Abstract
OBJECTIVE:
The aim of this study was to determine the erythrocyte antioxidant enzyme activity and the superoxide dismutase, catalase, glutathione peroxidase, and plasma malondialdehyde levels in aging mice and to evaluate how these measures are modulated by potential antioxidants, including the tocotrienol-rich fraction, Piper betle, and Chlorella vulgaris.
METHOD:
One hundred and twenty male C57BL/6 inbred mice were divided into three age groups: young (6 months old), middle-aged (12 months old), and old (18 months old). Each age group consisted of two control groups (distilled water and olive oil) and three treatment groups: Piper betle (50 mg/kg body weight), tocotrienol-rich fraction (30 mg/kg), and Chlorella vulgaris (50 mg/kg). The duration of treatment for all three age groups was two months. Blood was withdrawn from the orbital sinus to determine the antioxidant enzyme activity and the malondialdehyde level.
RESULTS:
Piper betle increased the activities of catalase, glutathione peroxidase, and superoxide dismutase in the young, middle, and old age groups, respectively, when compared to control. The tocotrienol-rich fraction decreased the superoxide dismutase activity in the middle and the old age groups but had no effect on catalase or glutathione peroxidase activity for all age groups. Chlorella vulgaris had no effect on superoxide dismutase activity for all age groups but increased glutathione peroxidase and decreased catalase activity in the middle and the young age groups, respectively. Chlorella vulgaris reduced lipid peroxidation (malondialdehyde levels) in all age groups, but no significant changes were observed with the tocotrienol-rich fraction and the Piper betle treatments.
CONCLUSION:
We found equivocal age-related changes in erythrocyte antioxidant enzyme activity when mice were treated with Piper betle, the tocotrienol-rich fraction, and Chlorella vulgaris. However, Piper betle treatment showed increased antioxidant enzymes activity during aging.
Keywords: Aging, Antioxidant Enzymes, Piper Betle, Tocotrienol-Rich Fraction, Chlorella Vulgaris
INTRODUCTION
Free oxygen radicals have been proposed as important causative agents of aging. The theory of aging associated with the accumulation of free radicals, which damage cells and tissues, was suggested by Harman (1,2). The univalent reduction of molecular oxygen results in reactive oxygen species (ROS) such as superoxide anions (O2−), hydrogen peroxide (H2O2) and hydroxyl radicals (OH−) (3). These cytotoxic species can cause irreversible oxidative damage to biologic molecules, such as lipids, proteins, and DNA in the cells, which affect enzyme activity and membrane function (4,5).
Fortunately, the accumulation of ROS is controlled in vivo by a wide spectrum of non-enzymatic antioxidant systems, such as bilirubin; glutathione (GSH); vitamins A, C, and E; and defense-related enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) (6). The activity of antioxidant enzymes in response to the accumulation of ROS is equivocal, and both increased and decreased activities have been reported during the aging processes (4). However, if this defense mechanism fails to control the increased levels of ROS, oxidative stress occurs and is capable of injuring membrane lipids, proteins and nucleic acids (7). Increased ROS react with polyunsaturated fatty acids to induce the release of toxic and reactive aldehyde metabolites, such as MDA, which is one of the end products of the lipid peroxidation process (8,9). Lipid peroxidation is associated with aging and a variety of chronic health diseases, such as cancer and atherosclerosis (10-12).
Numerous studies suggest that antioxidants can prevent the oxidation of various macromolecules, such as DNA, proteins, and lipids, thus preventing the aging process and increasing the lifespan of the organism (13,14). In this study, we investigated three types of antioxidants: Piper betle (PB), the tocotrienol-rich fraction (TRF), and Chlorella vulgaris (CV). Piper betle L. (Piperaceae) or Sireh leaves have a strong, pungent aromatic flavor and are widely used as masticatory agents in Asia. PB has a high antioxidant capacity and contains several bioactive compounds, such as hydroxychavicol, eugenol, chavibetol, and allylpyrocatechol (15,16). PB also has significant antioxidant activity and can modulate antioxidant enzyme activity, such as that of SOD, GPx, and CAT, in radiation-induced lipid peroxidation in Swiss albino mice (17,18).
Tocotrienols extracted from crude palm oil consist mainly of a mixture of α, β, γ, and δ tocotrienols and some α tocopherols; this extract is referred to as the tocotrienol-rich fraction (TRF). Treatment with the TRF results in lifespan extension and a reduction in the age-dependent accumulation of protein carbonyls in Caenorhabditis elegans (19). The TRF also prevents oxidative damage by inhibiting protein and lipid peroxidation in rat liver microsomes (20). Chin et al. (21) reported that long-term supplementation with the TRF can modulate antioxidant enzymes, such as SOD, GPx, and CAT, in older humans, reduce protein damage and improve levels of advanced glycosylation end (AGE) products.
Chlorella vulgaris, a unicellular green algae, has been widely used as a food additive (22) and has nutritive value (23) in addition to other medicinal benefits. An in vitro study revealed the antitumor effects of CV, which inhibits the proliferation of HepG2 liver cancer cells and induces apoptosis (24). CV reduced the number of tumors in ethionine-induced liver cancer in rats and modulated the antioxidant enzyme activity and blood malondialdehyde (MDA) levels (25).
