Abstract
Amygdala glutamatergic neurotransmission regulates withdrawal induced anxiety-like behaviors following chronic ethanol exposure. The lateral/basolateral amygdala receives multiple glutamatergic projections that contribute to overall amygdala function. Our lab has previously shown that rat cortical (external capsule) afferents express postsynaptic alterations during chronic intermittent ethanol exposure and withdrawal. However, thalamic (internal capsule) afferents also provide crucial glutamatergic input during behavioral conditioning, and they have not been studied in the context of chronic drug exposure. We report here that these thalamic inputs express altered presynaptic function during withdrawal from chronic ethanol exposure. This is characterized by enhanced release probability, as exemplified by altered paired-pulse ratios and decreased failure rates of unitary events, and increased concentrations of synaptic glutamate. Quantal analysis further implicates a withdrawal-dependent enhancement of the readily-releasable pool of vesicles as a probable mechanism. These functional alterations are accompanied by increased expression of vesicle associated protein markers. These data demonstrate that chronic ethanol modulation of glutamate neurotransmission in the rat lateral/basolateral amygdala is afferent-specific. Further, presynaptic regulation of lateral/basolateral amygdala thalamic inputs by chronic ethanol may be a novel neurobiological mechanism contributing to the increased anxiety-like behaviors that characterize withdrawal.
Keywords: Strontium Substitution, Coefficient of Variation, Whole-cell patch clamp electrophysiology, Paired Pulse Ratio, Soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE), γ-D-glutamylglycine (DGG)
1. Introduction
Emotion-related signal processing occurs in the amygdala’s lateral/basolateral subdivisions (BLA; Sah et al., 2003) which form the primary input nuclei of this brain region. Information flow into the BLA occurs via anatomically distinct glutamatergic afferents relaying cortical and subcortical information (Rainnie et al., 1991) onto glutamatergic principal neurons. Following local processing, these pyramidal cells drive physiological and psychological manifestations of anxiety and fear (Davis et al., 1994). Thus, the regulation of glutamate signaling arising from different afferents can have a profound impact on fear learning and anxiety (Bauer et al., 2002). For example, qualitatively distinct types of information carried by cortical (external capsule, EC) and subcortical/thalamic (internal capsule, IC) afferents suggests they make differential contributions to BLA neuron activity. In support of this, in vivo fear conditioning induces afferent-specific alterations of synaptic transmission at EC and IC inputs (Boatman and Kim, 2006). Afferent-specific pre- and postsynaptic mechanisms also govern the initiation and expression of BLA glutamatergic synaptic plasticity in vitro and in vivo (Sah et al., 2008; Sigurdsson et al., 2007).
Pathological conditions can also target input-specific mechanisms at BLA glutamatergic synapses. Chronic intermittent ethanol (CIE) exposure significantly increases the postsynaptic function of N-Methyl-D-aspartate (NMDA) (Läck et al., 2007), kainate (Läck et al., 2009), and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) (Christian et al., 2012) glutamate receptors. And withdrawal (WD) from CIE exposure produces treatment-specific increases of anxiety-like behavior regulated by BLA glutamatergic signaling (Läck et al., 2007). Recent data suggests glutamatergic signaling alterations may be input-specific. For example, the external capsule (cortical) glutamatergic afferents exhibit only CIE/WD-dependent increases in post-synaptic AMPAR function (Christian et al., 2012) without any presynaptic alterations (Christian et al., 2012; Läck et al., 2007). However, nothing is known about the impact of CIE/WD on thalamic IC-inputs. Since IC (thalamic) inputs express presynaptic forms of plasticity (Zinebi et al., 2002), we hypothesized that CIE and WD could enhance presynaptic function at IC-BLA glutamatergic afferents.
2. Methods
2.1. Animals
Male Sprague Dawley rats (Harlan, Indianapolis, IN, USA) ~5–6 weeks of age (100–150g) were housed in groups for all experiments. Animals were given five days to recover from the shipping and were then placed into our experimental groups (below). At the time of tissue preparation, animals were ~7–8weeks of age (170–200g). All experimental procedures conformed to NIH Guidelines for the Care and Use of Laboratory Animals and were reviewed and approved by the WFUSM Animal Care and Use Committee.
2.2. CIE and WD exposure
CIE and WD exposures were similar to previous reports (Christian et al., 2012). Ethanol vapor (~37 mg/L) or room air exposures (CON) were conducted during the light-phase of the light/dark cycle for 10 consecutive days, 12 hours/day (Läck et al., 2007). Ethanol vapor exposed animals were euthanized either while still intoxicated (CIE) or 24 hours after the last ethanol exposure (WD). CIE animal trunk blood was collected and analyzed using a commercially available alcohol dehydrogenase assay kit (Genzyme, Middleton WI, U.S.A.). Mean blood-ethanol concentrations were 185.34±5.75mg/deciliter.
2.3 Electrophysiology methods
2.3.1. Slice Preparation
Coronal brain slices containing the amygdala were taken for electrophysiology experiments from anesthetized animals (3% isoflurane) following decapitation in accordance with an approved Wake Forest Baptist Health Institutional ACUC protocol. Brains were incubated and sliced (400μm) on a Leica VT1200S (Leica, Germany) or Vibratome Series 3000 (Vibratome, St. Louis, MO) in ice-cold sucrose modified artificial cerebral spinal fluid (aCSF) containing (in mM): 180 sucrose, 30 NaCl, 4.5 KCl, 1 MgCl2·6H2O, 26 NaHCO3, 1.2 NaH2PO4, 0.10 ketamine, and 10 glucose, equilibrated with 95% O2 and 5% CO2. Slices were incubated for ~1 hour in room temperature (~25°C), oxygenated standard aCSF containing (in mM): 126 NaCl, 3 KCl, 1.25 NaH2PO4, 2 MgSO4, 26 NaHCO3, 10 glucose, and 2 CaCl2·2H2O before initiation of recordings (1–5 hours post preparation). Sigma-Aldrich (St. Louis, MO) and Tocris (Ellisville, MO) purveyed all chemical reagents.
2.3.2. Whole-cell patch-clamp recording
BLA slices were transferred to a submersion-type recording chamber and perfused with room temperature aCSF (2.0 ml/min) for whole-cell voltage clamp recordings similar to previously published reports (Christian et al., 2012). Recording electrodes were filled with an internal solution containing (in mM): 145 K-Gluconate, 5 NaCl, 1 MgCl2, 10 EGTA, 10 HEPES, 2 Mg-ATP, 0.1 Na-GTP, pH 7.25, osmolarity 280–290; pipette open tip resistances were 6–12 MΩ. Data were acquired via Axopatch 700b or 200b amplifiers (Molecular Devices, Foster City, CA) and analyzed offline via pClamp software (Molecular Devices). Inclusion criteria for presumptive principal neurons included high membrane capacitances (>100pF) and low access resistances in the whole-cell configuration (<20MΩ) (Washburn and Moises, 1992) leading to the inclusion of ~80% of all cells analyzed. Synaptic responses were electrically evoked using either concentric bipolar stimulating electrodes (FHC Inc, Bowdoin, ME) or electrodes fabricated from theta tube borosilicate glass (World Precision Instruments, INC.; Sarasota, FL) with constant-voltage stimulation as previously described (Christian et al., 2012; Läck et al., 2009). Glutamatergic synaptic currents were pharmacologically isolated by continuous perfusion of slices with 100μM picrotoxin.
