Abstract
Agonists and positive allosteric modulators (PAMs) of α7 nicotinic acetylcholine receptors (nAChRs) are currently being considered as novel therapeutic approaches for managing cognitive deficits in schizophrenia and Alzheimer’s disease. Though α7 agonists were recently found to possess antinociceptive and anti-inflammatory properties in rodent models of chronic neuropathic pain and inflammation, the effects of α7 nAChRs PAMs on chronic pain and inflammation remain largely unknown. The present study investigated whether PAMs, by increasing endogenous cholinergic tone, potentiate α7 nAChRs function to attenuate inflammatory and chronic neuropathic pain in mice. We tested two types of PAMS, type I (NS1738) and type II (PNU-120596) in carrageenan-induced inflammatory pain and chronic constriction injury (CCI) neuropathic pain models. We found that both NS1738 and PNU-120596 significantly reduced thermal hyperalgesia, while only PNU-120596 significantly reduced edema caused by a hind paw infusion of carrageenan. Importantly, PNU-120596 reversed established thermal hyperalgesia and edema induced by carrageenan. In the CCI model, PNU-120596 had long-lasting (up to 6 hrs), dose-dependent anti-hyperalgesic and anti-allodynic effects after a single injection, while NS1738 was inactive. Systemic administration of the α7 nAChR antagonist MLA reversed PNU-120596’s effects, suggesting the involvement of central and peripheral α7 nAChRs. Furthermore, PNU-120596 enhanced an ineffective dose of selective agonist PHA-543613 to produce anti-allodynic effects in the CCI model. Our results indicate that the type II α7 nAChRs PAM PNU-120596, but not the type I α7 nAChRs PAM NS1738, shows significant anti-edematous and anti-allodynic effects in inflammatory and CCI pain models in mice.
Keywords: nicotinic receptors, chronic pain, inflammation, mice, allosteric modulators
Introduction
Chronic neuropathic pain arguably arises due to long-term plasticity changes in somatosensory pathways from the periphery to the cortex. These plasticity changes often occur after nerve injury and/or dysfunction in the central nervous system (CNS), resulting in significantly enhanced pain sensation (hyperalgesia) or in otherwise non-noxious stimuli to cause pain (allodynia) (Wang et al., 2011; Zhuo, 2007; Harden, 2005). Increased pain sensitivity, one of the most common signs of an inflammatory disorder, is mediated by a host of different factors, including enzymes, neuropeptides, eicosanoids, chemokines and cytokines (Dray and Bevan, 1993; Sandkuhler, 2009; Wang et al., 2011). To date, several drugs, such as opioids and anti-inflammatory, anti-seizure and antidepressant agents, used to treat chronic neuropathic pain have major adverse effects and/or incomplete pain relief for patients. Thus, development of drugs possessing increased efficacy and safety is needed.
Previous studies suggest utility of nicotinic acetylcholine receptor (nAChR) agonists to treat chronic pain conditions (Bannon et al., 1998; Khan et al., 2003; Miao et al., 2004; Vincler, 2005; Pacini et al., 2010). Multiple subtypes of nAChRs are expressed in pain transmission pathways (Khan et al., 2003). For example, α4β2* and α7 subtypes are expressed in the spinal cord dorsal horn (Cordero-Eraudquin et al., 2004; Cordero-Eraudquin and Changeux, 2001; Marubio et al., 1999). Recent work has focused on the role of the α7 nAChRs in modulating inflammation and nociception (Westman et al., 2010; Marrero and Bencherif, 2009; Medhurst et al., 2008; de Jonge and Ulloa, 2007). In addition to their neuronal presence, α7 nAChRs are expressed on macrophages (Tracy et al., 2002; Wang and Wang, 2003; Ulloa et al., 2005), which are key immune cells involved in the initiation, maintenance, and resolution of inflammation (Fujiwara and Kobayashi, 2005). Previous studies have demonstrated the importance of acetylcholine (ACh) directly interacting with α7 nAChRs expressed on macrophages and other cytokine-producing cells in down-regulating proinflammatory cytokine synthesis and preventing tissue damage (Tracey, 2002; De Rosa et al., 2009; Wang et al., 2009). In addition, Xiao et al. (2002) showed an up-regulation of α7 nAChR subunit expression in the rat dorsal root ganglion fourteen days after sciatic nerve axotomy. Moreover, α7 nAChRs agonists elicited significant anti-inflammatory and antinociceptive effects in rodent models of chronic neuropathic pain and inflammation (Damaj et al., 2000; Wang et al., 2005; Hamurtekin and Gurun, 2006; Medhurst et al., 2008; Gurun et al., 2009; Rowley et al., 2010). Therefore, the α7 nAChR represents a promising target for the development of analgesic and anti-inflammatory agents. However, concerns regarding α7 nAChR agonists as clinical candidates persist. For example, α7 nAChRs desensitize rapidly in response to high agonist concentration in vitro followed by a long period of desensitization (Bertrand et al., 1992). Furthermore, an agonist-based therapeutic approach would disrupt endogenous cholinergic tone (Papke et al., 2009).
One alternative approach to selectively enhance activity of the α7 nAChRs is via positive allosteric modulation. As reported previously (Faghih et al., 2007), positive allosteric modulators (PAMs) facilitate endogenous neurotransmission and/or enhance the efficacy and potency of an agonist without directly stimulating the agonist-binding sites. In principle, PAMs do not exhibit intrinsic activity at the receptor, however they can reinforce endogenous cholinergic neurotransmission without directly activating α7 nAChRs (Albuquerque et al., 2001; Faghih et al., 2007; Faghih et al., 2008). PAMs have been classified as either type I, such as NS1738, or type II, such as PNU-120596, on the basis of their distinct effects on desensitization (Bertrand and Gopalakrishnan, 2007; Bertrand et al., 2008; Timmermann et al., 2007). PNU-120596, but not NS1738, modifies the equilibrium among active and desensitized states resulting in significantly prolonged responses, even promoting the activation of previously desensitized receptors (Gronlien et al., 2007; Hurst et al., 2005; Roncarati et al., 2008). Initially, allosteric modulators of the α7 nAChRs were developed for the treatment of cognitive disorders such as Alzheimer’s disease and schizophrenia, however their effects in pain models have not been reported (Ahring et al., 2007; Faghih et al., 2007; Conejero-Goldberg et al., 2008; McLean et al., 2011).
Therefore, in the present study, we evaluated whether potentiating the endogenous α7 cholinergic system through the allosteric modulation of α7 nAChRs produces anti-inflammatory, anti-hyperalgesic and anti-allodynic effects in mouse models of inflammation and chronic neuropathic pain. Accordingly, NS1738 (a type I α7 nAChR PAM) and PNU-120596 (a type II α7 nAChR PAM) were evaluated in the carrageenan short-term inflammatory pain and the chronic constriction injury (CCI) neuropathic pain models. In addition, we evaluated whether PNU-120596 enhances the antinociceptive effects of a selective α7 nAChRs agonist, PHA-543611, in these models.
2. Materials and Methods
Subjects
Naïve male adult ICR (Harlan Laboratories; Indianapolis, IN) mice weighing between 20 and 30 g served as subjects. Mice were housed 4–5 per cage in a temperature-controlled (20–22°C) environment with a 12-h light-dark cycle and were given unlimited access to food and water in their home cages. All animals were maintained in a facility approved by the American Association for Accreditation of Laboratory Animal Care and the study was approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University. At the end of each experiment, the animals were euthanized by way of CO2 inhalation. We attest that all efforts made to minimize the number of animals used and their suffering.
Drugs
Methyllycaconitine citrate (MLA), was purchased from Sigma-Aldrich Inc. (St. Louis, MO). PNU 120596 [1-(5-Chloro-2,4-dimethoxy-phenyl)-3-(5-methyl-isoxazol-3-yl)] and PHA-543613 were obtained from the National Institute on Drug Abuse (NIDA) supply program (Bethesda, MD). NS 1738 [N-(5-Chloro-2-hydroxyphenyl)-N'-[2-chloro-5-(trifluoromethyl)phenyl] was purchased from Tocris Biosciences (Minneapolis, MN). All drugs except for PNU-120596 and NS1738 were dissolved in physiological saline (0.9% sodium chloride) and injected subcutaneously (s.c.) in a volume of 1ml/100 g body weight unless noted otherwise. PNU 120596 and NS1738 were dissolved in vehicle consisting of 1 volume ethanol, 1 volume Emulphor-620 (Rhone-Poulenc, Inc., Princeton, NJ) and 18 volumes distilled water, and injected via the (i.p.) route of administration. Doses of NS1738 and PNU-120596 were chosen based on their activity in in vivo models of memory and cognition (Timmermann et al., 2007; Christensen et al., 2010). Lambda-carrageenan was purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in saline. All doses are expressed as the free base of the drug.
