The Johns Hopkins Hospital is a 982-bed tertiary care teaching hospital located in Baltimore, Maryland. The hospital offers a wide range of inpatient medical services, including medicine, surgery, obstetrics and gynecology, neurology, psychiatry, and is one of only a few hospitals with a dedicated inpatient HIV/AIDS service. The HIV/AIDS service has a clinical pharmacist, but other services do not consistently include pharmacists on the rounding team. Monday through Friday, during medical rounds, the HIV/AIDS service pharmacist reviews all medications administered on the prior day for only those patients admitted to the HIV/AIDS service. In addition, inpatient ART regimens are reconciled with outpatient pharmacy records. If regimens are deemed suboptimal or inappropriate, primary care providers are notified by the inpatient team. In 2004, the Johns Hopkins Hospital implemented Eclipsys Sunrise Clinical Manager, a CPOE system. This system is equipped with a number of clinical support tools which alert providers to potential drug-drug interactions and calculate creatinine clearance at the time of order entry. However, the system does not flag incomplete antiretroviral regimens or identify ART medication dosing or scheduling errors.
Data Collection and Variables
For each patient, data collected include: demographics (age, sex, race/ethnicity, and HIV transmission risk factor), laboratory (measurement of CD4 T-cell count and HIV-1 RNA level within 90 days of each hospitalization and estimated glomerular filtration rate [GFR] at each hospitalization), inpatient utilization (admission date, discharge date, and admitting service for each hospitalization), and inpatient medications (name, dose, route, frequency, start date, and end date for all medications ordered on the first 2 days of each hospitalization). All data were abstracted from the JHHCC database, except for inpatient medication orders which were obtained from a comprehensive Johns Hopkins Hospital inpatient pharmacy and billing system.
Age was divided into 3 groups, 18–39, 40–49, and ≥50 years. Patients’ race/ethnicity was categorized as white, black, or other. HIV transmission risk factors were grouped into men who have sex with men, heterosexual, injection drug use, and other or unknown. The CD4 T-cell and HIV-1 RNA laboratory values used in this analysis were the closest values obtained within 90 days of each hospitalization and categorized as ≤ or >200 cells/mm3 for CD4 T-cell counts and ≤ or >400 copies/mL for HIV-1 RNA levels. GFR was estimated at the time of hospitalization using the Modification of Diet in Renal Disease formula. Inpatient services were categorized as HIV/AIDS, other internal medicine and medical subspecialties, general surgery and surgical subspecialties, obstetrics and gynecology, and neurology/psychiatry.
Identification of Medication Errors
We identified all eligible patients who received ART medication orders during hospitalization between 1 January and 31 December 2009. Two clinical pharmacists specializing in infectious diseases/HIV (J. M. M., D. C.) divided and reviewed all medication orders on the first 2 days of hospitalization to identify ART medication and drug interaction errors, taking into account GFR. Errors were classified as (1) incomplete regimen, (2) incorrect dosage, (3) incorrect schedule, or (4) nonrecommended drug-drug combinations. The Supplementary Appendix describes the nonrecommended drug-drug combinations examined, based on “do not coadminister” or “contraindicated” recommendations made in the Department of Health and Human Services Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents [25].
Data Analysis
Pearson's χ2 test was used to determine whether hospitalized patients with or without ART prescriptions differed in demographic or clinical characteristics. For each hospital admission in which ART medications were ordered, we calculated the total number of errors on the first and second days of hospitalization. We evaluated the duration of errors by comparing the proportion of hospital admissions with errors on day 1 with that on day 2. Errors in dosage and schedule were further analyzed by antiretroviral drug class. Multivariable logistic regression models were fit to identify patient and hospital factors associated with the occurrence of any error on admission. The primary outcome variable was ART medication errors, defined as any error belonging to 1 of the 4 classes listed above. Independent variables included in the model were age, sex, race/ethnicity, HIV transmission risk factor, measurement of CD4 T-cell count and HIV-1 RNA level within 90 days of hospitalization, and admitting hospital service. In secondary models, we evaluate the additional effect of GFR on ART medication errors.
