Abstract
CD147, also named basigin (Bsg) or extracellular matrix (ECM) metalloproteinase inducer (EMMPRIN), is a highly glycosylated protein first identified as a tumor cell surface molecule. In cancer, it is well established that CD147 promotes metastasis by stimulating the production of MMPs. Recent studies have also suggested that it may be associated with tumor growth and angiogenesis. Interestingly, CD147 is expressed in germ cells of different development stages in the testis and its knockout mice are infertile, indicating an essential role of CD147 in spermatogenesis. While the detailed involvement of CD147 in spermatogenesis remains elusive, our recent findings have revealed a dual role of CD147 in germ cell development. On the one hand, it regulates the migration of spermatogonia and spermatocytes via the induction of MMP-2 production; on the other hand, it specifically regulates the survival/apoptosis of spermatocytes but not spermatogonia through a p53-independent pathway. In this review, we aim to provide an overview on the functions of CD147, comparing its roles in cancer and the testis, thereby providing new insights into the regulatory mechanisms underlying the process of spermatogenesis.
Keywords: CD147, cancer metastasis, spermatogenesis, apoptosis, germ cell
Introduction
Spermatogenesis is a multi-step process involving a number of cellular events including mitosis, meiosis, cell migration, apoptosis and differentiation, which enable the germ cells to undergo several developmental stages, from spermatogonia to primary and secondary spermatocytes, round spermatids and eventually spermatozoa. Germ cells simultaneously undergo differentiation and migration from the basal compartment toward the adluminal compartment during spermatogenesis. The migration of germ cells requires a highly orchestrated network that involves the endocrine/paracrine signaling and restructuring of the cell adhesion complex, namely, the ectoplasmic specialization (ES). The ES is a very important structure between Sertoli cells or Sertoli cells and germ cells during spermatogenesis, which is prominently identified at the blood-testis barrier (BTB, also known as basal ES) and Sertoli-germ cell adhesion junctions (apical ES).1-3 The migration of germ cells in the testis is accompanied with various differentiation processes. During the first meiotic division, the cell cycle progression is prolonged and DNA double strand breaks (DSBs) are being generated in order to allow the genetic recombination through chromosome crossover. The primary spermatocytes survive the cell cycle arrest and the naked DNA through the acquisition of autocrine/paracrine survival signals4,5 and the activation of DNA repairing machinery.6,7 To ensure the integrity of the genome, spermatocytes that failed to repair the DNA mismatch and double strand breaks are eliminated through apoptosis.8 Therefore, the differentiation of germ cells is tightly coupled to germ cell migration as well as apoptotic activity during spermatogenesis.
Over the past decade, there has been a tremendous augmentation in our understanding of the regulation of ES formation and restructuring, which has been extensively reviewed.2,9-11 However, little is known about the molecules on germ cells that regulate the germ cell migration process. Interestingly, emerging evidence has indicated an important role of CD147, a membrane protein originally found in cancer, in both germ cell migration and survival/apoptosis. This review aims to provide an overview on the function of CD147 and discuss its emerging roles in spermatogenesis and the underlying mechanisms.
Structures and Expression of CD147
CD147, also known as EMMPRIN and Basigin,12 is a member of the immunoglobulin superfamily (IgSF). The protein contains two (short isoform) or three (long isoform) extracellular Ig domains at the N terminus, a highly conserved transmembrane domain and a short cytoplasmic tail at the C terminus. The atypical charged amino acid, glutamic acid, present in the transmembrane domain has been shown to be important for the interaction of CD147 with other proteins in mediating its function.13 Multiple glycosylation sites have been found in the Ig domain and the protein is expressed as a highly glycosylated transmembrane protein.14,15 Glycosylation appears to be important for the function of CD147 and may serve as a regulatory mechanism.16-18
The expression of CD147 in normal tissue was found mainly restricted to the reproductive tract, brain, eye and muscle.19-22 However, reactivation of CD147 was observed in different tumors including the brain,23 lung, breast,24 colon,25,26 bladder27 and liver,28 and found to be associated with the invasiveness of the cancers. Therefore, CD147 has been considered a biomarker for cancer diagnosis and prognostic molecule.29
Role of CD147 in Cancer Metastasis
The pathological roles of CD147 in tumorigenesis and cancer metastasis are well documented to be associated with its ability to induce the expression of matrix metalloproteinases (MMPs).12,19,29 It is well established that the migration and metastasis of cancer require the degradation of extracellular matrix (ECM), the degeneration of cell adhesion molecules on cancer cells, and the formation of new blood or lymph vessels. The degradation of ECM requires a number of different proteases including the MMPs family and the urokinase plasminogen activator (uPA) system.30 CD147 was originally identified as a tumor cell surface molecule, which possesses the ability to induce MMP-1 expression.31 Lim et al. reported the high expression of MMP-1 in the stromal fibroblasts which were adjacent to the CD147-expressed tumor cells in lung cancer. Moreover, activation of p38 was found to induce the CD147 upregulated expression of MMP-1.32 Subsequent studies further demonstrated that CD147 was able to stimulate the expression of MMP-2, MMP-3, MMP-9 and MMP-11 in fibroblast cells as well as in tumor cells, which was associated with tumor cell migration and invasion.12,33-35 The mechanisms by which CD147 upregulates MMPs family appear to depend on its interaction with other proteins. Interestingly, blocking the interaction between CD147 and α6β1 integrin by antibody treatment and knockdown of CD147 by siRNA both result in significant reduction in MMP-2 and MMP-9 secretion, indicating that CD147-α6β1 integrin interaction may be an upstream mechanism regulating MMPs activity.36 Moreover, the interaction between CD147 and integrin has been reported to be associated with cellular architecture37 and invasion potential in human hepatocellular carcinoma (HCC) cells.38-40
The role of CD147 in cancer migration may not limit to the regulation of MMPs expression. Lescaille et al. have demonstrated that CD147 stimulates the invasion of oral squamous cancer cell carcinoma by upregulating uPA expression,41 indicating that CD147 may be able to activate other proteases besides MMPs. Dai et al. reported that CD147 interacted with α6β1 integrin in human HCC cells and stimulated the tumor invasion process via PI3K pathway.36 Zhao et al. also revealed that CD147 enhanced the mesenchymal movement by activating integrin-FAK-PI3K signaling pathway and inhibiting the phosphorylation of annexin II and amoeboid movement in HCC cells.42 The ability of CD147 in regulating the expression of both MMPs family and uPA system, together with its ability to regulate cell adhesion dynamics through protein-protein interactions, have made CD147 one of the most important regulators of cancer migration and metastasis and a strong candidate for anti-tumor therapy.19,29
Compared with the better characterized downstream targets and signaling, the molecules that are involved in regulating the expression and function of CD147 in cancers are poorly defined. A recent study has shown that TGF-β, a key regulator of epithelial mesenchymal transition (EMT), can upregulate the expression of CD147 through PI3K/Akt/GSK3β/Snail/Slug signaling cascade whereas ectopic overexpression of CD147 can also result in elevated level of TGF-β 43, suggesting a positive feed-back loop in regulation of EMT and cancer metastasis in HCC. Taken together, CD147 is well documented to play a key role in cancer cell migration and metastasis.