Pain models
Carrageenan model of short inflammatory pain
These procedures have been previously described by (Lichtman et al., 2004). Briefly, edema was induced by giving an intraplantar injection of 0.5% lambda-carrageenan in a 20 µl volume into the hind right paw using a 301/2 gauge needle. Saline (20 µl) was injected into the left hind paw.
Measurement of paw edema
The thickness of the carrageenan-treated and control paws were measured both before and after carrageenan injection at the time points indicated in Results section, using digital calipers (Traceable Calipers, Friendswood, TX). Data were recorded to the nearest ± 0.01mm and expressed as change in paw thickness ΔPT = right paw thickness − left paw thickness.
Measurement of thermal hyperalgesia
Mice were placed in clear plastic chambers (7 cm × 9 cm × 10 cm) on an elevated glass surface and allowed to acclimatize for at least 30 min before testing. The infrared beam of a radiant heat source was directed at the plantar surface of each hind paw, in the area immediately proximal to the toes. A 20-second cut-off time was used. Three measures of paw withdrawal latency were taken and averaged for each hind-paw using the Hargreaves test (Yalcin et al., 2011). The paw withdrawal latency was defined as the time from the onset of radiant heat to withdrawal of the animal’s hind paw (Lichtman et al., 2004). Withdrawal thresholds were measured in each hind paw. Results were expressed either as withdrawal latency for each paw or as ΔPWL (s) = contralateral latency − ipsilateral latency.
NS1738, PNU-120596 or vehicle was administered 15 min prior to an intraplantar injection of carrageenan and then mice were tested 6 h after the injection for paw withdrawal latencies and paw diameters. For the antagonist study, s.c. MLA was injected 10 min prior to a PNU-120596 or NS1738 injection, which was followed 15 min later with carrageenan and then mice were tested 6 h after the last injection.
To determine whether PNU-1209596 can reduce established thermal hyperalgesia and paw edema after carrageenan injection, paw withdrawal latency and paw diameter were measured after the establishment of thermal hyperalgesia or paw edema, respectively (3 h after carrageenan administration). Subsequently, a treatment of either vehicle or PNU-120596 (8 mg/kg, i.p.) was administered 3 h after carrageenan, and paw withdrawal latency and paw diameter were measured 15 min, 1 h, and 3 h after either treatment. The dose of 8 mg/kg of PNU-1209596 was chosen since it is generally thought that reversal of inflammation usually requires higher doses than development.
Chronic constriction injury (CCI)
Mice were anesthetized with pentobarbital (45 mg/kg, i.p.). An incision was made just below the hipbone, parallel to the sciatic nerve. The right common sciatic nerve was exposed at the level proximal to the sciatic trifurcation, and a nerve segment 3–5 mm long was separated from surrounding connective tissue. Two loose ligatures of 6-0 silk suture, spaced 1.0–1.5 mm apart, were made around the nerve. Skin and muscles were closed with suture. This procedure resulted in chronic constrictive injury of the ligated nerve. In sham-operated controls, an identical surgical incision was performed on the same paw, except that the sciatic nerve was not ligated. For the purposes of this paper, the paw that underwent surgery will be referred to as the ipsilateral paw, and the paw that did not undergo surgery will be referred to as the contralateral paw. After surgery, mice were allowed to recover in a warmed cage on clean paper towels and then returned to their home cage after regaining consciousness. Any suture that remained after two weeks was removed from the healed surgical wound. We assessed both thermal hyperalgesia and mechanical allodynia in CCI mice via the Hargreaves test and von Frey filaments test, respectively.
Thermal hyperalgesia
Thermal hyperalgesia was measured via the Hargreaves test as described earlier, in the context of carrageenan. In the CCI model of neuropathic pain, mice were pretreated with either vehicle or NS1738, and then tested 15 min and 1, 3 and 6h later in the Hargreaves plantar stimulator test. The other group of mice was pretreated with vehicle or different doses of PNU-120596 and then tested 15 min and 1, 6 and 24 h later in the Hargreaves plantar stimulator test. In regard to the sham group; mice were pretreated with either vehicle or PNU-120596 and then tested 3 h later in the Hargreaves plantar stimulator test. For the antagonist study, mice were pretreated with MLA s.c. and then, 10 min later, were administered PNU-120596, and were tested in the Hargreaves plantar stimulator test 15 min later. The hyperalgesia scores were recorded in a blinded fashion.
Mechanical allodynia in mice
Mechanical allodynia thresholds were determined according to the method of Chaplan et al. (1994). Mice were placed in a Plexiglas cage with mesh metal flooring and allowed to acclimate for 30 min before testing. A series of calibrated von Frey filaments (Stoelting, Wood Dale, IL) with logarithmically incremental stiffness ranging from 2.83 to 5.88 expressed dsLog10 of [10 £ force in (mg)] were applied to the paw with a modified up-down method (Dixon, 1965). In the absence of a paw withdrawal response to the initially selected filament, a thicker filament corresponding to a stronger stimulus was presented. In the event of paw withdrawal, the next weaker stimulus was chosen. Each hair was presented perpendicularly against the paw, with sufficient force to cause slight bending, and held 2–3 s. The stimulation of the same intensity was applied 5 times to the hind paw at intervals of a few seconds. The mechanical threshold was expressed as Log10 of [10 £ force in (mg)], indicating the force of the Von Frey hair to which the animal reacted (paw withdrawn, licking or shaking). Mechanical stimuli thresholds were determined for each animal after 15 min, 3 and 6 h after the injection of NS1738, PNU-120596 and PHA-543613 in the mice that had CCI surgery. To determine whether PNU-120596 enhances the effects of the selective α7 agonist PHA-543613, PHA-543613-treated mice were given a 15 min pretreatment of PNU-120596 and were tested 30 min, 3 and 6 h after the PHA-543613 injection. The allodynia scores were recorded in a blinded fashion.
Locomotor activity
Mice were placed into individual Omnitech (Columbus, OH) photocell activity cages (28 × 16.5 cm) after 15 min after the i.p. administration of vehicle, NS1738 or PNU-120596 at different doses. Interruptions of the photocell beams (two banks of eight cells each), which assess walking and rearing, were then recorded for the next 30 min. Data were expressed as the number of photocell interruptions.
Motor coordination
We also evaluated the effect of PNU-120596, at doses that produce antinociceptive effects (8mg/kg, i.p.), in the rotarod test. In order to measure motor coordination, we used the rotarod test (IITC Inc. Life Science). The animals are placed on textured drums (1¼ inch diameter) to avoid slipping. When an animal falls onto the individual sensing platforms, test results are recorded. Five mice were tested at a time using a rate of 4 rpm. Naive mice were trained until they remained on the rotarod for 5 min. Animals that failed to meet this criterion within three trials were discarded. Fifteen min after the injection of vehicle or drugs, mice were placed on the rotarod for 5 min. If a mouse fell from the rotarod during this time period, it was scored as motor impaired. Percent impairment was calculated as follows: % impairment = [(180-test time/180*100]. Mice were pretreated with either i.p. vehicle or PNU-120596 15 min before the test.
Data analysis
The data obtained were analyzed using a GraphPad software program and expressed as the mean ± S.E.M. Statistical analyses were done using student’s t test and one-way or repeated measures two-way analysis of variance (ANOVA) tests. Tukey’s or Bonferroni’s tests were used for post hoc analysis. P-values less than 0.05 (p < 0.05) were considered significant.