Because the same patient could be hospitalized more than once throughout the year, data from different admission are not fully independent. In multivariate analyses, we therefore used generalizing estimating equations, with each patient as a cluster, independence working correlation, and robust standard errors, to deal with the correlation across admissions for individual patients [26]. All analyses were conducted with Stata software (version 11.2).
RESULTS
In 2009, 702 hospital admissions were identified among 388 HIV-infected patients. Of these, 380 admissions had ART medications prescribed on the first day of hospitalization (230 patients). Seventy-two admissions had a length of stay <24 hours, leaving 308 admissions with ART medication orders on the second hospital day (188 patients). The majority of hospitalized patients were male, ≥50 years of age, black, and had injection drug use as an HIV transmission risk. Twenty-seven percent of the sample had a CD4 T-cell count ≤200 cells/mm3 within 90 days of hospitalization. Most patients were admitted to the HIV/AIDS service (64%), followed by other medical services (14%) and surgery (8%) (Table 1). As expected, a higher CD4 T-cell count and lower HIV-1 RNA level within 90 days of admission were associated with receipt of ART.
Table 1.
Demographic and Clinical Characteristics of Hospitalized HIV-Infected Patients in 2009
| Hospitalized Patients, No. (%) |
|||
|---|---|---|---|
| Variable | All Patients (N = 388) (%) | Patients Prescribed ART (n = 230) | Patients Not Prescribed ART (n = 158) |
| Age, years | |||
| 18–39 | 54 (14) | 26 (11) | 28 (18) |
| 40–49 | 151 (39) | 89 (39) | 62 (39) |
| ≥50 | 183 (47) | 115 (50) | 68 (43) |
| Race/ethnicity | |||
| White | 54 (14) | 38 (17) | 16 (10) |
| Black | 328 (84) | 189 (82) | 139 (88) |
| Other | 6 (2) | 3 (1) | 3 (2) |
| Sex | |||
| Female | 160 (41) | 96 (42) | 64 (41) |
| Male | 228 (59) | 134 (58) | 94 (59) |
| HIV transmission | |||
| MSM | 43 (11) | 26 (11) | 17 (11) |
| Heterosexual | 122 (32) | 75 (33) | 47 (30) |
| IDU | 202 (52) | 116 (50) | 86 (54) |
| Other or unknown | 21 (5) | 13 (6) | 8 (5) |
| CD4 T-cell count within 90 days of admission, cells/mm3 | |||
| ≤200 | 146 (38) | 64 (27) | 82 (52) |
| >201 | 239 (61) | 165 (72) | 74 (47) |
| Missing | 3 (1) | 1 (1) | 2 (1) |
| HIV-1 RNA level within 90 days of admission, copies/mL | |||
| ≤400 | 207 (53) | 174 (75) | 33 (21) |
| >400 | 178 (46) | 55 (24) | 123 (78) |
| Missing | 3 (1) | 1 (1) | 2 (1) |
| Admitting service | |||
| HIV/AIDS | 258 (66) | 148 (64) | 110 (70) |
| Medicine | 53 (14) | 32 (14) | 21 (13) |
| Surgery | 27 (7) | 20 (8) | 7 (4) |
| Obstetrics and gynecology | 18 (5) | 15 (7) | 3 (2) |
| Neurology/psychiatry | 32 (8) | 15 (7) | 17 (11) |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; IDU, injection drug use; MSM, men who have sex with men.
