Abstract
Left ventricular hypertrophy (LVH) is an independent, modifiable risk factor for cardiovascular disease. However, current screening strategies are limited. In 2478 participants without clinical disease from the Dallas Heart Study, we evaluated a multi-marker screening strategy that complements electrocardiographic (ECG) criteria for LVH with two biomarkers, amino-terminal pro-B-type natriuretic peptide (NT-proBNP) and highly sensitive cardiac troponin T (cTnT). An integer LVH risk score from 0 to 3 was determined as the sum of: (1) LVH by Sokolow-Lyon ECG, (2) NT-proBNP in the highest sex-specific quartile, and (3) detectable cTnT. Cardiac MRI-determined LVH served as the primary outcome.
The probability of LVH increased from 2% with an LVH risk score of 0 to 50% with a score of 3 (p < 0.001). S-L ECG afforded low sensitivity (26%, 95% CI 17–32%) and high specificity (96%, 95% CI 95–97%), while a risk score ≥2 offered higher sensitivity (44%, 95% CI 34–51%) with good specificity (90%, 95% CI 89–93%), a score threshold of 1 offered reasonable sensitivity (76%, 95% CI 67–83%) with lower specificity (55%, 95% CI 53–61%) and high negative predictive value (98%, 95% CI 97–98%). AUC improved from 0.760 (95% CI 0.716–0.804) for ECG alone to 0.798 (95% CI 0.754–0.842) for the LVH risk score (p = 0.0012) consistent with modest improvement in overall discrimination. Better screening for LVH may be achieved by combining simple tests, which collectively provide additional information compared to ECG alone. Further studies are needed to evaluate the impact and cost-effectiveness of a multi-marker screening strategy.
Keywords: left ventricular hypertrophy screening, diagnostic performance, amino-terminal B-type natriuretic peptide, highly sensitive troponin T, electrocardiographic Sokolow-Lyon criteria
Introduction
Mounting evidence has associated a pathological increase in left ventricular (LV) mass with higher rates of cardiovascular (CV) morbidity and mortality even after adjustment for potential confounders.1–3 Evidence stemming from meta-analyses and randomized trials suggests that angiotensin converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB) are more effective in reversing LVH than other anti-hypertensive therapies and are associated with improved clinical outcomes, independent of blood pressure control.4–6 These studies suggest LVH is an independent, modifiable, and potentially overlooked risk factor, with management implications more complex than simply maintaining effective blood pressure control. Therefore, screening for LVH may be a valuable target in the effort to improve CV outcomes.
LVH screening is hindered by low disease prevalence, poor test characteristics of ECG-based detection methods,7 and the high cost of universal screening with transthoracic echocardiography (TTE).8 Amino-terminal B-type natriuretic peptide (NTproBNP) and cardiac troponin T (cTnT) are validated biomarkers associated with LVH9–11 and have test characteristics complementary to the ECG; adding these assays to the ECG may improve LVH screening, especially in subgroups such as the obese, where the prevalence of LVH is high and ECG criteria perform poorly.12
Using the Dallas Heart Study (DHS), a probability-based population sample of Dallas County residents, we evaluated a multi-marker strategy for LVH screening. For each DHS participant, an integer LVH risk score from 0 to 3 was calculated as the sum of following criteria: (1) LVH by Sokolow-Lyon ECG criteria, (2) NTproBNP in the top sex-specific quartile, and (3) detectable cTnT using a highly sensitive assay. Using cardiac MRI-determined LVH as the primary outcome, we characterized the performance of each test and the combined LVH risk score.
Methods
Study Group
The DHS is a probability-based population sample of 6101 Dallas County residents ages 18–65. Details of the study design and participant selection have been described previously.13 Blacks were intentionally oversampled to comprise 50% of the DHS study cohort. All participants provided written informed consent and the study was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center. We included all subjects who participated in all three phases of DHS data collection, including cardiac magnetic resonance imaging (cMRI, n=2501). We excluded 23 subjects with myocardial infarction, heart failure, coronary artery bypass graft surgery, percutaneous coronary intervention, or ischemic cerebrovascular disease, or with bundle branch block, or Q-wave evidence of prior MI, as these could interfere with the interpretation of LVH criteria by ECG or prompt cardiac imaging. No subjects had atrial fibrillation on their 12-lead ECG. This left a final sample of 2478 subjects for the present analysis.
MRI and ECG
ECG-gated cine magnetic resonance images were obtained from 2 comparable 1.5-T MRI systems (Philips Medical Systems, Best, Netherlands). Standard 12-lead ECGs were recorded and interpreted by two DHS investigators blinded to demographic and clinical information. We assessed the test characteristics of three ECG criteria: Sokolow-Lyon voltage criteria,14 Cornell ECG criteria,15 Romhilt-Estes ECG criteria,16 and the Cornell/Strain index (C/S index)17 for detection of LVH in the DHS population.
Biomarker Assays
Based on prior work from our group, we used a well-validated, commercially available NT-proBNP assay (Roche Diagnostics)18 and chose a cut point of 7.82 pmol/L in women and 3.42 pmol/L in men which corresponds to the gender-specific 75th percentile in a healthy, phenotypically normal subpopulation of the DHS cohort.19. We measured cTnT levels using a highly sensitive assay on an automated platform (Roche Diagnostics). Based on previous analyses by our group11 and others10, we used the minimal detectable concentration (MDC) of the cTnT assay (0.003 mcg/L) as our threshold for LVH screening.