Results
Effect of NS1738 and PNU-120596 in the Carrageenan test
Mice were given an intraplantar injection of carrageenan and then tested for hyperalgesia and edema 6 h later. Anti-hyperalgesic and anti-edematous effects of the α7 nAChRs PAMs were determined after i.p. administration of the drugs. The administration of 10 and 30 mg/kg of NS1738 15 min before intraplantar carrageenan blocked the development of hyperalgesic responses [F(2,27) = 9.07, p < 0.01] (Figure 1A). The anti-hyperalgesic effect of NS1738 at 30 mg/kg was blocked by pretreating the animals with MLA, a α7 nAChRs antagonist. A single s.c. injection of MLA (10 mg/kg, s.c.) given 10 min before i.p. NS1738 completely blocked the anti-hyperalgesic effects of NS1738 (30 mg/kg) [ΔPWL of Vehicle/Vehicle = 9.1 ± 0.6 s; Vehicle/NS1738 (30) = 3.1 ± 0.4 s; MLA/Vehicle = 8.5 ± 0.3 s; MLA/NS1738 = 8.7 ± 0.8 s]. However, none of the doses NS1738 tested did diminish the magnitude of paw edema [F(2,27) = 0.05, p = 0.95] (Figure 1B).
Figure 1. Effects of α7 nAChR PAMs NS1738 and PNU-120596 on carrageenan-induced hyperalgesia and edema in mice.
Animals were given an intraplantar injection of carrageenan, and hyperalgesia and paw-edema were assessed 6 h later. NS1738 (30mg/kg) at 15 min before carrageenan injection significantly reduced (A) thermal hyperalgesia, but not paw-edema (B). PNU-120596 (4mg/kg) significantly reduced both (C) thermal hyperalgesia and (D) paw edema induced by carrageenan. Data are expressed as means ± S.E.M of ΔPWL (s) or ΔPT (mm) of 6–8 mice. *p < 0.05 when compared to mice that were given an intraplantar injection of carrageenan and an i.p. injection of vehicle.
In contrast, administration of PNU-120596 15 min before an intraplantar carrageenan injection blocked hyperalgesic responses [F(2,15) = 17.26, p < 0.001] (Figure 1C) and significantly diminished the magnitude of paw edema [F(2,15) = 20.46, p < 0.001] (Figure 1D) at 6 h after intraplantar carrageenan administration. The anti-hyperalgetic effects of PAMs much later that the time points shown in Figure 2 such as 8, 12, 24 and 48 hours after carrageenan injection. PNU-120506 does reverses carrageenan-induced thermal hyperalgesia at all of these times. However, because of space limitations and simplicity of presentation, the results were not shown. We now include a statement about these times in the Results section.
Figure 2. Blockade of PNU-120596 and NS1738 effects on carrageenan-induced hyperalgesia and edema in mice by the α7 nAChR antagonist MLA.
(A) PNU-120596 (4mg/kg, i.p., 15 min treatment) significantly attenuated carrageenan-induced hyperalgesia. This anti-hyperalgesic effect was blocked by a pretreatment of α7 nAChR antagonist MLA (10 mg/kg, s.c.) 10 min before PNU-120596 administration. (B) PNU-120596 (4mg/kg) administration also significantly attenuated carrageenan-induced paw edema, and this effect was blocked by 10 min pretreatment with MLA, (10 mg/kg, s.c.) before PNU-120596. Data are expressed as means ± S.E.M of ΔPWL (s) or ΔPT (mm) of 6–8 mice. (C) NS1738 (30 mg/kg, i.p., 15 min treatment) significantly attenuated carrageenan-induced hyperalgesia. This anti-hyperalgesic effect was blocked by a pretreatment of α7 nAChR antagonist MLA (10 mg/kg, s.c.) 10 min before NS1738administration. *p < 0.05 when compared to mice that were given an intraplantar injection of carrageenan and an s.c. injection of vehicle. PNU = PNU-120596; NS = NS1738.
We next evaluated whether inhibition of α7 nAChRs would block PNU-120596’s effects on hyperalgesia and paw edema. A single s.c. injection of MLA (10 mg/kg, s.c.) given 10 min before i.p. PNU-120596 completely blocked the anti-hyperalgesic effects of PNU-120596 [F(3,36) = 12.84, p < 0.001] (Figure 2A). Similarly, s.c. MLA given 10 min before PNU-120596 completely antagonized PNU-120596 anti-edematous effects [F(3,36) = 11.44, p < 0.001] (Figure 2B). Additionally, MLA (10 mg/kg, s.c.) given 10 min before i.p. NS1738 completely blocked the anti-hyperalgesic effects of NS1738 (Figure 2C).
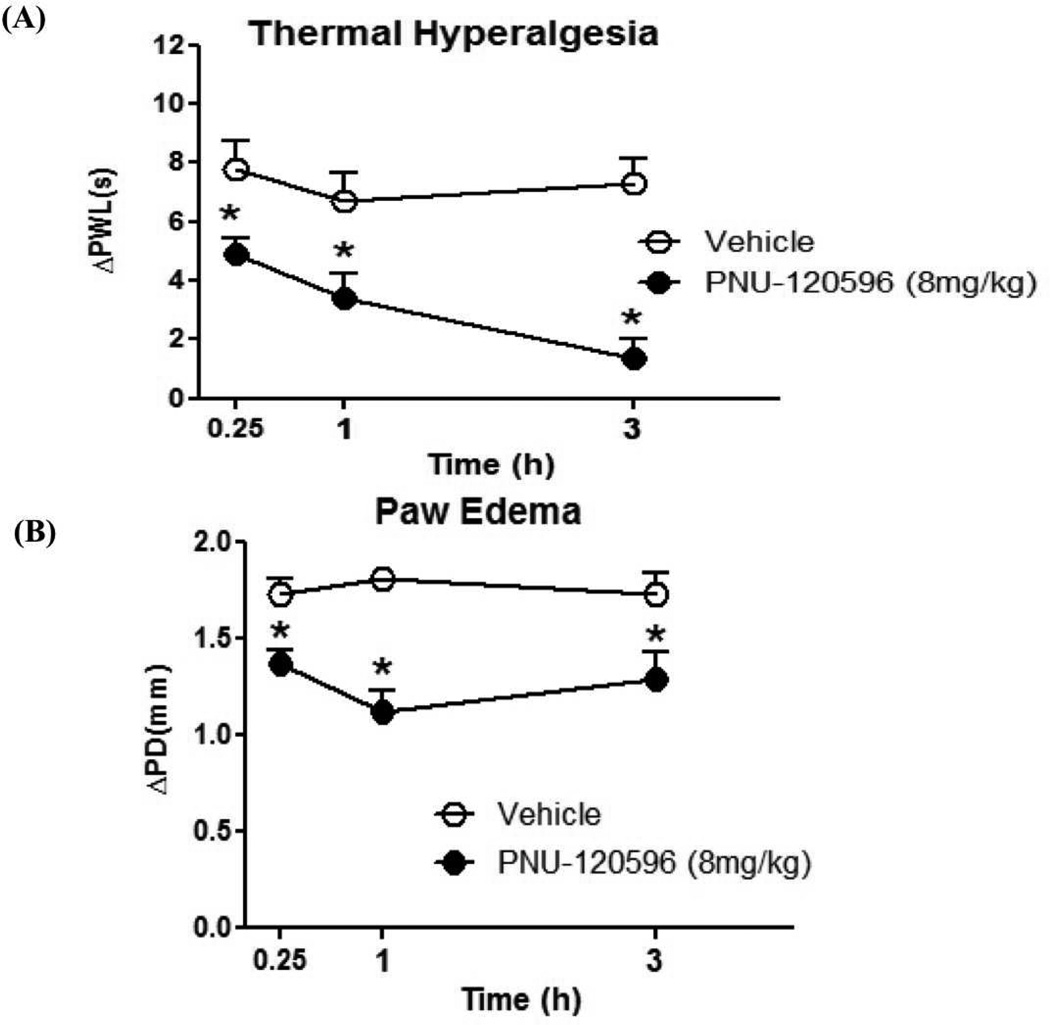
We then addressed the clinically relevant question of whether PNU-120506 can reverse carrageenan-induced thermal hyperalgesia and paw edema after they have been established. This is a particularly important question as anti-inflammatory agents are most often administered after inflammation has already occurred. Mice were administered intraplantar carrageenan and then, 3 h later, administered PNU-120596 (i.p.). Subsequently, mice were tested for thermal hyperalgesia and for paw thickness, at 15 min, 1 h and 3 h post-PNU-120596 treatment. As shown in Figure 3A, PNU-120596 reversed carrageen-induced thermal hyperalgesia at 15 min [t(10) = 2.542, p < 0.05], 1 h [t(10) = 2.58, p < 0.05] and 3 h [t(10) = 5.36, p < 0.001] after the carrageenan injection. Likewise, as shown (Figure 3B) PNU-120596 attenuated carrageenan-induced paw edema at 15 min [t(10) = 3.10, p < 0.05], 1h [t(10) = 5.55, p < 0.001] and 3h [t(10) = 2.47, p < 0.05] after the establishment of carrageenan-induced thermal hyperalgesia.