2.3.3. Paired-pulse ratio
Two stimuli of equal intensity were evoked with a range of inter-pulse intervals (25–500 ms), with evoked excitatory post synaptic current (EPSC) amplitudes being used to calculate paired-pulse ratio’s (PPR). A conservative estimate of amplitudes to minimize contamination of second response amplitude by the first response decay at short time intervals ((peak 2 – peak 1)/peak 1) was utilized for all PPR calculations (Schulz et al., 1995). Several studies utilized bath application of pharmacological agents during the paired-pulse experiments to further characterize presynaptic mechanisms associated with alterations of glutamatergic synaptic function following CIE and WD.
2.3.4. Collision protocol
Paired electrical stimuli of equal intensity were delivered at an inter-pulse interval of 500ms paired at a single afferent (e.g. EC1 – EC2; IC1 – IC2) or mixed at multiple afferents (e.g. EC1 – IC2; IC1 – EC2). Response decay did not influence amplitudes at these longer interpulse intervals, peak EPSC amplitudes were used to calculate PPR’s (second amplitude – first amplitude, divided by the first) at both IC and EC afferents. Ratios were calculated across all afferent stimulation patterns and examined for facilitation or depression during the second stimulation at each input (EC1 – EC2; IC1-IC2) independent of stimulation pattern.
2.3.5. SR2+ evoked responses
Some electrophysiology experiments were conducted in aCSF where strontium (2.0mM) was substituted for calcium (2.0mM) to allow the characterization of electrically evoked asynchronous EPSC (aEPSC) responses (Choi and Lovinger, 1997a; Miledi, 1966) at specific afferents.
2.3.6. Synaptic Fatigue
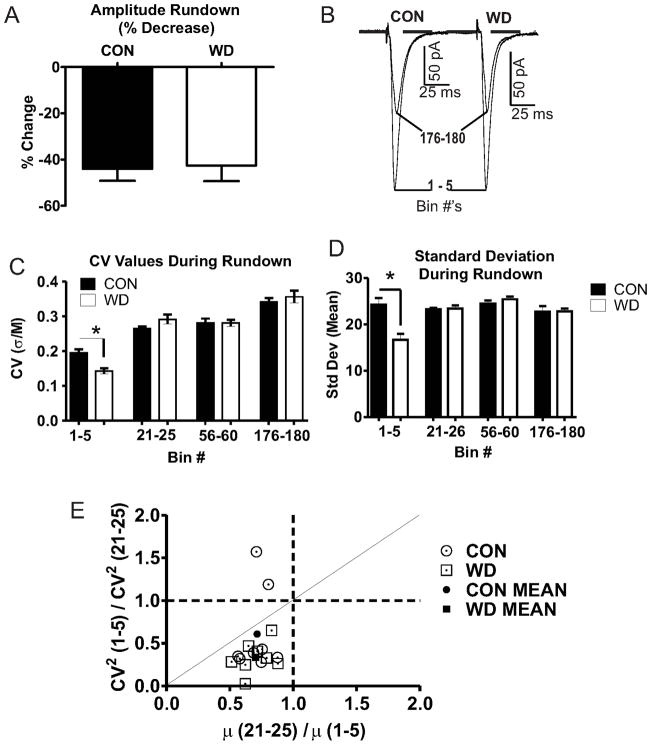
2.0 Hz stimulation (15min) was applied to the IC-BLA input following a stable baseline period to generate synaptic rundown. EPSC amplitudes were analyzed for standard deviations and utilized in a Coefficient of Variance (CV) analysis (Deng et al., 2010; Faber and Korn, 1991) to characterize alterations of synaptic function. Standard deviation and mean amplitude values were calculated from 10 consecutive EPSC events grouped into bins of 5 consecutive event (50 sweeps total/bin) groups over the duration of the protocol.
2.4. Western Blot Methods
Western Blot methods are similar to those previously reported (Christian et al., 2012). Importantly, single blots contained BLA protein from single animals (1 per lane) containing CON, CIE, and WD groups (4 per group). Within each blot, group specific expression (4 lanes) was averaged and normalized to CON mean expression (percent CON). Several experiments were performed in duplicate to increase power. Antibodies to protein targets (primary concentration utilized) exhibited specificity as indicated by immunoreactive bands at expected molecular weights: Vglut1 (1:40,000), Vglut2 (1:7000) from Synaptic Systems (Goettingen, Germany), synaptobrevin I (0.5 μg/ml), synaptobrevin II (1:1000), syntaxin 1 (1:3000), synaptotagmin I (1.0 μg/ml) and synaptotagmin II (1:750) from Abcam (Cambridge, MA), and SNAP25 (1:1000) from Chemicon (Now Millipore, Billerica, MA). Vglut1/2 protein samples were run as above with the exception that protein samples were not heated prior to loading per manufacturer’s instructions.
2.5. Statistics
All values are expressed as mean ± SEM. Primary statistical analyses were conducted using 2-way ANOVA (SigmaPlot, Systat Software Inc, San Jose, CA), one-way ANOVA, or t-tests (Graph Pad, GraphPad Software Inc, La Jolla, CA) depending on the experimental design. p < 0.05 was considered statistically significant. Significant between-group differences in the ANOVAs were measured using Newman-Keuls post-hoc tests.
3. Results
3.1. Anatomically distinct glutamatergic inputs maintain functional independence independent of treatment
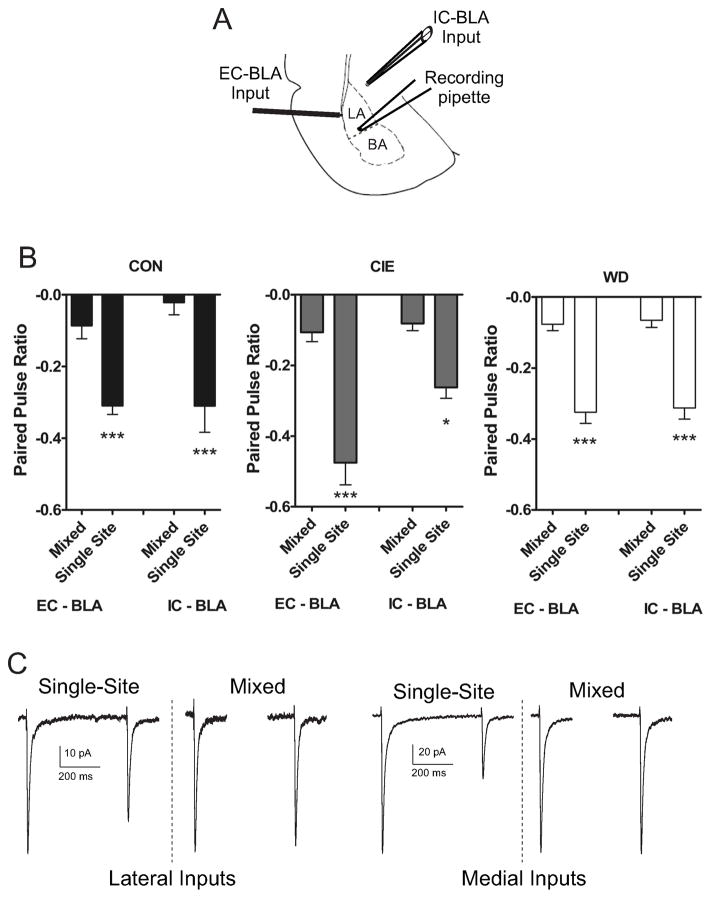
Previous studies indicate EC–BLA and IC-BLA afferents are functionally independent using in vitro recording techniques (Tsvetkov et al., 2004). Using paired stimuli (500 ms) at mixed (IC1 – EC1; EC2 – IC2) or single afferents (EC1 – EC2; IC1 – IC2) we tested if this independence was maintained across CIE and WD (Fig. 1). A two-way ANOVA (treatment condition vs. stimulation site) indicated significant main effects for stimulation site [F(3,60) = 48.748, p < 0.05] but not treatment condition [F(2,60) = 1.84, p > 0.05] (Fig. 1). These data coupled with no significant interaction [F (6,60) = 1.781, p > 0.05] suggests CIE and WD exposure do not impact the functional independence of EC and IC afferents.