Although many studies have demonstrated the antioxidant potential of PB, TRF, and CV, limited studies have addressed their effects in association with aging. Therefore, we embarked on this study to evaluate whether PB, TRF, and CV can modulate oxidative stress that is induced by the accumulation of ROS and MDA during aging in mice.
METHODS
Animal model and experimental design
A total of 120 male C57BL/6 inbred mice were purchased from bioLASCO, Taiwan Co., Ltd (Taipei, Taiwan). The mice were maintained in polycarbonate cages placed in a room with a controlled temperature, controlled humidity and a 12 h light-dark cycle. The study was approved by the Animal Ethics Committee of the Faculty of Medicine, UKM (FP/BIOK/2008/YASMIN/13-FEB/216-FEB-2008-DEC-2010) and was conducted at the Department of Biochemistry, Faculty of Medicine, Universiti Kebangsaan Malaysia (UKM), Kuala Lumpur, Malaysia.
The mice were randomly divided into three main groups according to their age. These groups were 6-month-old (young) mice, 12-month-old (middle-aged) mice, and 18-month-old (old) mice. Each group was further divided into four groups, which were two control and three treatment groups. Each group consisted of eight mice. The mice in the control group for the PB and the CV groups were given distilled water, and the mice in the control group for the TRF group were given olive oil. Both controls were given to rats by gavage. Mice in the treatment groups were given PB (50 mg/kg body weight), CV (50 mg/kg body weight), and TRF (30 mg/kg body weight) each day via gavage (forced feeding). Each treatment was administered for two months for each age group. For each age group, blood was collected from the mice, after the two-month treatment period to analyze antioxidant enzymes and oxidative stress marker analysis (SOD, GPx, CAT and MDA). Blood was collected from the orbital sinus and maintained on ice in tubes containing heparin (an anticoagulant) followed by centrifugation at 3,000 x g for 15 min at 4°C. The top yellow plasma layer was pipetted without disturbing the white buffy layer and was stored at -20°C for later analysis of MDA. The white buffy layer (leukocytes) was removed. The erythrocytes (red blood cells) were washed with normal saline and centrifuged at 3,000 x g for 15 min at 4°C, which was repeated four times until the supernatant was clear. The erythrocytes were aliquoted into several microcentrifuge tubes and stored at -20°C for later determination of antioxidant enzyme activity.
Preparation of PB extract
Dried PB leaves were purchased from Ethno Resources Company (Sungai Buloh, Selangor, Malaysia). The identification and voucher number (UKMB 29768) of the plants was obtained from Herbarium, UKM, Bangi. The dried PB leaves were grounded into powder, and a 10% concentration of PB was prepared. Aqueous PB extractions were prepared using a Soxhlet Extractor (Eyela, Japan) according to the method of Pin et al. (26) with some modifications. Finally, the aqueous PB extract was dried into a powdered form using a freeze dryer (Labconco, Kansas City, MO, USA). The powdered PB was diluted in distilled water to yield a concentration of 50 mg/kg body weight before being used throughout the experiment.
Preparation of the TRF
The TRF (70%) was purchased from Golden Hope Bioganic (Selangor, Malaysia). The TRF consists of α-tocopherol (149.2 mg/g), α- tocotrienol (164.7 mg/g), β-tocotrienol (48.6 mg/g), γ-tocotrienol (213.2 mg/g), and δ-tocotrienol (171.9 mg/g). The TRF was diluted in olive oil to yield a concentration of 30 mg/kg body weight before being used throughout the experiment. This concentration was used based on our earlier laboratory findings that long-term supplementation of 30 mg/kg body weight of palm oil vitamin E reduced elevated levels of MDA in rats during aging (27).
Preparation of CV
The stock CV Beijerinck (strain 072) was obtained from the University of Malaya Algae Culture Collection (UMACC, Malaysia) and was grown in Bold Basal Media (BBM) (12-h light-dark cycle). The algae slurry was centrifuged three times at 3,000 rpm for 10 min at 4°C to separate the algae from the medium. The pelleted algae were then diluted in distilled water at a concentration of 50 mg/kg body weight before being used throughout the experiment. This concentration was used based on our previous study in rats in which 50 mg/kg body weight of CV exhibited sufficient anti-tumor and antioxidant effects in liver cancer-induced rats (25).
Measurement of antioxidant enzyme activity
SOD activity was measured following the method proposed by Beyer & Fridovich (28). The assay mixture contained 50 mmol/l potassium phosphate buffer at a pH of 7.8, 0.1 mmol/l EDTA, 9.9 mmol/l L-methionine, 5.7×10−5 mol/l nitro blue tetrazolium (NBT), and 2.5×10−2% (w/v) Triton X-100. Approximately 0.02 ml of the sample (red blood cells) was added to 1.0 ml of the assay mixture. Riboflavin (0.01 ml of 4.4%) was added last to initiate the reaction. The reduction of NBT was measured at 560 nm in a Shimadzu-UV 160A Spectrometer (Kyoto, Japan). The SOD activity was expressed in units of enzyme/gram hemoglobin. One unit of SOD corresponds to the enzyme concentration required to inhibit the chromogen produced (NBT) by 50% in 1 min under standard conditions.
