Abstract
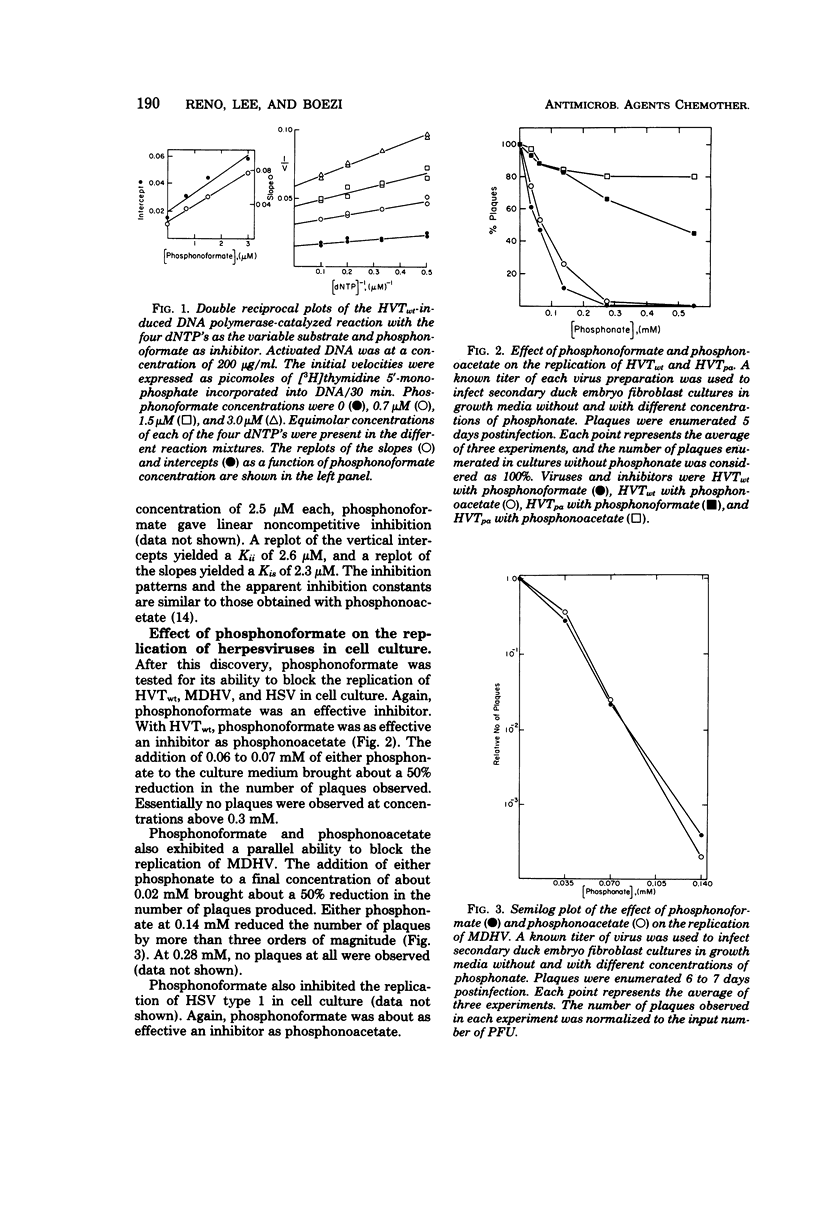
Phosphonoformate was found to be an inhibitor of the deoxyribonucleic acid polymerase induced by the herpesvirus of turkeys. The apparent inhibition constants were 1 to 3 μM. Phosphonoformate was also able to block the replication in cell culture of Marek's disease herpesvirus, the herpesvirus of turkeys, and herpes simplex virus. It was as effective as phosphonoacetate. Phosphonoformate was not an effective inhibitor of a phosphonoacetate-resistant mutant of the herpesvirus of turkeys nor of its induced deoxyribonucleic acid polymerase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- BANDURSKI R. S., AXELROD B. The chromatographic identification of some biologically important phosphate esters. J Biol Chem. 1951 Nov;193(1):405–410. [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- Fitzwilliam J. F., Griffith J. F. Experimental encephalitis caused by herpes simplex virus: comparison of treatment with tilorone hydrochloride and phosphonoacetic acid. J Infect Dis. 1976 Jun;133 (Suppl):A221–A225. doi: 10.1093/infdis/133.supplement_2.a221. [DOI] [PubMed] [Google Scholar]
- Gerstein D. D., Dawson C. R., O J. O. Phosphonoacetic acid in the treatment of experimental herpes simplex keratitis. Antimicrob Agents Chemother. 1975 Mar;7(3):285–288. doi: 10.1128/aac.7.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay J., Subak-Sharpe J. H. Mutants of herpes simplex virus types 1 and 2 that are resistant to phosphonoacetic acid induce altered DNA polymerase activities in infected cells. J Gen Virol. 1976 Apr;31(1):145–148. doi: 10.1099/0022-1317-31-1-145. [DOI] [PubMed] [Google Scholar]
- Herrin T. R., Fairgrieve J. S., Bower R. R., Shipkowitz N. L. Synthesis and anti-herpes simplex activity of analogues of phosphonoacetic acid. J Med Chem. 1977 May;20(5):660–663. doi: 10.1021/jm00215a008. [DOI] [PubMed] [Google Scholar]
- Huang E. S. Human cytomegalovirus. IV. Specific inhibition of virus-induced DNA polymerase activity and viral DNA replication by phosphonoacetic acid. J Virol. 1975 Dec;16(6):1560–1565. doi: 10.1128/jvi.16.6.1560-1565.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern E. R., Richards J. T., Overall J. C., Jr, Glasgow L. A. Genital Herpesvirus homonis infection in mice. II. Treatment with phosphonoacetic acid, adenine arabinoside, and adenine arabinoside 5'-monophosphate. J Infect Dis. 1977 Apr;135(4):557–567. doi: 10.1093/infdis/135.4.557. [DOI] [PubMed] [Google Scholar]