Role of CD147 in the Reproductive System
CD147 has been reported to be expressed in both the male and female reproductive tracts. In the female reproductive tract, CD147 is expressed in the ovary,44 uterine endometrium,45-47 placenta and fetal membranes.48 Increased expression of CD147 has been observed in the endometrium and the trophoectoderm of the embryo during blastocyst implantation.45,49 In mouse testis, the expression of CD147 is reported to first appear at 7 days postpartum (dpp) and gradually increase in a developmental-dependent manner.50 Several studies have demonstrated that CD147 is localized in mouse leptotene spermatocytes and step 9–11 spermatids51 and on the head region of capacitated spermatozoa.52 In our recent study, we further demonstrated that CD147 was also expressed in spermatogonia in both human and mouse testes.50
Knockout of CD147 leads to subfertility in female mice while the male mice are infertile.53 The subfertility in female mice was proposed to be resulted from fertilization and implantation failure due to reduced MMPs production.45 It should be noted that CD147 knockout embryos undergo normal early preimplantation development, suggesting that CD147 is dispensable for the process.45 Male CD147 knockout mice were reported to be infertile with germ cells arrested at meiotic metaphase and degenerating germ cells observed within the seminiferous tubules.49,53 However, the detailed mechanism underlying the CD147 knockout-induced infertility in males had not been delineated until recently.
CD147 is Required for the Migration of Spermatogonia and Spermatocytes
Although germ cell migration is a characteristic feature of spermatogenesis and CD147 is known to be expressed in germ cells of all developmental stages in the testis, its role in spermatogenesis remained uninvestigated until recently. Early reports on the function of CD147 in male reproductive system have been focused on its function in spermatozoa. Saxena et al. reported that CD147 was glycosylated on spermatozoa, however, during their transit through the epididymis, CD147 underwent deglycosylation.52 Whether the deglycosylation is required for normal function of CD147 in spermatozoa remains elusive. Nonetheless, using immunodepletion approach, the researchers further demonstrated that CD147 might play a role in mediating the primary binding of spermatozoa to the zona pellucida during sperm-cumulus interaction.52,54
Early stage germ cell migration and cancer cell migration are similar in that both require the degradation of ECM and the degeneration of cell adhesion molecules. The dissociation of spermatogonia from the basement membrane of the testis is the first step in germ cell migration. Although the niche for spermatogonia is not well characterized, the basement membrane, which is considered a modified form of extracellular matrix (ECM), has been found to be mainly composed of laminin, collagen, and fibronectin.55 Laminin has been demonstrated to support the proliferation of spermatogonial stem cells.56 Cell adhesion molecule β1 integrin, which mediates the binding of the germ cells to laminin, is required for the homing of spermatogonial stem cell to the niche.57 It has been suggested that the detachment of spermatogonia from basement membrane may require the disruption of the β1 integrin-laminin interaction possibly through the activation of different kinds of ECM proteases.
MMPs family represents the largest class of proteases with 28 family members currently identified.55 MMP-2, 7 and 9 are the well-characterized members with known substrates. The substrates for MMP-2 include gelatin, laminin, fibronectin, collagen type I, II, IV, V and VII. MMP-7 and 9 share similar substrates including gelain, proteoglycans, fibronectin and collagen type IV.58,59 Of the 28 MMPs identified, 18 of them have been shown to be expressed in the testes.60 Since CD147 is known to regulate the expression of multiple MMPs in cancer cells, we have also investigated the possible involvement of CD147 in regulating MMPs in the testis.50 Among the eight MMPs that are known to be regulated by CD147, we have found that MMP-2, MMP-7, MMP-9 and MMP-23 are differentially expressed during testicular development. CD147 and MMP-2 are also co-localized on the surface of spermatogonia, spermatocytes and round spermatids in adult mouse seminiferous tube. Interfering CD147 function by immunodepletion in vitro results in a decrease in the migration rate of both GC-1 cells (immortalized spermatogonia) and GC-2 cells (immortalized spermatocytes), accompanied with the reduction of MMP-2 expression and activity, suggesting that CD147 may regulate the migration of spermatogonia and spermatocytes by regulating MMP-2 50. Whether CD147 may also regulate other MMPs in the testis requires further investigation.
After entering the meiotic cycles, the preleptotene spermatocyte will leave the basal compartment, transit through the recontructuring BTB and enter the adluminal compartment. Unlike other “blood-tissue barrier” which mainly comprise of tight junctions, the “blood-testis barrier” is formed by coexisting tight junctions, desmosome-gap junctions and basal ectoplasmic specialization (ES), a testis-specific actin-based anchoring junction.9,61 After completing the second meiotic cycle, round spermatids undergo spermiogenesis and give rise to elongated spermatids and spermatozoa. The development of germ cells of various stages is guided and nursed by Sertoli cells through apical ES, which unlike basal ES, exists in the germ cell-Sertoli cell interface.62 After spermiogenesis, the spermatozoa are released to the lumen through the dissolution of apical ES, a process known as spermiation. Protein networks involved in BTB, basal and apical ES are complicated. The cell-cell interaction is mediated by integral membrane proteins and adaptors or scaffolding proteins.10,62 These include the cadherin/catenin complex, the nectin/afadin complex and the α6β1-integrin/laminin γ3 complex in the ES; and the occluding/ZO-1 complex in the tight junction.62,63 Besides, a number of motor proteins, trafficking proteins, regulatory and signaling proteins, cytoskeletal proteins and proteins with specialized functions such as MMPs are also involved in the complex networks of ES.61,62 The presence of CD147 interacting protein β1 integrin and downstream target MMP-2 in the ES suggests that CD147 is likely to regulate the migration of more differentiated germ cells other than spermatogonia and spermatocytes through the BTB and basal ES. Indeed, co-localization of CD147 and MMP-2 has been found in round spermatids.50 However, the role of CD147 in migration of more differentiated germ cells remains elusive due to the lack of in vitro model and technical limitation for in vivo study.
CD147 as an Initiator of ES and BTB Restructuring?
The disassembly of basal ES and dissolution of apical ES are coupled events that are observed at Stage VIII of the seminiferous tubules. Recently, Yan et al. have shown that active protein fragments from laminin β3 domain I or laminin γ3 domain IV of the apical ES are able to perturb BTB functions in cultured Sertoli cells,64 suggesting that the coordination between basal and apical ES is mediated by these laminin fragments. Since laminin is expressed in chains in ECM, it is believed that a protease is required for the production of biologically active fragments through cleavage. Interestingly, co-localization and physical interaction between MMP-2 and laminin γ3 chains were observed at the apical ES at stage VII and early VIII of the seminiferous tubules and chemical induction of MMP-2 was shown to disrupt Sertoli-germ cell adhesion,65 indicating that MMP-2 may mediate the production of active laminin fragments. Since CD147 is a well- known physiological inducer of MMP-2 and their co-localization was observed in round spermatid and spermatozoa,50 it is plausible that CD147 may act as an initiator for the production of active laminin fragments required for dissolution of apical ES.
