Abstract
We have previously identified and purified multipotent mesenchymal stromal cell (MSC)-like cells in the highly regenerative endometrial lining of the human uterus (eMSC) as CD140b+CD146+ cells. Due to ease of accessibility with minimal morbidity via biopsy, we are proposing to use eMSC in cell-based therapies; however, culture conditions compliant with Good Manufacturing Practice have not been established for eMSC. The aim of this study was to optimize serum-free and xeno-free culture conditions for expansion of eMSC for potential clinical use. Real-time cell assessment (Xcelligence) and MTS viability assays were used to measure attachment and proliferation of freshly isolated, flow cytometry-sorted CD140b+CD146+ eMSC cultured in several commercially available and in-house serum-free and xeno-free media in combination with five attachment matrices (fibronectin, collagen, gelatin, laminin, and Cell Start-XF®). Comparisons were made with a standard serum-containing medium, DMEM/F-12/10% fetal bovine serum. Under all conditions examined, eMSC attachment and proliferation was greatest using a fibronectin matrix, with Lonza TP-SF® and our in-house DMEM/SF/FGF2/EGF serum-free xeno-product-containing medium similar to serum-containing medium. Hypoxia increased eMSC proliferation in the DMEM/SF/FGF2/EGF serum-free medium. Culture of eMSC for 7 days on a fibronectin matrix in DMEM/SF/FGF2/EGF serum-free media in 5% O2 maintained greater numbers of undifferentiated eMSC expressing CD140b, CD146, and W5C5 compared to culture under similar conditions in Lonza TP-SF medium. However, the percentage of cells expressing typical MSC phenotypic markers, CD29, CD44, CD73, and CD105, were similar for both media. EMSC showed greater expansion in 2D compared to 3D culture on fibronectin-coated microbeads using the optimized DMEM/SF/FGF2/EGF medium in 5% O2. In the optimized 2D culture conditions, eMSC retained CFU activity, multipotency, and MSC surface phenotype, representing the first steps in their preparation for potential clinical use.
Introduction
Mesenchymal stem cells, also known as multipotent stromal cells or mesenchymal stromal cells (MSC),1 were first discovered in the bone marrow as an adherent, clonogenic, nonhemopoietic cell population with capacity to undergo extensive proliferation in culture and differentiate into multiple mesodermal lineages.2 MSC have since been identified in adipose tissue and multiple human organs, including the pancreas, skeletal muscle, endometrium,3,4 and placenta,5–7 where they are postulated to generate connective tissue over the lifespan. Substantial evidence indicates that MSC are a subset of pericytes that line the blood vessels7,8 and secrete trophic and immunomodulatory factors in response to injury.9 MSC home to sites of tissue damage when infused intravenously, and secrete bioactive molecules that promote tissue repair, with little evidence of engraftment.10 Transplanted MSC act in a paracrine manner secreting large quantities of angiogenic, antifibrotic, antiapoptotic, immunosuppressive factors and other molecules inducing endogenous tissue-specific progenitor cell mitosis to promote cellular replacement, angiogenesis, and limit scarring, cell death, immunosurveillance, and chronic inflammation—processes that collectively repair damaged tissue.9 These important characteristics warrant MSC as a highly attractive cell source for regenerative medicine. Indeed, clinical trials using allogeneic bone marrow MSC for treatment of spinal cord injury, heart disease, stroke, and cartilage repair have already commenced.11,12
We have previously identified MSC-like cells in human endometrium,4,8 a highly regenerative tissue undergoing >400 cycles of growth, differentiation and shedding during a woman's reproductive years. We have demonstrated that a large, single human endometrial stromal colony forming unit (CFU) undergoes ∼30 population doublings yielding ∼6.1×1012 cells, can be subcloned or replated at clonal level at least three times, and differentiates into mesodermal lineages, smooth muscle, osteocytes, adipocytes, and chondrocytes in vitro4, indicating that endometrial stromal CFU possess key adult stem cell properties of self-renewal, differentiation, and high proliferative capacity. Endometrial MSC (eMSC) can be prospectively isolated from hysterectomy8 and endometrial biopsy13 tissues as CD140b+CD146+ cells, which have similar phenotype, proliferative capacity, and differentiation potential as other MSC. eMSC are enriched 10-fold in the CD140b+CD146+ subpopulation over freshly isolated endometrial stromal cells.8 These eMSC are found in a perivascular location, suggesting their role in endometrial repair and angiogenesis each menstrual cycle. Cultured stromal cells derived from endometrial biopsies also expressed CD140b and CD146 and differentiated into functional dopaminergic neurons14 and insulin-producing pancreatic cells,15 demonstrating the broad potential of eMSC for cell-based therapies.
Key advantages of using endometrial tissue as a source of MSC for cell-based therapies are the ease of accessibility via biopsy without the need for anesthetic, the lack of scarring, and the minimal pain incurred during their harvest. Therefore, we hypothesize that human eMSC are a readily available source of autologous cells that may provide a novel cell-based therapy for potential clinical applications. As eMSC comprise approximately 1% of endometrial stromal cells,4 it will be necessary to first expand these cells in culture to obtain sufficient numbers for clinical use. This requires serum-free and xeno-free (animal product-free) culture conditions compliant with Good Manufacturing Practice (GMP).16,17
A potential autologous application for eMSC is pelvic organ prolapse (POP), a major hidden disease burden affecting millions of women.18 POP is the herniation of the uterus, bladder, and/or bowel into the vagina, which can result in externalization of one or more these organs.19 The symptoms of POP include urinary and bowel incontinence and sexual dysfunction; 25% of all women have one or more symptoms of POP.20 The major risk factor for POP is vaginal delivery due to pelvic floor tissue damage after childbirth; however, aging, chronic constipation, chronic asthma, and obesity increase the risk of POP development.19 Current treatment for POP is reconstructive surgery, frequently involving augmentation with synthetic mesh.21 Nineteen percent of women have POP surgery, and of these, 15% will have further operations due to surgical failure or complications associated with mesh usage.22 We are currently designing tissue engineering constructs incorporating human eMSC into novel scaffolds as an autologous cell-based therapy to regenerate the lost and damaged fascia of the vaginal wall and provide support for the pelvic organs.23 In this study, we aim to optimize eMSC culture under serum-free or xeno-free conditions and scale up eMSC in 3D culture in preparation for potential clinical use in POP surgery.
Materials and Methods
Patient samples
Human endometrial tissue was collected from 38 premenopausal women aged 23–47 (35.9±1.2) years undergoing Pipelle biopsy (n=29) or hysterectomy (n=9) for nonendometrial pathologies and who had not taken exogenous hormones for 3 months before surgery. eMSC have been purified and characterized in both hysterectomy8 and biopsy 13 tissues. Written informed consent was obtained from each patient and human ethics approval was obtained from the Southern Health and Monash University Human Research Ethics committees. Tissues were collected via our departmental tissue bank.
Endometrial stromal cell isolation
Endometrial tissue from hysterectomy tissue was scrapped off from the underlying myometrial layer and dissociated to single cells using enzymatic and mechanical digestion as previously described.3,4 Biopsy tissue was similarly dissociated. Briefly, endometrium was finely minced and dissociated in Ca2+- and Mg2+-free DMEM/F-12/10% fetal bovine serum (FBS) medium (Invitrogen) containing 0.5% (wt/vol) collagenase type 1 (Worthington Biochemical Corporation) and 40 μg/mL deoxyribonuclease type 1 (Roche Diagnostics) in a rotating MacsMix (Miltenyi Biotech) at 37°C for 90 min. The cell suspension was then filtered using a 40-μm sterile sieve (Becton-Dickinson Labware) to separate stromal cells from epithelial gland fragments and undigested tissue. The stromal cells in the filtrate were resuspended in HEPES-buffered DMEM/F-12/5% newborn calf serum (Bench medium), erythrocytes removed by Ficoll-Paque (Pharmacia Biotechnology) density gradient centrifugation, and then washed to produce single cell suspensions of endometrial stromal cells.