Variable definitions
For this study, cMRI-derived LV mass (LVM) was indexed to body-surface area (BSA) and the presence of LVH was defined as at or above the gender-specific 99th percentile (95 g/m2 in women and 117 g/m2 in men), of the healthy, phenotypically normal subpopulation of the DHS cohort.19 This data-derived threshold closely approximates previously published echo-derived cut points shown to be predictors of adverse CV events,20 and was thus chosen a priori as the outcome for the primary analysis. The components of the LVH risk score were: (1) an ECG which met S-L ECG criteria ([S amplitude in V1 + maximum R amplitude in V5 or V6] > 3.5mV),14 (2) NT-proBNP greater than the gender-specific 75th percentile,18 and (3) detectable cTnT using the high-sensitivity assay. For each participant, an integer LVH risk score ranging from 0 to 3 was calculated by summing the number of above criteria that were met.
Statistical Analysis
The diagnostic performance characteristics of ECG, cTnT and NT-proBNP were evaluated as separate tests and in combination. We compared the area under the receiver operator characteristic (ROC) curve (AUC) for the individual and combined tests. Categorical analyses compared differences for individual and combined groups using the Jonckheere-Terpstra test. Stratified analyses using chi-square and Kruskal-Wallis tests were performed for continuous and categorical variables across gender, ethnicity, hypertension and BMI categories21. We defined “number needed to screen” as the number of patients who would need to be screened in order to detect one more case of LVH. This was calculated by dividing 1 over the positive predictive value. All statistical analyses were performed using SAS Version 9.2 (Cary, NC, USA), two-sided p values <0.05 were considered significant. Please see online supplement for additional methods detail.
Results
Study group
Our cohort (55% women, 47% Black, 29% having hypertension) had a mean (± SD) age of 44 ± 9.6 and BMI 30 ± 7.1 kg/m2 (Table 1). All subjects had an estimated GFR ≥60 ml/min/1.73 m2. The prevalence of LVH was 5.4%, with higher prevalence in Blacks (9.8%) and in participants with BMI > 35 kg/m2 (6.7%) (p < 0.05 for each). Participants with hypertension had a higher prevalence of LVH (14.3%) than those without (2.0%) (p < 0.0001). ECG with positive S-L criteria for LVH was seen in 4.8% of participants, cTnT was detectable in 24.3%, and by construction 25% had an NT-proBNP in the top sex-specific quartile. Supplemental Table S1 (online) shows the number of patients in each testing group.
Table 1.
Patient characteristics stratified by LVH risk score.
| Group n |
Overall 2478 |
Score 0 1330 (54%) |
Score ≥ 1 1148 (46%) |
Score ≥ 2 282 (11%) |
Score 3 24 (1%) |
p-value |
|---|---|---|---|---|---|---|
| Age (years) | 44 (10) | 41 (9) | 46 (10) | 49 (9) | 51 (8) | <.0001 |
| Men | 1115 (45%) | 467 (35%) | 648 (56%) | 200 (71%) | 19 (79%) | <.0001 |
| Black | 1169 (47%) | 604 (45%) | 565 (49%) | 174 (62%) | 22 (92%) | <.0001 |
| Hypertension | 690 (29%) | 294 (22%) | 396 (35%) | 150 (53%) | 19 (79%) | <.0001 |
| Diabetes | 237 (10%) | 106 (7%) | 131 (12%) | 47 (19%) | 3 (13%) | <.0001 |
| BMI (kg/m2) | 30 (7) | 30 (7) | 29 (7) | 29 (7) | 28 (8) | 0.03 |
| BMI >30 | 1081 (44%) | 607 (46%) | 474 (41%) | 111 (39%) | 9 (38%) | 0.06 |
| LVEF (%) | 72 (7) | 73 (7) | 72 (8) | 70 (10) | 65 (15) | 0.0014 |
| LV Mass (g) | 161 (43) | 153 (38) | 171 (47) | 196 (39) | 242 (55) | <.0001 |
| GFR * | 101 (23) | 103 (21) | 99 (24) | 94 (25) | 92 (30) | <.0001 |
| Sokolow-Lyon voltage (mm) | 22 (7) | 21 (6) | 24 (8) | 28 (9) | 43 (7) | <.0001 |
| NTproBNP (pmol/L) | 7.2 (46.5) | 2.8 (2.1) | 13 (70.8) | 31.2 (145.2) | 73 (138.9) | <.0001 |
| cTnT (mcg/L) | 0.003 (0.008) | Below detectable | 0.006 (0.012) | 0.010 (0.022) | 0.012 (0.012) | <.0001 |
Data shown are mean (±standard deviation) for continuous variables and n (%) for categorical variables.
GFR stands for glomerular filtration rate, measured in mL/min/1.73m2.
Individual test performance
The S-L ECG voltage criteria had sensitivity of 26% (95% CI 17–32%) with specificity of 96% (95% CI 95–97%) and an AUC of 0.760 (95% CI 0.716–0.804); the Cornell criteria had a sensitivity of 35% (95% CI 27–44%) with AUC of 0.738 (95% CI 0.689–0.788), the C/S index criteria had a sensitivity of 44% (95% CI 36–53%) and an AUC of 0.754 (0.708–0.800). The R-E point score system had lower sensitivity and AUC in our study cohort. We selected the Sokolow-Lyon voltage criteria as our ECG standard for LVH because it had the highest AUC and is ubiquitous in clinical practice. Table 2 shows a comparison of the diagnostic characteristics of each ECG criteria tested. Note that the AUC of the S-L criteria was only modestly higher than the Cornell criteria, the C/S index criteria and the Romhilt-Estes ECG criteria in the DHS. Supplemental Table S2 (online) shows the AUCs of each ECG criteria tested individually, as well as incorporated in the LVH risk score. Since the C/S index criteria had the highest sensitivity of the various ECG criteria, we repeated our analysis using this as the alternate ECG comparator; these analyses are shown as supplemental Tables S3 to S6 (online).
Table 2.