Apart from laminin α3, β3 and γ3 chains, which are restricted to elongated spermatids, a number of different laminin chains are also observed in the basement membrane and basal ES.66 Although active laminin fragments from β3 and γ3 chains are found in the apical ES and are able to perturb BTB functions, leading to the hypothesis that disassembly of basal ES is coordinated by active laminin fragments from apical ES, the possible contribution from laminin of the basement membrane and basal ES has not been examined. The observed co-localization of CD147 with MMP-2 in spermatogonia and spermatocytes and the demonstrated requirement of CD147 for MMP-2 activity in these germ cells50 suggest that CD147/MMP-2 may also produce active laminin fragments in the basement membrane and basal ES which could also contribute to the disassembly of basal ES. Further studies are required to demonstrate the role of CD147 in the initiation of disassembly of basal and apical ES. It should be noted that TGF-β has been found in the apical ES and BTB62 and overexpression or local administration of TGF-β has been shown to perturb BTB functions in vitro and in vivo.67 Interestingly TGF-β is known to upregulate the expression of CD147 in HCC metastasis.43 Therefore, it is plausible that TGF-β upregulates the expression of CD147 in the testis, which in turn induces MMP-2 activity and subsequent active laminin fragment production that leads to the disassembly of basal and apical ES and restructuring of BTB. Of note, CD147 has also been implicated in blood-brain barrier function and some associated brain diseases.68,69 Further studies are required to investigate the exact role and mechanism of CD147 in the regulation of restructuring of ES during spermatogenesis.
CD147 Regulates Spermatocyte-Specific Survival/Apoptosis
Apart from its well-established role in cell migration, recent studies have demonstrated that knockdown of CD147 by siRNA or blocking functional CD147 with specific antibody inhibits the cancer growth and tumorigenecity of multiple cancer cells.70-72 Further functional studies reveal that the massive cell death is due to enhanced cell apoptosis induced by CD147 inhibition,73-76 indicating CD147 regulates survival/apoptotic process in cancer cells. While the mechanisms underlying CD147-mediated apoptosis regulation are largely unknown, it has been suggested that CD147 blocking induces apoptosis through a caspase-dependent pathway.77 More interestingly, a recent study on HCC revealed that CD147 played pivotal roles in modulating the unfolded protein response (UPR)-related anti-apoptosis and chemo-sensitivity mechanism in HCC through Bip (also called glucose-regulated protein 78), which provides an opportunity to enhance the efficacy of anti-tumor drugs in the treatment of HCC by CD147 inhibition.78
In adult testes, apoptosis in germ cells is mainly involved in maintaining the dynamic balance of germ cells in order to eliminate defective germ cells that fail to repair endogenous DNA mismatch or exposed to exogenous stimuli such as heat, chemotherapeutic agents and environmental toxicants.79 De-regulation of germ cell apoptosis might lead to testicular cancer or infertility,80 highlighting the significance of tight control of apoptosis in spermatogenesis. The control of male germ cell apoptosis in the testis is a highly preserved process, which is regulated by both extrinsic (also known as death receptor pathway) and intrinsic pathway (mitochondrial pathway).7 The intrinsic pathway is triggered by the release of cytochrome c from the mitochondria when germ cells are under hypoxia or other stresses, resulting in the activation of caspase 9 in apoptosome complex with subsequent caspase cascades. In contrast, the extrinsic apoptosis pathway is triggered by activation of death receptors (TNFR1, Fas/CD95, DR3, DR4 and DR5) and their corresponding ligands (TNF-α and FasL), thereby triggering the activation of caspase-8 as the initiator of the downstream cleavage of procaspase effectors (such as 3, 6, 7 and 10), eventually leading to germ cell apoptosis.7 Additional pathway includes the perforin/granzyme pathway that induces apoptosis via either granzyme B or granzyme A.81 p53 has been demonstrated as a key player in the cellular response to multiple stresses and promotes apoptosis in both extrinsic and intrinsic pathways during spermatogenesis.82 While p53-induced intrinsic apoptotic pathway has been elucidated to target on the pro-apoptotic proteins of bcl-2 family, including bcl-2, bax, bid, noxa and puma,82-84 p53-mediated extrinsic apoptosis has been suggested to activate two death receptors (Fas/CD95, DR5) and PARP in response to Gamma-irradiation, DNA damage and other stresses.85-87 Of particular importance, p53 dependent apoptosis has been reported to be cell type specific during germ cell development.7,88
Interestingly, it has long been observed that the CD147 knockout male mice are infertile with arrested and degenerated germ cells observed in the testis,53 indicating possible involvement of CD147 in the apoptotic process of germ cells. Indeed, interfering with CD147 function by neutralizing antibody in vivo resulted in significant reduction in testicular size and weight, which was attributed to accumulated apoptosis-related germ cell loss, as indicated by a significant increase in apoptotic spermatocytes observed in stage XI-XII seminiferous tubules.89 Furthermore, immunodepletion of CD147 in spermatocytes cell line GC-2, but not spermatogonia cell line GC-1, results in a significant increase in the number of apoptotic cells.89 These findings have revealed a novel germ cell apoptosis regulation mechanism involving CD147, which provides a possible mechanism underlying the long observed germ cell arrest and degeneration in CD147 knockout mice model.53 These findings also indicate that CD147 may specifically regulate apoptosis in spermatocytes but not spermatogonia, suggesting a distinct CD147-mediated stage-specific apoptotic regulation program in spermatogensis. Interestingly, this CD147-mediated apoptotic mechanism appears to be through a novel p53-independent pathway, involving the activation of caspase-3 and PARP,89 details of which await further investigation. These results suggest that CD147 may be important in promoting the survival of differentiated germ cells during their development in spermatogenesis, downregulation of which may lead to germ cell apoptosis.
Dual Role of CD147 in Spermatogenesis: A Surveillance Mechanism for Germ Cell Development?
Spermatogenesis in mammals is a highly ordered process, which includes six consecutive mitotic cycles of differentiating spermatogonia synchronized with the meiotic cycle of spermatocytes, and followed by spermatid differentiation to spermatozoa.90 On top of that, during spermatogenesis, developing germ cells must traverse from the basal lamina to the adluminal compartment of the seminiferous epithelium, at which terminally developed spermatozoa can be released into the tubular lumen. Accordingly, these processes must under precise regulation through an intricate network of signaling pathways to ensure the quality and quantity of mature spermatozoa62,80. Based on the findings that CD147 is involved in the regulation of both migration and survival/apoptosis of germ cells, we propose that the dual functionality of CD147 during spermatogenesis may provide a quality control surveillance mechanism directing the germ cells to survive and migrate for further development or undergo apoptosis.