GPx activity was measured according to the method proposed by Paglia & Valentine (29). The activity was assayed with a coupled enzyme system in which the oxidized GSH reduction was coupled to nicotinamide adenine dinucleotide phosphate (NADPH) oxidation by GSH reductase. The oxidation of NADPH was measured spectrometrically at 340 nm. The assay mixture contained 0.05 mol/l phosphate buffer at a pH of 7.0, 5 mmol/l EDTA, 2 mmol/l NaN3, 1 mmol/l GSH, 0.2 mmol/l NADPH, and 4 μg GSH reductase. The sample (0.02 ml) was added to 2.88 ml of the assay mixture. The reaction was initiated by the addition of 0.1 ml of 2.5 mmol/l H2O2. A blank assay with buffer instead of a sample was used to correct for any non-enzymatic oxidation of GSH and NADPH by peroxide. GPx activity was expressed in milliunits/milligram hemoglobin. One specific unit is defined as 1 μmol of NADPH needed with 1 g of hemoglobin to be converted to NADP in 1 min of reaction.
CAT activity was measured according to the method proposed by Aebi (30). In the ultraviolet range, H2O2 shows a continual increase in absorption with a decreasing wavelength. The decomposition of H2O2 can be followed directly by the decrease in the absorbance at 240 nm. The difference in absorbance (ΔA240) per unit time is a measure of CAT activity. The reagents used were a phosphate buffer (50 mmol/l, pH 7.0) and 30 mmol/l H2O2 in a phosphate buffer, which was prepared fresh before each assay. Hemolysate was obtained by adding 0.1 ml of red blood cells to 0.4 ml of distilled water. A 1:500 dilution of this concentrated hemolysate was prepared with phosphate buffer immediately before the assay was performed. The reaction was initiated by the addition of 1 ml of 30 mmol/l H2O2 to 2 ml of diluted hemolysate. A blank assay with buffer instead of substrate and 2 ml of hemolysate was used to correct for any non-enzymatic reaction. The absorbance was observed for approximately 30 sec. The CAT activity is defined in specific units/milligram hemoglobin. One unit of CAT corresponds to the amount of enzyme needed to decompose H2O2 in phosphate buffer, at pH 7.0, in 1 sec of reaction.
Measurement of hemoglobin
The hemoglobin levels were determined through the quantitative determination of cyanmethemoglobin in the blood. Ferricyanide oxidizes oxyhemoglobin to methemoglobin, and cyanide converts methemoglobin to cyanmethemoglobin. The absorbance measurements were recorded at 540 nm. The cyanmethemoglobin reagent (Eagle Diagnostic, St. Louis, USA) contains a surfactant to promote rapid hemolysis and to accelerate the formation of cyanmethemoglobin. The reaction was completed in 3 min.
Measurement of plasma MDA
The LPO product, MDA, was quantified using the method proposed by Pilz et al. (31) with some modifications by Sim et al. (32). The principle of the test is based on the derivation of MDA with 2,4-dinitrophenylhydrazine and its subsequent conversion into pyrazole and hydrazone derivatives, which are then separated using high-performance liquid chromatography (HPLC). This method allows for a more specific estimation of MDA compared to the thiobarbituric acid reactive substances (TBARS) method. HPLC analyses were performed on a LC-10 AT VP Shidmadzu (Kyoto, Japan) liquid chromatography system equipped with a diode array detector and an auto injection valve. An α-bond C18 125A column (3.9×150 mm) with a 5 μm particle size (Alltech, Deerfield, Illinois, USA) was used. A Shidmadzu Class-VP software system controlled the equipment and was utilized for data processing.
Elution was performed isocratically with a mixture of 0.2% (v/v) acetic acid in MilliQ water and acetonitrile (62:38) (v/v) at a flow rate of 0.6 ml/min at room temperature. The chromatograms were acquired at 310 nm.
Standard and sample preparation for MDA
A standard of 1,1,3,3-tetraetoxypropane (TEP) was used. A stock solution of MDA was obtained as follows: 25 μl TEP was dissolved in 100 ml of deionized water to yield a 1 mmol/l stock solution. The MDA was prepared by hydrolysis of 1 ml TEP stock solution in 50 ml of 1% sulfuric acid and was incubated for 2 h at room temperature. The solution was stored at 40°C and used within four weeks. The resulting MDA standard of 20 nmol/ml was further diluted with 1% sulfuric acid to yield different concentrations from 1 to 20 nmol/ml of MDA.
