Abstract
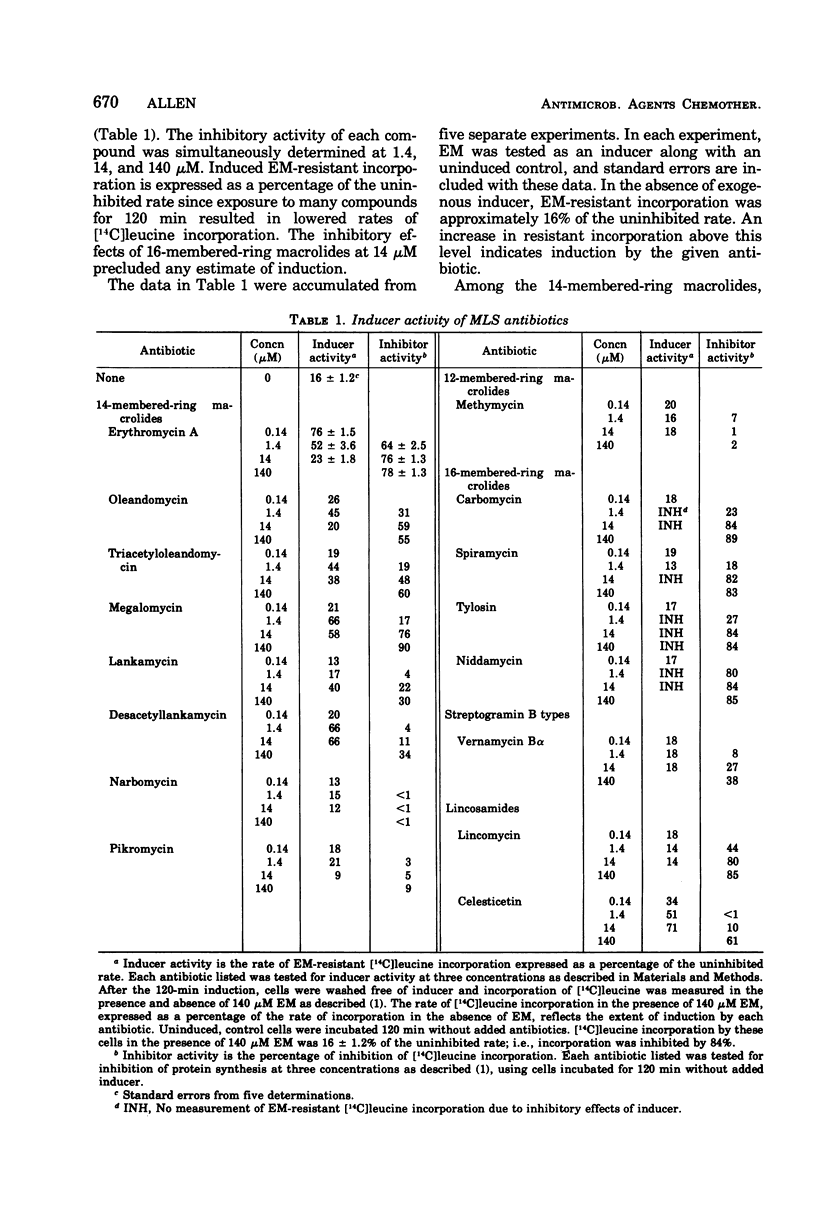
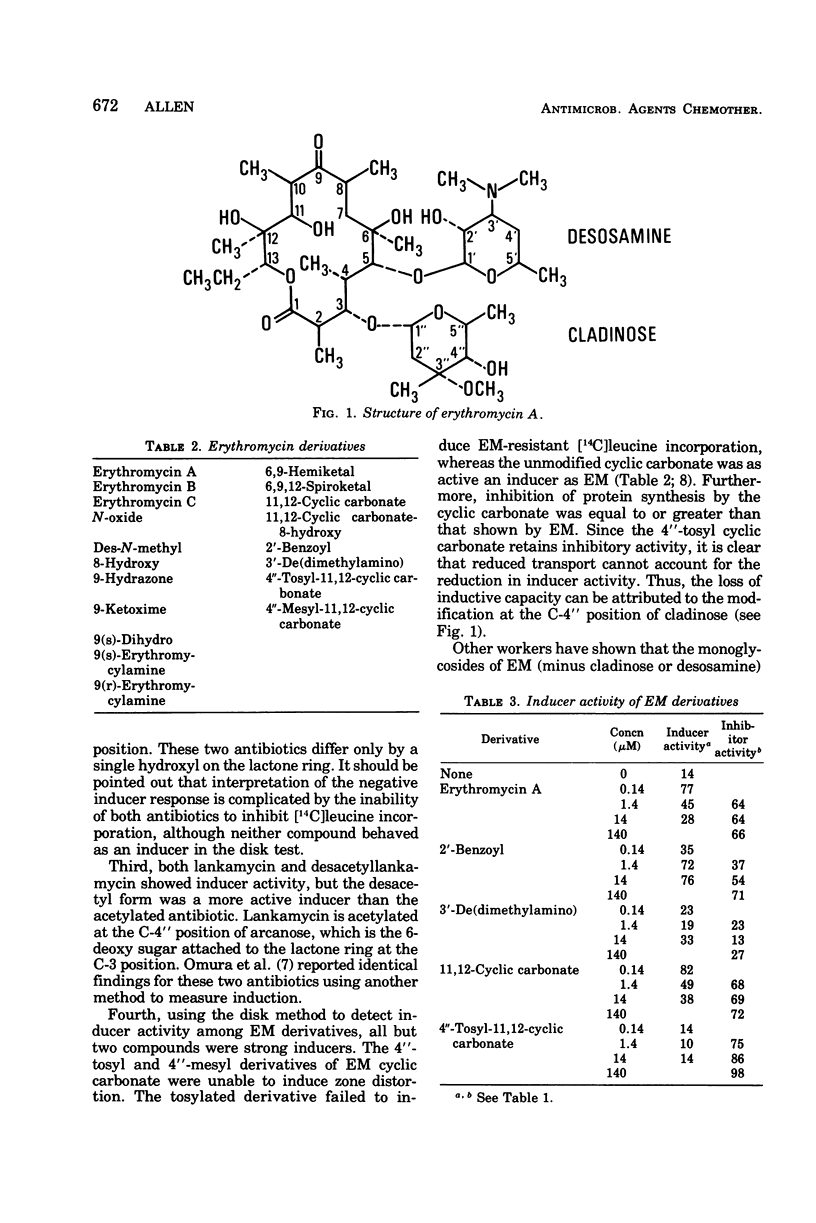
Several macrolide-, lincosamide-, and streptogramin B-type (MLS) antibiotics were tested as inducers of erythromycin A (EM)-resistant [14C]leucine incorporation. Only 14-membered-ring macrolides having a glycosidically linked 6-deoxy sugar at the C-3 position of the lactone ring and the structurally dissimilar lincosamide, celesticetin, showed inducer activity. Modifications of EM at the C-4″ position of cladinose can apparently destroy the inducer property but do not affect the inhibitory properties of the antibiotic. The findings clearly show that inducer and inhibitor activities can be dissociated and are consistent with the concept that distinct binding/receptor sites are utilized for inhibition of ribosome function and induction of resistance.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen N. E. Macrolide resistance in Staphylococcus aureus: induction of macrolide-resistant protein synthesis. Antimicrob Agents Chemother. 1977 Apr;11(4):661–668. doi: 10.1128/aac.11.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F. N., Weisblum B. The specificity of lincomycin binding to ribosomes. Biochemistry. 1967 Mar;6(3):836–843. doi: 10.1021/bi00855a025. [DOI] [PubMed] [Google Scholar]
- Hyder S. L., Streitfeld M. M. Inducible and constitutive resistance to macrolide antibiotics and lincomycin in clinically isolated strains of Streptococcus pyogenes. Antimicrob Agents Chemother. 1973 Sep;4(3):327–331. doi: 10.1128/aac.4.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malke H. Genetics of resistance to macrolide antibiotics and lincomycin in natural isolates of Streptococcus pyogenes. Mol Gen Genet. 1974;135(4):349–367. doi: 10.1007/BF00271149. [DOI] [PubMed] [Google Scholar]
- Omura S., Namiki S., Shibata M., Muro T., Sawada J. Studies on the antibiotics from Streptomyces spinichromogenes var. kujimyceticus. V. Some antimicrobial characteristics of kujimycin A and kujimycin B against macrolide resistant staphylococci. J Antibiot (Tokyo) 1970 Sep;23(9):448–460. doi: 10.7164/antibiotics.23.448. [DOI] [PubMed] [Google Scholar]
- Ono H., Inoue M., Mao J. C., Mitsuhashi S. Drug resistance in Staphylococcus aureus. Induction of macrolide resistance by erythromycin, oleandomycin and their derivatives. Jpn J Microbiol. 1975 Oct;19(5):343–347. [PubMed] [Google Scholar]
