Abstract
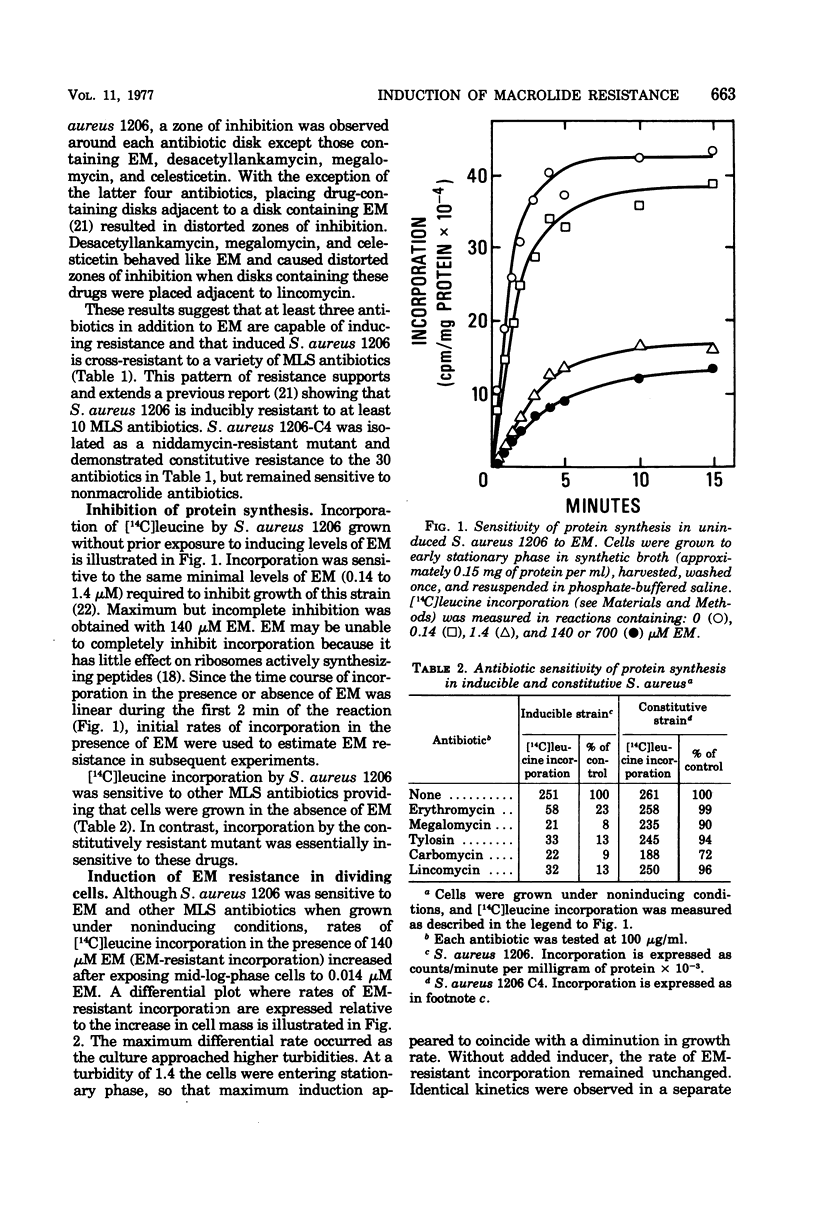
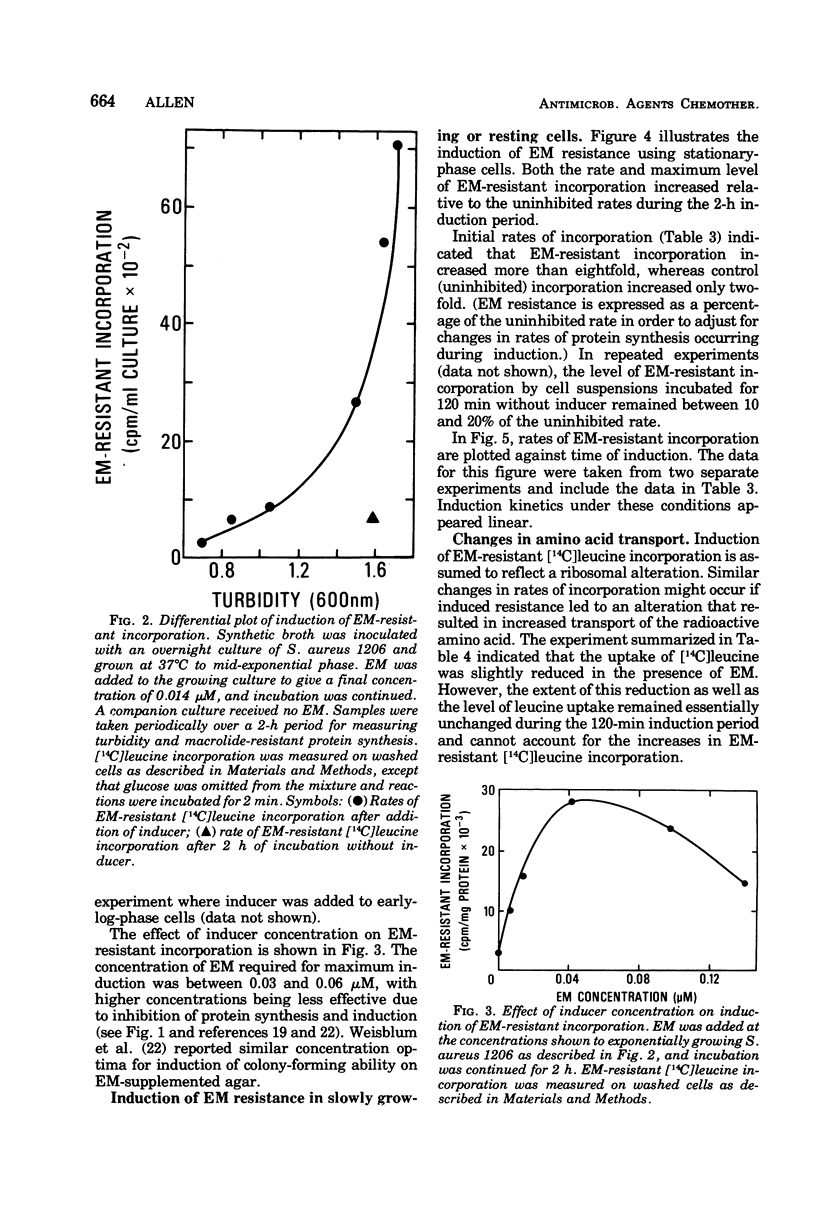
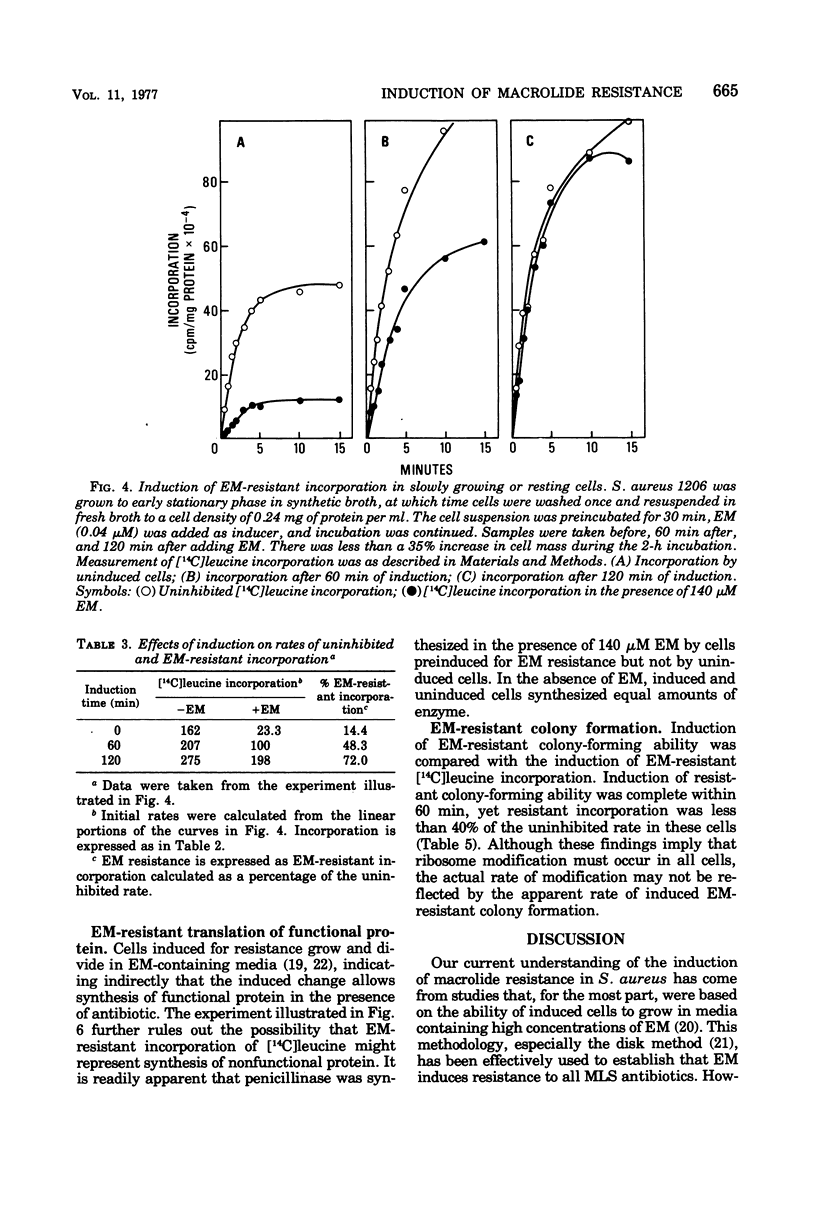
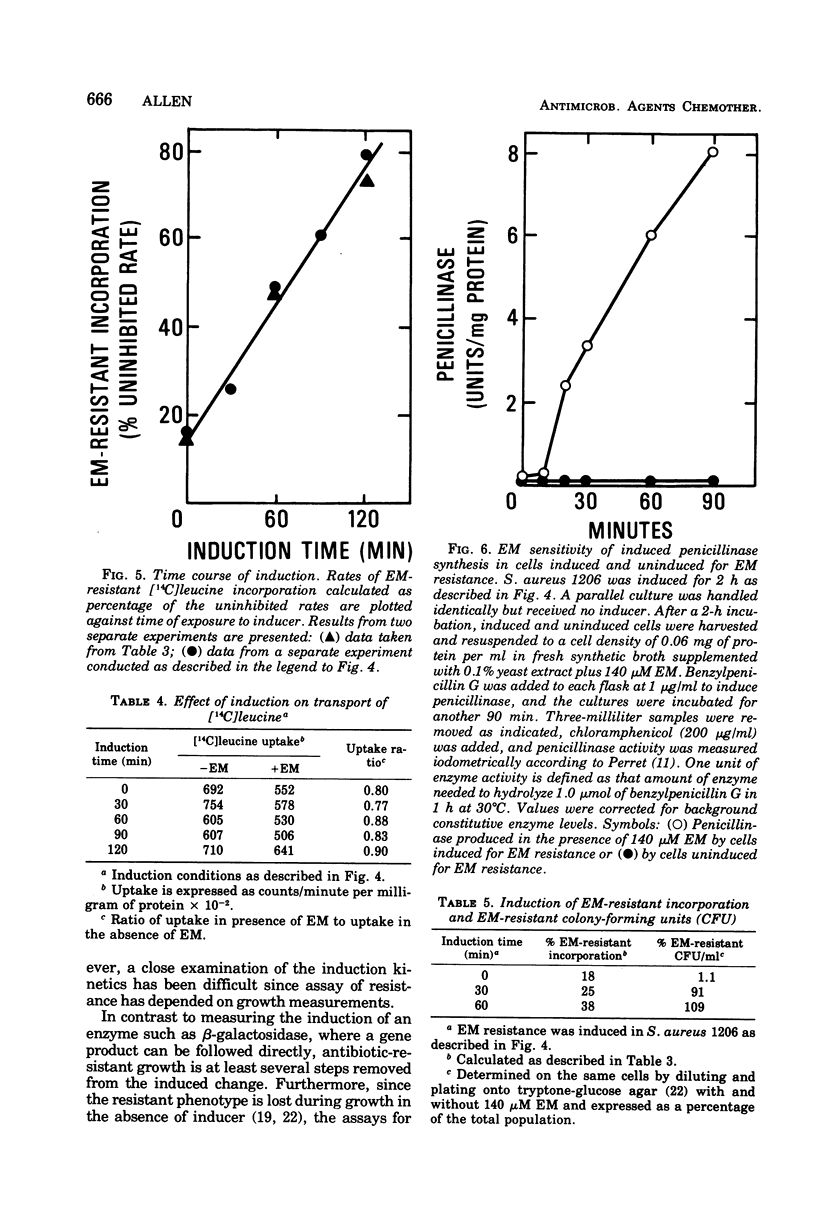
Induction of resistance to macrolide-, lincosamide-, and streptogramin B-type antibiotics in Staphylococcus aureus was studied by monitoring the appearance of erythromycin A (EM)-resistant [14C]leucine incorporation. Examination of the induction process revealed saturation kinetics and a time course much like that reported for penicillinase in gram-positive bacteria. Induction kinetics in exponentially growing cells were sigmoidal and appeared to reach a maximum and constant rate when growth reached stationary phase. Since the induction of EM-resistant colony-forming ability was complete within 60 min, ribosome modification cannot be limited to a fraction of the population and must occur in essentially every cell. However, EM-resistant growth was expressed in cells where less than half the [14C]leucine-incorporating activity was resistant to EM. This suggests that resistance requires that only a threshold level of ribosome modification be exceeded and that, once exceeded, resistance is dominant to sensitivity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen N. E. Macrolide resistance in Staphylococcus aureus: inducers of macrolide resistance. Antimicrob Agents Chemother. 