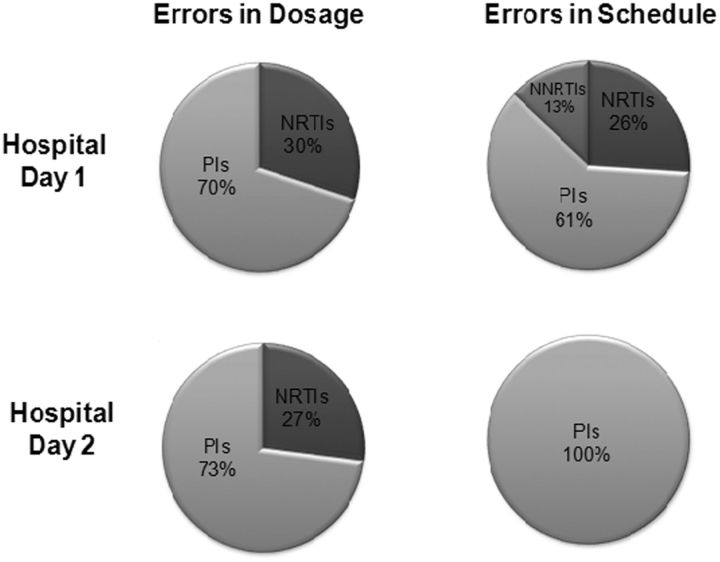
A total of 145 ART medication errors in 110 hospital admissions (83 patients) were identified on the first hospital day (29% of eligible admissions) (Table 2). The most common error was an incomplete regimen (58%), followed by incorrect dosage (38%), incorrect schedule (23%), and nonrecommended drug-drug combinations (13%). Analysis of dosing errors revealed that 70% occurred in protease inhibitors (PIs), and 30% involved nucleoside/nucleotide reverse-transcriptase inhibitors (NRTIs). Errors in scheduling occurred mostly with PIs (61%); followed by NRTIs (26%) and nonnucleoside reverse-transcriptase inhibitors (NNRTIs) (13%) (Figure 1). Review of nonrecommended drug-drug interactions revealed that 46% involved the combination of fluticasone and ritonavir-boosted PIs, and the remaining 54% were equally divided between the combination of midazolam and PIs and the combination of proton pump inhibitors and atazanavir.
Table 2.
Description of Inpatient Antiretroviral Medication Errors
| Error Classification | Errors, No. (%) |
Examples of Errors |
||
|---|---|---|---|---|
| Hospital Day 1, (n = 145) | Hospital Day 2, (n = 22) | Prescription as Ordered | Specific Error Identified | |
| Incomplete regimen | 64 (58) | 0 (0) | Darunavir 600 mg twice a day and ritonavir 100 mg twice a day | Addition antiretroviral agents (eg, tenofovir disoproxil fumarate and emtricitabine) were missing from the regimen |
| Incorrect dosage | 42 (38) | 11 (50) | Darunavir 600 mg twice a day, ritonavir 100 mg twice a day, abacavir sulfate 600 mg/d, and lamivudine 50 mg/d | Lamivudine should have been ordered at a dosage of 150 mg/d based on a creatinine clearance of 40 mL/min |
| Incorrect schedule | 25 (23) | 11 (50) | Kaletra (lopinavir/ritonavir) 2 200/50 mg tablets daily and Truvada (emtricitabine/tenofovir disoproxil fumarate) 1 tablet daily | Kaletra should have been ordered to be taken twice daily |
| Nonrecommended drug-drug combinations | 14 (13) | 0 (0) | Atazanavir sulfate 300 mg/d, ritonavir 100 mg/d, Truvada (emtricitabine/tenofovir disoproxil fumarate) 1 tablet daily, and inhaled fluticasone daily | Coadministration of ritonavir-boosted PIs with fluticasone can cause Cushing's syndrome and secondary adrenal insufficiency and is not recommended |
Abbreviation: PIs, protease inhibitors.
Figure 1.
Distribution of errors in dosage and scheduling by antiretroviral drug class. Abbreviations: NNRTIs, nonnucleoside reverse-transcriptase inhibitor; NRTIs, nucleoside/nucleotide reverse-transcriptase Inhibitors; Pls, protease inhibitors.
On the second day of hospitalization, a total of 22 ART medication errors were identified in 21 hospital admissions (7% of eligible admissions) (Table 2). Half of all errors were due to incorrect dosing, with the reminder caused by incorrect scheduling. PIs accounted for all scheduling errors and 73% of dosing errors (Figure 1). No incomplete regimens or nonrecommended drug-drug interactions were identified. Twelve of the 22 ART medication errors (55%) initially occurred on the first day of hospitalization and were not corrected by hospital day 2 (ie, persistent errors), whereas 10 errors (45%) initially occurred on the second day of hospitalization (ie, new errors). Among new errors, 70% were due to incorrect scheduling, whereas 67% of persistent errors were caused by incorrect dosing.