Diagnostic performance for ECG criteria tested.
| Diagnostic Characteristic |
Sokolow- Lyon |
Cornell | Romhilt- Estes |
C/S Index |
|---|---|---|---|---|
| Sensitivity | 26% (16.8–31.8) |
35% (27.5–44.2) |
13% (8.1–20.3) |
44% (36.1–52.8) |
| Specificity | 96% (95.4–97) |
94% (93.9–95.7) |
98% (98.2–99.1) |
93% (91.6–93.7) |
| PPV | 27% (19–35.5) |
28% (21.9–36) |
37% (24–52.6) |
26% (20.3–31.6) |
| NPV | 96% (94.7–96.4) |
96% (95.4–97) |
95% (94.3–96) |
97% (95.9–97.4) |
| AUC | 0.760 (0.716–0.804) |
0.738 (0.689–0.788) |
0.651 (0.603–0.699) |
0.754 (0.708–0.800) |
Parentheses indicate 95% confidence interval.
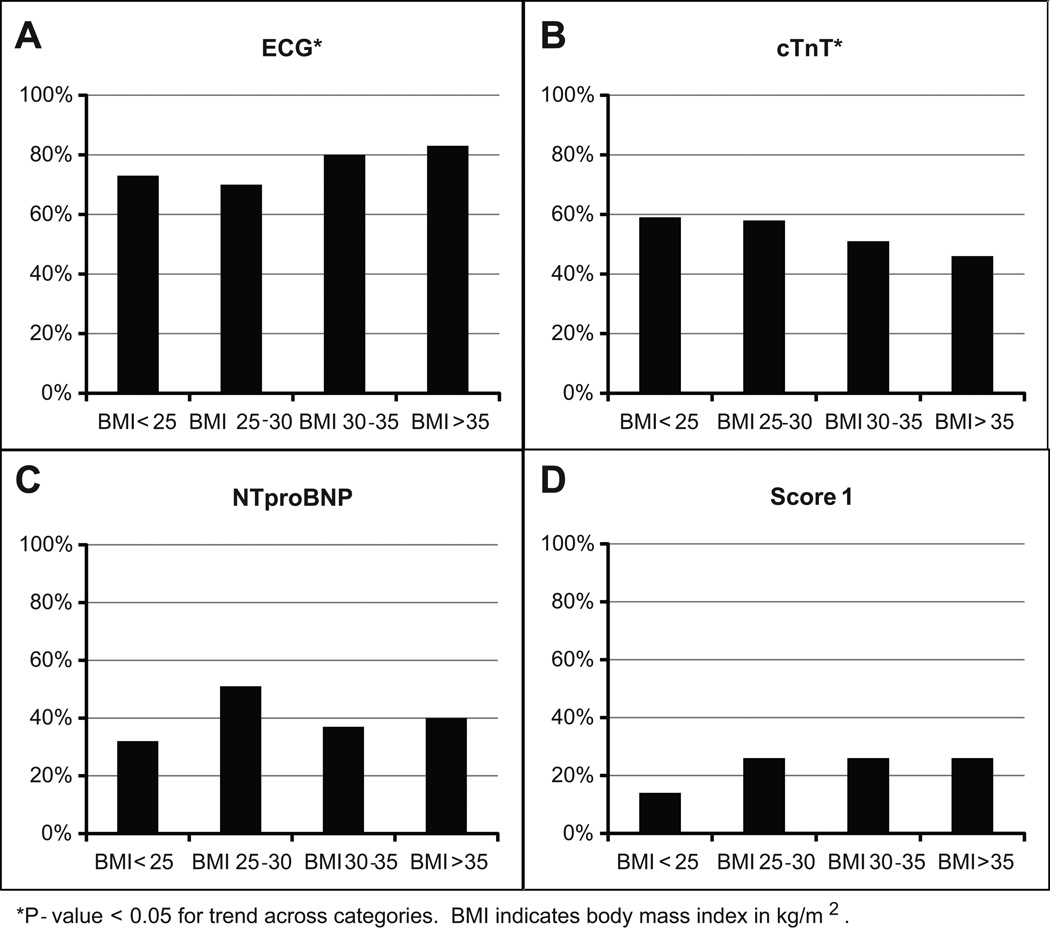
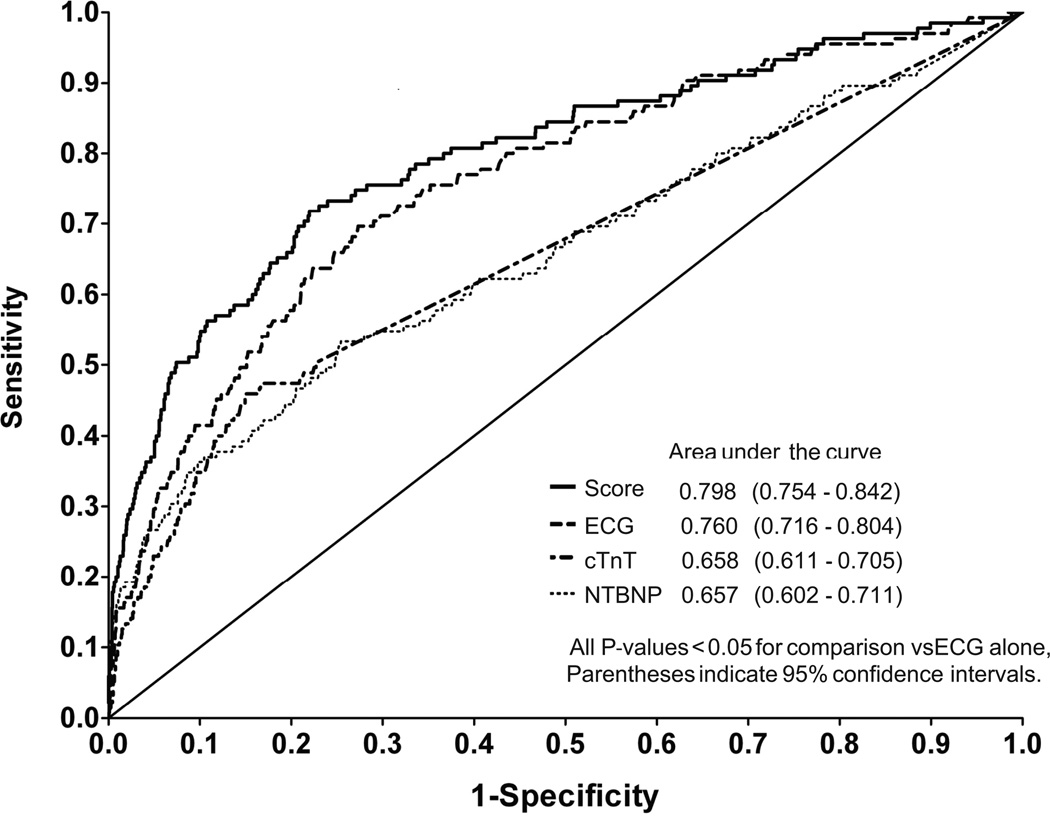
NT-proBNP and cTnT had higher sensitivity (55%, 95% CI 44–61% and 50%, 95% CI 42–59%, respectively) and lower specificity (72%, 95% CI 76–78% and 77%, 95% CI 76–79%, respectively) compared to ECG alone (Table 3). S-L ECG criteria (Figure 1A) had a significantly higher false negative rate as BMI increased (83% in highest BMI category, p < 0.05 for trend), contrasting with cTnT (Figure 1B) which had a trend toward lower rate of false negatives at higher BMI categories, but still had high false negative rates overall (46% in highest cTnT category, p < 0.05 for trend); NT-proBNP (Figure 1C) remained similar across BMI categories, while LVH risk score ≥1 had the lowest false negative rate of 26% at the highest BMI group (Figure 1D). Overall, the highest AUC individual test (Figure 2) was the S-L ECG, with an AUC of 0.760 (95% CI 0.716–0.804); higher than either NT-proBNP (AUC 0.657, 95% CI 0.602–0.711, p=0.0017 vs ECG alone) or cTnT (AUC 0.658, 95% CI 0.602–0.711, p=0.0013 vs ECG alone).
Table 3.
Diagnostic performance for individual tests and LVH risk score.
| Diagnostic Characteristic |
ECG | NTBNP | cTnT | Score ≥ 1 | Score ≥ 2 | Score 3 |
|---|---|---|---|---|---|---|
| Sensitivity | 26% (16.8–31.8) |
55% (43.8–61.2) |
50% (41.6–59.1) |
76% (67.4–82.5) |
44% (33.8–51) |
9% (4.7–15) |
| Specificity | 96% (95.4–97) |
72% (70.6–78) |
77% (75.5–78.9) |
55% (52.7–61.2) |
90% (88.8–93.1) |
99% (98.2–99.8) |
| PPV | 27% (19–35.5) |
10% (9.3–14.6) |