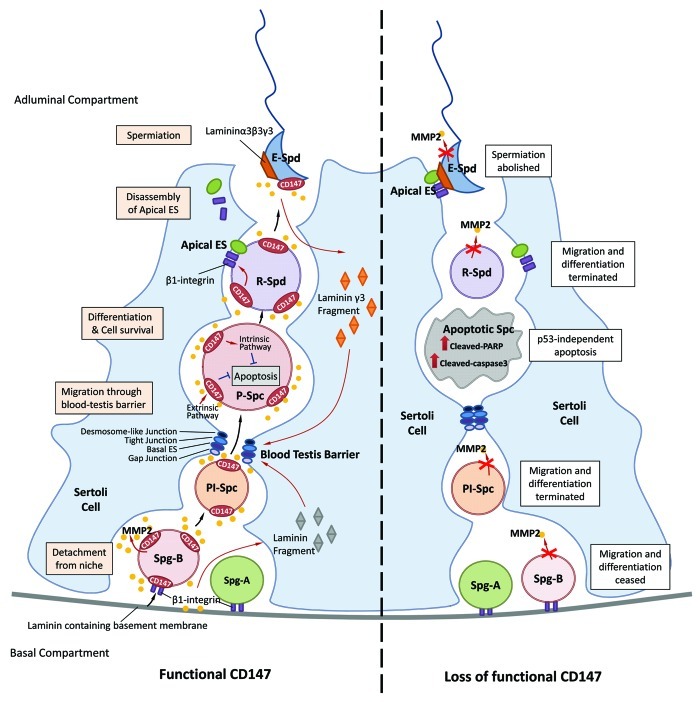
On the one hand, CD147 facilitates the migration of the developing germ cells from the basal compartment toward the adluminal compartment which is important for the differentiation of germ cells. On the other hand, CD147 acts as an anti-apoptotic factor in spermatocytes through a p53-independent pathway (Fig. 1). This newly identified surveillance mechanism may monitor both external and internal conditions to ensure the successful completion of upstream events before the germ cells proceed to the next phase. Of note, the expression of CD147 increases along with the differentiation and migration of germ cells in the testis. While CD147 is expressed at all stages during mouse testicular development, its expression gradually increases in intensity during the differentiation of spermatocytes and spermatids in adult mouse and human testes.50 More importantly, CD147 is colocalized with MMP-2 in adult mouse seminiferous tube, indicating that CD147 is associated with germ cell migrative mechanisms. Direct evidence supporting this notion comes from our recent study showing that CD147 regulates migration of spermatogonia and spermatocytes via induction of MMP-2 production. Given that MMP-2 disrupts Sertoli-germ cell adhesion which could be mediated by active laminin fragments, and laminin fragments are required for the restructure of ES,65 it is very likely that CD147 functions as a critical migration regulator through induction of MMPs, which subsequently rearrange the structure of ES and facilitate the embedded migration and differentiation program of germ cells (Fig. 1). Indeed, immuno-electron microscopic analysis has demonstrated that CD147 is also localized on the plasma membrane of the Sertoli cells which are in close contact with spermatocytes and spermatids, indicating that CD147 is involved in the crosstalk between germ cells and their surrounding environment, such as Sertoli cells and ES.
Figure 1. Schematic diagram showing the dual role of CD147 in spermatogenesis. CD147 is expressed in spermatogonia (Spg),spermatocytes (Spc) and spermatids (Spd) and facilitates the production and activation of MMP-2 that is required for the migration of the developing germ cells (left panel). Defects or mis-regulation of CD147 would cease the migration and probably the differentiation of developing germ cells and spermiation of spermatids (right panel). CD147 may interact with β1-integrin and activate MMP-2 in regulating the production of laminin γ3 fragment from the apical ES and other subtypes of laminin fragments from the basement membrane, which may involve in restructuring of the BTB and ES. CD147 also acts as an anti-apoptotic factor in spermatocytes via a p53-independent pathway, downregulation or defect of which leads to apoptosis of spermatocytes. The dual role of CD147 provides a quality control surveillance mechanism directing the germ cells to survive and migrate for further development or undergo apoptosis. Key: Spg-A, type A spermatogonia; Spg-B, type B spermatogonia; Pl-Spc, preleptotene spermatocyte; P-Spc, pachytene spermatocyte; R-Spd, round spermatid; E-Spd, elongated spermatid; ES, ectoplasmic specialization.
Our recent in vitro study has demonstrated that CD147 deprivation induces apoptosis specifically in spermatocytes.89 These findings are consistent with the in vivo observation that the TUNEL positive signals accumulate at the spermatocytes rather than other germ cell types after CD147 antibody treatment in mice.89 After mitosis, the differentiated spermatogonia detach from the basement membrane and enter into the meiotic cycle. Further differentiation of primary spermatocytes is associated with the migration toward the lumen of the seminiferous tubules. The tightly-controlled apoptosis during these processes plays a major role in spermatogonial density regulation as well as in the maintenance of the required homeostasis among various germ cell types. Thus, it appears that CD147 is critical for the synchronization between the spermatogonial and the spermatocyte cycles and acts as a major molecular machinery controlling spermatocyte progression for further differentiation through its dual functionality: promoting migration and cell survival. In agreement with this hypothesis, we have observed a remarkable increase in the expression of CD147 at 21 dpp when spermatocytes are the main germ cell type in the seminiferous tubules50. The dynamic changes in the expression profile of CD147, along with its anti-apoptosis role, are consistent with the notion that upregulation of CD147 promotes the survival and migration of spermatocytes, failure of which could result in apoptosis and degeneration of germ cells (Fig. 1), as observed in CD147 knockout mice.
Concluding Remark
Although studies on the role of CD147 in spermatogenesis are limited, they have revealed novel mechanisms governing germ cell apoptosis and migration, providing new insights into the complex and delicate regulation of spermatogenesis. CD147 appears to be a key player in checking the differentiation stage and quality of germ cells and facilitating the migration of the checked germ cells for further differentiation. Apart from the regulatory role of CD147, we have learned several lessons on spermatogenesis as well as cancer from these studies: (1) germ cell apoptosis regulation during spermatogenesis could be cell type- or stage specific; (2) the process of germ cell survival and migration during spermatogenesis may be linked and governed by a single molecule; (3) certain molecular machinery controlling cell migration and/or cell survival are shared by male germ cells and cancer cells.