Figure 3. Reversal of carrageenan-induced hyperalgesia and paw edema by PNU-120596 in mice.
Animals were given an intraplantar injection of carrageenan and 3 h later they received received PNU-120596 (8mg/kg, i.p). Thermal hyperalgesia and paw diameter were then measured 0.25, 1 and 3 h after administration of the drug. PNU-120596 significantly reversed carrageenan-induced (A) thermal hyperalgesia and (B) paw edema at the different times tested. Data are expressed as means ± S.E.M of ΔPWL (s) or ΔPT (mm) of 6–8 mice per group. *p < 0.05 when compared to the correspondent vehicle group.
Effects of NS1738 and PNU-120596 in the CCI model
Effects of on thermal hyperalgesia in the CCI mice
In the CCI model of neuropathic pain, NS1738 (30mg/kg) did not attenuate thermal hyperalgesia at any time point tested (Figure 4). Ten days after CCI surgery, Δ paw withdrawal latencies in the NS1738-treated CCI group were not significantly different than thresholds recorded from the vehicle-treated CCI group [F(3,4) = 0.69, p = 0.60].
Figure 4. The α7 nAChR type I PAM NS1738 did not attenuate chronic constriction injury (CCI)-induced thermal hyperalgesia in mice.
Ten days after chronic constriction injury, mice were pretreated with either vehicle or NS1738, and then tested 15, 60, 180 and 360 min later in the Hargreaves plantar stimulator test. NS1738 (30mg/kg, i.p.) did not attenuate CCI-induced thermal hyperalgesia. Sham group did not differ between vehicle and drug treatment groups (data not shown). Results were expressed as means ± SEM paw latencies in s from ipsilateral and contralateral paws (n = 6–8 mice per group). *p < 0.05 when compared to contralateral paw latency. Veh = vehicle; NS = NS1738.
The effects of PNU-120596 (1, 2 and 4 mg/kg, i.p.) on the thermal sensitivity in sham mice group were measured 3 h after injection As shown in Table 1, PNU-120596 did not produce significant [F(3,20) = 0.84, p = 0.49] differences in thermal sensitivity in control mice.. In contrast, PNU-120596 dose-dependently reversed thermal hyperalgesia in injured mice as shown in Figure 5. These anti-hyperalgesic effects were seen at 15 min [F(3,20) = 9.78, p < 0.001], 3 h [F(3,20) = 8.28, p < 0.001] and 6 h [F(3,20) = 5.13, p < 0.01], but disappeared 24 h after PNU-120596 administration [t(10) = 0.11, p = 0.92]. The anti-hyperalgesic effects of PNU-120596 were also evident when the results were expressed as AUC values (Table 2).
Table 1. The Effect PNU-120596 on Paw Withdraw Latency in Sham Mice.
PNU-120596 at various doses did not alter thermal sensitivity as measured in the Hargreaves plantar stimulator in sham groups. Data are expressed as means ± S.E.M. of 6–8 mice per group.
| Treatment | Dose (mg/kg) | PWL (s) ± S.E.M. |
|---|---|---|
| Vehicle contralateral | 0 | 2.52 ± 0.26 |
| Vehicle ipsilateral | 0 | 2.26 ± 0.31 |
| PNU-120596 | 1 | 2.30 ± 0.25 |
| PNU-120596 | 2 | 2.53 ± 0.32 |
| PNU-120596 | 4 | 2.32 ± 0.23 |
Figure 5. The α7 nAChR type II PAM PNU-120596 did attenuate chronic constriction injury (CCI)-induced thermal hyperalgesia in mice.
Ten days after chronic constriction injury, mice were pretreated with either vehicle or different doses of PNU-120596, and then tested 0.25, 3, 6 and 24 h later in the Hargreaves plantar stimulator test. PNU-120596 (1, 2 and 4 mg/kg, i.p.) did attenuate CCI-induced thermal hyperalgesia in mice in a dose- and time-related manner; *p < 0.05 when compared to vehicle contralateral paw latency and #p < 0.05 when compared to vehicle to ipsilateral paw latency. Data are expressed as means ± S.E.M. of 6–8 mice per group. Veh = vehicle; PNU = PNU-120596.
Table 2. The Effect of PNU-120596 on Thermal Hyperalgesia in the CCI Mice.
The anti-hyperalgesic effect of PNU-120596 was measured as the area under the curve (AUC) of ΔPWL values. Data are expressed as means and S.E.Ms of 6–8 mice per group.
| Treatment | Dose (mg/kg) | AUC ± S.E.M. |
|---|---|---|
| Vehicle | 0 | 11.13 ± 0.33 |
| PNU-120596 | 1 | 9.45 ± 0.4 |
| PNU-120596 | 2 | 4.25 ± 0.48* |
| PNU-120596 | 4 | 4.25 ± 0.33* |
p < 0.05 when compared to the zero dose (vehicle).
To determine whether the anti-hyperalgesic effects of PNU-120596 were dependent on α7 nAChRs, mice were administered MLA (10mg/kg, s.c.), an α7 nAChRs antagonist, 10 min before PNU-120596 and thermal hyperalgesia was measured 15 min after PNU-120596 (4mg/kg, i.p.) administration. MLA significantly blocked the anti-hyperalgesic effect of PNU-120596 (4 mg/kg) [F(2,10) = 13.35, p < 0.01]. Indeed, a single s.c. injection of MLA (10 mg/kg, s.c.) given 10 min before i.p. PNU-120596 completely blocked the anti-hyperalgesic effects of the drug [ΔPWL of Vehicle/Vehicle = 8.5 ± 0.9 s; Vehicle/PNU-120596(4) = 1.8 ± 0.5 s; MLA/PNU-120596 = 7.8 ± 0.8 s].
Effects of NS1738 and PNU-120596 on mechanical allodynia in CCI mice
Fifteen days after the CCI surgery, mice were given an i.p. injection of NS1738, PNU-120596, or vehicle. In mice that received vehicle or NS1738, ipsilateral paws displayed significantly lower thresholds than contralateral paws after 15 min [t(10) = 4.19, p < 0.01], 3 h [t(10) = 4.34, p < 0.01] and 6 h [t(10) = 4.90, p < 0.001] as shown in Figure 6A. NS1738 (30mg/kg i.p.) treatment did not significantly reverse mechanical allodynia thresholds produced by a vehicle (i.p.) treatment at 15 min [t(10) = 1.76, p = 0.11], 3 h [t(10) = 1.33, p = 0.21], or 6 h [t(10) = 2.21, p = 0.05] (Figure 6A).
Figure 6. CCI-induced mechanical allodynia in mice was attenuated by PNU-120596 but not by NS1738 treatment.
Ten days after chronic constriction injury, mice were pretreated with either vehicle, NS1738 or PNU-120596 and then tested 0.25, 3 and 6 h later in the mechanical allodynia test. (A) NS1738 (30mg/kg, i.p.) did not attenuate CCI-induced allodynia at any of the times tested. (B) PNU-120596 (1, 4 and 8 mg/kg, i.p.) significantly attenuated CCI-induced mechanical allodynia in a dose-related manner. Mechanical sensitivity in sham mice did not differ between drug treatments (data not shown). Each point represents the mean ± SE of 6 – 8 mice. Data are presented as means ± S.E.M. of paw withdrawal thresholds. *p < 0.05 when compared to vehicle contralateral paw withdrawal thresholds and #p < 0.05 when compared to vehicle to ipsilateral paw withdrawal thresholds. Veh = vehicle; PNU = PNU-120596.