11% (8.9–14.1) |
9% (7.9–11.6) |
21% (18.2–29.2) |
50% (30.6–73.2) |
| NPV | 96% (94.7–96.4) |
97% (95.7–97.4) |
96% (95.5–97.2) |
98% (96.8–98.4) |
97% (95.7–97.2) |
95% (94.1–95.8) |
| Positive LR | 6.3 (4.38–9.1) |
2.0 (1.95–2.77) |
2.2 (1.84–2.66) |
1.7 (1.66–2.06) |
4.6 (4.16–6.73) |
17.4 (8.5–42.1) |
| Negative LR | 0.8 (0.72–0.87) |
0.6 (0.51–0.73) |
0.6 (0.54–0.76) |
0.4 (0.31–0.56) |
0.6 (0.54–0.73) |
0.9 (0.87–0.97) |
PPV = positive predictive value, NPV = negative predictive value, LR= likelihood ratio, NT-proBNP= Amino-terminal B-type natriuretic peptide, cTnT= highly sensitive cardiac troponin T.
Figure 1.
Probability of false negative for diagnosis of left ventricular hypertrophy, stratified by BMI category.
Figure 2.
ROC curves and AUCs for the individual and combined test strategies.
Combined test performance
A total of 1330 (54%) participants had a score of 0, while 1148 (46%) had a score ≥ 1, 282 (11%) a score ≥ 2 and 24 (1%) a score of 3. An LVH risk score ≥1 offered higher sensitivity (76%, 95% CI 67.4–82.5) than any individual test, with moderate specificity (55%, 95% CI 52.7–61.2), whereas LVH risk scores ≥ 2 or 3 had progressively higher specificities (90 and 99% respectively) with lower sensitivities (44 and 9% respectively). All testing groups had a very high negative predictive value (≥ 95%) (Table 3).
Compared with ECG S-L voltage alone (AUC 0.760, 95% CI 0.716–0.804), the addition of cTnT (AUC 0.780, 95% CI 0.738–0.822, p = 0.0074 vs ECG alone) or NT-proBNP (AUC 0.796, 95% CI 0.752–0.840, p = 0.0023 vs ECG alone) significantly improved discrimination of LVH. The combination of all three tests demonstrated the highest discrimination (AUC 0.798, 95% CI 0.754–0.842, p = 0.0012 vs ECG alone) (Figure 2). The combination of both biomarkers without ECG had a significantly lower AUC than ECG alone (0.623 vs 0.760 p <0.0001). Stratified analyses showed that the observed improvements in AUC over ECG alone were statistically significant in subjects older than 50 years, men, Black subjects, and in those with hypertension. We also observed a consistent and significant improvement across BMI categories (Table 4).