Figure 1. Anatomically distinct glutamatergic BLA afferents are functionally distinct across CIE and WD.
A) Diagram representing relative placement of recording and stimulating electrode placements in External Capsule (EC) and Internal Capsule (IC) BLA afferents for electrophysiology experiments (adopted from (Paxinos and Watson, 1997)). B) Paired electrical stimuli (500 ms) were delivered to EC or IC afferents in either a single afferent (EC-EC; IC-IC) or mixed afferent (EC-IC; IC-EC). Paired, single site stimulation elicited robust, but similar paired pulse depression at both afferents across all treatment conditions that did not [(EC1 – EC2), Kruskal-Wallis = 5.34, p > 0.05; (IC1 – IC2) Kruskal-Wallis = 0.36, p > 0.05]. Mixed afferent stimulation elicited little paired pulse depression that did not differ across treatment [(EC1– EC2), Kruskal-Wallis = 1.13, p > 0.05; (IC1 – IC2), Kruskal-Wallis = 2.43, p > 0.05]. ANOVA (treatment condition vs. stimulation site) indicated a significant main effect for stimulation site [EC- EC paired, (−0.37 ± 0.02 n = 18); EC - EC unpaired, (−0.08 ± 0.01 n = 18); IC - IC paired, (−0.29 ± 0.02 n = 18); IC - IC unpaired (−0.05 ± 0.01 n = 18); F(3,60) = 48.748, p < 0.05] but not treatment [CON (−0.18 ± 0.03, n = 24); CIE (−0.23 ± 0.03, n = 24); WD (−0.19 ± 0.02, n = 24); F(2,60) = 1.84, p > 0.05] with no significant interaction [F (6,60) = 1.781, p > 0.05]. This lack of functional interaction indicates that these anatomically distinct afferent pathways are functionally distinct, independent of treatment. C) Representative traces showing paired pulse depression during single afferent stimulation at both EC/IC afferents. Mixed afferent stimulation results in little paired pulse depression at either input.
3.2. WD increases pre-synaptic release probability at IC-BLA afferents
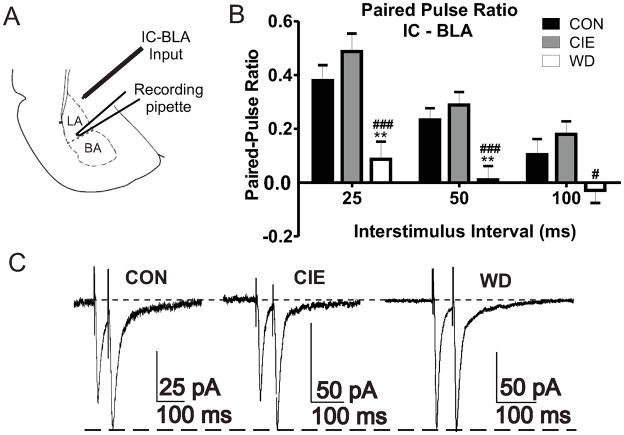
We utilized paired electrical stimulations across a range of inter pulse intervals (25 – 100ms) to characterize treatment effects on presynaptic function at IC-BLA inputs (Fig. 2). Previous reports indicate no treatment dependent alterations of presynaptic function at EC-BLA afferents (Christian et al., 2012) and were not investigated further. WD but not CIE significantly decreased paired pulse ratios intervals at IC-BLA afferents (Fig. 2B) suggesting a WD-dependent increase of presynaptic glutamate release probability. Our data demonstrate treatment dependent differences in paired pulse facilitation but no effect on paired pulse depression at IC-BLA synapses. Importantly, facilitation at short inter-stimulus intervals and depression at longer inter-stimulus intervals was previously reported at this afferent pathway (McKernan and Shinnick-Gallagher, 1997; Zinebi et al., 2002) with independent regulation of paired pulse facilitation and depression occurring at single synapses (Chen et al., 2004).
Figure 2. WD increases presynaptic function at IC-BLA afferents.
A) Diagram representing relative placements of stimulating (IC-BLA) and recording electrodes (BLA) for electrophysiology experiments. B) Decreased PPRs during WD indicate increased release probability at multiple interstimulus intervals: 25ms [CON, 0.37 ± 0.05, n = 16; CIE, 0.48 ± 0.06, n = 10; WD, 0.08 ± 0.06, n = 11; F(2,34) = 9.464, p < 0.05], 50ms [CON, 0.23 ± 0.04, n = 16; CIE, 0.28 ± 0.04, n = 14; WD, 0.01 ± 0.05, n = 15; F(2,42) = 9.488 p > 0.05], 100ms [CON, 0.10 ± 0.05, n = 15; CIE, 0.17 ± 0.04, n = 13, WD, −0.02 ± 0.04 n =15; F(2,40) = 4.117 p > 0.05)], One Way ANOVA’s, Newman-Keuls post hoc tests. ### = significant vs. CON; # ,** = significant vs. CIE. C) Representative traces of PPR recordings (50 ms) scaled to second peak amplitude during each treatment condition.