1977 Apr;11(4):669–674. doi: 10.1128/aac.11.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHABBERT Y. Antagonisme in vitro entre l'érythromycine et la spiramycine. Ann Inst Pasteur (Paris) 1956 Jun;90(6):787–790. [PubMed] [Google Scholar]
- Hashimoto H., Oshima H., Mitsuhashi S. Drug resistance of staphylococci. IX. Inducible resistance to macrolide antibiotics in Staphylococcus aureus. Jpn J Microbiol. 1968 Sep;12(3):321–327. [PubMed] [Google Scholar]
- Hyder S. L., Streitfeld M. M. Inducible and constitutive resistance to macrolide antibiotics and lincomycin in clinically isolated strains of Streptococcus pyogenes. Antimicrob Agents Chemother. 1973 Sep;4(3):327–331. doi: 10.1128/aac.4.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imsande J. Regulation of penicillinase synthesis: evidence for a unified model. J Bacteriol. 1970 Jan;101(1):173–180. doi: 10.1128/jb.101.1.173-180.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lai C. J., Dahlberg J. E., Weisblum B. Structure of an inducibly methylatable nucleotide sequence in 23S ribosomal ribonucleic acid from erythromycin-resistant Staphylococcus aureus. Biochemistry. 1973 Jan 30;12(3):457–460. doi: 10.1021/bi00727a015. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Weisblum B. Altered methylation of ribosomal RNA in an erythromycin-resistant strain of Staphylococcus aureus. Proc Natl Acad Sci U S A. 1971 Apr;68(4):856–860. doi: 10.1073/pnas.68.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Weisblum B., Fahnestock S. R., Nomura M. Alteration of 23 S ribosomal RNA and erythromycin-induced resistance to lincomycin and spiramycin in Staphylococcus aureus. J Mol Biol. 1973 Feb 15;74(1):67–72. doi: 10.1016/0022-2836(73)90355-0. [DOI] [PubMed] [Google Scholar]
- Leggate J., Holms W. H. Gratuitous synthesis of beta-lactamase in Staphylococcus aureus. J Bacteriol. 1968 Dec;96(6):2110–2117. doi: 10.1128/jb.96.6.2110-2117.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRET C. J. Iodometric assay of penicillinase. Nature. 1954 Nov 27;174(4439):1012–1013. doi: 10.1038/1741012a0. [DOI] [PubMed] [Google Scholar]
- SWALLOW D. L., SNEATH P. H. Studies on staphylococcal penicillinase. J Gen Microbiol. 1962 Jul;28:461–469. doi: 10.1099/00221287-28-3-461. [DOI] [PubMed] [Google Scholar]
- Saito T., Hashimoto H., Mitsuhashi S. Drug resistance of staphylococci. Foation of erythromycin-ribosome complex. Decrease in the formation of erythromycin-ribosome complex in erythromycin resistant strains of Staphylococcus aureus. Jpn J Microbiol. 1969 Mar;13(1):119–121. doi: 10.1111/j.1348-0421.1969.tb00441.x. [DOI] [PubMed] [Google Scholar]