Table 4.
Area under the curve values for individual and combined diagnostic test groups.
| Test Group | n | LVH Prevalence |
ECG | cTnT | NTBNP | ECG + cTnT |
ECG + NTBNP |
ECG + NTBNP + cTnT |
|---|---|---|---|---|---|---|---|---|
| Overall | 2478 | 5.4% | 0.760 (0.716–0.804) |
0.658 (0.611–0.705) |
0.657 (0.602–0.711) |
0.780* (0.738–0.822) |
0.796* (0.752–0.840) |
0.798*† (0.754–0.842) |
| Age < 50 | 1786 | 4.6% | 0.770 (0.716–0.824) |
0.649 (0.590–0.707) |
0.622 (0.551–0.693) |
0.785 (0.733–0.838) |
0.797 (0.744–0.851) |
0.798 (0.745–0.851) |
| Age >50 | 692 | 7.7% | 0.757 (0.683–0.832) |
0.650 (0.570–0.731) |
0.690 (0.605–0.774) |
0.784 (0.712–0.856) |
0.794* (0.719–0.870) |
0.801* (0.727–0.876) |
| Male | 1115 | 6.4% | 0.719 (0.657–0.781) |
0.697 (0.629–0.765) |
0.709 (0.636–0.782) |
0.761* (0.703–0.819) |
0.794* (0.735–0.853) |
0.795*‡ (0.736–0.854) |
| Female | 1363 | 4.7% | 0.792 (0.728–0.856) |
0.614 (0.554–0.674) |
0.630 (0.546–0.713) |
0.794 (0.730–0.858) |
0.802 (0.736–0.867) |
0.803 (0.738–0.869) |
| HTN no | 1788 | 2.0% | 0.782 (0.698–0.866) |
0.578 (0.496–0.660) |
0.509 (0.389–0.628) |
0.797 (0.714–0.880) |
0.790 (0.704–0.876) |
0.789 (0.703–0.876) |
| HTN yes | 690 | 14.3% | 0.733 (0.678–0.789) |
0.640 (0.581–0.698) |
0.680 (0.620–0.740) |
0.745*† (0.691–0.799) |
0.773*‡ (0.718–0.827) |
0.773*‡ (0.718–0.827) |
| Black | 1169 | 9.8% | 0.704 (0.653–0.756) |
0.642 (0.590–0.694) |
0.695 (0.639–0.751) |
0.730*† (0.681–0.780) |
0.762* (0.711–0.813) |
0.763* (0.712–0.813) |
| Other | 1309 | 1.6% | 0.744 (0.612–0.876) |
0.640 (0.520–0.762) |
0.581 (0.419–0.744) |
0.751 (0.617–0.884) |
0.763 (0.636–0.890) |
0.763 (0.636–0.891) |
| BMI | ||||||||
| <25 | 590 | 3.7% | 0.820 (0.744–0.897) |
0.626 (0.512–0.740) |
0.711 (0.564–0.858) |
0.852† (0.788–0.916) |
0.893*‡ (0.840–0.945) |
0.896* (0.841–0.951) |
| 25–30 | 807 | 5.3% | 0.765 (0.687–0.842) |
0.605 (0.521–0.690) |
0.611 (0.512–0.710) |
0.776 (0.699–0.851) |
0.782 (0.704–0.859) |
0.791* (0.715–0.867) |
| 30–35 | 562 | 6.2% | 0.748 (0.651–0.845) |
0.713 (0.623–0.803) |
0.671 (0.569–0.772) |
0.764 (0.667–0.860) |
0.787 (0.693–0.882) |
0.792* (0.697–0.886) |
| >35 | 519 | 6.7% | 0.765 (0.678–0.852) |
0.680 (0.588–0.773) |
0.693 (0.590–0.796) |
0.820* (0.739–0.900) |
0.796 (0.705–0.886) |
0.822* (0.740–0.905) |
p-value <0.05 vs ECG alone,
p-value <0.05 vs ECG+NTproBNP,
p-value <0.05 vs ECG+hsTnT.
Parentheses indicate 95% confidence interval.
LVH risk score diagnostic efficiency
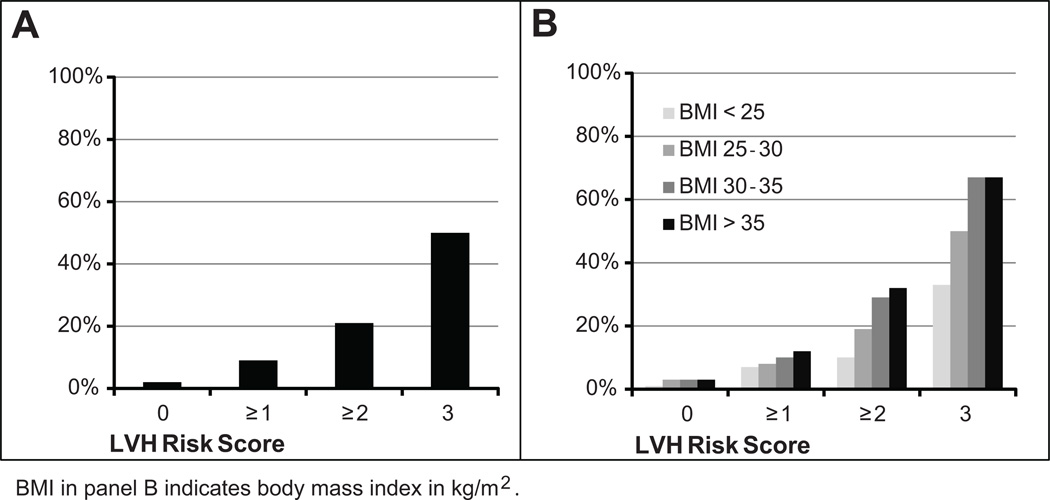
The probability of having LVH increased markedly from 2% in those with an LVH risk score of 0 to 50% in those with an LVH risk score of 3 (p < 0.0001), although the absolute number of people with a risk score of 3 was small (Figure 3A). The number needed to screen (NNS) to detect one case of LVH was 50 in the group with LVH risk score of 0, 11 for those with score ≥ 1, 5 for a score ≥ 2, and 2 for a score of 3. The association of the score with the presence of LVH became more pronounced at higher BMI categories (p < 0.05 for trend; Figure 3B). In our cohort, the triple test strategy picked up more LVH cases than any individual test, including 71 additional LVH cases over ECG alone, with notable improvement in sensitivity for groups where the prevalence of LVH is high, these include those aged >50, men, Blacks, and subjects with hypertension or obesity (Table 5).