Multicolor flow cytometry sorting to obtain eMSC
eMSC were obtained as previously described.8 Briefly, purified endometrial stromal cell suspensions (>5×106 cells/mL) were labeled with CD146 antibody (CC9 clone, IgG2a) supernatant24 (gift from P. Simmons; previously Peter MacCallum Cancer Centre), CD140b (PDGFR-β, 20 μg/mL, clone PR72 112, IgG1, R&D Systems), or isotype-matched negative control IgG2a and IgG1 for 30 min, followed by washing and then fluorescein isothiocynanate (FITC)-conjugated anti-mouse IgG2a (50 μg/mL) and phycoerythrin (PE)-conjugated anti-mouse IgG1 (10 μg/mL) (Becton Dickinson). Cells were then incubated with allophycocyanin (APC)-conjugated anti-CD45 (10 μg/mL; Caltag Laboratories), washed, and resuspended in 2% fetal calf serum/phosphate buffered saline (FCS/PBS) containing 5 μM SytoxBlue cell viability marker (Invitrogen).
Flow cytometry cell sorting (FACS) was then performed to isolate CD140b+CD146+ eMSC on a MoFlo® XDP cell sorter (Beckman Coulter) using established protocols for setting gates8 and Summit software (version 5.2; Cytomation, Inc.). Flow cytometry-sorted eMSC cells were cultured in DMEM/F-12/10%FBS medium containing 10 ng/mL human fibroblast growth factor-2 (FGF2) (Millipore) in fibronectin-coated flasks (10 μg/mL; Becton Dickinson Biosciences) for 2 passages to generate sufficient numbers for use in subsequent experiments as done previously.25 Cells subject to serum-free and xeno-free conditions were weaned off serum by transfer from 10%FBS to 5%FBS to 1%FBS-supplemented DMEM/F-12 media for 24–48 h each, and then serum-starved in DMEM/0.5% bovine serum albumin, AlbuMAX I (derived from BSE-free animals, Invitrogen) medium for 24 h before experimental use.
Xcelligence real-time cell assessment
The Xcelligence Real-time Cell Assessment System (Roche Diagnostics) dynamically monitors cell activity by measuring changes in impedance, detected by the electrode sensor surface of the wells.26 96-well Xcelligence E-plates (ACEA Biosciences, Roche Diagnostics) were coated with various attachment matrices; 10 μg/mL fibronectin (Becton Dickinson Biosciences), 0.05% gelatin in PBS (Sigma Aldrich), collagen type-IV (1:100 dilution; Roche, gift from Dr. Ursula Manuelpillai, Monash Institute of Medical Research, Melbourne, Australia), Cell Start-XF® (1:50 dilution; xeno-free matrix; Invitrogen), and laminin (1:50 dilution; Invitrogen gift from Dr. Terry Johns, Monash Institute of Medical Research, Melbourne, Australia) for 1 h at 37°C. Serum- and xeno-free media were added in 50-μL volumes to appropriate wells and equilibrated at room temperature for 15 min before a background reading was taken on the Xcelligence Real-Time Cell Assessment System. Media examined were Lonza Therapeak chemically defined serum-free medium containing human serum albumin (Lonza TP-SF®), DMEM/SF/FGF2/EGF in-house serum-free xeno product-containing medium with 10 ng/mL FGF2, 10 ng/mL epidermal growth factor (EGF), 0.5% BSA (AlbuMAX I, Invitrogen), ITS (10 μg/mL insulin, 5.5 μg/mL transferrin, 6.7 ng/mL sodium selenite, 11 μg/mL sodium pyruvate; Invitrogen), 50 μM 2-mercaptoethanol (Sigma-Aldrich), 100 μM L-ascorbic acid-2-phosphate (Sigma-Aldrich), 100 μg/mL heparin (Sigma-Aldrich), 10 nM linoleic acid (Sigma-Aldrich), 2 mM glutamine, and antibiotic-antimycotic solution as previously described),3 StemPro-XF® (xeno-free, Invitrogen), Mesencult-XF® (StemCell Technologies), and DMEM/F-12/10%FBS (Gibco Invitrogen) (standard serum medium containing 1% glutamine and antibiotics). Endometrial stromal cells or eMSC cell suspensions were seeded in triplicate for each condition into E-plate wells at 1000 cells/well in a final volume of 100 μL. Initial attachment and spreading was monitored continuously by measuring real-time cell index (CI) every 5 min for the first 2 h. Thereafter, CI was measured every 30 min for 7–10 days, with medium changes every 2–3 days. Rate of attachment (CI/h) and CI doubling time for proliferating cells (between second and third media change) were calculated using Xcelligence RTCA software (version 1.2; ACEA Biosciences, Inc.).
MTS proliferation assay
The MTS-based colorimetric assay measuring cell viability was used to assess cell proliferation as described previously.27 Endometrial stromal cells or eMSC cell suspensions were seeded into wells of a 96-well culture plate at 1000 cells/well in 100-μL volumes in triplicate for each condition and incubated in normoxia (20% O2, 5% CO2, 37°C) or hypoxia (5% O2, 5% CO2, 37°C) (Thermo Scientific Australia, Trigas incubator), MTS reagent (Cell Titer 96 Aqueous One Solution; Promega) was added 2 h after seeding (time 0) and then after 4, 7, and 10 days of culture, and incubated for 2 h. Absorbance (490 nm) was measured on a microplate reader (SpectraMax Plus384; Molecular Devices) using SoftMax Pro software (version 4.8; Molecular Devices). Regular medium changes were every 2–3 days.