While we have learned important lessons from CD147, it also raises several important questions, which may lead to exciting research on spermatogenesis, If CD147 is an important check-point molecule in spermatogenesis, what signal(s) triggers its up or downregulation? CD147 appears to regulate germ cell survival/apoptosis and migration via distinctive mechanisms, does CD147 interact with different proteins to activate different signaling pathways? If yes, what are its interacting molecules? Does CD147 also regulate apical ES as demonstrated for basal ES? If yes, does it involve the same mechanism involving MMPs only or other proteases, such as uPA, as well? The CD147-mediated apoptosis seems to be distinctively different from the conventional extrinsic and intrinsic germ cell apoptotic pathways since it is p53-independent. What is the exact signaling pathway for this CD147-dependent p53-independent pathway? Does it have any cross-talk with the extrinsic and intrinsic pathways? The answers to these questions will undoubtedly and significantly advance our understanding of the regulatory mechanisms underlying spermatogenesis. Further studies comparing CD147 expression profile and related signaling pathways between testicular cancer and azoospermic patients will not only confirm a key role of CD147 in spermatogenesis but also provide a new potential diagnostic marker for cancer and a novel target for male contraceptives.
Acknowledgments
The authors wish to thank Dr. Yechun Ruan for her artwork. The work was supported in parts by National Basic Research Program of China (2012CB944903), National Natural Science Foundation of China (No. 31100841), Shenzhen city science and technology project: medicine and health (No. 201102002), China Postdoctoral Science Foundation funded project (No. 20110490920), Foundation for Distinguished Young Talents in Higher Education of Guangdong (No. LYM11112), the Focused Investment Scheme, Li Ka Shing Institute of Health Sciences and GRF2010/2011 (CUHK466710) of Hong Kong University Grants Committee.
Footnotes
Previously published online: www.landesbioscience.com/journals/spermatogenesis/article/22014
References
- 1.Toyama Y, Maekawa M, Yuasa S. Ectoplasmic specializations in the Sertoli cell: new vistas based on genetic defects and testicular toxicology. Anat Sci Int. 2003;78:1–16. doi: 10.1046/j.0022-7722.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- 2.Wong EW, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochim Biophys Acta. 2008;1778:692–708. doi: 10.1016/j.bbamem.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan HH, Mruk DD, Lee WM, Cheng CY. Ectoplasmic specialization: a friend or a foe of spermatogenesis? Bioessays. 2007;29:36–48. doi: 10.1002/bies.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan W, Samson M, Jégou B, Toppari J. Bcl-w forms complexes with Bax and Bak, and elevated ratios of Bax/Bcl-w and Bak/Bcl-w correspond to spermatogonial and spermatocyte apoptosis in the testis. Mol Endocrinol. 2000;14:682–99. doi: 10.1210/me.14.5.682. [DOI] [PubMed] [Google Scholar]
- 5.Chang IY, Kim JH, Park KH, Yoon SP. Experimental varicocele induces p53-dependent germ cell apoptosis through activation of γ-H2AX. Urol Int. 2010;85:216–20. doi: 10.1159/000316356. [DOI] [PubMed] [Google Scholar]
- 6.Matulis S, Handel MA. Spermatocyte responses in vitro to induced DNA damage. Mol Reprod Dev. 2006;73:1061–72. doi: 10.1002/mrd.20508. [DOI] [PubMed] [Google Scholar]
- 7.Aitken RJ, Findlay JK, Hutt KJ, Kerr JB. Apoptosis in the germ line. Reproduction. 2011;141:139–50. doi: 10.1530/REP-10-0232. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Gu H, Lin H, Chi T. Essential roles of the chromatin remodeling factor BRG1 in spermatogenesis in mice. Biol Reprod. 2012;86:186. doi: 10.1095/biolreprod.111.097097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee NP, Cheng CY. Ectoplasmic specialization, a testis-specific cell-cell actin-based adherens junction type: is this a potential target for male contraceptive development? Hum Reprod Update. 2004;10:349–69. doi: 10.1093/humupd/dmh026. [DOI] [PubMed] [Google Scholar]
- 10.Yan HH, Mruk DD, Lee WM, Cheng CY. Cross-talk between tight and anchoring junctions-lesson from the testis. Adv Exp Med Biol. 2008;636:234–54. doi: 10.1007/978-0-387-09597-4_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng CY, Wong EW, Yan HH, Mruk DD. Regulation of spermatogenesis in the microenvironment of the seminiferous epithelium: new insights and advances. Mol Cell Endocrinol. 2010;315:49–56. doi: 10.1016/j.mce.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iacono KT, Brown AL, Greene MI, Saouaf SJ. CD147 immunoglobulin superfamily receptor function and role in pathology. Exp Mol Pathol. 2007;83:283–95. doi: 10.1016/j.yexmp.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirk P, Wilson MC, Heddle C, Brown MH, Barclay AN, Halestrap AP. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J. 2000;19:3896–904. doi: 10.1093/emboj/19.15.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shirozu M, Tada H, Tashiro K, Nakamura T, Lopez ND, Nazarea M, et al. Characterization of novel secreted and membrane proteins isolated by the signal sequence trap method. Genomics. 1996;37:273–80. doi: 10.1006/geno.1996.0560. [DOI] [PubMed] [Google Scholar]
- 15.Vitiello A, Sette A, Yuan L, Farness P, Southwood S, Sidney J, et al. Comparison of cytotoxic T lymphocyte responses induced by peptide or DNA immunization: implications on immunogenicity and immunodominance. Eur J Immunol. 1997;27:671–8. doi: 10.1002/eji.1830270315. [DOI] [PubMed] [Google Scholar]
- 16.Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996;56:5309–18. [PubMed] [Google Scholar]
- 17.Sun J, Hemler ME. Regulation of MMP-1 and MMP-2 production through CD147/extracellular matrix metalloproteinase inducer interactions. Cancer Res. 2001;61:2276–81. [PubMed] [Google Scholar]
- 18.Tang W, Chang SB, Hemler ME. Links between CD147 function, glycosylation, and caveolin-1. Mol Biol Cell. 2004;15:4043–50. doi: 10.1091/mbc.E04-05-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weidle UH, Scheuer W, Eggle D, Klostermann S, Stockinger H. Cancer-related issues of CD147. Cancer Genomics Proteomics. 2010;7:157–69. [PubMed] [Google Scholar]