Figure 3.
Probability of left ventricular hypertrophy (LVH) stratified by LVH risk score overall (A) and further stratified by BMI category (B).
Table 5.
Screening efficiency comparison between S-L ECG criteria and LVH risk score 1.
| True Positives | False Positives | Sensitivity | ||||||
|---|---|---|---|---|---|---|---|---|
| Test Group | n | Cases* | ECG | Score 1 | ECG | Score 1 | ECG | Score 1 |
| Overall | 2478 | 135 | 32 | 103 | 88 | 955 | 26% (16.8–31.8) |
76% (67.4–82.5) |
| Age >50 | 692 | 53 | 14 | 45 | 18 | 372 | 26% (15.3–40.3) |
85% 72.4–93.3) |
| HTN | 690 | 99 | 21 | 80 | 27 | 300 | 21% (17–33.5) |
81% 70.5–87.2) |
| Black | 1169 | 114 | 28 | 88 | 75 | 452 | 25% (17–33.5) |
77% (68.4–84.5) |
| Men | 1115 | 71 | 16 | 59 | 66 | 539 | 23% (13.5–34) |
83% (70.7–89.9) |
| Women | 1363 | 64 | 16 | 44 | 22 | 416 | 25% (15–37.4) |
69% (55.9–79.8) |
|
BMI 30–35 |
562 | 35 | 7 | 26 | 10 | 205 | 20% (8.4–36.9) |
74% (53.7–85.4) |
| BMI >35 | 519 | 35 | 6 | 26 | 8 | 181 | 17% (6.6–33.6) |
74% (56.7–87.5) |
Total number of subjects identified as having LVH by cMRI. Parentheses indicate 95% confidence interval.
Discussion
The ECG is the least expensive and most widely available method for the detection of LVH; however, several studies have shown the various ECG criteria perform poorly as a screening test. A 2007 meta-analysis of 21 studies comparing the diagnostic performance of various criteria revealed the Sokolow-Lyon index to have a sensitivity of 21%,7 similar to that observed in the present study (26%). ECG criteria consistently underperform in obese individuals with correspondingly high rates of false negatives, particularly troublesome due to the higher prevalence of LVH in this subgroup.14 In the context of high obesity rates in modern clinical practice, this severely limits the potential of the ECG as a screening tool for LVH, in fact, the 2007 European Society of Hypertension and European Society of Cardiology guidelines now explicitly indicate that the ECG alone should not be used to evaluate for LVH.22
In the present study, diagnostic performance improved when using a combinatory testing strategy. The proposed LVH risk score at a threshold of 1 provides increase in sensitivity (76%, 95% CI 67–83) over standard S-L ECG criteria (26%, 95% CI 17–32), at the cost of lower specificity (55%, 95% CI 53–61). However, the risk score provides more information than any individual dichotomous split. For example, the risk score would have very high specificity for LVH in patients with a risk score ≥ 2 and a very high negative predictive value (98%) for those with a score of 0. While the sensitivity of the LVH risk score is lower compared with routine echocardiography in the general population, we observe that the sensitivity of the risk score begins to approach the screening performance of limited echocardiography (which has been reported in the range of 75% to 95%)8,23 when applied to subgroups where the prevalence of LVH is higher. Observed sensitivity rates for the LVH risk score were found to be 85% in those aged > 50, 81% in those with hypertension, 83% in men, 77% in Black subjects and 74% in individuals with BMI >35. Screening these populations with echocardiography has several limitations, one of the most important being high cost,8 current Medicare reimbursement for a routine TTE is well over $500,24 while reimbursement for an ECG or for the NT-proBNP assay are one tenth as high,25 with costs for the cTnT assay expected to stabilize at a similar level. We speculate that future cost-effectiveness analyses could reveal a multi-marker strategy offers significant cost savings over echocardiography. In addition, ultrasound imaging requires the presence of specialized infrastructure and trained personnel to perform and interpret the findings, and is also subject to technical difficulties, especially in obese subjects where image quality may suffer.26
Both NT-proBNP and cTnT have been positively associated with LV strain, renin-angiotensin-aldosterone system activation, myocardial fibrosis, and myocyte necrosis.9–11 Serum NTproBNP levels significantly correlate with LV wall thickness and LV mass index even in patients with significant renal disease.9,10 cTnT is also a marker of cardiac structural abnormalities even in the presence of hypertension and renal dysfunction.10,11 Despite limited utility as stand-alone screening tests for LVH,18, 26 these two biomarkers have important characteristics which make them attractive complements to the ECG in a multi-marker strategy: low assay costs, higher sensitivity, and better performance in obese patients.12,18,27 We define LVH using cardiac MRI, which is more accurate than echocardiography,28 and examine a multi-ethnic, population-based cohort adding to the validity and generalizability of these observations.