Evaluation of eMSC phenotype cultured in optimized serum-free medium
Paired cultures of passage 3 eMSC were cultured in Lonza-TP-SF and our in-house DMEM/SF/FGF2/EGF serum-free xeno-product containing medium for 7 days until 80% confluency and lifted with TrypLE™ Express. The surface phenotype for typical MSC markers was examined by flow cytometry as described previously.8 Briefly, cells were incubated with antibodies against CD29 (1 μg/mL, clone mAb 13, rat IgG2a; Becton Dickinson), CD44 (1 μg/mL, clone G44-26, mouse IgG2b; Becton Dickinson), CD73 (20 μg/mL, clone AD2, mouse IgG1; Becton Dickinson), CD105 (10 μg/mL, clone 266, mouse IgG1; Becton Dickinson), CD146 (CC9 supernatant), and their respective isotype IgGs at the same concentration and followed by either Alexa Fluor 488-conjugated anti-rat IgG (10 μg/mL; Molecular Probes), anti-mouse FITC conjugated IgG2b (Dako), or PE-conjugated anti-mouse Ig F(ab’)2 fragment (10 μL/mL; Chemicon Australia). Some cells were incubated directly with PE-Cy5.5-conjugated anti-CD34 (50 μL/mL, clone 581, mouse IgG1; Southern Biotech), APC-conjugated anti-CD45 (as above), FITC-conjugated anti-CD90 (1 μg/mL, clone 5E10, mouse IgG1; Becton Dickinson), PE-CD140b (20 μg/mL, clone PR72112, IgG1, R&D Systems), or PE-anti-human W5C5 (BioLegend). Conjugated isotype-matched controls were included for each antibody. Cells were then incubated with 5 μM Flow SYTOX Blue and viable cells were analyzed on a MoFlo cytometer as described above.8
Evaluation of eMSC properties in optimized DMEM/SF/FGF2/EGF serum-free medium
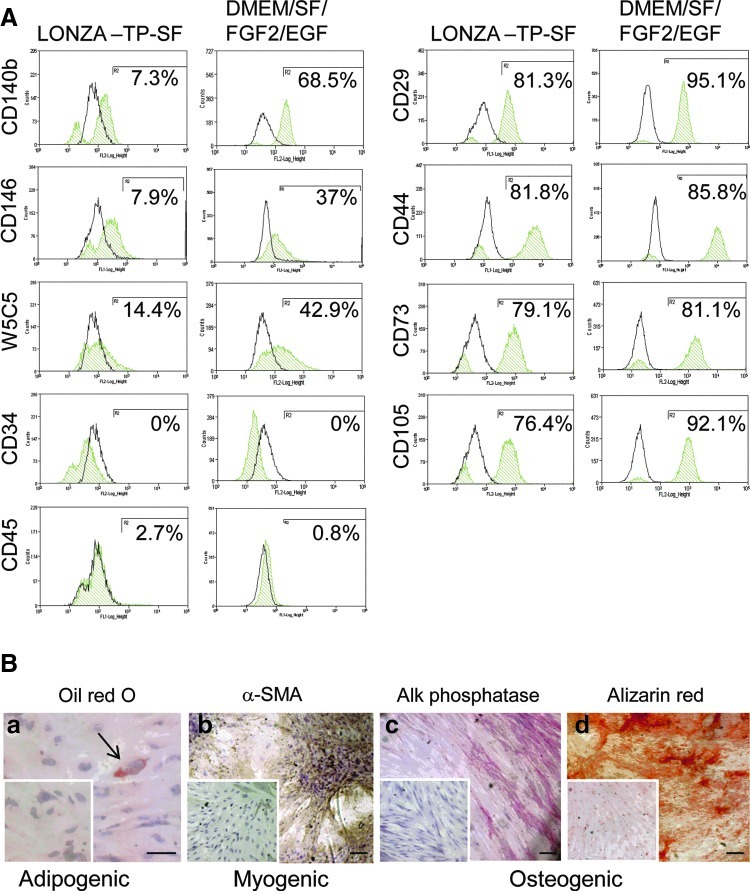
To assess CFU activity, passage 3 eMSC were cultured in DMEM/SF/FGF2/EGF serum-free xeno-product containing medium for 7 days until 80% confluency and were lifted with TrypLE Express. About 2000 cells were seeded at 100 cells/cm2 in fibronectin-coated 6-cm culture dishes for 15 days, and cloning efficiency was calculated from formalin-fixed, hematoxylin-stained plates as reported previously.3 To assess differentiation capacity, the remaining cells were cultured in standard adipogenic, myogenic, and osteogenic induction media and control medium (1% serum) on 13-mm coverslips for 3 weeks as described previously8 and were assessed by Oil Red O or 1% Alizarin Red (pH 4.1) staining or immunostained using an alkaline phosphatase kit (Sigma-Aldrich) or α smooth muscle actin (α SMA; 3.6 μg/mL, clone 1A4; Dako) for adipogenic, osteogenic, and myogenic differentiation, respectively. Stained cells were examined under an Olympus microscope (Olympus Corporation) and images were captured using a digital video camera (Fujix; Fuji).
3D culture of human eMSC
Human eMSC were cultured on CultiSpher-S gelatin beads (Percell Biolytica, Sweden)28 with and without fibronectin (50 μg/mL) coating in DMEM/SF/FGF2/EGF serum-free xeno product-containing medium and DMEM/F-12/10%FBS media. Cells were seeded at 500,000 cells/0.45 mL hydrated settled CultiSpher-S beads (75 mg dry) as described previously29,30 in 125-mL spinner flasks with a fluid level of 50 mL with intermittent stirring, 25 rpm for 2 min every 30 min for the first 21 h, and then stirred continuously at 25 rpm thereafter. Cell viability was assessed at various time points from 2 to 21 days using the Live/Dead® cell viability assay (Molecular Probes). Bead/cell suspensions were washed in warm PBS and incubated in the Live/Dead stain (2 μM calcein and 4 μM ethidium homodimer-1) for 15 min at 37°C. Live cells were stained green by Calcein-AM and dead cells red by ethidium homodimer-1. Viability was assessed using Nikon TE-2000U inverted fluorescence microscope. Cell production was determined by counting eMSC cultured for 6 and 12 days and harvested by Pronase (5 mg/mL, 6 min) (Calbiochem; EMD Biosciences) and counted by hemocytometer. For phenotyping, cells were harvested using xeno-free TrypLE Express (Gibco, Life Technologies) and then examined by flow cytometry.
Statistical analysis
Rate of stromal cell and eMSC attachment and CI doubling time (real-time Xcelligence data obtained using RTCA software) and MTS cell proliferation data were analyzed using GraphPad Prism software (version 5; GraphPad Software, Inc.). As data were not normally distributed by D'Agostino-Pearson test, nonparametric Friedman analysis and Dunn's post-hoc test were used to determine significant differences (p<0.05). Data are reported as means±SEM of n=3 experiments from three different patients' stromal cells or eMSC.
Results
Optimal serum-free and xeno-free culture conditions for human endometrial stromal cells
Unsorted endometrial stromal cells were initially cultured on five different matrices and uncoated plastic in six different culture media in a checkerboard manner using the Xcelligence system in order to identify key culture conditions for subsequent testing on eMSC. Our initial experiments focused on testing in-house serum-free medium previously used for eMSC clonal culture31 and several commercially available serum-free and xeno-free media produced under GMP conditions and designed for bone marrow MSC culture. The serum-containing medium3 was used as a comparator. Given that serum contains factors promoting cell attachment, it was necessary to identify the optimal matrix for cell attachment for use with serum-free and xeno-free media. Initial Xcelligence real-time experiments revealed that endometrial stromal cell attachment was most rapid for fibronectin-coated surfaces when cultured in each of the media examined, although this was only significant between the other surfaces for Lonza TP-SF and serum-containing medium (Supplementary Fig. S1A-a, b; Supplementary Data are available online at www.liebertpub.com/tec). Similar rates of stromal cell attachment and spreading on fibronectin were observed between StemPro-XF, Lonza TP-SF, and our in-house DMEM/SF/FGF2/EGF (Supplementary Fig. S1A-f). Stromal cells cultured in the xeno-free medium StemPro-XF showed a similar rapid attachment on the xeno-free matrix Cell Start XF as on fibronectin and gelatin (Supplementary Fig. S1A-c). Stromal cells showed poor rates of attachment to all matrices in Mesencult-XF medium (Supplementary Fig. S1A-d), and in general attached more rapidly on collagen than gelatin or laminin matrices and least on uncoated plastic in the various media, although these were not significantly different (Supplementary Fig. S1A).
We next investigated stromal cell proliferation in the various media. Xcelligence real-time cell assessment was used to measure proliferation as the CI doubling time, where the shortest doubling times indicate the most rapid cell proliferation, observed as the lowest positive value. In conditions where cells survived but did not proliferate or eventually detached and died, the CI doubling time was reported as zero.32 As expected, endometrial stromal CI doubling times were lowest (∼25 h) in serum-containing DMEM/F-12/10%FBS irrespective of matrix, indicating that rapid proliferation was promoted by the serum content of the medium (Supplementary Fig. S1B-a). Similar short CI doubling times were also observed on fibronectin coating for Lonza TP-SF (16.3±6.8 h) and in-house DMEM/SF/FGF2/EGF (28.4±12.4 h) media (Supplementary Fig. S1B-b, d), superior to all other matrix/media combinations (Supplementary Fig. S1B-e). In Lonza TP-SF medium, doubling times were considerably longer for collagen (119±11.2 h), gelatin (106±13.2), and laminin (109±1.4 h), but only significantly different from uncoated surfaces for gelatin (p<0.05). StemPro-XF only supported stromal cell proliferation on gelatin-coated (62.1±5.0 h) and collagen-coated (52.5±16.2 h) surfaces, but not on its partner matrix Cell Start-XF (Supplementary Fig. S1B-c). The CI doubling times for Mesencult XF were zero for all matrices (data not shown), indicating cell death and its failure to support longterm growth of endometrial stromal cells.