In a separate group of injured mice treated with vehicle, ipsilateral paws displayed significantly lower thresholds than contralateral paws after at 15 min [t(8) = 5.84, p < 0.001], 3 h [t(8) = 6.03, p < 0.001] and 6 h [t(8) = 6.05, p < 0.001] as shown (Figure 6B). In contrast to NS1738, i.p. PNU-120596 dose-dependently produced significant anti-allodynic effects [F(3,17) = 6.15, p < 0.01], compared to those produced by an i.p. vehicle treatment, when tested 15 min post-treatment (Figure 6B). The anti-allodynic effects of PNU-120596 (4 mg/kg) persisted for 3 h but no longer significantly differed from vehicle at 6h [F(3,17) = 3.20, p = 0.05] (Figure 6B). Thresholds produced by PNU-120596-treated mice in the sham group (the control) did not differ from those produced by vehicle-treated mice (data not shown).
We next tested the effects of combination of PHA-543613, a selective α7 agonist, and PNU-120596 in the CCI model. As depicted in Figure 7A, PHA-543613 on its own reverses mechanical allodynia at 3 h after s.c. administration in a dose-related manner. Figure 7B show the effects of combination of low inactive doses of PNU-120596 (1mg/kg, i.p.) and PHA-543613 (2mg/kg) in the CCI test. Low doses of PNU-120596 or PHA-543613 did not significantly block mechanical allodynia at any time point. However, the co-administration of these subthreshold doses of PNU-120596 and PHA-543613 significantly reversed mechanical allodynia at 3 h post-treatment compared to the administration of vehicle then vehicle [t(8) = 2.72, p < 0.05]. However, no significant anti-allodynic effects were found at 30 min [t(8) = 1.57, p = 0.16] or 6 h [t(8) = 0.91, p = 0.39] post-treatment (Figure 7B).
Figure 7. The α7 nAChR type II PAM PNU-120596 enhanced the anti-allodynic effects of the α7 nAChR agonist PHA-543613.
(A) PHA-543613 (1, 2 and 6 mg/kg, s.c.) on its own significantly attenuated CCI-induced mechanical allodynia in a dose-related manner 3h after injection of the drug. The anti-allodynic effect of PHA-543613 was significant at the dose of 6 mg/kg. (B) PNU-120596 pretreatment enhanced PHA-543613 in the CCI-induced mechanical allodynia test. Mice were pretreated with a low dose of PNU-120596 (1 mg/kg, i.p.) and 15 min later they received an inactive dose of PHA-543613 (2 mg/kg, s.c.). Mice were then tested at 0.5, 3 and 6 h after treatment in the mechanical allodynia test. Neither PNU-120596 nor PHA-543613 attenuated CCI-induced allodynia at 30 min, 3 h, or 6 h; allodynia thresholds were significantly different in ipsilateral paws (with CCI) than contralateral paws (without CCI). PNU-120596 pretreatment before PHA-543613 administration significantly attenuated CCI-induced allodynia at the same doses mentioned before at 3 h, but not at 30 min or 6 h, post-treatment. Data are presented as means ± S.E.M. of paw withdrawal thresholds of 6–8 mice. *p < 0.05 vs. vehicle contralateral paw withdrawal thresholds; #p < 0.05 when compared to vehicle to ipsilateral paw withdrawal thresholds. Veh = vehicle; PHA = PHA-543613; Contra = contralateral; Ipsi = ipsilateral.
In order to infer whether motor impairment may have contributed to the effects of NS1738 and PNU-120596 in the pain tests, we evaluated NS1738 and PNU-120596 on spontaneous activity and motor coordination in mice. Mice treated with an active dose of NS1738 (30mg/kg, i.p.) or PNU-120596 (8 mg/kg, i.p.) did not show significant changes in locomotor activity [F(2,10) = 2.91, p = 0.10] 15 min after injection [Vehicle = 1508 ± 230 interrupts; NS1738(30) = 1750 ± 210 interrupts; PNU-120596(8) = 1230 ± 310 interrupts]. We also evaluated whether an effective antinociceptive dose of PNU-120596 would impair performance in the rotarod test. Mice treated with PNU-120596 (8 mg/kg, i.p.) did not show deficits in motor coordination [F(2,12) = 0.09, p = 0.92] 15 min after testing [Vehicle = 10 ± 7% impairment; PNU-120596(8) = 12 ± 8 % impairment].
Discussion
The objective of the present study was to examine the effects of type I (NS1738) and II (PNU-120596) α7 nAChR PAMs in murine inflammatory and chronic neuropathic pain models after acute administration. Overall, our results showed that while both NS1738 and PNU-120596 attenuated hyperalgesia associated with inflammation, only PNU-120596 decreased the hyperalgesia and allodynia in the chronic neuropathic pain model. Whereas PNU-120596 produced consistent effects in carrageenan and CCI models, there was a surprising disparity of the effects of NS1738 on hyperalgesia and edema. In addition, PNU-120596’s effects were blocked by MLA, suggesting that α7 nAChRs play a critical role in its action. Importantly, the antinocieptive effects of PNU-120596 occurred at doses that had no effect on motor function and coordination in mice.
One possible explanation for the difference between these two drugs in the present study is may be due to their mechanisms of modulation of α7 nAChRs. As mentioned earlier, type I and II PAMs enhance endogenous α7 neurotransmission through different mechanisms. PNU-120596, a type II PAM, via α7 nAChR activation, increases channel conductance and permeability by increasing channel mean open time while not altering the physiological response pattern, whereas NS1738, a type I PAM, does not have this ability (Hurst et al., 2005). Indeed, type I PAMs reduce the height of energy barriers for transitions into active states immediately resulting in potentiated responses with conserved kinetics. In contrast, type II PAMs not only increase the degree of activation immediately after agonist applications, but also alter the equilibrium between active and desensitized states resulting in dramatically prolonged responses, even promoting activation of previously desensitized receptors (Grønlien et al., 2007). More specifically, PNU-120596’s anti-inflammatory (anti-edema) activity may be explained by the sustained α7 nAChR activation leading to the induction of anti-inflammatory pathways. A possible pathway is the inhibition of nuclear factor-kB (NF-kB), which induces macrophage activation and pro-inflammatory cytokine production (Lee et al., 1994; Baeuerle & Henkel, 1994). Support for this mechanism lays in the fact that NF-kB has been shown to reliably occur after carrageenan-induced paw edema and has been associated with nicotine’s anti-inflammatory action (Bhattacharyya et al., 2008; Borthakur et al., 2007; Menegazzi et al., 2008; Min et al., 2009; Wang et al., 2004). It will be important in future studies to compare the effects of PNU-120596 and NS1738 in affecting the NF-kB pathway and pro-inflammatory cytokine production.
PNU-120596 significantly reduced carrageenan-induced paw edema and thermal hyperalgesia after onset. This reversal of nociceptive and edematous responses has potential clinical significance, because anti-inflammatory drugs are typical given to patients after the onset of inflammation. Several explanations may account for PNU-120596’s effects. First, the difference between PNU-120596 and NS1738 effects suggest that desensitization may play an important in PNU-120596’s efficacy. The slower rate of desensitization induced by PNU-120596 could be enhancing Ca2+ influx, therefore magnifying downstream effects. Although it has been reported that Ca2+ influx through a nAChR channel itself seems to contribute very little to the cytosolic concentration of calcium (Zhou and Neher, 1993), other studies report that Ca2+ permeability through α7 nAChRs is important in cellular processes (Seguela et al., 1993). A recent study showed that α7 nAChR-mediated calcium signaling and catecholamine release in bovine chromaffin cells requires PNU-120596 (del Barrio et al., 2011). There is also evidence for α7 nAChRs-mediated noradrenaline release (Rowley and Flood, 2008) in the lumbar spinal cord as well as serotonin release (Cordero-Erausquin & Changeux, 2001). Second, PNU-120596, in the presence of endogenous ACh and choline, may activate α7 nAChRs expressed in the nociceptive system and on macrophages, consequently inhibiting TNF-α synthesis (Bernik et al., 2002). In line with this suggestive mechanism, Munson et al. (2012) recently showed that PNU-120596 produces anti-hyperalgesic and anti-inflammatory effects in the formalin, carrageenan or complete Freund's adjuvant (CFA) tests in rats through a decrease in TNF-α and IL-6 levels.