- 20.Juel C, Halestrap AP. Lactate transport in skeletal muscle - role and regulation of the monocarboxylate transporter. J Physiol. 1999;517:633–42. doi: 10.1111/j.1469-7793.1999.0633s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanyenda LJ, Verdile G, Boulos S, Krishnaswamy S, Taddei K, Meloni BP, et al. The dynamics of CD147 in Alzheimer’s disease development and pathology. J Alzheimers Dis. 2011;26:593–605. doi: 10.3233/JAD-2011-110584. [DOI] [PubMed] [Google Scholar]
- 22.Ochrietor JD, Linser PJ. 5A11/Basigin gene products are necessary for proper maturation and function of the retina. Dev Neurosci. 2004;26:380–7. doi: 10.1159/000082280. [DOI] [PubMed] [Google Scholar]
- 23.Sameshima T, Nabeshima K, Toole BP, Yokogami K, Okada Y, Goya T, et al. Expression of emmprin (CD147), a cell surface inducer of matrix metalloproteinases, in normal human brain and gliomas. Int J Cancer. 2000;88:21–7. doi: 10.1002/1097-0215(20001001)88:1<21::AID-IJC4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 24.Polette M, Gilles C, Marchand V, Lorenzato M, Toole B, Tournier JM, et al. Tumor collagenase stimulatory factor (TCSF) expression and localization in human lung and breast cancers. J Histochem Cytochem. 1997;45:703–9. doi: 10.1177/002215549704500508. [DOI] [PubMed] [Google Scholar]
- 25.Abraham D, Zins K, Sioud M, Lucas T, Aharinejad S. Host CD147 blockade by small interfering RNAs suppresses growth of human colon cancer xenografts. Front Biosci. 2008;13:5571–9. doi: 10.2741/3100. [DOI] [PubMed] [Google Scholar]
- 26.Lecona E, Olmo N, Turnay J, Santiago-Gómez A, López de Silanes I, Gorospe M, et al. Kinetic analysis of butyrate transport in human colon adenocarcinoma cells reveals two different carrier-mediated mechanisms. Biochem J. 2008;409:311–20. doi: 10.1042/BJ20070374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muraoka K, Nabeshima K, Murayama T, Biswas C, Koono M. Enhanced expression of a tumor-cell-derived collagenase-stimulatory factor in urothelial carcinoma: its usefulness as a tumor marker for bladder cancers. Int J Cancer. 1993;55:19–26. doi: 10.1002/ijc.2910550105. [DOI] [PubMed] [Google Scholar]
- 28.Jiang JL, Zhou Q, Yu MK, Ho LS, Chen ZN, Chan HC. The involvement of HAb18G/CD147 in regulation of store-operated calcium entry and metastasis of human hepatoma cells. J Biol Chem. 2001;276:46870–7. doi: 10.1074/jbc.M108291200. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Xu J, Chen L, Zhong WD, Zhang Z, Mi L, et al. HAb18G (CD147), a cancer-associated biomarker and its role in cancer detection. Histopathology. 2009;54:677–87. doi: 10.1111/j.1365-2559.2009.03280.x. [DOI] [PubMed] [Google Scholar]
- 30.Pepper MS. Role of the matrix metalloproteinase and plasminogen activator-plasmin systems in angiogenesis. Arterioscler Thromb Vasc Biol. 2001;21:1104–17. doi: 10.1161/hq0701.093685. [DOI] [PubMed] [Google Scholar]
- 31.Biswas C. Tumor cell stimulation of collagenase production by fibroblasts. Biochem Biophys Res Commun. 1982;109:1026–34. doi: 10.1016/0006-291X(82)92042-3. [DOI] [PubMed] [Google Scholar]
- 32.Lim M, Martinez T, Jablons D, Cameron R, Guo H, Toole B, et al. Tumor-derived EMMPRIN (extracellular matrix metalloproteinase inducer) stimulates collagenase transcription through MAPK p38. FEBS Lett. 1998;441:88–92. doi: 10.1016/S0014-5793(98)01474-4. [DOI] [PubMed] [Google Scholar]
- 33.Biswas C, Zhang Y, DeCastro R, Guo H, Nakamura T, Kataoka H, et al. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995;55:434–9. [PubMed] [Google Scholar]
- 34.Guo H, Zucker S, Gordon MK, Toole BP, Biswas C. Stimulation of matrix metalloproteinase production by recombinant extracellular matrix metalloproteinase inducer from transfected Chinese hamster ovary cells. J Biol Chem. 1997;272:24–7. doi: 10.1074/jbc.272.1.24. [DOI] [PubMed] [Google Scholar]
- 35.Gabison EE, Hoang-Xuan T, Mauviel A, Menashi S. EMMPRIN/CD147, an MMP modulator in cancer, development and tissue repair. Biochimie. 2005;87:361–8. doi: 10.1016/j.biochi.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 36.Dai JY, Dou KF, Wang CH, Zhao P, Lau WB, Tao L, et al. The interaction of HAb18G/CD147 with integrin alpha6beta1 and its implications for the invasion potential of human hepatoma cells. BMC Cancer. 2009;9:337. doi: 10.1186/1471-2407-9-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curtin KD, Meinertzhagen IA, Wyman RJ. Basigin (EMMPRIN/CD147) interacts with integrin to affect cellular architecture. J Cell Sci. 2005;118:2649–60. doi: 10.1242/jcs.02408. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Wu J, Song F, Tang J, Wang SJ, Yu XL, et al. Extracellular membrane-proximal domain of HAb18G/CD147 binds to metal ion-dependent adhesion site (MIDAS) motif of integrin β1 to modulate malignant properties of hepatoma cells. J Biol Chem. 2012;287:4759–72. doi: 10.1074/jbc.M111.277699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang J, Wu YM, Zhao P, Yang XM, Jiang JL, Chen ZN. Overexpression of HAb18G/CD147 promotes invasion and metastasis via alpha3beta1 integrin mediated FAK-paxillin and FAK-PI3K-Ca2+ pathways. Cell Mol Life Sci. 2008;65:2933–42. doi: 10.1007/s00018-008-8315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu YM, Tang J, Zhao P, Chen ZN, Jiang JL. Enhanced expression of Hab18g/CD147 and activation of integrin pathway in HCC cells under 3-D co-culture conditions. Cell Biol Int. 2009;33:199–206. doi: 10.1016/j.cellbi.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 41.Lescaille G, Menashi S, Cavelier-Balloy B, Khayati F, Quemener C, Podgorniak MP, et al. EMMPRIN/CD147 up-regulates urokinase-type plasminogen activator: implications in oral tumor progression. BMC Cancer. 2012;12:115. doi: 10.1186/1471-2407-12-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao P, Zhang W, Wang SJ, Yu XL, Tang J, Huang W, et al. HAb18G/CD147 promotes cell motility by regulating annexin II-activated RhoA and Rac1 signaling pathways in hepatocellular carcinoma cells. Hepatology. 2011;54:2012–24. doi: 10.1002/hep.24592. [DOI] [PubMed] [Google Scholar]