The MTS viability (endpoint) assay was used to verify the proliferation rates of freshly isolated human endometrial stromal cells in the same media and matrix combinations as for Xcelligence over a similar 7-day period. As shown in Supplementary Figure S1C, cells cultured in-house DMEM/SF/FGF2/EGF medium (Fig. 1C-e) and commercially available Lonza TP-SF medium (Fig. 1C-b) showed similar proliferation rates as serum-containing medium (DMEM/F-12/10%FBS) (Fig. 1C-a) on fibronectin coating (p>0.05). However, cells showed greater proliferation in the in-house DMEM/SF/FGF2/EGF medium on fibronectin (A490nm day 7, 2.9±0.6) compared with the uncoated surface (1.93±0.3) (p<0.05) (Supplementary Fig. S1C-e). Similar proliferation rates were also observed for endometrial stromal cells cultured in the Lonza TP-SF medium on Cell Start-XF, collagen, gelatin, and laminin matrices (p>0.05), and slightly lower on an uncoated surface, although this was not significant (Supplementary Fig. S1C-b). Low proliferation rates were observed for xeno-free StemPro-XF (p<0.05) (Supplementary Fig. S1C-c) and Mesencult-XF (p<0.05) (Supplementary Fig. S1C-d) on all matrices compared with serum-containing and serum-free Lonza TP-SF and in house DMEM/SF/FGF2/EGF media on their respective matrices.
FIG. 1.
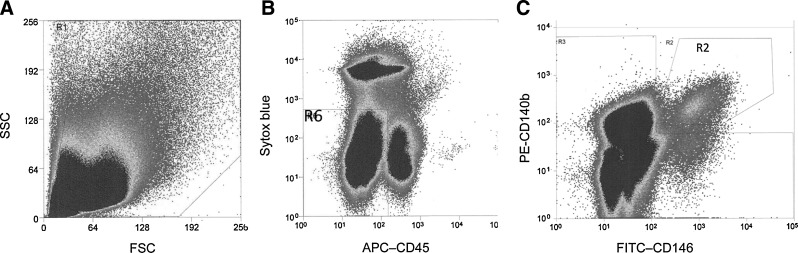
Flow cytometry sorting of human endometrial mesenchymal stromal cells (MSC). (A) Scatterplot of human endometrial cells. (B) Electronic gating used to obtain viable (Sytox Blueneg) and exclude CD45+ leukocytes. Region R6 was then analyzed for (C) coexpression of CD140 and CD146. Double-stained CD140b+CD146+ cells in R2 gate were then sorted.
Human eMSC attachment and spreading on various matrices
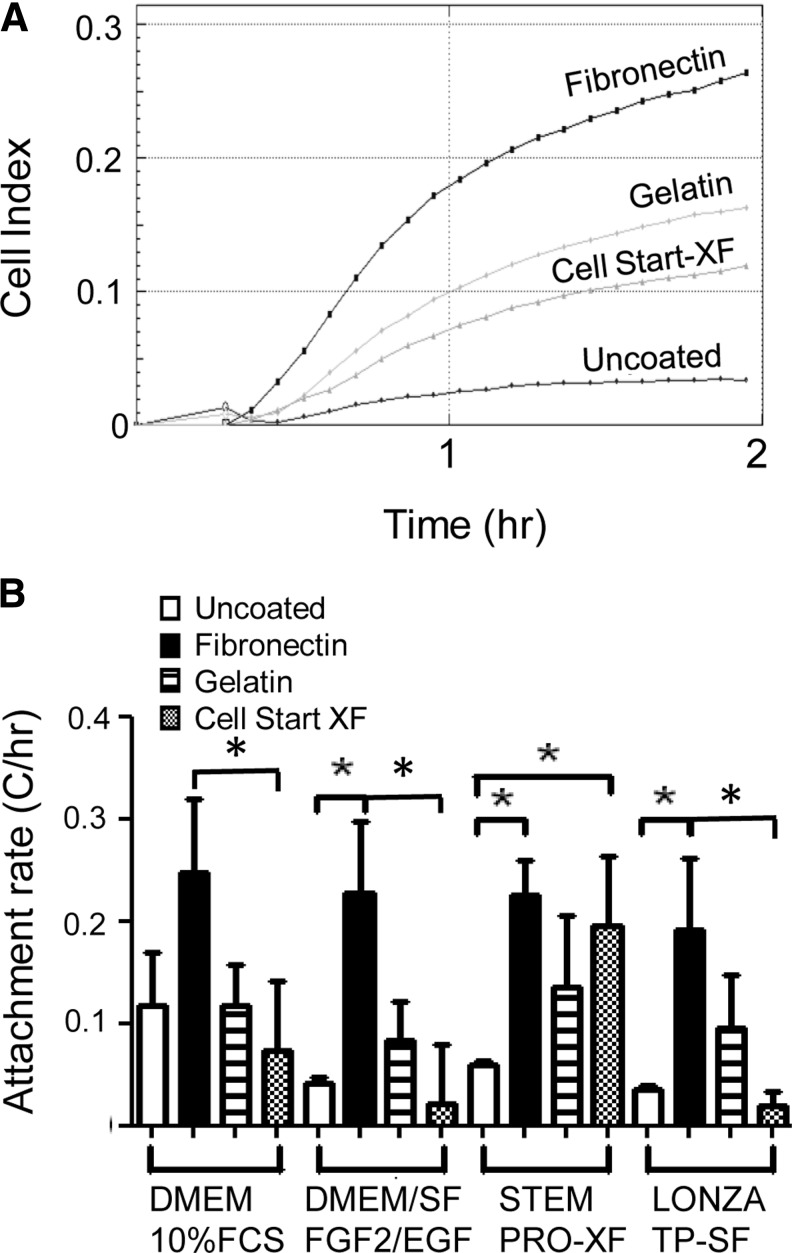
Having identified key culture conditions on unsorted stromal cells that contain a small population of eMSC, we then examined flow cytometry-sorted endometrial cells to obtain purified eMSC as previously published.8 Isolated stromal cells were first gated on size on the scatterplot (Fig. 1A), dead cells and CD45+ leukocytes then were excluded (Fig. 1B), and the CD140b+CD146+ double-positive eMSC (1%–1.5%) were sorted from each patient (n=12) (Fig. 1C) and expanded in culture. eMSC attachment and spreading was determined by Xcelligence real-time cell assessment. Figure 2A shows a representative real-time CI curve for the initial 2 h of culturing CD140b+CD146+ eMSC in the serum-containing medium (DMEM/10% FBS). The rate of eMSC attachment (CI/h) was significantly increased on fibronectin matrix (black bars) compared with culture on uncoated surfaces for in-house serum-free DMEM/SF/FGF2/EGF (fibronectin 0.23±0.07 vs. uncoated 0.042±0.006 p<0.05, n=3) and Lonza TP-SF serum-free media (fibronectin 0.19±0.07 vs. uncoated 0.036±0.003, p<0.05, n=3), and for StemPro-XF xeno-free medium (fibronectin 0.26±0.02 vs. uncoated 0.063±0.003, p<0.05, n=3) (Fig. 2B). There was a trend for a higher rate of eMSC attachment in the serum medium on uncoated surfaces (white bars) compared to serum-free and xeno-free media, likely due to fibronectin present in serum, but this was not significant (p>0.05) (Fig. 2B). Although eMSC consistently showed an apparent lower rate of attachment on gelatin-coated wells compared to fibronectin and appeared greater than uncoated surfaces, these differences were not significant (p>0.05) (Fig. 2B). However, eMSC attachment to fibronectin was significantly greater than to the xeno-free matrix, Cell Start-XF in all culture media examined (p<0.05) except for xeno-free medium, StemPro-XF. In fact, eMSC cultured in StemPro-XF showed similar rates of attachment on Cell start-XF as on fibronectin, and was significantly increased compared with no coating (Cell Start-XF 0.19±0.12 vs. uncoated 0.06±0.003) (p<0.05, n=3 separate isolates) (Fig. 2B).