Limitations
The absolute increase in AUC we achieved is modest (0.760 to 0.798, p=0.0012), however, even small improvements in this AUC range are difficult to achieve. In Figure 2, the shape of the ROC curve for the risk score compared to the ECG, the curves converge for specificity <50%, so most of the improvement comes in the most meaningful range of specificity. In addition, the AUC, based on individual voltage components as continuous variables, is potentially misleading about the utility of the ECG in diagnosing LVH in clinical practice; specific ECG criteria have low sensitivity and high specificity.
In addition, the population prevalence of LVH strongly influences the positive and negative predictive values, suggesting the risk score would be most useful in selected subpopulations, such as the obese, where LVH is more common and the test characteristics of other screening methods are less favorable.29
The cTnT assay studied in this analysis can detect much lower concentrations (~10-fold) than the conventional commercial assays currently available for clinical use in the US. They are currently available in several countries and are expected to enter routine clinical use in the US in the near future. Our population did not include enough subjects with chronic kidney disease to allow extrapolation to these subjects. Finally, this is a cross-sectional study and therefore is not able to address clinical outcomes.
Perspectives
Our results suggest that a testing strategy that adds NT-proBNP and cTnT (using a highly sensitive assay) to the 12 lead ECG could serve as a simple, inexpensive screen for LVH in selected populations, and may help reduce unnecessary echocardiography. Further studies determining the cost-effectiveness of this approach may help clarify the clinical role of combined biomarker and ECG testing for the purpose of screening for LVH.
Supplementary Material
Novelty and significance.
-
What is new?
This study uses a large, multi-ethnic probability-based population sample of Dallas County, Texas, USA, residents to evaluate a novel screening strategy for left ventricular hypertrophy as determined by cardiac MRI. This is the first study to evaluate adding the results of commonly used biomarker (NT-proBNP, cTnT) assays to the standard 12-lead electrocardiogram.
-
What is relevant?
LVH is an independent, modifiable risk factor that may require specific therapies besides adequate blood pressure control. Current LVH screening strategies have very low sensitivity (ECG) or require significant infrastructure, highly trained personnel, and high cost (echocardiography). Our approach represents a simple, accessible screening strategy that can effectively improve upon ECG-based screening strategies without the higher resource requirements of broad-based echocardiographic screening.
-
Summary.
Screening for LVH may be a worthwhile target in selected populations, but current methods are inadequate for routine clinical use. In 2478 participants without clinical CV disease from the multi-ethnic, population-based Dallas Heart Study, we evaluated a novel screening strategy that complements ECG criteria for LVH with two well validated biomarkers, NT-proBNP and cardiac Troponin T. We found that the sensitivity of this three test panel begins to approach the performance of limited echocardiography when applied to subgroups where the prevalence of LVH is high. This multi-marker strategy could serve as an effective screen for LVH in selected populations where LVH prevalence is high and ECG based screening is ineffective, such as the obese, without incurring the overhead and expense that would be associated with more widespread echocardiographic screening.
Acknowledgments
Funding/support:
Grant support for the Dallas Heart Study was provided by the Donald W. Reynolds Foundation and by US Public Health Service General Clinical Research Center grant M01-RR00633 from National Institutes of Health (NIH)/NCRR-CR. Measurements of cardiac troponin T, N-terminal pro-brain-type natriuretic peptide were provided by Roche Diagnostics (Indianapolis, Indiana). Roche Diagnostics and the NIH had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 2.Verdecchia P, Carini G, Circo A, Dovellini E, Giovannini E, Lombardo M, Solinas P, Gorini M, Maggioni AP MAVI (MAssa Ventricolare sinistra nell’Ipertensione) Study Group. Left ventricular mass and cardiovascular morbidity in essential hypertension: the MAVI study. J Am Coll Cardiol. 2001;38:1829–1835. doi: 10.1016/s0735-1097(01)01663-1. [DOI] [PubMed] [Google Scholar]
- 3.Verdecchia P, Porcellati C, Reboldi G, Gattobigio R, Borgioni C, Pearson TA, Ambrosio G. Left ventricular hypertrophy as an independent predictor of acute cerebrovascular events in essential hypertension. Circulation. 2001;104:2039–2044. doi: 10.1161/hc4201.097944. [DOI] [PubMed] [Google Scholar]
- 4.Mathew J, Sleight P, Lonn E, Johnstone D, Pogue J, Yi Q, Bosch J, Sussex B, Probstfield J, Yusuf S Heart Outcomes Prevention Evaluation (HOPE) Investigators. Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation. 2001;104:1615–1621. doi: 10.1161/hc3901.096700. [DOI] [PubMed] [Google Scholar]
- 5.Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS, Snapinn S, Harris KE, Aurup P, Edelman JM, Wedel H, Lindholm LH, Dahlöf B LIFE Study Investigators. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004;292:2343–2349. doi: 10.1001/jama.292.19.2343. [DOI] [PubMed] [Google Scholar]
- 6.Devereux RB, Dahlöf B, Gerdts E, Boman K, Nieminen MS, Papademetriou V, Rokkedal J, Harris KE, Edelman JM, Wachtell K. Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial. Circulation. 2004;110:1456–1462. doi: 10.1161/01.CIR.0000141573.44737.5A. [DOI] [PubMed] [Google Scholar]