- Saito T., Shimizu M., Mitsuhashi S. The problems of drug-resistant pathogenic bacteria. Macrolide resistance in staphylococci. Ann N Y Acad Sci. 1971 Jun 11;182:267–278. doi: 10.1111/j.1749-6632.1971.tb30663.x. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Saito T., Mitsuhashi S. Macrolide resistance in Staphylococcus aureus. Correlation between spiramycin-binding to ribosomes and inhibition of polypeptide synthesis in cell-free system. Jpn J Microbiol. 1970 May;14(3):215–219. doi: 10.1111/j.1348-0421.1970.tb00512.x. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Saito T., Mitsuhashi S. Macrolide resistance in Staphylococcus aureus. Decrease of spiramycin-binding to 50S ribosomal subunit in macrolide resistant strains of staphylococci. J Antibiot (Tokyo) 1970 Sep;23(9):467–468. [PubMed] [Google Scholar]
- Tai P. C., Wallace B. J., Davis B. D. Selective action of erythromycin on initiating ribosomes. Biochemistry. 1974 Oct 22;13(22):4653–4659. doi: 10.1021/bi00719a029. [DOI] [PubMed] [Google Scholar]
- WEAVER J. R., PATTEE P. A. INDUCIBLE RESISTANCE TO ERYTHROMYCIN IN STAPHYLOCOCCUS AUREUS. J Bacteriol. 1964 Sep;88:574–580. doi: 10.1128/jb.88.3.574-580.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum B., Demohn V. Erythromycin-inducible resistance in Staphylococcus aureus: survey of antibiotic classes involved. J Bacteriol. 