FIG. 2.
Attachment of human endometrial MSC to various matrices when cultured in serum-containing, and serum- and xeno-free media measured using Xcelligence. (A) Representative real-time traces of CI in DMEM/10% FCS on four different matrices. (B) Rate of endometrial MSC (eMSC) attachment (CI/h). Data are mean±SEM (n=3 samples from three different patients). *p<0.05. CI, cell index; FCS, fetal calf serum; SF, serum-free, XF, xeno-free; FGF2, fibroblast growth factor 2; EGF, epidermal growth factor.
Human eMSC proliferation on several matrixes in various media
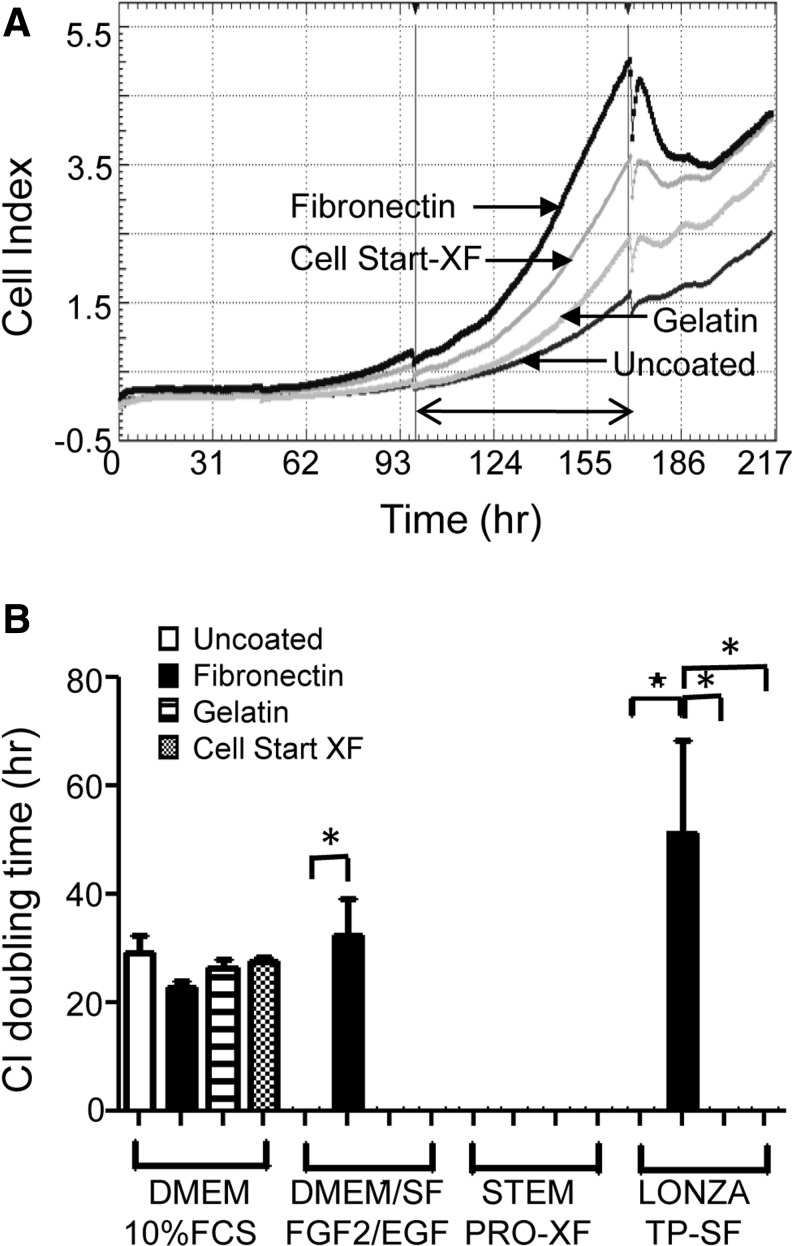
Having established that fibronectin was the best attachment factor for CD140b+CD146+ eMSC, we then investigated eMSC proliferation by determining the CI doubling times using Xcelligence real-time cell assessment. Figure 3A shows representative real-time CI traces over 7 days with repeated measurements every 30 min. Figure 3B shows that eMSC cultured in in-house DMEM/SF/FGF2/EGF (fibronectin 32.5±6.7 h vs. uncoated 0 h) and in Lonza TP-SF serum-free media (fibronectin 51.4±17.1 h vs. uncoated 0 h) proliferated significantly more on fibronectin compared with no coating (p<0.05, n=3) as indicated by the lowest positive CI doubling times. However, on gelatin and Cell Start-XF matrices, neither medium supported eMSC proliferation, nor did the StemPro-XF medium as indicated the zero CI doubling times, suggesting that under these conditions, there was a loss of cell viability as cells lifted off the surfaces (Fig. 3B). This was prevented in serum-containing medium (DMEM/F-12/10%FBS), which showed positive CI doubling times for eMSC on uncoated plastic, gelatin, and Cell Start-XF (Fig. 3B), suggesting that fibronectin in the serum contributed to this growth-enhancing effect.
FIG. 3.
Growth rate of human endometrial MSC cultured on various matrices in serum-containing, and serum- and xeno-free media measured using Xcelligence. (A) Real-time CI traces of a single representative sample of eMSC cultured in DMEM/10%FCS for over 200 h. Second and third media changes are observed by the interruption in the traces. (B) CI doubling time was calculated for the period between the second and third media change as shown by the arrow for DMEM/10%FCS, DMEM/SF/FGF2/EGF/Stem Pro-XF®, and Lonza TP-SF®. Lowest positive value is the most rapid doubling time. Zeros indicate loss of cell viability. Data are mean±SEM (n=3 samples from three different patients). *p<0.05.
eMSC proliferation in SF medium is enhanced under hypoxic culture conditions
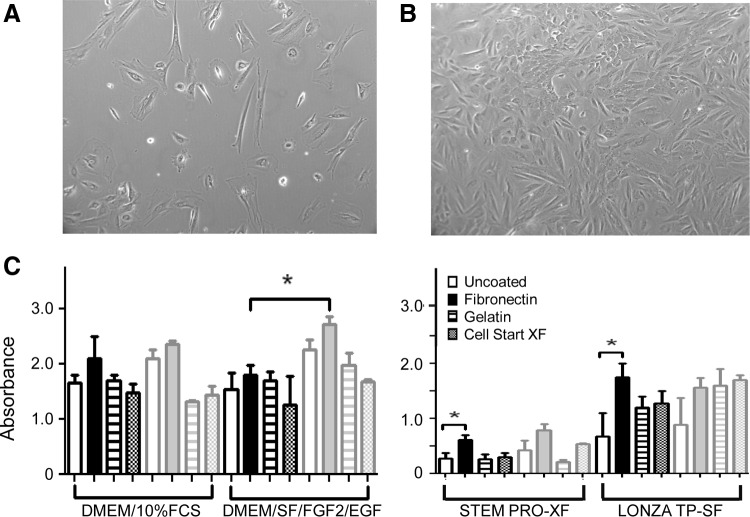
Xcelligence CI doubling time data for eMSC were next verified using MTS proliferation assays. The effect of hypoxia (5% O2) was also examined. Figure 4B shows that when CD140b+CD146+ eMSC were cultured in in-house DMEM/SF/FGF2/EGF serum-free medium in hypoxia, they grew more rapidly compared to typical normoxic (atmospheric) conditions (Fig. 4A). CD140b+CD146+ eMSC cultured for 7 days in the four media on three different matrices and uncoated surfaces showed significantly increased proliferation in the in-house DMEM/SF/FGF2/EGF SF medium on fibronectin in hypoxia compared with normoxia (hypoxia 2.72±0.19 vs. normoxia 2.17±0.26) (p<0.05, n=3 separate isolates) (Fig. 4C). eMSC proliferation also increased significantly in Lonza TP-SF and StemPro-XF media when cultured on fibronectin compared with uncoated surfaces in normoxia (p<0.05, n=3), although significance for these media was not observed when cultured under hypoxic conditions (Fig. 4C).