- 7.Pewsner D, Jüni P, Egger M, Battaglia M, Sundström J, Bachmann LM. Accuracy of electrocardiography in diagnosis of left ventricular hypertrophy in arterial hypertension: systematic review. BMJ. 2007;335:711. doi: 10.1136/bmj.39276.636354.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuspidi C, Meani S, Valerio C, Fusi V, Sala C, Zanchetti A. Left ventricular hypertrophy and cardiovascular risk stratification: impact and cost-effectiveness of echocardiography in recently diagnosed essential hypertensives. J Hypertens. 2006;24:1671–1677. doi: 10.1097/01.hjh.0000239305.01496.ca. [DOI] [PubMed] [Google Scholar]
- 9.Choi SY, Lee JE, Jang EH, Kim MO, Baek H, Ki CS, Park SW, Kim DJ, Huh WS, Oh HY, Kim YG. Association between changes in N-terminal pro-brain natriuretic peptide levels and changes in left ventricular mass index in stable hemodialysis patients. Nephron Clin Pract. 2008;110:c93–c100. doi: 10.1159/000157622. [DOI] [PubMed] [Google Scholar]
- 10.Wang AY, Lam CW, Wang M, Chan IH, Lui SF, Zhang Y, Sanderson JE. Diagnostic potential of serum biomarkers for left ventricular abnormalities in chronic peritoneal dialysis patients. Nephrol Dial Transplant. 2009;24:1962–1969. doi: 10.1093/ndt/gfp067. [DOI] [PubMed] [Google Scholar]
- 11.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okin PM, Jern S, Devereux RB, Kjeldsen SE, Dahlöf B LIFE Study Group. Effect of obesity on electrocardiographic left ventricular hypertrophy in hypertensive patients: the losartan intervention for endpoint (LIFE) reduction in hypertension study. Hypertension. 2000;35:13–18. doi: 10.1161/01.hyp.35.1.13. [DOI] [PubMed] [Google Scholar]
- 13.Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, Staab JM, Hobbs HH Dallas Heart Study Investigators. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–1480. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 14.Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37:161–186. doi: 10.1016/0002-8703(49)90562-1. [DOI] [PubMed] [Google Scholar]
- 15.Molloy TJ, Okin PM, Devereux RB, Kligfield P. Electrocardiographic detection of left ventricular hypertrophy by the simple QRS voltage-duration product. J Am Coll Cardiol. 1992:1180–1186. doi: 10.1016/0735-1097(92)90376-x. [DOI] [PubMed] [Google Scholar]
- 16.Romhilt DW, Estes EH., Jr A point-score system for the ECG diagnosis of left ventricular hypertrophy. Am Heart J. 1968;75:752–758. doi: 10.1016/0002-8703(68)90035-5. [DOI] [PubMed] [Google Scholar]
- 17.Verdecchia P, Angeli F, Reboldi G, Carluccio E, Benemio G, Gattobigio R, Borgioni C, Bentivoglio M, Porcellati C, Ambrosio G. Improved cardiovascular risk stratification by a simple ECG index in hypertension. Am J Hypertens. 2003;16:646–652. doi: 10.1016/s0895-7061(03)00912-9. [DOI] [PubMed] [Google Scholar]
- 18.de Lemos JA, McGuire DK, Khera A, Das SR, Murphy SA, Omland T, Drazner MH. Screening the population for left ventricular hypertrophy and left ventricular systolic dysfunction using natriuretic peptides: results from the Dallas Heart Study. Am Heart J. 2009;157:746–753. doi: 10.1016/j.ahj.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 19.Drazner MH, Dries DL, Peshock RM, Cooper RS, Klassen C, Kazi F, Willett D, Victor RG. Left ventricular hypertrophy is more prevalent in Blacks than whites in the general population: the Dallas Heart Study. Hypertension. 2005;46:124–129. doi: 10.1161/01.HYP.0000169972.96201.8e. [DOI] [PubMed] [Google Scholar]
- 20.de Simone G, Devereux RB, Daniels SR, Koren MJ, Meyer RA, Laragh JH. Effect of growth on variability of left ventricular mass: assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol. 1995;25:1056–1062. doi: 10.1016/0735-1097(94)00540-7. [DOI] [PubMed] [Google Scholar]
- 21.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6:51S–209S. [PubMed] [Google Scholar]
- 22.2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2007;28:1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 23.Devereux RB. Detection of left ventricular hypertrophy by M-mode echocardiography. Anatomic validation, standardization, and comparison to other methods. Hypertension. 1987;9:II19–II26. doi: 10.1161/01.hyp.9.2_pt_2.ii19. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Medicare and Medicaid Services. Medicare Ambulatory Payment Classification. [Accessed October 10th, 2011];2011 https://www.cms.gov/HospitalOutpatientPPS.
- 25.Centers for Medicare and Medicaid Services. Clinical laboratory fee schedule database. [Accessed October 10th, 2011];2011 https://www.cms.gov/ClinicalLabFeeSched.
- 26.Vasan RS, Benjamin EJ, Larson MG, Leip EP, Wang TJ, Wilson PW, Levy D. Plasma natriuretic peptides for community screening for left ventricular hypertrophy and systolic dysfunction: the Framingham heart study. JAMA. 2002;288:1252–1259. doi: 10.1001/jama.288.10.1252. [DOI] [PubMed] [Google Scholar]
- 27.Das SR, Alexander KP, Chen AY, Powell-Wiley TM, Diercks DB, Peterson ED, Roe MT, de Lemos JA. Impact of body weight and extreme obesity on the presentation, treatment, and in-hospital outcomes of 50,149 patients with ST-Segment elevation myocardial infarction results from the NCDR (National Cardiovascular Data Registry) J Am Coll Cardiol. 2011;58:2642–2650. doi: 10.1016/j.jacc.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myerson SG, Bellenger NG, Pennell DJ. Assessment of left ventricular mass by cardiovascular magnetic resonance. Hypertension. 2002;39:750–755. doi: 10.1161/hy0302.104674. [DOI] [PubMed] [Google Scholar]
- 29.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286:1195–1200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.