1969 May;98(2):447–452. doi: 10.1128/jb.98.2.447-452.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum B., Siddhikol C., Lai C. J., Demohn V. Erythromycin-inducible resistance in Staphylococcus aureus: requirements for induction. J Bacteriol. 1971 Jun;106(3):835–847. doi: 10.1128/jb.106.3.835-847.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winshell E., Shaw W. V. Kinetics of induction and purification of chloramphenicol acetyltransferase from chloramphenicol-resistant Staphylococcus aureus. J Bacteriol. 1969 Jun;98(3):1248–1257. doi: 10.1128/jb.98.3.1248-1257.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yag Y., Franke A. E., Clewell D. B. Plasmid-determined resistance to erythromycin: comparison of strains of streptococcus faecalis and streptococcus pyogenes with regard to plasmid hmology and resistance inducibility. Antimicrob Agents Chemother. 1975 Jun;7(6):871–873. doi: 10.1128/aac.7.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi S., Nakajima Y., Inoue M., Oka Y. Decrease in accumulation of macrolide antibiotics as a mechanism of resistance in Staphylococcus aureus. Jpn J Microbiol. 1971 Jan;15(1):39–52. doi: 10.1111/j.1348-0421.1971.tb00549.x. [DOI] [PubMed] [Google Scholar]