FIG. 4.
Effect of hypoxia on endometrial MSC proliferation in serum-free media. Endometrial MSC cultured in DMEM/SF/FGF2/EGF on fibronectin in (A) normoxia (20% O2) and (B) hypoxia (5% O2). (C) Proliferation of endometrial MSC cultured in serum-containing, serum-free, and xeno-free media on various matrices in hypoxia (gray bars) or normoxia (black bars) as measured by MTS viability assay on day 7. Data are mean±SEM (n=3 samples from three different patients). Values for each condition for each patient sample were means of triplicate wells. *p<0.05.
eMSC retain MSC properties and phenotype after culture expansion in optimized serum-free medium
Having identified optimal conditions for serum-free culture of eMSC, we next investigated the phenotype of passage 3 eMSC following 7 days culture in both Lonza TP-SF and our in house serum-free xeno-containing DMEM/SF/FGF2/EGF media on fibronectin-coated flasks in 5% O2. Figure 5A shows that the percentage of cells expressing the surface markers used for isolating eMSC from endometrial tissues, CD140b, CD1468, or W5C525, was consistently higher in the DMEM/SF/FGF2/EGF medium than in the Lonza TP-SF medium, indicating less spontaneous differentiation in the former. However, the MSC phenotype (CD29+, CD44+, CD73+, CD105+, CD34−, and CD45−) was similar for both serum-free media (Fig. 5A). Further evaluation of eMSC function was undertaken on eMSC cultured in the DMEM/SF/FGF2/EGF medium. The cloning efficiency of passage 3 eMSC was 1.32%±0.65% (n=5), which is similar to our previously published 1.25% for freshly isolated endometrial stromal cells cultured in serum-containing medium.3 Passage 3 eMSC previously cultured in serum-free DMEM/SF/FGF2/EGF medium for 7 days before differentiation induction media, retained capacity to differentiate into (Fig. 5B-a) adipocytes stained with the fat soluble dye, Oil Red O, (Fig. 5B-b) αSMA-expressing smooth muscle cells, and (Fig. 5B-c) alkaline phosphatase-expressing osteocytes, which generated mineral calcium as detected by Alizarin Red (Fig. 5B-d).
FIG. 5.
Phenotype and differentiation of eMSC cultured in serum-free medium. Passage 3 eMSC (n=2 separate samples) were each cultured in Lonza TP-SF and DMEM/SF/FGF2/EGF media on fibronectin-coated flasks in 5% O2 for 7 days and then (A) examined for surface phenotype by single-color flow cytometry for multiple MSC markers (green histograms are positive cells; black histograms are isotype controls), and (B) for differentiation potential by culture in (a) adipogenic, (b) myogenic, and (c) osteogenic media (controls were in 1% serum-containing medium) for 3 weeks. (a) Oil Red O was used to visualize fat droplets after adipogenic induction, ↑, adipocyte (b) αSMA immunohistochemistry for myogenic induction and (c) alkaline phosphatase and (d) Alizarin Red for osteogenic induction. Controls cultures shown as insets were similarly treated. Results are from a single-experiment representative of two independent experiments on two separate patient eMSC samples. Scale bars 100 μm. Color images available online at www.liebertpub.com/tec
eMSC 3D culture for scale-up cell production
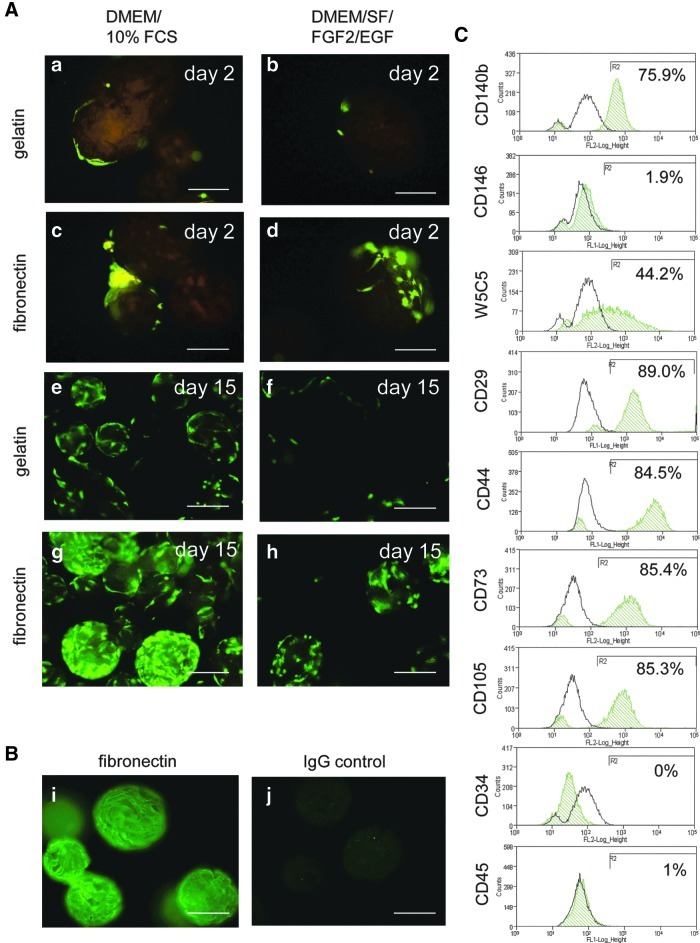
Culture expansion of cells in a 3D environment promotes cell–cell interactions resulting in rapid proliferation and cell production of a more physiological extracellular matrix (ECM).28 We therefore assessed the 3D culture of eMSC on matrix-coated microbeads. Given that eMSC culture was superior on fibronectin-coated surfaces, CD140b+CD146+eMSC were attached onto Cultispher-S gelatin beads with and without fibronectin coating and cultured in serum-containing DMEM/F-12/10%FBS (Fig. 6A-a, c, e, g) and serum-free DMEM/SF/FGF2/EGF media (Fig. 6A-b, d, f, h). Fibronectin coating was confirmed by antifibronectin immunohistochemistry (Fig. 6B-i, j). Live/Dead staining 2 days after seeding showed increased eMSC attachment and spreading on fibronectin-coated beads in both serum and serum-free media (Fig. 6A-c, d) compared with gelatin beads (Fig. 6A-a, b). Live/Dead stain on day 15 showed that eMSC underwent greater proliferation on fibronectin-coated than gelatin beads in DMEM/SF/FGF2/EGF medium (Fig. 6A-f, h). In DMEM/F-12/10%FBS, there was even greater eMSC proliferation on fibronectin (Fig. 6A-e, g).
FIG. 6.