In multivariable analysis, no patient demographic or clinical characteristics were associated with an ART medication error on the first day of hospitalization. Compared with patients admitted to the HIV/AIDS service, those admitted to a surgical service were at increased risk of ART medication errors (adjusted odds ratio, 3.10; 95% confidence interval, 1.18–8.18). (Table 3) Among errors identified on the surgical service, medication omissions were the most common (45%). Adjusting for other variables, admission GFR was not associated with ART medication errors. Multivariable analysis of errors occurring on the second day of admission did not produce any significant associations.
Table 3.
Multivariate Associations with Antiretroviral Medication Errors on Hospital Day 1
| Variable | Adjusted Odds Ratio (95% CI) |
|---|---|
| Age, years | |
| 18–39 | 1.00 (reference) |
| 40–49 | 1.94 (.49–7.61) |
| ≥50 | 2.34 (.61–8.92) |
| Race/ethnicity | |
| White | 1.00 (reference) |
| Black | 1.24 (.54–2.84) |
| Sex | |
| Female | 1.00 (reference) |
| Male | 1.03 (.51–2.07) |
| HIV transmission | |
| MSM | 1.00 (reference) |
| Heterosexual | 0.60 (.20–1.85) |
| IDU | 0.77 (.29–2.07) |
| Other or unknown | 0.53 (.11–2.49) |
| CD4 T-cell count within 90 days of admission, cells/mm3 | |
| ≤200 | 1.00 (reference) |
| >200 | 0.96 (.43–2.14) |
| HIV-1 RNA within 90 days of admission, copies/mL | |
| ≤400 | 1.00 (reference) |
| >400 | 0.69 (.30–1.57) |
| Admitting service | |
| HIV/AIDS | 1.00 (reference) |
| Medicine | 1.65 (.69–3.95) |
| Surgery | 3.10a (1.18–8.18) |
| Obstetrics and gynecology | 0.51 (.06–4.14) |
| Neurology/psychiatry | 0.76 (.18–3.14) |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; IDU, injection drug use; MSM, men who have sex with men.
a P < .05.
DISCUSSION
This study demonstrated that ART medication errors are common among hospitalized patients with HIV infection in the first 24 hours of admission, but at our hospital are quickly corrected by the second day of hospitalization. Twenty-nine percent of hospitalizations had an antiretroviral prescription error on the first day of admission, which decreased to 7% by hospital day 2. This initial error rate is somewhat higher than that in previous studies, which estimated rates between 21% and 26% [12, 14]. Multiple factors could contribute to this high initial rate, including provider experience and adverse effects of CPOE, whereas review of medication orders by clinical pharmacists may account for the relatively short duration of prescription errors.
Improvements in ART and transformation of HIV from an acute illness to a chronic disease have considerable decreased use of inpatient services by PLWH. Between 2002 and 2007, hospitalization rates significantly declined, from 35 to 27 per 100 persons [27]. Consequently, inpatient providers today may be less likely to manage PLWH. Exposure to PLWH directly affects HIV knowledge. In a survey of 379 physicians caring for PLWH, Landen et al demonstrated that HIV caseload was significantly associated with HIV-specific knowledge [28]. Similarly, a multistate study of 1233 physicians examining choice of ART for HIV-infected patients noted that physicians with little or no previous HIV experience were less likely to choose ART consistent with current guidelines [29]. The importance of HIV experience, combined with decreasing exposure to HIV-infected patients, may be contributing to ART medication errors.
Although many studies note that CPOE decreases medication errors [2, 18, 19], in recent years a growing number of studies demonstrate that CPOE may actually contribute to medication errors [20–23]. In a mixed qualitative and quantitative study of 261 CPOE users at a large tertiary-care teaching hospital, the CPOE system facilitated 22 types of medication errors. Those that may be highly relevant to hospitalized PLWH receiving ART include reliance on CPOE displays to determine the range of doses for infrequently used medications; loss of data, time, and focus when CPOE is nonfunctional; problems ordering off-formulary medications; and failure to provide medications after surgical or other procedures [20]. Hospitals should recognize both the advantages and disadvantages of CPOE and should actively strive to fix features that facilitate medication errors. Additional research is needed to determine the impact of CPOE implementation on ART medication errors.