- 43.Wu J, Ru NY, Zhang Y, Li Y, Wei D, Ren Z, et al. HAb18G/CD147 promotes epithelial-mesenchymal transition through TGF-β signaling and is transcriptionally regulated by Slug. Oncogene. 2011;30:4410–27. doi: 10.1038/onc.2011.149. [DOI] [PubMed] [Google Scholar]
- 44.Smedts AM, Curry TE., Jr. Expression of basigin, an inducer of matrix metalloproteinases, in the rat ovary. Biol Reprod. 2005;73:80–7. doi: 10.1095/biolreprod.104.036145. [DOI] [PubMed] [Google Scholar]
- 45.Kuno N, Kadomatsu K, Fan QW, Hagihara M, Senda T, Mizutani S, et al. Female sterility in mice lacking the basigin gene, which encodes a transmembrane glycoprotein belonging to the immunoglobulin superfamily. FEBS Lett. 1998;425:191–4. doi: 10.1016/S0014-5793(98)00213-0. [DOI] [PubMed] [Google Scholar]
- 46.Xiao LJ, Chang H, Ding NZ, Ni H, Kadomatsu K, Yang ZM. Basigin expression and hormonal regulation in mouse uterus during the peri-implantation period. Mol Reprod Dev. 2002;63:47–54. doi: 10.1002/mrd.10128. [DOI] [PubMed] [Google Scholar]
- 47.Noguchi Y, Sato T, Hirata M, Hara T, Ohama K, Ito A. Identification and characterization of extracellular matrix metalloproteinase inducer in human endometrium during the menstrual cycle in vivo and in vitro. J Clin Endocrinol Metab. 2003;88:6063–72. doi: 10.1210/jc.2003-030457. [DOI] [PubMed] [Google Scholar]
- 48.Li W, Alfaidy N, Challis JR. Expression of extracellular matrix metalloproteinase inducer in human placenta and fetal membranes at term labor. J Clin Endocrinol Metab. 2004;89:2897–904. doi: 10.1210/jc.2003-032048. [DOI] [PubMed] [Google Scholar]
- 49.Igakura T, Kadomatsu K, Kaname T, Muramatsu H, Fan QW, Miyauchi T, et al. A null mutation in basigin, an immunoglobulin superfamily member, indicates its important roles in peri-implantation development and spermatogenesis. Dev Biol. 1998;194:152–65. doi: 10.1006/dbio.1997.8819. [DOI] [PubMed] [Google Scholar]
- 50.Chen H, Fok KL, Yu S, Jiang J, Chen Z, Gui Y, et al. CD147 is required for matrix metalloproteinases-2 production and germ cell migration during spermatogenesis. Mol Hum Reprod. 2011;17:405–14. doi: 10.1093/molehr/gar013. [DOI] [PubMed] [Google Scholar]
- 51.Maekawa M, Suzuki-Toyota F, Toyama Y, Kadomatsu K, Hagihara M, Kuno N, et al. Stage-specific localization of basigin, a member of the immunoglobulin superfamily, during mouse spermatogenesis. Arch Histol Cytol. 1998;61:405–15. doi: 10.1679/aohc.61.405. [DOI] [PubMed] [Google Scholar]
- 52.Saxena DK, Oh-Oka T, Kadomatsu K, Muramatsu T, Toshimori K. Behaviour of a sperm surface transmembrane glycoprotein basigin during epididymal maturation and its role in fertilization in mice. Reproduction. 2002;123:435–44. doi: 10.1530/rep.0.1230435. [DOI] [PubMed] [Google Scholar]
- 53.Toyama Y, Maekawa M, Kadomatsu K, Miyauchi T, Muramatsu T, Yuasa S. Histological characterization of defective spermatogenesis in mice lacking the basigin gene. Anat Histol Embryol. 1999;28:205–13. doi: 10.1046/j.1439-0264.1999.00194.x. [DOI] [PubMed] [Google Scholar]
- 54.Saxena DK, Toshimori K. Molecular modifications of MC31/CE9, a sperm surface molecule, during sperm capacitation and the acrosome reaction in the rat: is MC31/CE9 required for fertilization? Biol Reprod. 2004;70:993–1000. doi: 10.1095/biolreprod.103.021667. [DOI] [PubMed] [Google Scholar]
- 55.Siu MK, Cheng CY. Extracellular matrix: recent advances on its role in junction dynamics in the seminiferous epithelium during spermatogenesis. Biol Reprod. 2004;71:375–91. doi: 10.1095/biolreprod.104.028225. [DOI] [PubMed] [Google Scholar]
- 56.Kanatsu-Shinohara M, Miki H, Inoue K, Ogonuki N, Toyokuni S, Ogura A, et al. Long-term culture of mouse male germline stem cells under serum-or feeder-free conditions. Biol Reprod. 2005;72:985–91. doi: 10.1095/biolreprod.104.036400. [DOI] [PubMed] [Google Scholar]
- 57.Kanatsu-Shinohara M, Takehashi M, Takashima S, Lee J, Morimoto H, Chuma S, et al. Homing of mouse spermatogonial stem cells to germline niche depends on beta1-integrin. Cell Stem Cell. 2008;3:533–42. doi: 10.1016/j.stem.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 58.Overall CM. Molecular determinants of metalloproteinase substrate specificity: matrix metalloproteinase substrate binding domains, modules, and exosites. Mol Biotechnol. 2002;22:51–86. doi: 10.1385/MB:22:1:051. [DOI] [PubMed] [Google Scholar]
- 59.Amălinei C, Căruntu ID, Bălan RA. Biology of metalloproteinases. Rom J Morphol Embryol. 2007;48:323–34. [PubMed] [Google Scholar]
- 60.Nuttall RK, Sampieri CL, Pennington CJ, Gill SE, Schultz GA, Edwards DR. Expression analysis of the entire MMP and TIMP gene families during mouse tissue development. FEBS Lett. 2004;563:129–34. doi: 10.1016/S0014-5793(04)00281-9. [DOI] [PubMed] [Google Scholar]
- 61.Wong CH, Cheng CY. The blood-testis barrier: its biology, regulation, and physiological role in spermatogenesis. Curr Top Dev Biol. 2005;71:263–96. doi: 10.1016/S0070-2153(05)71008-5. [DOI] [PubMed] [Google Scholar]
- 62.Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol. 2010;6:380–95. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheng CY, Mruk DD. An intracellular trafficking pathway in the seminiferous epithelium regulating spermatogenesis: a biochemical and molecular perspective. Crit Rev Biochem Mol Biol. 2009;44:245–63. doi: 10.1080/10409230903061207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan HH, Mruk DD, Wong EW, Lee WM, Cheng CY. An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:8950–5. doi: 10.1073/pnas.0711264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Siu MK, Cheng CY. Interactions of proteases, protease inhibitors, and the beta1 integrin/laminin gamma3 protein complex in the regulation of ectoplasmic specialization dynamics in the rat testis. Biol Reprod. 2004;70:945–64. doi: 10.1095/biolreprod.103.023606. [DOI] [PubMed] [Google Scholar]
- 66.Yan HH, Mruk DD, Cheng CY. Junction restructuring and spermatogenesis: the biology, regulation, and implication in male contraceptive development. Curr Top Dev Biol. 2008;80:57–92. doi: 10.1016/S0070-2153(07)80002-0. [DOI] [PubMed] [Google Scholar]