3D culture of endometrial MSC on (A) gelatin- and fibronectin-coated microbeads in serum-containing (DMEM/10%FCS) (left panel) or serum-free (DMEM/SF/FGF2/EGF) (right panel) media. Endometrial MSC cultured for (a–d) 2 days on (a, b) gelatin or (c, d) fibronectin-coated microbeads, (e–h) 15 days on (e, f) gelatin or (g, h) fibronectin-coated microbeads. Cells are stained with Live/Dead® stain (2 μM calcein and 4 μM ethidium homodimer-1) to detect viable (green) and dead (red) cells. (B) Immunostained fibronectin-coated microbeads (i) with anti-fibronectin antibody (FITC) and (j) negative control IgG. Scale bars 100 μm. (C) After 12 days in culture, cells were recovered from the microbeads and examined for surface phenotype by single color flow cytometry for multiple markers (green histograms are positive cells, black histograms are isotype controls). Color images available online at www.liebertpub.com/tec
We next compared 2D and 3D culture of eMSC in our in house DMEM/SF/FGF2/EGF medium using passage 3 eMSC seeded onto fibronectin-coated flasks and Cultispher microbeads at similar seeding densities (2000 cells/cm2) and cultured for 12 days under hypoxic conditions (5% O2). Cells in the culture flasks were passaged once on day 6 when 80% confluent. The eMSC cultured in 2D underwent 6.4 PD (mean, n=2 samples) generating 27.3×106 (mean, n=2 samples) from an initial 0.32×106 cells. In comparison, paired cultures of eMSC cultured for the same time at the same initial seeding density (2000 cells/cm2) underwent of 2.2 PD (mean, n=2 samples) on fibronectin coated microbeads generating 3.53×106 (mean, n=2 samples) from an initial 0.5×106 cells, indicating a trend to greater expansion on 2D compared to 3D surfaces. While the phenotype of the eMSC cultured on the microbeads retained typical MSC markers, there was a reduction in the percentages of CD146+cells (Fig. 6C) compared with 2D culture (Fig. 5A center left panels), suggesting that 3D culture promoted fibroblast differentiation.
Discussion
Published guidelines33 recommend that stem cells for regenerative medicine applications should meet the following criteria: (1) harvested by minimally invasive procedures, (2) differentiated along multiple cell lineage pathways in a regulatable and reproducible manner, and (3) manufactured in accordance with current GMP guidelines (using serum-free and xeno-free (animal product-free) reagents). In compliance with these guidelines, eMSC are an attractive candidate for regenerative medicine applications. In this study we investigated the scale-up culture of eMSC in serum-free and xeno-free culture conditions on various matrices using high-throughput real-time Xcelligence screening of cellular function as a first step in defining GMP conditions for this novel and readily available source of MSC. We identified two serum-free conditions that promoted the most rapid adhesion and proliferation of eMSC, notably our in-house DMEM/SF/FGF2/EGF and commercially available Lonza TP-SF media when using a fibronectin matrix. Culture expansion of eMSC in serum-free media in physiological oxygen concentration was also identified as an important condition for optimal eMSC growth in the DMEM/SF/FGF2/EGF medium. Our studies identified fibronectin as the superior matrix and highly important for eMSC attachment and growth in both 2D and 3D culture expansion protocols. The commercially available Cell Start-XF matrix is unlikely to contain fibronectin as it failed to support eMSC attachment or growth in the range of media and matrices tested. Enhanced cell attachment through the use of attachment factors is particularly important in tissue engineering applications using biological or synthetic scaffolds aiming to incorporate and grow MSC for cell delivery to damaged tissues.
Importantly, we demonstrated that culture of CD140+CD146+ eMSC8 in our optimized serum-free xeno-product containing DMEM/SF/FGF2/EGF medium on fibronectin matrix in relative hypoxia (5% O2) retained their relative numbers, phenotype, cloning efficiency, and multilineage differentiation capacity. Expansion of eMSC was superior on 2D surfaces in culture flasks compared to 3D on microbeads, which appeared to promote eMSC fibroblast differentiation. Thus, we have now identified the first set of serum-free conditions enabling the culture expansion of human eMSC for use in tissue engineering applications.
Fibronectin is an important ECM protein that interacts with MSC integrins in their microenvironmental niche to regulate attachment, migration, proliferation, and differentiation.34 Fibronectin has key roles during development and in mediating wound repair. Interestingly, fibronectin and its major ligand α5β1 integrin are upregulated during monthly endometrial remodeling and repair,35 a process likely mediated by PDGFR-β activation.36 eMSC express high levels of PDGFR-β8 (this study) and are likely key cells mediating endometrial repair. It was therefore not surprising that fibronectin was identified as a critical factor in promoting eMSC attachment and proliferation in our in vitro culture protocols. Our data are consistent with a recent report on enhanced growth of human endometrial stromal cells encapsulated in alginate microbeads functionalized with multimeric fibronectin.37
As for all MSC, establishment of optimal ex vivo growth conditions for eMSC is an important prerequisite for their potential use in cell-based therapies, since low numbers retrieved from endometrium necessitates substantial ex vivo expansion. We have previously identified four growth factors, FGF2, EGF, TGFα, and PDGF-BB, that individually supported CFU activity of freshly isolated human endometrial stromal cells in serum-free media.31 However, CFU activity in these single growth factor media formulations was less than in the serum medium. In this study we therefore combined both EGF and FGF2 in our in-house DMEM/SF/FGF2/EFG serum-free medium and showed similar proliferative activity of endometrial stromal cells and flow cytometry sorted CD140b+CD146+ eMSC populations. However, endometrial stromal cells and eMSC proliferated poorly if at all in the two commercially available xeno-free media formulated for bone marrow MSC. Similarly umbilical cord MSC only proliferated in the Stem Pro XF® medium when 2% human serum was included, although the Mesencult-XF medium supported their growth.38 This indicates the importance in defining optimal media/matrix combinations compliant with GMP for ex vivo culture expansion of each MSC source.
Hypoxia is another important condition influencing ex vivo expansion of bone marrow-derived MSC. Similarly, we showed that in serum-free conditions (DMEM/SF/FGF2/EGF on fibronectin) eMSC proliferation and long-term viability in vitro was significantly enhanced under low oxygen (5% O2) compared with normoxia (20% O2). Similar findings were observed for human endometrial stromal side population cells, likely closely related to the eMSC population, which showed greater proliferation when cultured in hypoxia compared to normoxia.39 These findings are consistent with previous studies showing increased efficiency in bone marrow MSC expansion in hypoxic compared with normoxic conditions.40–42 Culture expansion of bone marrow MSC under hypoxic conditions mimicking in vivo tissue O2 tension promotes proliferation through alteration of cellular metabolism, maintaining CFU activity, self-renewal, and undifferentiated phenotypes, and prolonging MSC lifespan.40,41,43 The effect of hypoxia in preserving the progenitor phenotype and function of MSC is more pronounced in serum-free conditions compared to serum medium40 (this study). In conditions of low O2 tension, bone marrow MSC synthesize more fibronectin in both 2D and 3D culture42 as well as angiogenic and growth promoting growth factors (VEGF, FGF2, HGF, and IGF-1). It is possible that hypoxia induces similar beneficial effects on eMSC. Thus, culture of eMSC in hypoxia will be an important consideration for minimizing ex vivo expansion times and generating sufficient numbers of cells for clinical use.
This study also showed that eMSC can be scaled up in 3D culture in serum-free medium on fibronectin-coated beads consistent with 2D culture results. Culture expansion of MSC in 3D using matrix-coated beads is a well-established method for tissue engineering applications as it provides a biochemical and physiological microenvironment more similar to in vivo conditions than 2D monolayer culture.42,44,45 In particular, 3D culturing allows MSC to adopt their native morphology by facilitating cell–cell and cell–ECM interactions. Cell size decreases,45 cell signaling changes,44 and MSC surface antigen expression alters in a reversible manner45 and subsequent differentiation capacity of MSC is enhanced.44–46 Collectively, our data suggest that 2D culture expansion protocols in serum-free medium on fibronectin matrix under hypoxic conditions promotes eMSC viability, proliferation, and maintains the eMSC phenotype to a greater extent than 3D culture, pointing to some differences in MSC growth characteristics between bone marrow and eMSC in vitro.