3.3. WD increases synaptic glutamate levels
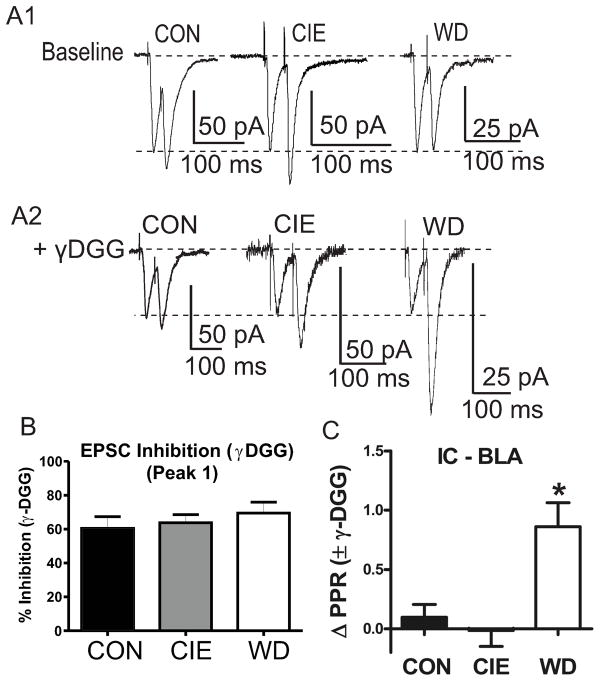
To confirm a treatment-dependent shift in release probability at IC-BLA synapses, we indirectly measured relative synaptic glutamate concentrations following treatment using PPR’s (25ms) in the presence (Fig. 3A2) or absence (Fig. 3A1) of gamma-D-glutamylglycine (γDGG, 1.0mM), a low affinity competitive antagonist at AMPAR (Fig. 3). Application of γDGG inhibited EPSC’s generated by the first stimulus similarly across treatment groups (Fig. 3B); suggesting no treatment-related change in the acute sensitivity of AMPA receptors to the antagonist. This indicates that γDGG can be used as a probe to measure treatment-related alterations of presynaptic function.
Figure 3. WD increases synaptic glutamate concentration.
A1) Representative traces of baseline paired pulse stimulation (25 ms) across treatment conditions. Traces are scaled to first response amplitudes in all cases. A2) Representative traces following bath application of γDGG (1.0 mM) illustrate decreased second pulse inhibition by γDGG during WD. B) Inhibition of first-response amplitude by γDGG was not different across treatment groups [CON 60.49 ± 6.81%, n =7; CIE, 63.70 ± 4.82, n = 10; WD, 69.48 ± 6.49, n =7; F(2,21) = .052; p > 0.05]. C) WD slices exhibit significantly greater PPR values following bath application of γDGG due to decreased pulse to pulse inhibition [ΔPPR; CON 0.09 ± 0.10, n = 7; CIE, −0.01 ± 0.13, n = 10; WD, 0.86 ± 0.20, n = 7; F(2,21) = 9.475, p < 0.05 Newman Keuls Post Hoc Test * = significant from CON and CIE].
We next compared paired-pulse ratios prior to and following γDGG application to slices. To better illustrate the effect of γDGG, we quantified the difference between baseline and drug ratios as a change in PPR values (Δ PPR). Application of γDGG onto WD cells significantly increased ΔPPR compared to baseline values but did not alter ΔPPR values in either CON or CIE cells (Fig. 3C), due to a decrease in inhibition of second response amplitudes. These data are consistent with increased release probability at IC-BLA synapses suggesting that synaptic concentrations of glutamate increase during WD.
3.4. WD decreases failure rates at IC-BLA synapses
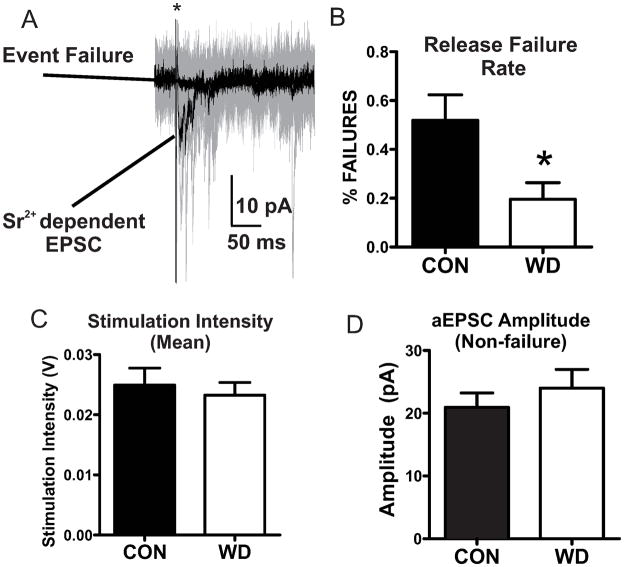
As CIE treatment did not measurably alter presynaptic function in our experiments on the cortical afferents (Christian et al., 2012), we chose to focus further functional investigations on WD-dependent alterations at the IC/thalamic inputs. To further characterize presynaptic functional alterations, we examined synaptic responses utilizing minimal stimulation techniques that activate very low numbers of synapses to observe release probability at individual synaptic inputs (Isaac et al., 1998). However, under our standard recording conditions we were unable to consistently observe mixtures of failures and successful responses suggesting a relatively high release probability at IC-BLA synapses (data not shown). To artificially reduce release probability, we utilized a modified aCSF containing strontium instead of calcium (Dodge et al., 1969). Under these recording conditions similar stimulation intensities (Fig. 4C) evoked a mixture of both Sr2+ dependent responses and non-release events (failures) (Fig. 4A). WD cells demonstrated a significant decrease in failure rates (# of sweeps with failure/# of total sweeps) compared to CON cells (Fig. 4B). Post-synaptic response amplitudes measured during non-failure events did not differ between groups (Fig. 4D). These data support the hypothesis of WD-dependent increases in presynaptic function at IC-BLA synapses.
Figure 4. Decreased rates of vesicle release failures during WD.
A) Representative traces showing mean Sr2+ dependent EPSC responses and non release event failures (Black traces). Representative raw data (20 sweeps) from exemplar cell (Gray traces). * indicates truncated stimulus artifact. B) WD decreases release failure rates [CON (8); WD (7); t = 2.492, df = 13, * = p < 0.05]. C) Stimulation intensities did not differ between treatment conditions [CON (8); WD (7); t = 0.47 df = 13, p > 0.05]. D) Non-failure EPSC amplitude events do not differ with treatment [CON (8), WD (7); t =0.824, df = 13, p > 0.05].
3.5. WD decreases CV values at IC-BLA synapses
To further confirm a presynaptic localization for WD-dependent effects on release we utilized variance analysis to attribute functional alterations in synaptic transmission to specific synaptic compartments (Choi and Lovinger, 1997b; Faber and Korn, 1991). Given the unknown number of synaptic connections at the synapses we are measuring, we utilized the coefficient of variation (CV = Standard deviation (σ)/mean EPSC amplitude (M)) as a comparison measure as it allows for indirect analysis of quantal parameters (Faber and Korn, 1991). Our data demonstrating increased release probability (Fig. 2) and synaptic glutamate (Fig. 3) suggest there are treatment-related alterations within distinct synaptic vesicle pools. We therefore utilized a ‘synaptic fatigue’ stimulation protocol similar to previously reports to elicit quantal synaptic responses from distinct vesicular pools (see (Scheuss and Neher, 2001)). Standard deviations (σ) and mean EPSC amplitudes (M) were calculated for all bins and used to calculate CV values. Our stimulation protocol induced synaptic ‘rundown’ in both treatment groups with WD reaching maximum rundown more quickly than CON cells. However, the absolute percent decrease in EPSC amplitudes across the stimulation series did not differ between treatment conditions (Fig. 5A,B), again suggesting no treatment-dependent alteration in postsynaptic function. Analysis indicated that WD responses exhibited more pronounced decreases in CV values compared to CON, and this difference was specific to the initial rundown phase (Bin 1–5) while CV values did not differ at any other bin interval (Fig. 5C). This WD dependent decrease of CV values at Bins 1–5 is attributable to a more dramatic decrease in σ during WD (Fig. 5D). Post-hoc analysis using CV2 values (Faber and Korn, 1991) localized the functional changes specifically to presynaptic compartments (Fig. 5E). This π/R plot [Fig. 5; (π = M (21–25)/M (1–5)); (R = CV2 (1–5)/CV2 (21–25)] is strongly suggestive of a presynaptic localization for the measured functional alterations (Choi and Lovinger, 1997b; Faber and Korn, 1991).