- Lee L. F., Boezi J. A., Blakesley R. W., Koenig M., Towle H. C. Marek's disease herpesvirus-induced DNA polymerase. J Virol. 1974 Nov;14(5):1209–1219. doi: 10.1128/jvi.14.5.1209-1219.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. F., Nazerian K., Leinbach S. S., Reno J. M., Boezi J. A. Effect of phosphonoacetate on Marek's disease virus replication. J Natl Cancer Inst. 1976 Apr;56(4):823–827. doi: 10.1093/jnci/56.4.823. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Robishaw E. E. Mode of inhibition of herpes simplex virus DNA polymerase by phosphonoacetate. Biochemistry. 1975 Dec 16;14(25):5475–5479. doi: 10.1021/bi00696a015. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Robishaw E. E., Overby L. R. Inhibition of DNA polymerase from herpes simplex virus-infected wi-38 cells by phosphonoacetic Acid. J Virol. 1975 May;15(5):1281–1283. doi: 10.1128/jvi.15.5.1281-1283.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overby L. R., Robishaw E. E., Schleicher J. B., Rueter A., Shipkowitz N. L., Mao J. C. Inhibition of herpes simplex virus replication by phosphonoacetic acid. Antimicrob Agents Chemother. 1974 Sep;6(3):360–365. doi: 10.1128/aac.6.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchase H. G. Immunofluorescence in the study of Marek's disease. I. Detection of antigen in cell culture and an antigenic comparison of eight isolates. J Virol. 1969 Jun;3(6):557–565. doi: 10.1128/jvi.3.6.557-565.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROIZMAN B., AURELIAN L. ABORTIVE INFECTION OF CANINE CELLS BY HERPES SIMPLEX VIRUS. I. CHARACTERIZATION OF VIRAL PROGENY FROM CO-OPERATIVE INFECTION WITH MUTANTS DIFFERING IN CAPACITY TO MULTIPLY IN CANINE CELLS. J Mol Biol. 1965 Mar;11:528–538. doi: 10.1016/s0022-2836(65)80008-0. [DOI] [PubMed] [Google Scholar]
- Shipkowitz N. L., Bower R. R., Appell R. N., Nordeen C. W., Overby L. R., Roderick W. R., Schleicher J. B., Von Esch A. M. Suppression of herpes simplex virus infection by phosphonoacetic acid. Appl Microbiol. 1973 Sep;26(3):264–267. doi: 10.1128/am.26.3.264-267.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers W. C., Klein G. Inhibition of Epstein-Barr virus DNA synthesis and late gene expression by phosphonoacetic acid. J Virol. 1976 Apr;18(1):151–155. doi: 10.1128/jvi.18.1.151-155.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter R. L., Nazerian K., Purchase H. G., Burgoyne G. H. Isolation from turkeys of a cell-associated herpesvirus antigenically related to Marek's disease virus. Am J Vet Res. 1970 Mar;31(3):525–538. [PubMed] [Google Scholar]