The dominant errors identified in this study, incomplete regimen, incorrect dosage, and incorrect schedule, are consistent with previous findings in the literature [11, 12, 14, 15]. Unlike investigators in prior studies, we further divided errors by antiretroviral drug class, noting over both hospital days that 71%–73% of dosing and scheduling errors occurred in PIs. This is not unexpected, because popular NNRTI and NRTI drugs often have simple dosing schedules. Atripla (efavirenz/tenofovir/emtricitabine), Truvada (emtricitabine/tenofovir disoproxil fumarate), and Epzicom (lamivudine/abacavir) are each dispensed as 1 tablet daily. In comparison, PIs are available in many different dosage forms and strengths, often requiring adjustment based on antiretroviral experience, PI mutations, drug-drug interactions, and drug schedule (ie, once vs twice daily) [25]. Furthermore, many PIs are dispensed twice daily. These data indicate that inpatient providers should be especially careful when entering medication orders for PIs, and pharmacists should be aware of the potential for medication errors when dispensing this particular drug class.
In this study, patients admitted to surgical services were at increased risk of ART medication errors on the first day of hospitalization, compared with those admitted to an HIV/AIDS service. In an attempt to identify the underlying causes of ART medication errors, Synder et al interviewed providers who had committed errors [30]. Most errors were caused by a lack of expertise with prescribing antiretroviral medications, failure to reconcile home or transfer medications, poor or no written or verbal communication between providers, overlooking decision support software alerts, and/or lack of ownership for HIV therapy when treating patients’ primary problem [30]. To overcome these obstacles, it would be advantageous for institutions to provide education and resources regarding ART, have clinical pharmacists review medications orders, and encourage consultation from infectious diseases practitioners when HIV-infected patients with complicated conditions are admitted to the hospital.
ART medication errors dramatically declined on the second day of hospitalization, which may be a consequence of clinical pharmacy review of medication orders. Multiple studies have shown that clinical pharmacists are effective at decreasing ART medication errors [14, 16, 31–33]. Heelon and colleagues documented that review of inpatient antiretroviral medication orders by a clinical pharmacist reduced the median length of time until error correction from 84 hours (range, 24–7584 hours) to 15.5 hours (range, 1–216 hours) [14]. This time frame is consistent with our data. Furthermore, a recent study noted that a pharmacist-driven medication reconciliation process within 24 hours of admission was associated with a decrease in ART medication errors from 52% to 5% [33]. Although the involvement of clinical pharmacists in medication reconciliation may not be feasible at all hospitals, such programs should be considered in high-risk patient populations, including PLWH.
There are several potential limitations to this analysis. It is based on data from a single large tertiary teaching hospital. Although our results may not generalize to all inpatient settings, they are likely applicable to many teaching hospitals. Only patients enrolled in the JHHCC were evaluated; we did not review records of hospitalized patients with HIV infection receiving care at outside institutions. Although we believe this number to be small, excluding these individuals may affect our results. We also focused our analysis on the first 2 days of hospitalization. It is possible that errors detected early in the admission may be corrected later in the hospital course, conversely new errors may arise as patients transfer between services, undergo surgical or other procedures, or are discharged. Analyses did not distinguish medication errors occurring in new ART prescriptions from those arising in continued therapy; differentiating between new and continuing ART prescriptions may help target future interventions. This study focused on prescribing errors, and did not evaluate dispensing and administration errors. As such, our results may underestimate the number of actual medication errors. In addition, we evaluated only antiretroviral-related nonrecommended drug-drug combinations, further studies are needed to evaluate other drug-drug interactions. We identified a large number of PLWH who were not prescribed ART in the first 24 hours of hospitalization; these may have included patients with newly diagnosed HIV infection, patients in whom ART was not prescribed by their outpatient provider, or patients who were not adherent to prescribed therapy. In addition, medical teams may have mistakenly not continued outpatient ART or intentionally withheld ART owing to particular medical or surgical conditions (eg, renal failure, drug toxicity, surgery). Further research is necessary to determine the reasons why these individuals were not receiving ART.
In summary, our findings confirmed that inpatient ART medication errors are common on the first day of hospitalization but are typically corrected within 48 hours. Incomplete regimens and errors in dosing were the most common errors, with patients admitted to surgical services at greatest risk. New interventions, both educational and technological, and clinical pharmacist coverage during evening and weekend hours are needed to safeguard patients and prevent the serious complications of antiretroviral medication errors especially during the first 24 hours of admission.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the patients, providers, investigators, and staff of the Johns Hopkins HIV Clinical Cohort.