Figure 5. CV analysis localizes WD dependent functional alterations to presynaptic compartments.
A) Absolute EPSC rundown are not different between groups (t = 0.17, df = 14, p > 0.05). B) Representative traces scaled to Bin 1–5 peak values show no change in mean amplitudes. C) CV values (σ/M) decrease in WD (n=8) relative to CON (n=8) during initial rundown [Bin 1–5; t = 4.30 df = 8, * = p < 0.05], but not across extended time points [bins 21–25, t = 1.741, df = 8; bins 56–60, t = 0.02, df = 8; bins 176–180, t = 0.70, df = 8; p > 0.05 for all comparisons]. D) WD decreases σ values during Bins 1–5 [bins 1–5, t = 3.88, df = 8, * = p < 0.05] but not at extended Bin intervals [bins 21–25, t = 0.21, df = 8, p > 0.05; bins 56–60, t = 1.06, df = 8, p > 0.05; bins 176–180, t = 0.03, df = 8, p > 0.05]. E) CV2 analysis characterizing localization of increased WD dependent glutamate function with values on or below the diagonal line suggesting a presynaptic mechanism of action.
3.6. V-Snare, but not T-Snare Proteins are dynamically regulated during WD
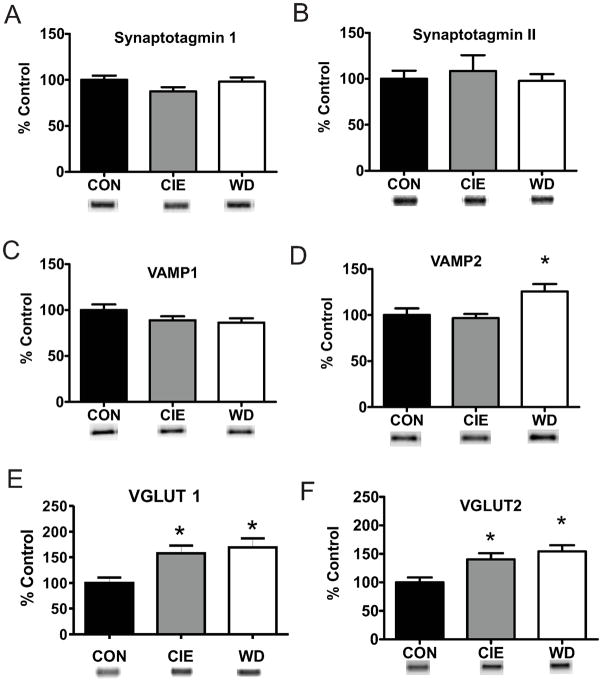
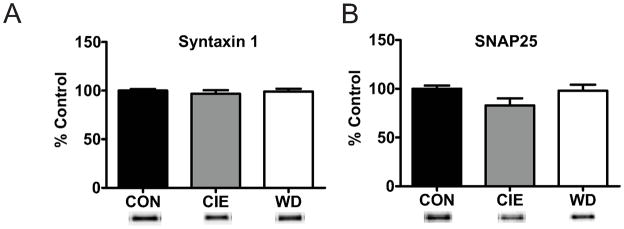
Given the multiple measures of presynaptic localization for functional alterations during WD we hypothesized that alterations in vesicular protein or release machinery contribute to increased release probability, enhanced synaptic glutamate concentrations, and more dramatic effects on vesicle release in this treatment group. We utilized western blot techniques to examine protein expression of known presynaptic and vesicular protein markers following CIE and WD treatment. Several V-SNARE proteins exhibited dynamic regulation in protein expression by WD, but not CIE. Synaptobrevin (VAMP) is critical for SNARE complex formation and vesicular release (Liu et al., 2011) and demonstrated WD induced increases for total protein levels of VAMP2 (Fig. 6D) but not for VAMP1 expression (Fig. 6C). Vesicular glutamate transporter isoforms 1 (Vglut1; Fig. 6E) and 2 (Vglut2; Fig. 6D) protein, critical for vesicular glutamate packaging (Fremeau Jr et al., 2001), were also increased following both CIE and WD (Fig. 4E,F). However, expression of V-SNARE proteins synaptotagmin I (Fig. 4A) and II (Fig. 4B), which function as calcium sensors for synchronous vesicle release (Young Jr and Neher, 2009) and recycling (Yao et al., 2012), were not altered by treatment. The total protein levels of T-SNARE proteins syntaxin1 (Fig. 7A) and SNAP25 (Fig. 7B) were not altered by CIE or WD suggesting that vesicle release site numbers are not altered by treatment. Together these protein expression data suggest a treatment-dependent increase in V-SNARE/vesicle associated proteins with no alteration of T-SNARE/release site proteins.
Figure 6. WD dependent alterations of vesicle proteins during WD.
A, B) Protein expression of Synaptotagmin I [CON, 100.00 ± 4.72, n = 8; CIE, 87.47 ± 4.56, n = 9; WD, 98.11 ± 4.51, n = 8] and II [CON, 100.00 ± 8.83, n = 4; CIE, 108.38 ± 17.33; n = 5; WD, 97.73 ± 7.33, n = 4] are unaltered following WD. C) VAMP1 protein expression is unaltered by CIE or WD [100.00 ± 6.16, n = 12; CIE, 88.99 ± 4.17, n = 12; WD, 86.44 ± 4.61, n = 12]. D) WD but not CIE increases VAMP2 protein expression [CONE, 100.00 ± 7.33, n = 11; CIE, 96.70 ± 4.48, n = 12; WD, 125.68 ± 7.96, n = 12]. E, F) VGlut protein expression increases during both CIE and WD VGlut1: [CON, 100.00 ± 10.48, n = 8; CIE, 157.56 ± 15.31, n = 9; WD, 168.98 ± 17.83, n = 9; F(2,23) = 5.697, p < 0.05 * indicates significant difference from CON]; VGlut2: [CON, 100.00 ± 8.74, n = 8; CIE, 140.24 ± 10.80, n = 9; WD, 154.32 ± 10.82, n = 9; F(2,23) = 7.226, p < 0.05 * indicates significant difference from CON)]. Increased protein expression of vesicle associated protein suggest in increase in vesicle size or number. VAMP2 and VGlut2 protein data suggests a pathway specific increase in synaptic vesicles.