An aliquot of 250 μl diluted standard or plasma was placed in a 1.5-ml Eppendorf tube, and 50 μl of 6 M aqueous sodium hydroxide was added. This mixture was incubated in a 60°C water bath for 30 min to achieve the alkaline hydrolysis of the protein-bound MDA. The protein was precipitated by adding 125 μl of 35% (v/v) perchloric acid, and the mixture was centrifuged at 6000×g for 10 min. The entire volume of the supernatant was transferred to an Eppendorf vial and mixed with 50 μl 2,4-dinitrophenylhydrazine prepared as a 5 mM solution in 2 M hydrochloric acid. Finally, this reaction mixture was incubated for 30 min at room temperature and was protected from light. An aliquot of 40 μl of this reaction mixture was injected into the HPLC system. The retention time for the MDA was detected at the 8th minute, and the area under the peak represented the amount of MDA in 40 μl of the reaction mixture. A serial concentration of the standard was generated as a reference curve.
Statistical analysis
The results are expressed as the means±standard error of the mean (SEM) and were analyzed using a one-way ANOVA with Statistical Package for Social Sciences (SPSS) version 13.0. p<0.05 was accepted as statistically significant.
RESULTS
SOD, GPx and CAT activities in the erythrocytes during aging in response to PB treatment
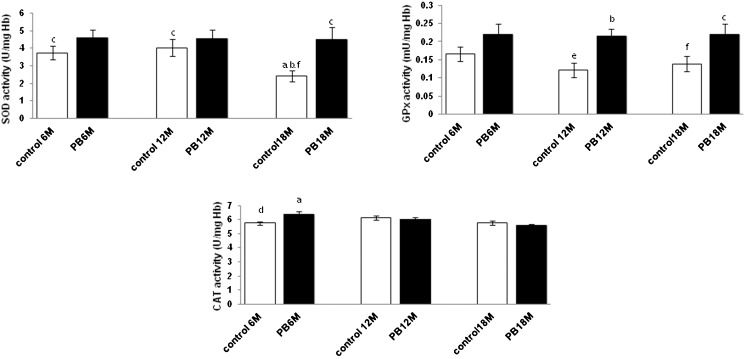
In the distilled water control group, the SOD activity decreased significantly (p<0.05) in the old age group, while no significant changes were observed for GPx and CAT activities (p>0.05) as the mice aged. When treated with PB, SOD activity increased in the old age group (p<0.05), and the GPx activity increased significantly in the middle and old age groups (p<0.05). In contrast, CAT activity was significantly increased in the young age group (p<0.05) when compared with the distilled water control group (Figure 1).
Figure 1.
Effect of Piper betle (PB) on SOD, GPx and CAT levels in the erythrocytes of mice with different ages. Values are the mean ± S.E.M. for 8 mice. a-c: significantly different (p<0.05) from the control at 6 M, 12 M, and 18 M, respectively. d-f: significantly different (p<0.05) from PB at 6 M, 12 M, and 18 M, respectively. Superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), hemoglobin (Hb), 6 month old (6 M), 12 month old (12 M), and 18 month old (18 M).
SOD, GPx, and CAT activities in the erythrocytes during aging in response to TRF treatment.
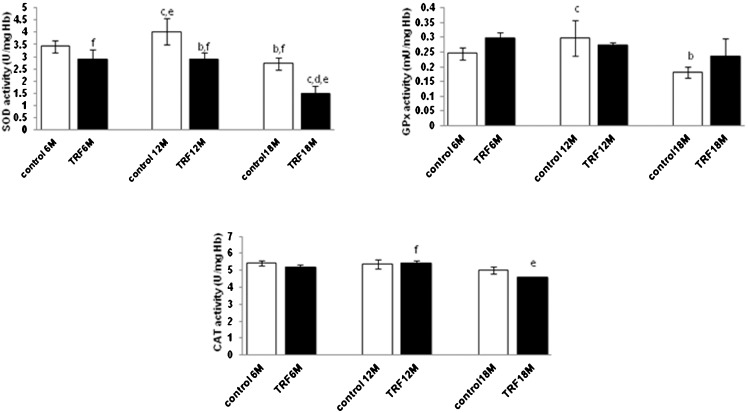
Total SOD and GPx activities decreased significantly in the old age group (p<0.05), while no significant changes were observed in the CAT activity (p>0.05) in the olive oil control group (olive oil was used as the vehicle for the TRF). When treated with the TRF, SOD activity decreased significantly in the middle and old age groups (p<0.05), while no significant changes were found in the GPx and CAT activities (p>0.05) when compared with the control group (Figure 2).
Figure 2.
Effect of the tocotrienol-rich fraction (TRF) on erythrocyte SOD, GPx and CAT levels in mice with different ages. Values are the mean ± S.E.M. for 8 mice. a-c: significantly different (p<0.05) from the control at 6 M, 12 M, and 18 M, respectively. d-f: significantly different (p<0.05) from TRF at 6 M, 12 M, and 18 M, respectively.
SOD, GPx, and CAT activities in the erythrocytes during aging in response to CV treatment.