Financial support. This work was supported by the National Institutes of Health (grants K24 DA 00432, R01DA11602, and R01 AA 16893 to R. D. M. and Mid-Career Award K24 AI073957 J. P. M.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Committee on Identifying and Preventing Medication Errors. Preventing medication errors. Washington, DC: National Academy Press; 2007. [Google Scholar]
- 2.Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995;274:29–34. [PubMed] [Google Scholar]
- 3.Bates DW, Spell N, Cullen DJ, et al. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. JAMA. 1997;277:307–11. [PubMed] [Google Scholar]
- 4.Raschke RA, Gollihare B, Wunderlich TA, et al. A computer alert system to prevent injury from adverse drug events: development and evaluation in a community teaching hospital. JAMA. 1998;280:1317–20. doi: 10.1001/jama.280.15.1317. [DOI] [PubMed] [Google Scholar]
- 5.Salanitro AH, Osborn CY, Schnipper JL, et al. Effect of patient- and medication-related factors on inpatient medication reconciliation errors. J Gen Intern Med. 2012 doi: 10.1007/s11606-012-2003-y. Epub ahead of print 15 February 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Budnitz DS, Shehab N, Kegler SR, Richards CL. Medication use leading to emergency department visits for adverse drug events in older adults. Ann Intern Med. 2007;147:755–65. doi: 10.7326/0003-4819-147-11-200712040-00006. [DOI] [PubMed] [Google Scholar]
- 7.Phillips DP, Barker GE. A July spike in fatal medication errors: a possible effect of new medical residents. J Gen Intern Med. 2010;25:774–9. doi: 10.1007/s11606-010-1356-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schnipper JL, Hamann C, Ndumele CD, et al. Effect of an electronic medication reconciliation application and process redesign on potential adverse drug events: a cluster-randomized trial. Arch Intern Med. 2009;169:771–80. doi: 10.1001/archinternmed.2009.51. [DOI] [PubMed] [Google Scholar]
- 9.Greenwald JL, Halasyamani LK, Greene J, et al. Making inpatient medication reconciliation patient centered, clinically relevant, and implementable: a consensus statement on key principles and necessary first steps. J Hosp Med. 2010;8:477–85. doi: 10.1002/jhm.849. [DOI] [PubMed] [Google Scholar]
- 10.Edelstein H, Wilson M. Antiretroviral medication errors were universal in hospitalized HIV-seropositive patients at a teaching hospital. J Acquir Immune Defic Syndr. 2001;28:496. doi: 10.1097/00042560-200112150-00015. [DOI] [PubMed] [Google Scholar]
- 11.Mok S, Minson Q. Drug-related problems in hospitalized patients with HIV infection. Am J Health Syst Pharm. 2008;65:55–9. doi: 10.2146/ajhp070011. [DOI] [PubMed] [Google Scholar]
- 12.Rastegar DA, Knight AM, Monolakis JS. Antiretroviral medication errors among hospitalized patients with HIV infection. Clin Infect Dis. 2006;43:933–8. doi: 10.1086/507538. [DOI] [PubMed] [Google Scholar]
- 13.DeLorenze GN, Follansbee SF, Nguyen DP, et al. Medication error in the care of HIV/AIDS patients: electronic surveillance, confirmation, and adverse events. Med Care. 2005;43:III63–8. doi: 10.1097/01.mlr.0000175622.81335.4d. [DOI] [PubMed] [Google Scholar]
- 14.Heelon M, Skiest D, Tereso G, et al. Effect of a clinical pharmacist's interventions on duration of antiretroviral-related errors in hospitalized patients. Am J Health Syst Pharm. 2007;64:2064–8. doi: 10.2146/ajhp070072. [DOI] [PubMed] [Google Scholar]
- 15.Purdy BD, Raymond AM, Lesar TS. Antiretroviral prescribing errors in hospitalized patients. Ann Pharmacother. 2000;34:833–8. doi: 10.1345/aph.19399. [DOI] [PubMed] [Google Scholar]
- 16.Carcelero E, Tuset M, Martin M, et al. Evaluation of antiretroviral-related errors and interventions by the clinical pharmacist in hospitalized HIV-infected patients. HIV Med. 2011;12:494–9. doi: 10.1111/j.1468-1293.2011.00915.x. [DOI] [PubMed] [Google Scholar]