Figure 7. T-Snare protein is not regulated by CIE or WD.
A) Total protein expression of T-Snare Syntaxin 1 is unaffected by CIE and WD exposures [CON, 100.00 ± 1.64, n =8; CIE, 96.75 ± 3.71, n = 8; WD, 99.12 ± 2.79, n = 8; F (2,21) = 0.348; p > 0.05]. B) T-Snare SNAP25 protein expression is unchanged following CIE and WD exposures [CON, 100.00 ± 3.26, n = 8; CIE, 82.86 ± 7.33, n = 8; WD, 97.94 ± 6.24, n = 8; F (2,21) = 2.537, p > 0.05]. No treatment dependent alterations in T-Snare protein expression levels suggest that the number of release sites at presynaptic terminals is unchanged.
4. Discussion
4.1. CIE and WD do not alter input independence
Previous data has demonstrated that EC and IC-BLA afferents into the amygdala are functionally distinct in drug-naïve animals (Tsvetkov et al., 2004). Our current data presented here indicate that neither CIE nor WD impact this functional independence of EC and IC-BLA afferents suggesting that functional alterations can be attributed to specific afferents. Our characterization of afferent specific alterations following CIE and WD further our understanding of how information processing occurs in the BLA. And the functional independence of afferent projections also suggests that brain region-dependent ‘upstream’ processing may differentially contribute to BLA signaling.
4.2. WD increases glutamatergic presynaptic functional measures at IC-BLA synapses
Paired-pulse ratios were reduced in WD slices at IC-BLA glutamatergic afferents similar to presynaptic effects of fear conditioning (Zinebi et al., 2002). Additional experiments identified WD dependent decreases of failure rates that support increased release probability at these thalamic inputs. Importantly, CON IC-BLA failure rates and non-failure EPSC amplitudes were similar to those previously reported in younger naïve animals (Shin et al., 2006), suggesting our recording techniques were able to quantify the pre- and post-synaptic components of unitary events. While previous studies suggest that decreased failure rates are sometimes attributable to increased postsynaptic function (Foster and McNaughton, 1991), the amplitudes of non-failure events did not differ between CON and WD suggesting postsynaptic AMPAR function was not altered. The paired-pulse ratio and decreased failure rate data together suggest WD increases presynaptic release probability/function.
Increased synaptic release probability should theoretically increase synaptic neurotransmitter concentrations. We examined relative glutamate concentrations using the low-affinity competitive AMPA receptor antagonist γ-DGG (Wu et al., 2007). Since the low-affinity antagonist is readily displaced by raising glutamate concentrations in the synapse, changes in the inhibition by γ-DGG during a train of stimuli are believed to reflect relative levels of glutamate in the synapse. γDGG inhibition following repetitive (in this case, paired-pulse) stimulation was greater in WD neurons, consistent with treatment-specific increases in synaptic glutamate levels. Importantly, γDGG affinity/efficacy did not differ between treatment groups supporting a presynaptic locus for CIE/WD effects at IC-BLA synapses. Previous studies interpret decreased γDGG inhibition as increased release in numbers of synaptic vesicles at individual release sites (Wadiche and Jahr, 2001). However, we cannot distinguish this interpretation and a variety of additional mechanisms could increase synaptic glutamate concentrations including increased vesicular neurotransmitter content.
To test the regulation of vesicle content/number, we utilized CV and CV2 analyses during a synaptic rundown procedure during prolonged repetitive stimulation. WD decreased CV values but only during the initial rundown phase. This was largely due to a decrease in standard deviation which represents the “pre-synaptic” component of CV. This is consistent with an increase in release probability (Deng et al., 2010) and in synaptic glutamate concentration (Oertner et al., 2002) during WD. The low CV values which characterize IC-BLA also indicate that multiple vesicles are likely released in response to single stimulations (Conti and Lisman, 2003). Together, our data are highly supportive of changes in presynaptic function, possibly due to an increase in vesicle number and/or increased function of the readily releasable pool of synaptic vesicles (Fioravante and Regehr, 2011); (Becherer and Rettig, 2006). Experimental alterations of readily releasable pools have been correlated to functional changes in paired pulse measures (Sullivan, 2007). Bins at longer time points likely reflect a multitude of processes such as vesicle recycling or reserve pool mobilization (Becherer and Rettig, 2006). These analyses also confirm that “post-synaptic” contributions to CV do not differ between groups (Choi and Lovinger, 1997b) further supporting a presynaptic localization for WD dependent functional alterations.
4.3. Protein alterations suggest increased vesicle numbers and support increased presynaptic function
One possible mechanism for increased presynaptic function characterized by enhancement of the readily-releasable pool would be a WD-dependent increase in the number of release sites. But there were no treatment dependent changes in SNAP25 or synatxin 1, two T-SNARE proteins that are required for functional docking and release of vesicles (Dun et al., 2010). Since functional approaches to test this directly (e.g. miniature EPSCs) lose input-specificity, we could not functionally confirm this. A complementary observation is that WD increases either vesicle number or vesicle content at IC-BLA synapses. The presence of multiple forms of vesicle fusion at central synapses (Zhang et al., 2009) and inconsistent reports about the prevalence of these different types of fusion mechanisms (Budzinski et al., 2009; Petukhov and Popov, 1986) complicate testing this hypothesis. Regardless, we show that WD increases levels of the vesicle associated proteins VAMP2 and VGlut2, but not VAMP1 or synaptotagmin (I/II). Larger vesicles may incorporate greater amounts of both VGlut2 and VAMP2 proteins which could support the interpretation of increased vesicular content and number. However, the distribution of these proteins within different presynaptic sub-compartments could also influence our interpretation, and we cannot rule out WD-dependent increases in the total number of vesicles per IC-BLA synapse. Increases in VGlut2 protein expression are particularly interesting given that the BLA does not express VGlut2 mRNA (Fremeau Jr et al., 2001); but this mRNA is highly expressed in the thalamus (Fremeau Jr et al., 2001). Since sensory thalamic nuclei send large numbers of glutamatergic afferents to the BLA (Ottersen and Ben-Ari, 1979), these data suggest that an increase in vesicle-specific protein levels may be specific to IC-BLA synapses and can help enhance presynaptic function at this specific input.
It is worth noting that increased presynaptic function has been linked to increases in vesicle docking (Murphy et al., 1997) and to multi-vesicle release (Bender et al., 2009; Oertner et al., 2002) which ultimately lead to decreased CV values (Choi and Lovinger, 1997b; Foster and McNaughton, 1991). These characteristics are very similar to the WD-dependent decreases in CV reported here and support the hypothesis that WD from chronic ethanol increases the number of vesicles released per stimulation event at IC-BLA synapses. Previous work with γDGG shows that increased synaptic glutamate levels follow artificial manipulations that enhance release probability (Wadiche and Jahr, 2001). Similar studies indicate that presynaptic LTP is accompanied by increased release probability attributable to the release of multiple vesicles (Bender et al., 2009). It is particularly noteworthy that IC-BLA synapses express presynaptic forms of LTP-like synaptic plasticity (Zinebi et al., 2002). Our γDGG data are therefore consistent with increased multi-vesicular release during withdrawal. An alternative explanation for increased presynaptic function could be the activation of ‘silent synapses’. But this has been more typically associated with the insertion of functional glutamate receptors into post-synaptic membranes (Liao et al., 2001). However, using multiple measures, CIE/WD do not induce measurable postsynaptic functional alterations at IC-BLA afferents. Our quantal analysis also did not identify changes in post-synaptic function (potency) consistent with a presynaptic locus for functional alterations (Stevens and Wang, 1994). Overall, the release of multiple vesicles in response to afferent stimulation is a likely mechanism for the WD dependent presynaptic functional increases resulting in increased synaptic glutamate concentration.