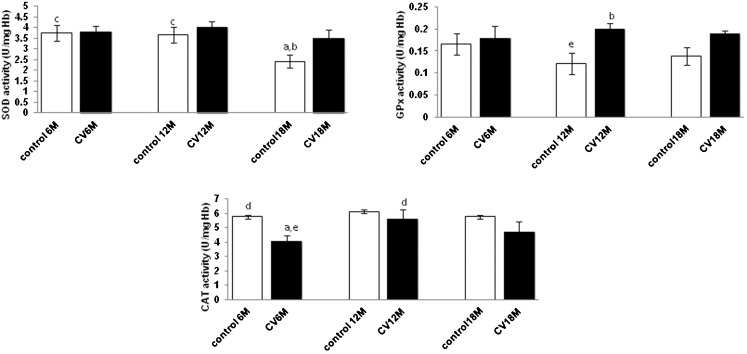
When treated with CV, no significant changes were observed for SOD activity for all age groups (p>0.05), while GPx activity was increased significantly in the middle age group (p<0.05). In contrast, CAT activity decreased significantly in the young age group (p<0.05) when compared with the control group (Figure 3).
Figure 3.
Effect of Chlorella vulgaris (CV) on erythrocyte SOD, GPx and CAT levels in mice with different ages. Values are the mean ± S.E.M. for 8 mice. a-c: significantly different (p<0.05) from the control at 6 M, 12 M and 18 M, respectively. d-f: significantly different (p<0.05) from CV at 6 M, 12 M, and 18 M, respectively.
Lipid peroxidation levels in plasma during aging in response to PB, TRF, and CV treatments.
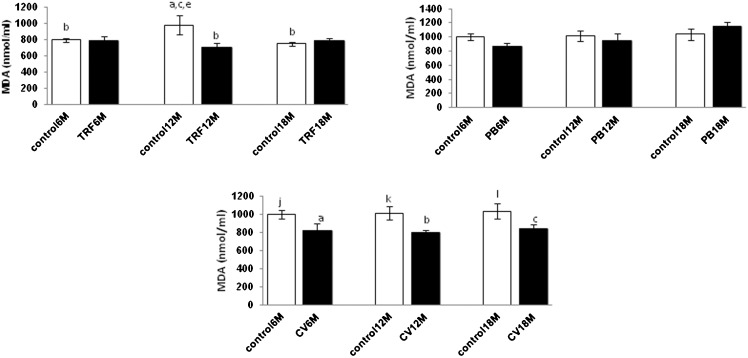
MDA is a measurement for lipid peroxidation of cell membranes. We noted no significant changes in MDA for all age groups in both distilled water and olive oil control groups and the PB treatment groups. However, CV treatment reduced lipid peroxidation in all age groups (p<0.05). The TRF treatment reduced lipid peroxidation in the middle-aged group (p<0.05) when compared with the control group (Figure 4).
Figure 4.
Effect of PB, TRF, and CV on the plasma malondialdehyde (MDA) level in mice with different ages. Values are the mean ± S.E.M. for 8 mice. a-c: significantly different (p<0.05) from the control at 6 M, 12 M, and 18 M, respectively. d-f: significantly different (p<0.05) from TRF at 6 M, 12 M, and 18 M, respectively. g-i: significantly different (p<0.05) from PB at 6 M, 12 M, and 18 M, respectively. j-l: significantly different (P<0.05) from CV at 6 M, 12 M, and 18 M, respectively.
DISCUSSION
Aging is normally associated with an increased level of reactive oxygen species (ROS), which cause severe damage to cells and tissues. An imbalance between the formation and elimination of ROS and the development of oxidative stress plays a vital role in age-associated diseases, such as cancer, Alzheimer's, and heart disease (33,34).
In the present study, we investigated three plant extracts (PB, TRF, and CV) in association with aging. These extracts possess certain degrees of antioxidant potential by scavenging ROS (25,35,36). Without any antioxidant treatment, we noted that SOD activities decreased with age in mice in both control groups (water and olive oil). Anderson et al. (37) reported an age-related decrease in SOD activity in human erythrocytes. The inactivation of SOD is also associated with aging in rats (38). The decreased SOD activity could be explained by an irreversible inactivation of SOD by its product, H2O2, in a concentration-dependent manner (39). H2O2 is usually removed by catalase and GPx in erythrocytes; however, the enzyme levels decreased with age (40). This decrease was also observed in our study, where GPx activity decreased with age, while CAT activity did not change with age. Apparently, CAT and GPx function in parallel pathways (21). A decrease in GPx's H2O2 salvaging activity is usually compensated for by CAT. Conversely, Inal et al. found that GPx and CAT activities increased during aging in healthy older adults (4). Chin et al. reported that GPx activity increased while CAT activity decreased in healthy older adults (21). These equivocal results in age-related antioxidant enzyme activity could be due to methodology, environmental and subject variation in both animal and human studies (4).
Human aging is affected by both genetic and lifestyle-related factors, such as diet and exercise. Nutrients can affect the rate of aging by altering the type and quantity of proteins (41), which alter the oxidative status of individuals (42). Dietary supplementation rich in antioxidants can reduce oxidative damage or boost the antioxidant system. In this study, we found that supplementation with PB, TRF, and CV as exogenous antioxidants improved the endogenous antioxidant function associated with removing accumulated ROS and improving the balance between ROS and antioxidants.