- 67.Xia W, Mruk DD, Lee WM, Cheng CY. Differential interactions between transforming growth factor-beta3/TbetaR1, TAB1, and CD2AP disrupt blood-testis barrier and Sertoli-germ cell adhesion. J Biol Chem. 2006;281:16799–813. doi: 10.1074/jbc.M601618200. [DOI] [PubMed] [Google Scholar]
- 68.Nahalkova J, Volkmann I, Aoki M, Winblad B, Bogdanovic N, Tjernberg LO, et al. CD147, a gamma-secretase associated protein is upregulated in Alzheimer’s disease brain and its cellular trafficking is affected by presenilin-2. Neurochem Int. 2010;56:67–76. doi: 10.1016/j.neuint.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 69.Gu J, Zhang C, Chen R, Pan J, Wang Y, Ming M, et al. Clinical implications and prognostic value of EMMPRIN/CD147 and MMP2 expression in pediatric gliomas. Eur J Pediatr. 2009;168:705–10. doi: 10.1007/s00431-008-0828-5. [DOI] [PubMed] [Google Scholar]
- 70.Zhu C, Pan Y, He B, Wang B, Xu Y, Qu L, et al. Inhibition of CD147 gene expression via RNA interference reduces tumor cell invasion, tumorigenicity and increases chemosensitivity to cisplatin in laryngeal carcinoma Hep2 cells. Oncol Rep. 2011;25:425–32. doi: 10.3892/or.2010.1088. [DOI] [PubMed] [Google Scholar]
- 71.Wang B, Xu YF, He BS, Pan YQ, Zhang LR, Zhu C, et al. RNAi-mediated silencing of CD147 inhibits tumor cell proliferation, invasion and increases chemosensitivity to cisplatin in SGC7901 cells in vitro. J Exp Clin Cancer Res. 2010;29:61. doi: 10.1186/1756-9966-29-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abraham D, Zins K, Sioud M, Lucas T, Aharinejad S. Host CD147 blockade by small interfering RNAs suppresses growth of human colon cancer xenografts. Front Biosci. 2008;13:5571–9. doi: 10.2741/3100. [DOI] [PubMed] [Google Scholar]
- 73.Hou Q, Tang X, Liu H, Tang J, Yang Y, Jing X, et al. Berberine induces cell death in human hepatoma cells in vitro by downregulating CD147. Cancer Sci. 2011;102:1287–92. doi: 10.1111/j.1349-7006.2011.01933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dean NR, Knowles JA, Helman EE, Aldridge JC, Carroll WR, Magnuson JS, et al. Anti-EMMPRIN antibody treatment of head and neck squamous cell carcinoma in an ex-vivo model. Anticancer Drugs. 2010;21:861–7. doi: 10.1097/CAD.0b013e32833d1a11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuang YH, Chen X, Su J, Wu LS, Liao LQ, Li D, et al. RNA interference targeting the CD147 induces apoptosis of multi-drug resistant cancer cells related to XIAP depletion. Cancer Lett. 2009;276:189–95. doi: 10.1016/j.canlet.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 76.Baba M, Inoue M, Itoh K, Nishizawa Y. Blocking CD147 induces cell death in cancer cells through impairment of glycolytic energy metabolism. Biochem Biophys Res Commun. 2008;374:111–6. doi: 10.1016/j.bbrc.2008.06.122. [DOI] [PubMed] [Google Scholar]
- 77.Intasai N, Mai S, Kasinrerk W, Tayapiwatana C. Binding of multivalent CD147 phage induces apoptosis of U937 cells. Int Immunol. 2006;18:1159–69. doi: 10.1093/intimm/dxl050. [DOI] [PubMed] [Google Scholar]
- 78.Tang J, Guo YS, Zhang Y, Yu XL, Li L, Huang W, et al. CD147 induces UPR to inhibit apoptosis and chemosensitivity by increasing the transcription of Bip in hepatocellular carcinoma. Cell Death Differ. 2012 doi: 10.1038/cdd.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang C, Cui YG, Wang XH, Jia Y, Sinha Hikim A, Lue YH, et al. transient scrotal hyperthermia and levonorgestrel enhance testosterone-induced spermatogenesis suppression in men through increased germ cell apoptosis. J Clin Endocrinol Metab. 2007;92:3292–304. doi: 10.1210/jc.2007-0367. [DOI] [PubMed] [Google Scholar]
- 80.Shaha C, Tripathi R, Mishra DP. Male germ cell apoptosis: regulation and biology. Philos Trans R Soc Lond B Biol Sci. 2010;365:1501–15. doi: 10.1098/rstb.2009.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martinvalet D, Zhu P, Lieberman J. Granzyme A induces caspase-independent mitochondrial damage, a required first step for apoptosis. Immunity. 2005;22:355–70. doi: 10.1016/j.immuni.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 82.Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene. 2003;22:9030–40. doi: 10.1038/sj.onc.1207116. [DOI] [PubMed] [Google Scholar]
- 83.Fauvet R, Dufournet C, Poncelet C, Uzan C, Hugol D, Daraï E. Expression of pro-apoptotic (p53, p21, bax, bak and fas) and anti-apoptotic (bcl-2 and bcl-x) proteins in serous versus mucinous borderline ovarian tumours. J Surg Oncol. 2005;92:337–43. doi: 10.1002/jso.20424. [DOI] [PubMed] [Google Scholar]
- 84.Li J, Lee B, Lee AS. Endoplasmic reticulum stress-induced apoptosis: multiple pathways and activation of p53-up-regulated modulator of apoptosis (PUMA) and NOXA by p53. J Biol Chem. 2006;281:7260–70. doi: 10.1074/jbc.M509868200. [DOI] [PubMed] [Google Scholar]
- 85.Yu J, Zhang L. The transcriptional targets of p53 in apoptosis control. Biochem Biophys Res Commun. 2005;331:851–8. doi: 10.1016/j.bbrc.2005.03.189. [DOI] [PubMed] [Google Scholar]
- 86.Ihrie RA, Bronson RT, Attardi LD. Adult mice lacking the p53/p63 target gene Perp are not predisposed to spontaneous tumorigenesis but display features of ectodermal dysplasia syndromes. Cell Death Differ. 2006;13:1614–8. doi: 10.1038/sj.cdd.4401871. [DOI] [PubMed] [Google Scholar]
- 87.Burns TF, Bernhard EJ, El-Deiry WS. Tissue specific expression of p53 target genes suggests a key role for KILLER/DR5 in p53-dependent apoptosis in vivo. Oncogene. 2001;20:4601–12. doi: 10.1038/sj.onc.1204484. [DOI] [PubMed] [Google Scholar]
- 88.Coates PJ. p53 and Mdm2: not all cells are equal. J Pathol. 2007;213:357–9. doi: 10.1002/path.2275. [DOI] [PubMed] [Google Scholar]
- 89.Chen H, Fok KL, Jiang X, Jiang J, Chen Z, Gui Y, et al. CD147 regulates apoptosis in mouse spermatocytes but not spermatogonia. Hum Reprod. 2012;27:1568–76. doi: 10.1093/humrep/des050. [DOI] [PubMed] [Google Scholar]
- 90.Blanco-Rodríguez J. A matter of death and life: the significance of germ cell death during spermatogenesis. Int J Androl. 1998;21:236–48. doi: 10.1046/j.1365-2605.1998.00133.x. [DOI] [PubMed] [Google Scholar]