5. Summary
The amygdala is an important brain region for the regulation of withdrawal induced anxiety. These studies demonstrate that anatomically distinct afferents maintain their functional independence during CIE or WD treatment. These studies also provide some of the first evidence for WD induced alterations of glutamate neurotransmission resulting from presynaptic functional increases at IC-BLA afferents. Biochemical and electrophysiological data presented here suggests WD increases vesicle number and subsequent multiple vesicular release, which serve to support increased presynaptic function. The time-course of exposure necessary to generate these presynaptic alterations will provide a critical clue to their role in withdrawal-related anxiety. Similarly, these alterations could contribute to persistent, withdrawal-associated anxiety as is seen with other chronic ethanol exposure paradigms (Rasmussen et al., 2001; Santucci et al., 2008). It is worth noting too that the robust presynaptic alterations in the ‘adolescent’ animals used in the current study may reflect a unique neurobiological sensitivity to chronic ethanol exposure in animals from this developmental age. This would parallel the unique behavioral sensitivity of young animals to ethanol exposure (Varlinskaya and Spear, 2012). Additional work with chronic ethanol exposure in adults would be needed to confirm this. Regardless, the unique functional and biochemical alterations identified here may provide treatment targets to reduce WD-induced anxiety following chronic ethanol.
Highlights.
Functional independence of anatomically distinct BLA afferents is maintained during CIE/WD.
IC-BLA synapses exhibit increased presynaptic glutamate function during WD.
WD dependent increase in presynaptic proteins support increased presynaptic function.
AMPA mediated IC-BLA postsynaptic receptor function is unchanged by CIE and WD.
Acknowledgments
We would like to acknowledge Dr. David Lovinger for his helpful comments during manuscript preparation and Mr. Eli McCool for his assistance with biochemical assays. We also thank NIH/NIAAA for supporting our research via grant award R01 AA014445 and institutional training grant T32 AA007565.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bauer EP, Schafe GE, LeDoux JE. NMDA receptors and L-type voltage-gated calcium channels contribute to long-term potentiation and different components of fear memory formation in the lateral amygdala. J Neurosci. 2002;22:5239–5249. doi: 10.1523/JNEUROSCI.22-12-05239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becherer U, Rettig J. Vesicle pools, docking, priming, and release. Cell and Tissue Research. 2006;326:393–407. doi: 10.1007/s00441-006-0243-z. [DOI] [PubMed] [Google Scholar]
- Bender VA, Pugh JR, Jahr CE. Presynaptically Expressed Long-Term Potentiation Increases Multivesicular Release at Parallel Fiber Synapses. The Journal of Neuroscience. 2009;29:10974–10978. doi: 10.1523/JNEUROSCI.2123-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatman JA, Kim JJ. A thalamo-cortico-amygdala pathway mediates auditory fear conditioning in the intact brain. European Journal of Neuroscience. 2006;24:894–900. doi: 10.1111/j.1460-9568.2006.04965.x. [DOI] [PubMed] [Google Scholar]
- Budzinski KL, Allen RW, Fujimoto BS, Kensel-Hammes P, Belnap DM, Bajjalieh SM, Chiu DT. Large Structural Change in Isolated Synaptic Vesicles upon Loading with Neurotransmitter. Biophysical Journal. 2009;97:2577–2584. doi: 10.1016/j.bpj.2009.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Harata NC, Tsien RW. Paired-pulse depression of unitary quantal amplitude at single hippocampal synapses. Proc Natl Acad Sci U S A. 2004;101:1063–1068. doi: 10.1073/pnas.0307149101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Lovinger DM. Decreased Frequency But Not Amplitude of Quantal Synaptic Responses Associated with Expression of Corticostriatal Long-Term Depression. J Neurosci. 1997a;17:8613–8620. doi: 10.1523/JNEUROSCI.17-21-08613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Lovinger DM. Decreased probability of neurotransmitter release underlies striatal long-term depression and postnatal development of corticostriatal synapses. Proc Natl Acad Sci U S A. 1997b;94:2665–2670. doi: 10.1073/pnas.94.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian DT, Alexander NJ, Diaz MR, Robinson S, McCool BA. Chronic intermittent ethanol and withdrawal differentially modulate basolateral amygdala AMPA-type glutamate receptor function and trafficking. Neuropharmacology. 2012;62:2430–2439. doi: 10.1016/j.neuropharm.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti R, Lisman J. The high variance of AMPA receptor- and NMDA receptor-mediated responses at single hippocampal synapses: Evidence for multiquantal release. Proceedings of the National Academy of Sciences. 2003;100:4885–4890. doi: 10.1073/pnas.0630290100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994;17:208–214. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Deng P-Y, Xiao Z, Jha A, Ramonet D, Matsui T, Leitges M, Shin H-S, Porter JE, Geiger JD, Lei S. Cholecystokinin Facilitates Glutamate Release by Increasing the Number of Readily Releasable Vesicles and Releasing Probability. J Neurosci. 2010;30:5136–5148. doi: 10.1523/JNEUROSCI.5711-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge FA, Jr, Miledi R, Rahamimoff R. Strontium and quantal release of transmitter at the neuromuscular junction. J Physiol. 1969;200:267–283. doi: 10.1113/jphysiol.1969.sp008692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun A, Rickman C, Duncan R. The t-SNARE Complex: A Close Up. Cellular and Molecular Neurobiology. 2010;30:1321–1326. doi: 10.1007/s10571-010-9599-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber DS, Korn H. Applicability of the coefficient of variation method for analyzing synaptic plasticity. Biophysical Journal. 1991;60:1288–1294. doi: 10.1016/S0006-3495(91)82162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioravante D, Regehr WG. Short-term forms of presynaptic plasticity. Current Opinion in Neurobiology. 2011;21:269–274. doi: 10.1016/j.conb.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, McNaughton BL. Long-term enhancement of CA1 synaptic transmission is due to increased quantal size, not quantal content. Hippocampus. 1991;1:79–91. doi: 10.1002/hipo.450010108. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The Expression of Vesicular Glutamate Transporters Defines Two Classes of Excitatory Synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Isaac JTR, Lüthi A, Palmer MJ, Anderson WW, Benke TA, Collingridge GL. An investigation of the expression mechanism of LTP of AMPA receptor-mediated synaptic transmission at hippocampal CA1 synapses using failures analysis and dendritic recordings. Neuropharmacology. 1998;37:1399–1410. doi: 10.1016/s0028-3908(98)00140-3. [DOI] [PubMed] [Google Scholar]