The most effective dose of PB on certain biological activities ranges from concentrations of 1 mg/kg to 1,000 mg/kg in experimental studies using mice and rats (18,43,44). We decided to use a lower dose of 50 mg/kg body weight in mice. This decision was based on two studies that noted that low doses of PB (1 mg/kg, 5 mg/kg, and 10 mg/kg) significantly modulated SOD, GPx, and CAT activity in radiation-induced lipid peroxidation in Swiss albino mice (18). This dose was considered toxicologically safe when fed orally to ICR mice when investigating antimalarial effects of PB (44). Chitra & Vidya (43) reported that high doses of PB extract (>200 mg/kg) produced toxic effects in the erythrocytes of experimental mice.
The administration of PB (50 mg/kg body weight) to mice markedly increased the levels of antioxidant enzymes by increasing CAT activity at a young age, increasing GPx activity in middle-aged mice, and increasing both SOD and GPx activities in the old age group. These results support the antioxidant potential of PB. PB treatment helps to produce more antioxidant enzymes, especially during old age, to cope with the overload of oxygen radicals and H2O2. The results from the present study concur with the findings of earlier investigation, in which PB elevated the antioxidant enzyme status in radiation-induced lipid peroxidation of Swiss albino mice (18). However, with TRF treatment, we noted a significant increase in SOD activity only in the old age group, while no changes were noted for the GPx or the CAT activity. One plausible explanation is that TRF is a powerful antioxidant that strongly scavenges most of the free radicals that accumulate in the body, thereby compensating the endogenous antioxidant potential in mice (21). However, CV treatment had no effect on SOD activity with age; conversely, it increased GPx activity in the middle-aged group and decreased CAT activity in the young group.
No significant changes in MDA were observed for either the controls or the PB treatment for all age groups. CV treatment reduced lipid peroxidation in all age groups. This result is in agreement with our previous finding that CV has a protective effect in rats induced with liver cancer by reducing elevated MDA levels (25). The TRF decreased the elevated MDA level in the middle-aged group, but no changes were found in the old age group. This result is in agreement with a previous study by Chin et al. (21), who found that long-term supplementation of TRF by older, healthy individuals did not reduce MDA levels compared with non-supplemented individuals. Conversely, long-term supplementation of TRF (60 mg/kg body weight) in rats reduced lipid peroxidation during aging (from six months up until 24 months) (45). PB and TRF appeared to have a lesser effect on lipid peroxidation compared to CV. This result may occur because the accumulation of oxidized proteins during aging is most likely linked to an age-related decline of antioxidant enzyme activity, whereas lipid peroxidation is less sensitive to the aging process (46).
In conclusion, PB, TRF, and CV, which are rich in antioxidants, can modulate the antioxidant enzymes activities of SOD, GPx, and CAT with different patterns during aging. CV had a greater effect compared with PB and TRF in reducing lipid peroxidation during aging.
ACKNOWLEDGMENTS
The authors wish to express their sincere gratitude to the National University of Malaysia (UKM) for full financial support of this project (UKM-GUP-SK-07-21-201). The authors are also grateful to the staff of the Department of Biochemistry for their technical support.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Harman D. The aging process. Proc Natl Acad Sci U S A. 1981;78(11):7124–8. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harman D. Free radicals in aging. Mol Cell Biochem. 1988;84(2):155–61. doi: 10.1007/BF00421050. [DOI] [PubMed] [Google Scholar]
- 3.Klauning JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–67. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 4.Inal ME, Kanbak G, Sunal E. Antioxidant enzyme activities and malondialdehyde levels related to aging. Clin Chim Acta. 2001;305(1):75–80. doi: 10.1016/s0009-8981(00)00422-8. [DOI] [PubMed] [Google Scholar]
- 5.Ceballos-Picot I, Trivier JM, Nicole A, Sinet PM, Thevenin M. Age-correlated modifications of copper–zinc superoxide dismutase and glutathione-related enzyme activities in human erythrocytes. Clin Chem. 1992;38(1):66–70. [PubMed] [Google Scholar]
- 6.Guemouri L, Artur Y, Herbeth B, Jeandel C, Cuny G, Siest G. Biological variability of superoxide dismutase, glutathione peroxidase, and catalase in blood. Clin Chem. 1991;37(11):1932–7. [PubMed] [Google Scholar]