In addition to differences between the effects of NS1738 and PNU-120596 in the carrageenan inflammatory pain model, both drugs produced differential effects in the chronic neuropathic pain (CCI) model. While NS1738 (30mg/kg) was inactive in this model, PNU-120596 dose-dependently reduced thermal hyperalgesia and mechanical allodynia via α7 nAChRs. Furthermore, PNU-120596 had no antinociceptive effect in contralateral paws (data not shown) or in sham-operated mice, indicating that it was antinociceptive under pain conditions, only. Considering NS1738’s lack of effect in the CCI model, poor blood-brain barrier permeability is improbable, since previous studies have shown that NS1738 was present at high enough concentrations in the brain after i.p. administration (Timmermann et al., 2007). Another possibility is receptor desensitization and α7 nAChR regulation, as discussed earlier with the carrageenan model (Hurst et al., 2005; Papke et al., 2009). Moreover, inflammatory and neuropathic pain might have different patho-mechanistic components (Walker et al., 1999), thus explaining NS1738’s different effects in carrageenan-induced and CCI-induced pain models.
As α7 nAChRs PAMs presumably have the capability of enhancing α7 nAChRs agonist response (Gronlien et al., 2007; Hurst et al., 2005), we investigated the interaction between PNU-120596 and the selective α7 nAChRs agonist PHA-543613. As predicted, the combination of PNU-120596 and PHA-543613 produced enhanced anti-allodynic effects in the CCI model compared to the effects of each drug given alone. Therefore, these results suggest that PNU-120596 potentiates both endogenous cholinergic tone and the effect of the exogenous α7 nAChRs agonist PHA-543613.
α7 nAChR-dependent neuroinflammatory processes may also be involved in the neuroprotective effects of α7 PAMs in neuropathic pain. This hypothesis is supported by α7 nAChR expression in anti-neuroinflammatory peripheral macrophages and murine microglia (Wang and Wang, 2003; Shytle et al., 2004; De Simone et al., 2005). A recent study showed that selective α7 nAChR agonist PNU-282987 could reduce hyperalgesia, edema and macrophagic infiltration in the sciatic nerve.
The exact mechanisms involved in the antinociceptive effects of PNU-120596 in the chronic pain models are currently unknown. Previous work has related CCI-induced neuropathic pain and DRG α7 nAChR up-regulation (Carnevale et al., 2007; Feuerbach et al., 2009; Fucile et al., 2005; Xiao et al., 2002). Thus, PNU-120596 could be enhancing endogenous cholinergic tone through α7 nAChRs in DRG neurons, reducing pain-related behaviors. PNU-120596 could also be acting through spinal α7 nAChRs (Genzen & McGehee, 2003), though other reports reported that central α7 nAChRs are not involved in thermal and mechanical nociceptive hypersensitivity in mice (Gao et al., 2010; Rashid et al., 2006). These discrepancies may be due to the differential modulation of synapses and nociceptive properties, such as pain intensity, in various pathways (Martin et al., 1990; Millan, 2002; Yoshimura & Furue, 2006; Gao et al., 2010) and variation in methodology (ex. testing apparatus). Furthermore, PNU-120596 may indirectly, through excitatory neurons, or directly, through inhibitory neurons, modulate inhibitory neurotransmitter, such as serotonin, release. Cordero-Erausquin and Changeux (2001) have shown that ACh from excitatory neurons may tonically activate inhibitory interneuron nAChRs, possibly allowing PNU-120596 to enhance α7 nAChR activation and indirectly activate inhibitory neurons.
Taken together, the results of the present study demonstrate, for the first time, that α7 nAChR PAMs have anti-hyperalgesic and anti-allodynic properties in inflammatory and chronic neuropathic pain models. Furthermore NS1738 and PNU-120596 differentially modulate endogenous cholinergic tone in these two models. Specifically, we found that PNU-120596 produced significant anti-hyperalgesic and anti-allodynic effects, but NS1738 was inactive in the chronic neuropathic pain model. In contrast to pain models, both type I and II PAMs improve memory and cognition in rodents (Thomsen et al., 2011; Hurst et al., 2005; Ng et al., 2007). Though PAMs are currently being developed for the treatment of cognitive deficits in patients with schizophrenia or Alzheimer’s disease (Thomsen et al., 2010), we demonstrate in our study that type II α7 nAChR PAMs may be potential candidates for treating chronic pain.
Highlights.
Alpha7 positive allosteric modulators are anti-inflammatory
PNU-120596 reversed carrageenan-induced thermal hyperalgesia and paw edema.
PNU-120595 anti-inflammatory effects were α7 receptor-mediated.
NS1738 did not attenuate hyperalgesia and allodynia in the CCI model.
PNU-120596 reversed thermal hyperalgesia and allodynia in the CCI model..
ABBREVIATIONS
- nAChR
nicotinic acetylcholine receptor(s)
- s.c.
subcutaneous injection
- i.p.
intraperitoneally
- CNS
central nervous system
- ACh
acetylcholine
- PAMs
positive allosteric modulators
- CCI
chronic constriction injury
- MIF
migration inhibitory factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahring PK, Peters D, Holst D, Christensen GK, et al. An Allosteric Modulator of the α7 Nicotinic Acetylcholine Receptor Possessing Cognition-Enhancing Properties in Vivo. J Pharmacol Exp Ther. 2007;323:294–307. doi: 10.1124/jpet.107.120436. [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Santos MD, Alkondon M, Pereira EF, Maelicke A. Modulation of nicotinic receptor activity in the central nervous system: a novel approach to the treatment of Alzheimer disease. Alzheimer Dis Assoc Disord. 2001;15(Suppl 1):S19–S25. doi: 10.1097/00002093-200108001-00004. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Bannon AW, Decker MW, Holladay MW, Curzon P, Donnelly-Roberts D, Puttfarcken PS, Bitner RS, Diaz A, Dickenson AH, Porsolt RD, et al. Broad-spectrum, non-opioid analgesic activity by selective modulation of neuronal nicotinic acetylcholine receptors. Science. 1998;279:77–81. doi: 10.1126/science.279.5347.77. [DOI] [PubMed] [Google Scholar]
- Bernik TR, Friedman SG, Ochani M, DiRaimo R, Ulloa L, Yang H, Sudan S, Czura CJ, Ivanova SM, Tracey KJ. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J Exp Med. 2002;195:781–788. doi: 10.1084/jem.20011714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand D, Bertrand S, Ballivet M. Pharmacological properties of the homomeric alpha 7 receptor. Neurosci Lett. 1992;146:87–90. doi: 10.1016/0304-3940(92)90179-b. [DOI] [PubMed] [Google Scholar]
- Bertrand D, Bertrand S, Cassar S, Gubbins E, Li J, Gopalakrishnan M. Positive allosteric modulation of the α7 nicotinic acetylcholine receptor: ligand interactions with distinct sites and evidence for a prominent role of the M2-M3 segment. Mol Pharmacol. 2008;74:1407–1416. doi: 10.1124/mol.107.042820. [DOI] [PubMed] [Google Scholar]
- Bertrand D, Gopalakrisshnan M. Allosteric modulation of nicotinic acetylcholine receptors. Biochem Pharmacol. 2007;74:1155–1163. doi: 10.1016/j.bcp.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Dudeja PK, Tobacman JK. Carrageenan-induced NFkappaB activation depends on distinct pathways mediated by reactive oxygen species and Hsp27 or by Bcl10. Biochim Biophys Acta. 2008;1780:973–982. doi: 10.1016/j.bbagen.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthakur A, Bhattacharyya S, Dudeja PK, Tobacman JK. Carrageenan induces interleukin-8 production through distinct Bcl10 pathway in normal human colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G829–G838. doi: 10.1152/ajpgi.00380.2006. [DOI] [PubMed] [Google Scholar]