- Läck AK, Christian DT, Diaz MR, McCool BA. Chronic ethanol and withdrawal effects on kainate receptor-mediated excitatory neurotransmission in the rat basolateral amygdala. Alcohol. 2009;43:25–33. doi: 10.1016/j.alcohol.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läck AK, Diaz MR, Chappell A, DuBois DW, McCool BA. Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. J Neurophysiol. 2007;98:3185–3196. doi: 10.1152/jn.00189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Scannevin RH, Huganir R. Activation of Silent Synapses by Rapid Activity-Dependent Synaptic Recruitment of AMPA Receptors. The Journal of Neuroscience. 2001;21:6008–6017. doi: 10.1523/JNEUROSCI.21-16-06008.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Sugiura Y, Lin W. The role of Synaptobrevin1/VAMP1 in Ca2+-triggered neurotransmitter release at the mouse neuromuscular junction. The Journal of Physiology. 2011;589:1603–1618. doi: 10.1113/jphysiol.2010.201939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- Miledi R. Strontium as a substitute for calcium in the process of transmitter release at the neuromuscular junction. Nature. 1966;212:1233– 1234. doi: 10.1038/2121233a0. [DOI] [PubMed] [Google Scholar]
- Murphy KP, Reid GP, Trentham DR, Bliss TV. Activation of NMDA receptors is necessary for the induction of associative long-term potentiation in area CA1 of the rat hippocampal slice. The Journal of Physiology. 1997;504:379–385. doi: 10.1111/j.1469-7793.1997.379be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertner TG, Sabatini BL, Nimchinsky EA, Svoboda K. Facilitation at single synapses probed with optical quantal analysis. Nat Neurosci. 2002;5:657–664. doi: 10.1038/nn867. [DOI] [PubMed] [Google Scholar]
- Ottersen OP, Ben-Ari Y. Afferent connections to the amygdaloid complex of the rat and cat. I. Projections from the thalamus. The Journal of Comparative Neurology. 1979;187:401–424. doi: 10.1002/cne.901870209. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; London: 1997. [DOI] [PubMed] [Google Scholar]
- Petukhov VV, Popov VI. Quantitative analysis of ultrastructural changes in synapses of the rat hippocampal field CA3 in vitro in different functional states. Neuroscience. 1986;18:823–835. doi: 10.1016/0306-4522(86)90103-x. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Excitatory transmission in the basolateral amygdala. J Neurophysiol. 1991;66:986–998. doi: 10.1152/jn.1991.66.3.986. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Mitton DR, Green J, Puchalski S. Chronic daily ethanol and withdrawal: 2. Behavioral changes during prolonged abstinence. Alcohol Clin Exp Res. 2001;25:999–1005. [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Sah P, Westbrook RF, Lüthi A. Fear Conditioning and Long-term Potentiation in the Amygdala. Annals of the New York Academy of Sciences. 2008;1129:88–95. doi: 10.1196/annals.1417.020. [DOI] [PubMed] [Google Scholar]
- Santucci AC, Cortes C, Bettica A, Cortes F. Chronic ethanol consumption in rats produces residual increases in anxiety 4 months after withdrawal. Behav Brain Res. 2008;188:24–31. doi: 10.1016/j.bbr.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Scheuss V, Neher E. Estimating Synaptic Parameters from Mean, Variance, and Covariance in Trains of Synaptic Responses. Biophysical Journal. 2001;81:1970–1989. doi: 10.1016/S0006-3495(01)75848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz PE, Cook EP, Johnston D. Using paired-pulse facilitation to probe the mechanisms for long-term potentiation (LTP) J Physiol Paris. 1995;89:3–9. doi: 10.1016/0928-4257(96)80546-8. [DOI] [PubMed] [Google Scholar]
- Shin RM, Tsvetkov E, Bolshakov VY. Spatiotemporal Asymmetry of Associative Synaptic Plasticity in Fear Conditioning Pathways. Neuron. 2006;52:883–896. doi: 10.1016/j.neuron.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson T, Doyère V, Cain CK, LeDoux JE. Long-term potentiation in the amygdala: A cellular mechanism of fear learning and memory. Neuropharmacology. 2007;52:215–227. doi: 10.1016/j.neuropharm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Stevens CF, Wang Y. Changes in reliability of synaptic function as a mechanism for plasticity. Nature. 1994;371:704–707. doi: 10.1038/371704a0. [DOI] [PubMed] [Google Scholar]
- Sullivan JM. A Simple Depletion Model of the Readily Releasable Pool of Synaptic Vesicles Cannot Account for Paired-Pulse Depression. J Neurophysiol. 2007;97:948–950. doi: 10.1152/jn.00554.2006. [DOI] [PubMed] [Google Scholar]
- Tsvetkov E, Shin RM, Bolshakov VY. Glutamate Uptake Determines Pathway Specificity of Long-Term Potentiation in the Neural Circuitry of Fear Conditioning. Neuron. 2004;41:139–151. doi: 10.1016/s0896-6273(03)00800-6. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Increases in anxiety-like behavior induced by acute stress are reversed by ethanol in adolescent but not adult rats. Pharmacol Biochem Behav. 2012;100:440–450. doi: 10.1016/j.pbb.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadiche JI, Jahr CE. Multivesicular Release at Climbing Fiber-Purkinje Cell Synapses. Neuron. 2001;32:301–313. doi: 10.1016/s0896-6273(01)00488-3. [DOI] [PubMed] [Google Scholar]
- Washburn MS, Moises HC. Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. J Neurosci. 1992;12:4066–4079. doi: 10.1523/JNEUROSCI.12-10-04066.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X-S, Xue L, Mohan R, Paradiso K, Gillis KD, Wu L-G. The Origin of Quantal Size Variation: Vesicular Glutamate Concentration Plays a Significant Role. J Neurosci. 2007;27:3046–3056. doi: 10.1523/JNEUROSCI.4415-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Kwon SE, Gaffaney JD, Dunning FM, Chapman ER. Uncoupling the roles of synaptotagmin I during endo- and exocytosis of synaptic vesicles. Nat Neurosci. 2012;15:243–249. doi: 10.1038/nn.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SM, Jr, Neher E. Synaptotagmin Has an Essential Function in Synaptic Vesicle Positioning for Synchronous Release in Addition to Its Role as a Calcium Sensor. Neuron. 2009;63:482–496. doi: 10.1016/j.neuron.2009.07.028. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Li Y, Tsien RW. The Dynamic Control of Kiss-And-Run and Vesicular Reuse Probed with Single Nanoparticles. Science. 2009;323:1448–1453. doi: 10.1126/science.1167373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinebi F, McKernan M, Shinnick-Gallagher P. Expression of fear-conditioning is accompanied by increased paired-pulse depression within the amygdala. Pharmacology Biochemistry and Behavior. 2002;71:393–400. doi: 10.1016/s0091-3057(01)00684-0. [DOI] [PubMed] [Google Scholar]