- 7.Ahmed RG. Is there a balance between oxidative stress and antioxidant defense system during development. Med J Islam World Acad Sci. 2005;15(2):55–63. [Google Scholar]
- 8.Kurata M, Suzuki M, Agar NS. Antioxidant systems and erythrocyte life-span in mammals. Comp Biochem Physiol. 1993;106(3):477–87. doi: 10.1016/0305-0491(93)90121-k. [DOI] [PubMed] [Google Scholar]
- 9.Hagihara M, Nishigaki I, Maseki M, Yagi K. Age-dependent changes in lipid peroxide levels in the lipoprotein fractions of human serum. J Gerontol. 1984;39(3):269–72. doi: 10.1093/geronj/39.3.269. [DOI] [PubMed] [Google Scholar]
- 10.Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000;21(3):361–70. doi: 10.1093/carcin/21.3.361. [DOI] [PubMed] [Google Scholar]
- 11.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci USA. 1994;91(23):10771–8. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bland JS. Oxidants and Antioxidants in Clinical Medicine: Past, Present and Future Potential. J Nutr Environ Med. 1995;5(3):255–80. [Google Scholar]
- 13.Taridi NM, Yahaya MF, Teoh SL, Latiff AA, Wan Ngah WZ, Das S. Tocotrienol Rich Fraction (TRF) supplementation protects against oxidative DNA damage and improves cognitive function in Wistar Rats. Clin Ter. 2011;162(2):93–8. [PubMed] [Google Scholar]
- 14.Makpol S, Durani LW, Chua KH, Mohd Yusof YA, Wan Ngah WZ. Tocotrienol-Rich Fraction Prevents Cell Cycle Arrest and Elongates Telomere Length in Senescent Human Diploid Fibroblasts. J Biomed Biotechnol. 2011(2011:506171. doi: 10.1155/2011/506171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pin KY, Luqman Chuah A, Abdull Rashih A, Mazura MP, Fadzureena J, Vimala S, et al. Anti oxidant and anti-inflammatory activities of extracts of betel leaves (Piper betle) from solvents with different polarities. J Trop For Sci. 2010;22(4):448–55. [Google Scholar]
- 16.Bhattacharya S, Banerjee D, Bauri AK, Chattopadhyay S, Bandyopadhyay SK. Healing property of the Piper betel phenol, allylpyrocatechol against indomethacin-induced stomach ulceration and mechanism of action. World J Gastroenterol. 2007;13(27):3705–13. doi: 10.3748/wjg.v13.i27.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dasgupta N, Bratati D. Antioxidant activity of Piper betle L. leaf extract in vitro. Food Chem. 2004;88:219–24. [Google Scholar]
- 18.Choudhary D, Kale RK. Antioxidant and nontoxic properties of Piper betle leaf extract: in vitro and in vivo studies. Phytother Res. 2002;16(5):461–6. doi: 10.1002/ptr.1015. [DOI] [PubMed] [Google Scholar]
- 19.Adachi H, Ishii N. Effects of tocotrienols on life span and protein carbonylation in Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci. 2000;55(6):280–5. doi: 10.1093/gerona/55.6.b280. [DOI] [PubMed] [Google Scholar]
- 20.Kamat JP, Sarma HD, Devasagayam TPA, Nesaretnam K, Basiron Y. Tocotrienols from palm oil as effective inhibitors of protein oxidation and lipid peroxidation in rat liver microsomes. Mol Cell Biochem. 1997;170(1-2):131–8. doi: 10.1023/a:1006853419214. [DOI] [PubMed] [Google Scholar]
- 21.Chin SF, Ibahim J, Makpol S, Abdul Hamid NA, Abdul Latiff A, Zakaria Z, et al. Tocotrienol Rich Fraction Supplementation Improved Lipid Profile and Oxidative Status in Healthy Older Adults: A Randomized Controlled Study. Nutr Metab (Lond) 2011;8(1):42. doi: 10.1186/1743-7075-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasegawa T, Kimura Y, Hiromatsu K, Kobayashi N, Yamada A, Makino M, et al. Effect of hot water extract of Chlorella vulgaris on cytokine expression patterns in mice with murine acquired immunodeficiency syndrome after infection with Listeria monocytogenes. Immunopharmacology. 1997;35(3):273–82. doi: 10.1016/s0162-3109(96)00150-6. [DOI] [PubMed] [Google Scholar]
- 23.Mohd Yusof YA, Hassan Basari J, Mukti NA, Sabuddin R, Muda AR, Sulaiman S, et al. Fatty acids composition of microalgae Chlorella vulgaris can be modulated by varying carbon dioxide concentration in outdoor culture. African J of Biotech. 2011;10(62):13536–42. [Google Scholar]
- 24.Mohd Yusof YA, Md Saad S, Makpol S, Shamaan NA, Wan Ngah WZ. Hot water extract of Chlorella vulgaris induced DNA damage and apoptosis. Clinics. 2010;65(12):1–7. doi: 10.1590/S1807-59322010001200023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suhaniza S, Nor Aripin S, Wan Zurinah WN, Yasmin Anum MY. Chemopreventive effect of Chlorella vulgaris in choline deficient diet and ethionine induced liver carcinogenesis in rats. Int J Cancer Res. 2006;2(3):234–41. [Google Scholar]