- 17.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 27 March 2012. Available at: http://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-treatment-guidelines/0/ Accessed 2 April 2012. [Google Scholar]
- 18.Bates DW, Leape LL, Cullen DJ, et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998;280:1311–6. doi: 10.1001/jama.280.15.1311. [DOI] [PubMed] [Google Scholar]
- 19.Bates DW, Kuperman G, Teich JM. Computerized physician order entry and quality of care. Qual Manag Health Care. 1994;2:18–27. [PubMed] [Google Scholar]
- 20.Koppel R, Metlay JP, Cohen A, et al. Role of computerized physician order entry systems in facilitating medication errors. JAMA. 2005;293:1197–203. doi: 10.1001/jama.293.10.1197. [DOI] [PubMed] [Google Scholar]
- 21.Berger RG, Kichak JP. Computerized physician order entry: helpful or harmful? J Am Med Inform Assoc. 2004;11:100–3. doi: 10.1197/jamia.M1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ash JS, Berg M, Coiera E. Some unintended consequences of information technology in health care: the nature of patient care information system-related errors. J Am Med Inform Assoc. 2004;11:104–12. doi: 10.1197/jamia.M1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaushal R, Shojania KG, Bates DW. Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review. Arch Intern Med. 2003;163:1409–16. doi: 10.1001/archinte.163.12.1409. [DOI] [PubMed] [Google Scholar]
- 24.Moore RD. Understanding the clinical and economic outcomes of HIV therapy: the Johns Hopkins HIV clinical practice cohort. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17(Suppl 1):S38–41. doi: 10.1097/00042560-199801001-00011. [DOI] [PubMed] [Google Scholar]
- 25.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 3 November 2008. Available at: http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL001226.pdf. Accessed 5 January 2012. [Google Scholar]
- 26.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 27.Yehia BR, Fleishman JA, Hicks PL, Ridore M, Moore RD, Gebo KA. Inpatient health services utilization among HIV-infected adult patients in care 2002–2007. J Acquir Immune Defic Syndr. 2010;53:397–404. doi: 10.1097/QAI.0b013e3181bcdc16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landon BE, Wilson IB, Wenger NS, et al. Specialty training and specialization among physicians who treat HIV/AIDS in the United States. J Gen Intern Med. 2002;17:12–22. doi: 10.1046/j.1525-1497.2002.10401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stone VE, Mansourati FF, Poses RM, Mayer KH. Relation of physician specialty and HIV/AIDS experience to choice of guideline-recommended antiretroviral therapy. J Gen Intern Med. 2001;16:360–8. doi: 10.1046/j.1525-1497.2001.016006360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Snyder AM, Klinker K, Orrick JJ, Janelle J, Winterstein AG. An in-depth analysis of medication errors in hospitalized patients with HIV. Ann Pharmacother. 2011;45:459–68. doi: 10.1345/aph.1P599. [DOI] [PubMed] [Google Scholar]
- 31.Bozek PS, Perdue BE, Bar-Din M, Weidle PJ. Effect of pharmacist interventions on medication use and cost in hospitalized patients with or without HIV infection. Am J Health Syst Pharm. 1998;55:1151–5. doi: 10.1093/ajhp/55.11.1151. [DOI] [PubMed] [Google Scholar]
- 32.de Maat MM, de Boer A, Koks CH, et al. Evaluation of clinical pharmacist interventions on drug interactions in outpatient pharmaceutical HIV-care. J Clin Pharm Ther. 2004;29:121–30. doi: 10.1111/j.1365-2710.2003.00541.x. [DOI] [PubMed] [Google Scholar]
- 33.Corrigan MA, Atkinson KM, Sha BE, Crank CW. Evaluation of pharmacy-implemented medication reconciliation directed at antiretroviral therapy in hospitalized HIV/AIDS patients. Ann Pharmacother. 2010;44:222–3. doi: 10.1345/aph.1M052. [DOI] [PubMed] [Google Scholar]