- Carnevale D, De Simone R, Minghetti I. Microglia-neuron interaction in inflammatory and degenerative diseases: role of cholinergic and noradrenergic systems. CNS Neurol Disord Drug Targets. 2007;6:388–397. doi: 10.2174/187152707783399193. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Christensen DZ, Mikkelsen JD, Hansen HH, Thomsen MS. Repeated administration of alpha7 nicotinic acetylcholine receptor (nAChR) agonists, but not positive allosteric modulators, increases alpha7 nAChR levels in the brain. J Neurochem. 2010;114:1205–1216. doi: 10.1111/j.1471-4159.2010.06845.x. [DOI] [PubMed] [Google Scholar]
- Conejero-Goldberg C, Davies P, Ulloa L. Alpha7 nicotinic acetylcholine receptor: a link between inflammation and neurodegeneration. Neurosci Biobehav Rev. 2008;32:693–706. doi: 10.1016/j.neubiorev.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson J. Side-effects of antidepressants. Br J Psychiatry Suppl. 1993;20:20–24. [PubMed] [Google Scholar]
- Cordero-Erausquin M, Changeux JP. Tonic nicotinic modulation of serotoninergic transmission in the spinal cord. Proc Nati Acad Sci USA. 2001;98:2803–2807. doi: 10.1073/pnas.041600698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero-Erausquin M, Pons S, Faure P, Changeux JP. Nicotine differentially activates inhibitory and excitatory neurons in the dorsal spinal cord. Pain. 2004;109:308–318. doi: 10.1016/j.pain.2004.01.034. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Meyer EM, Martin BR. The antinociceptive effects of alpha 7 nicotinic agonists in an acute pain model. Neuropharmacology. 2000;39:2785–2791. doi: 10.1016/s0028-3908(00)00139-8. [DOI] [PubMed] [Google Scholar]
- del Barrio L, Egea J, León R, Romero A, Ruiz A, Montero M, Alvarez J, López MG. Calcium signalling mediated through α7 and non-α7 nAChR stimulation is differentially regulated in bovine chromaffin cells to induce catecholamine release. Br J Pharmacol. 2011;162:94–110. doi: 10.1111/j.1476-5381.2010.01034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol. 2007;151:915–929. doi: 10.1038/sj.bjp.0707264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa MJ, Dionisio L, Agriello E, Bouzat C, Esandi MC. Alpha 7 nicotinic acetylcholine receptor modulates lymphocyte activation. Life Sci. 2009;85:444–449. doi: 10.1016/j.lfs.2009.07.010. [DOI] [PubMed] [Google Scholar]
- De Simone R, Ajmone-Cat MA, Carnevale D, Minghetti L. Activation of alpha7 nicotinic acetylcholine receptor by nicotine selectively up-regulates cyclooxygenase-2 and prostaglandin E2 in rat microglial cultures. J Neuroinflammation. 2005;2:4. doi: 10.1186/1742-2094-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Barrio L, Egea J, León R, Romero A, Ruiz A, Montero M, Alvarez J, López MG. Calcium signalling mediated through α7 and non-α7 nAChR stimulation is differentially regulated in bovine chromaffin cells to induce catecholamine release. Br J Pharmacol. 2011;162:94–110. doi: 10.1111/j.1476-5381.2010.01034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon W. The up-and-down method for small samples. J Am Stat Assoc. 1965;60:967–978. [Google Scholar]
- Dray, Bevan Inflammation and hyperalgesia: highlighting the team effort. Trends Pharmacol Sci. 1993;14:287–290. doi: 10.1016/0165-6147(93)90041-H. [DOI] [PubMed] [Google Scholar]
- Faghih R, Gfesser GA, Gopalakrishnan M. Advances in the discovery of novel positive allosteric modulators of the alpha7 nicotinic acetylcholine receptor. Recent Pat CNS Drug Discov. 2007;2:99–106. doi: 10.2174/157488907780832751. [DOI] [PubMed] [Google Scholar]
- Faghih R, Gopalakrishnan M, Briggs CA. Allosteric modulators of the alpha7 nicotinic acetylcholine receptor. J Med Chem. 2008;51:701–712. doi: 10.1021/jm070256g. [DOI] [PubMed] [Google Scholar]
- Feuerbach D, Lingenhoehl K, Olpe HR, Vassout A, Gentsch C, Chaperon F, Nozulak J, Enz A, Bilbe G, McAllister K, et al. The selective nicotinic actylcholine receptor alpha7 agonist JN403 is active in animal models of cognition, sensory gating, epilepsy and pain. Neuropharmacology. 2009;56:254–263. doi: 10.1016/j.neuropharm.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Fucile S, Sucapane A, Eusebi F. Ca2+ permeability of nicotinic acetylcholine receptors from rat dorsal root ganglion neurones. J Physiol. 2005;565:219–228. doi: 10.1113/jphysiol.2005.084871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:281–286. doi: 10.2174/1568010054022024. [DOI] [PubMed] [Google Scholar]
- Gao B, Hierl M, Clarkin K, Juan T, Nguyen H, Valk M, Deng H, Guo W, Lehto SG, Matson D, et al. Pharmacological effects of nonselective and subtype-selective nicotinic acetylcholine receptor agonists in animal models of persistent pain. Pain. 2010;149:33–49. doi: 10.1016/j.pain.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Genzen JR, McGehee DS. Nicotinic modulation of GABAergic synaptic transmission in the spinal cord dorsal horn. Brain Res. 2003;1031:229–237. doi: 10.1016/j.brainres.2004.10.042. [DOI] [PubMed] [Google Scholar]
- Grønlien JH, Håkerud M, Ween H, Thorin-hagene K, Briggs CA, Gopalakrishnan M, Malysz J. Distinct Profiles of α7 nAChR Positive Allosteric Modulation Revealed by Structurally Diverse Chemotypes. Mol Pharmacol. 2007;72:715–724. doi: 10.1124/mol.107.035410. [DOI] [PubMed] [Google Scholar]
- Gurun MS, Parker R, Eisenach JC, Vincler M. The effect of peripherally administered CDP-choline in an acute inflammatory pain model: the role of alpha7 nicotinic acetylcholine receptor. Anesth Analg. 2009;108:1680–1687. doi: 10.1213/ane.0b013e31819dcd08. [DOI] [PubMed] [Google Scholar]
- Hamurtekin E, Gurun MS. The antinociceptive effects of centrally administered CDP-choline on acute pain models in rats: the involvement of cholinergic system. Brain Res. 2006;1117:92–100. doi: 10.1016/j.brainres.2006.07.118. [DOI] [PubMed] [Google Scholar]
- Harden RN. Chronic neuropathic pain. Mechanisms, diagnosis, and treatment. Neurologist. 2005;11:111–122. doi: 10.1097/01.nrl.0000155180.60057.8e. [DOI] [PubMed] [Google Scholar]
- Hurst RS, Hajo M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, Rutherford-Root KL, Berkenpas MB, Hoffmann WE, Piotrowsqi DW, et al. A Novel Positive Allosteric Modulator of the α7 Neuronal Nicotinic Acetylcholine Receptor : In Vitro and In Vivo Characterization. J Neurosci. 2005;25:4396–4405. doi: 10.1523/JNEUROSCI.5269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan I, Osaka H, Stanislaus S, Calvo RM, Deerinck T, Yaksh TL, Taylor P. Nicotinic acetylcholine receptor distribution in relation to spinal neurotransmission pathways. J Comp Neurol. 2003;467:44–59. doi: 10.1002/cne.10913. [DOI] [PubMed] [Google Scholar]
- Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Shelton CC, Advani T, Cravatt BF. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain. 2004;109:319–327. doi: 10.1016/j.pain.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Marrero MB, Bencherif M. Convergence of alpha 7 nictonic acetylcholine receptor-activated pathways for anti-apoptosis and anti-inflammation: central role for JAK2 activation of STAT 3 and NF-kappaB. Brain Res. 2009;1256:1–7. doi: 10.1016/j.brainres.2008.11.053. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Gupta NK, Loo CM, Rohde DS, Basbaum AI. Differential effects of neurotoxic destruction of descending noradrenergic pathways on acute and persistent nociceptive processing. Pain. 1990;80:57–65. doi: 10.1016/s0304-3959(98)00194-8. [DOI] [PubMed] [Google Scholar]
- Marubio LM, del Mar Arroyo-Jimenez M, Cordero-Erausquin M, Léna C, Le Novère N, de Kerchove d'Exaerde A, Huchet M, Damaj MI, Changeux JP. Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature. 1999;398:805–810. doi: 10.1038/19756. [DOI] [PubMed] [Google Scholar]
- McLean SL, Idris N, Grayson B, Gendle D, Mackie C, Lesage A, Pemberton DJ, Neill J. PNU-120596, a positive allosteric modulator of α7 nicotinic acetylcholine receptors, reverses a sub-chronic phencyclidine-induced cognitive deficit in the attentional set-shifting task in female rats. J Psychopharmacol. 2011 doi: 10.1177/0269881111431747. In press. [DOI] [PubMed] [Google Scholar]