- 26.Pin KY, Chuah TG, Choong TSY, Abdull Rashih A, Rasadah MA, Laws CL. World Engineering Congress; Penang, Malaysia: 2007. Modelling of extraction kinetics of hydroxychavicol from betel (Piper betle L.) leaves. [Google Scholar]
- 27.Noor Aini AH, Illyana I, Wan Zurinah WN, Gapor MT, Musalmah M. Oxidants and Antioxidants in Biology. Cadiz; Spain: 2003. Effect of Long Term Palm Oil Vitamin E Supplementation on Rate of Wound Closure and Lipid Peroxidation at Different Age Groups in Rats. [Google Scholar]
- 28.Beyer WF , Jr, Fridovich I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem. 1987;161(2):559–66. doi: 10.1016/0003-2697(87)90489-1. [DOI] [PubMed] [Google Scholar]
- 29.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Clin Med. 1967;70(1):158–69. [PubMed] [Google Scholar]
- 30.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 31.Pilz J, Meineke I, Gleiter CH. Measurement of free and bound malondialdehyde in plasma by high-performance liquid chromatography as the 2,4-dinitrophenylhydrazine derivative. J Chromatogr B Biomed Sci Appl. 2000;742(2):315–25. doi: 10.1016/s0378-4347(00)00174-2. [DOI] [PubMed] [Google Scholar]
- 32.Sim SA, Salonikas C, Naidoo D, Wilcken DEL. Improved method for plasma malondialdehyde measurement by high-performance liquid chromatography using methyl malondialdehyde as an internal standard. J Chromatogr B Analyt Technol Biomed Life Sci. 2003 May;785(2):337–44. doi: 10.1016/s1570-0232(02)00956-x. [DOI] [PubMed] [Google Scholar]
- 33.Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreira PI, Duarte AI, Santos MS, Rego AC, Oliveira CR. An integrative view of the role of oxidative stress, mitochondria and insulin in Alzheimer's disease. J Alzheimers Dis. 2009;16(4):741–6. doi: 10.3233/JAD-2009-0972. [DOI] [PubMed] [Google Scholar]
- 35.Norfaizatul SO, Zetty Akmal CZ, Noralisa AK, Then SM, Wan Zurinah WN, Musalmah M. Dual Effects of Plant Antioxidants on Neuron Cell Viability. J Med Plants. 2010;9(6) Winter. [Google Scholar]
- 36.Arambewela L, Arawwawala M, Rajapaksa D. Piper betle: a potential natural antioxidant. Int J Food Sci Technol. 2006;41(1):10–14. [Google Scholar]
- 37.Andersen HR, Nielsen YB, Nielsen F, Grandjean P. Antioxidative enzyme activities in human erythrocytes. Clin Chem. 1997;43(4):562–8. [PubMed] [Google Scholar]
- 38.Glass GA, Gershon D. Enzymatic changes in rat erythrocytes with increasing cell and donor age: loss superoxide dismutase activity associated with increases in catalytically defective forms. Biochem Biophys Res Commun. 1981;103(4):1245–53. doi: 10.1016/0006-291x(81)90256-4. [DOI] [PubMed] [Google Scholar]
- 39.Salo DC, Pacifini RE, Davies KJN. Superoxide dismutase is preferentially degraded by a proteolytic stem from red blood cells following oxidative modification by hydrogen peroxide. Free Rad Biol Med. 1988;5(5-6):335–9. doi: 10.1016/0891-5849(88)90105-0. [DOI] [PubMed] [Google Scholar]
- 40.Alejendro DB, Martha SB, Nestor OB. Superoxide dismutase, catalase and glutathione peroxidase activities in human blood: influence of sex, age, and cigarette smoking. Clin Biochem. 1997;30(6):449–54. doi: 10.1016/s0009-9120(97)00047-7. [DOI] [PubMed] [Google Scholar]
- 41.Papet I, Dardevet D, Sornet C, Béchereau F, Prugnaud J, Pouyet C, et al. Acute phase protein levels and thymus, spleen and plasma protein synthesis rates differ in adult and old rats. J Nutr. 2003;133(1):215–9. doi: 10.1093/jn/133.1.215. [DOI] [PubMed] [Google Scholar]
- 42.Friel JK, Widness JA, Jiang T, Belkhode SL, Rebouche CJ, Ziegler EE. Antioxidant status and oxidant stress may be associated with vitamin E intakes in very low birth weight infants during the first month of life. Nutr Res. 2002;22(1-2):55–64. [Google Scholar]
- 43.Chitra S, Vidya N. Dose Dependent Effect of Piper betle Linn. Leaf Extract on Erythrocytes of Experimental Mice. Sri Ramachandra J Med. 2006;1(1) [Google Scholar]
- 44.Al-Adhroey AH, Nor ZM, Al-Mekhlafi HM, Amran AA, Mahmud R. Antimalarial Activity of Methanolic Leaf Extract of Piper betle L. Molecules. 2011;16(1):107–18. doi: 10.3390/molecules16010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noor Aini AH, Illyana I, Musalmah M, Wan Zurinah WN. Malondialdehyde level and antioxidant enzymes activities during aging in rats with and without vitamin E supplementation. Malaysian J Biochem Mol Biol. 2002;7:50–54. [Google Scholar]
- 46.Tian L, Cai Q, Wei H. Alterations of antioxidant enzymes and oxidative damage to macromolecules in different organs of rats during aging. Free Radical Biol Med. 1998;24(9):1477–84. doi: 10.1016/s0891-5849(98)00025-2. [DOI] [PubMed] [Google Scholar]