- Pestka S., Vince R., LeMahieu R., Weiss F., Fern L., Unowsky J. Induction of erythromycin resistance in Staphyloccus aureus by erythromycin derivatives. Antimicrob Agents Chemother. 1976 Jan;9(1):128–130. doi: 10.1128/aac.9.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Hashimoto H., Mitsuhashi S. Macrolide resistance in Staphylococcus aureus. Isolation of a mutant in which leucomycin is an active inducer. Jpn J Microbiol. 1970 Nov;14(6):473–478. [PubMed] [Google Scholar]
- Shimizu M., Saito T., Mitsuhashi S. Macrolide resistance in Staphylococcus aureus. Relation between spiramycin-binding to ribosome and inhibition of polypeptide synthesis in a heat inducible-resistant mutant. Jpn J Microbiol. 1970 Mar;14(2):155–162. [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Mutant of Staphylococcus aureus with lincomycin- and carbomycin-inducible resistance to erythromycin. Antimicrob Agents Chemother. 1974 May;5(5):538–540. doi: 10.1128/aac.5.5.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEAVER J. R., PATTEE P. A. INDUCIBLE RESISTANCE TO ERYTHROMYCIN IN STAPHYLOCOCCUS AUREUS. J Bacteriol. 1964 Sep;88:574–580. doi: 10.1128/jb.88.3.574-580.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum B., Davies J. Antibiotic inhibitors of the bacterial ribosome. Bacteriol Rev. 1968 Dec;32(4 Pt 2):493–528. [PMC free article] [PubMed] [Google Scholar]
- Weisblum B., Demohn V. Erythromycin-inducible resistance in Staphylococcus aureus: survey of antibiotic classes involved. J Bacteriol. 1969 May;98(2):447–452. doi: 10.1128/jb.98.2.447-452.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum B., Siddhikol C., Lai C. J., Demohn V. Erythromycin-inducible resistance in Staphylococcus aureus: requirements for induction. J Bacteriol. 1971 Jun;106(3):835–847. doi: 10.1128/jb.106.3.835-847.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yag Y., Franke A. E., Clewell D. B. Plasmid-determined resistance to erythromycin: comparison of strains of streptococcus faecalis and streptococcus pyogenes with regard to plasmid hmology and resistance inducibility. Antimicrob Agents Chemother. 1975 Jun;7(6):871–873. doi: 10.1128/aac.7.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]