- Medhurst SJ, Hatcher JP, Hille CJ, Bingham S, Clayton NM, Billinton A, Chessell IP. Activation of the alpha7-nicotinic acetylcholine receptor reverses complete freund adjuvant-induced mechanical hyperalgesia in the rat via a central site of action. J Pain. 2008;7:580–587. doi: 10.1016/j.jpain.2008.01.336. [DOI] [PubMed] [Google Scholar]
- Menegazzi M, Di Paola R, Mazzon E, Genovese T, Crisafulli C, Dal Bosco M, Zou Z, Suzuki H, Cuzzocrea S. Glycyrrhizin attenuates the development of carrageenan-induced lung injury in mice. Pharmacol Res. 2008;58:22–31. doi: 10.1016/j.phrs.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Miao FJ, Green PG, Benowitz N, Levine JD. Central terminals of nociceptors are targets for nicotine suppression of inflammation. Neuroscience. 2004;123:777–784. doi: 10.1016/j.neuroscience.2003.10.027. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Min SW, Kim NJ, Baek NI, Kim DH. Inhibitory effect of eupatilin and jaceosidin isolated from Artemisia princeps on carrageenan-induced inflammation in mice. J Ethnopharmacol. 2009;125:497–500. doi: 10.1016/j.jep.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Munro G, Hansen RR, Erichsen HK, Timmermann DB, Christensen JK, Hansen HH. The α7 nicotinic ACh receptor agonist compound B and positive allosteric modulator PNU-120596 both alleviate inflammatory hyperalgesia and cytokine release in the rat. Br J Pharmacol. 2012;167:421–435. doi: 10.1111/j.1476-5381.2012.02003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng HJ, Whittemore ER, Tran MB, Hogenkamp DJ, Broide RS, Johnstone TB, Zheng L, Stevens KE, Gee KW. Nootropic alpha 7 nicotinic receptor allosteric modulator derived from GABAA receptors modulators. Proc Natl Acad Sci USA. 2007;104:8059–8064. doi: 10.1073/pnas.0701321104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacini A, Di Cesare Mannelli L, Bonaccini L, Ronzoni S, Bartolini A, Ghelardini C. Protective effect of alpha7 nAChR: behavioural and morphological features on neuropathy. Pain. 2010;150:542–549. doi: 10.1016/j.pain.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Papke RL, Kem WR, Soti F, López-Hernández GY, Horenstein NA. Activation and desensitization of nicotinic alpha7-type acetylcholine receptors by benzylidene anabaseines and nicotine. J Pharmacol Exp Ther. 2009;329:791–807. doi: 10.1124/jpet.108.150151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid MH, Furue H, Yoshimura M, Ueda H. Tonic inhibitory role of alpha4beta2 subtype of nicotinic acetylcholine receptors on nociceptive transmission in the spinal cord in mice. Pain. 2006;125:125–135. doi: 10.1016/j.pain.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Roncarati R, Seredenina T, Jow B, Jow F, Papini S, Kramer A, Bothmann H, Dunlop J. Functional properties of alpha7 nicotinic acetylcholine receptors co-expressed with RIC-3 in a stable recombinant CHO-K1 cell line. Assay Drug Dev Technol. 2008;6:181–193. doi: 10.1089/adt.2007.120. [DOI] [PubMed] [Google Scholar]
- Rowley TJ, Flood P. Isoflurane prevents nicotine-evoked norepinephrine release from the mouse spinal cord at low clinical concentrations. Anesth Analg. 2008;107:885–889. doi: 10.1213/01.ane.0000287646.85834.1a. [DOI] [PubMed] [Google Scholar]
- Rowley TJ, Mckinstry A, Greenidge E, Smith W, Flood P. Antinociceptive and anti-inflammatory effects of choline in a mouse model of posperative pain. Br J Anaesth. 2010;105:201–207. doi: 10.1093/bja/aeq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandkuhler J. Models and mechanism of hyperalgesia and allodynia. Physiol Rev. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, Ehrhart J, Silver AA, Sanberg PR, Tan J. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem. 2004;89:337–343. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- Simler G, Lewis LG, et al. Potentiation of analgesic efficacy but not side effects: co-administration of an α4β2 neuronal nicotinic acetylcholine receptor agonist and its positive allosteric modulator in experimental models of pain in rats. Biochem Pharmacol. 2011;82:967–976. doi: 10.1016/j.bcp.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Timmermann DB, Grønlien JH, Kohlhaas KL, Nielsen EØ, Dam E, Jørgensen TD, et al. An Allosteric Modulator of the α7 Nicotinic Acetylcholine Receptor Possessing Cognition-Enhancing Properties in Vivo. J Pharmacol Exp Ther. 2007;323:294–307. doi: 10.1124/jpet.107.120436. [DOI] [PubMed] [Google Scholar]
- Thomsen MS, Hansen HH, Timmerman DB, Mikkelsen JD. Cognitive improvement by activation of alpha7 nicotinic acetylcholine receptors: from animal models to human pathophysiology. Curr Pharm Des. 2010;16:323–343. doi: 10.2174/138161210790170094. [DOI] [PubMed] [Google Scholar]
- Thomsen MS, El-Sayed M, Mikkelsen JD. Differential immediate and sustained memory enhancing effects of alpha7 nicotinic receptor agonists and allosteric modulators in rats. PLoS One. 2011;6:e27014. doi: 10.1371/journal.pone.0027014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nature Rev Drug Discov. 2005;4:673–684. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- Vincler M. Neuronal nicotinic receptors as targets for novel analgesics. Expert Opin Investig Drugs. 2005;14:1191–1198. doi: 10.1517/13543784.14.10.1191. [DOI] [PubMed] [Google Scholar]
- Walker K, Fox AJ, Urban LA. Animal models for pain research. Mol Med Today. 1999;5:319–321. doi: 10.1016/s1357-4310(99)01493-8. [DOI] [PubMed] [Google Scholar]
- Wang DW, Zhou RB, Yao YM. Role of cholinergic anti-inflammatory pathway in regulating host response and its interventional strategy for inflammatory diseases. Chin J Traumatol. 2009;12:355–364. [PubMed] [Google Scholar]
- Wang FZ, Xu SQ, Shen XF, Guo XR, Peng YZ, Yang J. Spinal macrophage migration inhibitory factor is a major contributor to rodent neuropathic pain-like hypersensitivity. Anesthesiology. 2011;114:643–659. doi: 10.1097/ALN.0b013e31820a4bf3. [DOI] [PubMed] [Google Scholar]
- Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Wang H, Metz C, Miller EJ, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- Wang LX, Wang ZJ. Animal and cellular models of chronic pain. Adv Drug Deliv Rev. 2003;55:949–965. doi: 10.1016/s0169-409x(03)00098-x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Su DM, Wang RH, Liu Y, Wang H. Antinociceptive effects of choline against acute and inflammatory pain. Neuroscience. 2005;132:49–56. doi: 10.1016/j.neuroscience.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Westman M, Saha S, Morshed M, Lampa J. Lack of acetylcholine nicotine alpha 7 receptor suppresses development of collagen-induced arthritis and adaptive immunity. Clin Exp Immunol. 2010;162:62–67. doi: 10.1111/j.1365-2249.2010.04204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao HS, Huang QH, Zhang FX, Bao L, Lu YJ, Guo C, Yang L, Huang WJ, Fu G, Xu SH, et al. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc Natl Acad Sci USA. 2002;12:8360–8365. doi: 10.1073/pnas.122231899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin I, Charlet A, Cordero-Erausquin M, Tessier L, Piciotto MR, Schlichter R, Poisbeau P, Freund-Mercier M, Barrot M. Nociceptive thresholds are controlled through spinal β2-subunit-containing nicotinic acetylcholine receptors. Pain. 2011;152:2131–2137. doi: 10.1016/j.pain.2011.05.022. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Furue H. Mechanisms for the anti-nociceptive actions of the descending noradrenergic and serotonergic systems in the spinal cord. J Pharmacol Sci. 2006;101:107–117. doi: 10.1254/jphs.crj06008x. [DOI] [PubMed] [Google Scholar]
- Zhou M. Neuronal mechanism for neuropathic pain. Mol Pain. 2007;3:14. doi: 10.1186/1744-8069-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Neher E. Calcium permeability of nicotinic acetylcholine receptor channels in bovine adrenal chromaffin cells. Pflugers Arch. 1993;425:511–517. doi: 10.1007/BF00374879. [DOI] [PubMed] [Google Scholar]