MSC are increasingly being used as a cell-based therapy in a number of clinical applications for treating a range of degenerative and inflammatory diseases.11,12,16,47 MSC have been reported in preclinical studies to improve disease outcomes, including enhanced myocardial function after infarction, in repairing liver damage and lung damage.48–50 Bone marrow MSC are currently the most common source for clinical use.11,51 Extensive studies of bone marrow-derived MSC have proven their multipotent differentiation potential and powerful immunosuppressive qualities. However, the collection of bone marrow is an invasive procedure requiring anesthesia. It involves significant discomfort to the donor and often results in low MSC yields, although bone marrow may also be harvested during orthopedic surgery.52 The quality of these cells is also variable with respect to both expansion, differentiation potential, and age of donor.53,54 Similarly, adipose tissue MSC are obtained from an invasive liposuction procedure also requiring anaesthia.55 In contrast eMSC can be obtained from endometrial biopsy, a minimally invasive office-based procedure without anesthetic.56 Others have obtained eMSC from menstrual blood57–59 and demonstrated their potential in tissue engineering applications in repairing ischemic heart and skeletal muscle in animal models.57,58 eMSC have been purified and characterized in both hysterectomy8,25 and biopsy13,39,60,61 tissues. CD140b+ CD146+ cells purified from both hysterectomy25 and biopsy13 have similar MSC properties and their perivascular location spans both functionalis and basalis layers; these indicate that eMSC derived from these sources are indeed the same subpopulation. Thus, the highly regenerative human endometrium provides a novel, readily available source of MSC, obtainable with minimal morbidity for future cell-based therapies.56
The poor ability of eMSC to grow in several commercially available xeno-free media designed for bone marrow MSC culture (Mesencult-XF and Stem Pro-XF) suggests some differences between these two sources of MSC, possible related to key nutritional requirements. However, these may be relatively minor because eMSC grew well in the Lonza Therapeak SF® medium also formulated for bone marrow MSC, perhaps indicating eMSC may be more sensitive to xeno-free conditions. Nevertheless, eMSC show similar features to bone marrow-derived MSC for the minimal defining characteristics of MSC.62 It is possible that eMSC originate from bone-marrow derived MSC as these cells circulate in low numbers and there is some evidence that bone marrow MSC incorporate into damaged human endometrium.63 Bone marrow MSC can partially differentiate into endometrial decidual cells in vitro.64 Thus, differences in culture requirements between bone marrow and eMSC may be related to their respective microenvironments and their relative turnover in vivo. eMSC are recruited to replace 5–10 mm of endometrial stroma each month, while bone marrow MSC would be a relatively stable population.
Findings from this study indicate that our in-house DMEM/SF/FGF2/EGF serum-free xeno product-containing medium provided optimal conditions for expansion of eMSC in culture, although the commercially available, GMP-compliant Lonza TP-SF serum-free medium also supported expansion of eMSC. Interestingly, several of the commercially available xeno-free GMP-compliant MSC media used to expand bone marrow-derived MSC, failed to support eMSC attachment and growth. Therefore, one of the next steps is to develop our DMEM/SF/FGF2/EGF medium under GMP-compliant conditions in preparation for clinical use. To this end we will modify our isolation procedure by replacing reagents containing animal products with GMP-compliant reagents. We also plan to simplify the purification of eMSC using our recently discovered single marker, W5C525, and magnetic beads rather than flow cytometry sorting. We will then expand our selected eMSC population in our in house serum-free xeno-product containing DMEM/SF/FGF2/EGF medium in 2D on fibronectin-coated culture flasks in 5% O2 for at least four passages. In conclusion, this study represents the first steps in preparing eMSC for potential clinical use. Collectively, the findings warrant further investigation of eMSC and substantiate the potential clinical use of these cells in regenerative medicine applications for clinical unmet needs. In particular, autologous or allogeneic eMSC may be a novel cell source for tissue engineering applications for the treatment of common conditions affecting large numbers of women such as pelvic organ proplapse.23,56
Supplementary Material
Acknowledgments
The authors acknowledge Frances Walker and Pamela Mamers for collection of the tissue, and Dr. Mark Lawrence and Dr. Tony Lawrence from Waverley Private Hospital for the provision of hysterectomy tissue. This study was supported by the Australian Stem Cell Centre (SDF-05) (CEG, JW, AR), NHMRC Project grant (1021126) (CEG, JW, AR), NHMRC RD Wright Career Development Award 465121 (CEG), and Victorian Government's Operational Infrastructure Support Program.
Disclosure Statement
The authors indicate no potential conflicts of interest.
References
- 1.Nombela-Arrieta C. Ritz J. Silberstein L.E. The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol. 2011;12:126. doi: 10.1038/nrm3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedenstein A.J. Chailakhyan R.K. Latsinik N.V. Panasyuk A.F. Keiliss-Borok I.V. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Chan R.W.S. Schwab K.E. Gargett C.E. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70:1738. doi: 10.1095/biolreprod.103.024109. [DOI] [PubMed] [Google Scholar]
- 4.Gargett C.E. Schwab K.E. Zillwood R.M. Nguyen H.P.T. Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80:1136. doi: 10.1095/biolreprod.108.075226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D. Moorman M.A. Simonetti D.W. Craig S. Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 6.Zuk P.A. Zhu M. Ashjian P. De Ugarte D.A. Huang J.I. Mizuno H. Alfonso Z.C. Fraser J.K. Benhaim P. Hedrick M.H. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crisan M. Yap S. Casteilla L. Chen C.W. Corselli M. Park T.S. Andriolo G. Sun B. Zheng B. Zhang L. Norotte C. Teng P.N. Traas J. Schugar R. Deasy B.M. Badylak S. Buhring H.J. Giacobino J.P. Lazzari L. Huard J. Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Schwab K.E. Gargett C.E. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod. 2007;22:2903. doi: 10.1093/humrep/dem265. [DOI] [PubMed] [Google Scholar]
- 9.Caplan A.I. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prockop D.J. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther. 2009;17:939. doi: 10.1038/mt.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parekkadan B. Milwid J.M. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin I. Baldomero H. Bocelli-Tyndall C. Slaper-Cortenbach I. Passweg J. Tyndall A. The survey on cellular and engineered tissue therapies in Europe in 2009. Tissue Eng Part A. 2011;17:2221. doi: 10.1089/ten.tea.2011.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spitzer T.L. Rojas A. Zelenko Z. Aghajanova L. Erikson D.W. Barragan F. Meyer M. Tamaresis J.S. Hamilton A.E. Irwin J.C. Giudice L.C. Perivascular human endometrial mesenchymal stem cells express pathways relevant to self-renewal, lineage specification, and functional phenotype. Biol Reprod. 2012;86:58. doi: 10.1095/biolreprod.111.095885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolff E.F. Gao X.B. Yao K.V. Andrews Z.B. Du H. Elsworth J.D. Taylor H.S. Endometrial stem cell transplantation restores dopamine production in a parkinson's disease model. J Cell Mol Med. 2010;15:747. doi: 10.1111/j.1582-4934.2010.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santamaria X. Massasa E.E. Feng Y. Wolff E. Taylor H.S. Derivation of insulin producing cells from human endometrial stromal stem cells and use in the treatment of murine diabetes. Mol Ther. 2011;19:2065. doi: 10.1038/mt.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bieback K. Kinzebach S. Karagianni M. Translating research into clinical scale manufacturing of mesenchymal stromal cells. Stem Cells Int. 2011;2010:193519. doi: 10.4061/2010/193519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sensebe L. Bourin P. Mesenchymal stem cells for therapeutic purposes. Transplantation. 2009;87:S49. doi: 10.1097/TP.0b013e3181a28635. [DOI] [PubMed] [Google Scholar]
- 18.Delancey J.O. The hidden epidemic of pelvic floor dysfunction: achievable goals for improved prevention and treatment. Am J Obstet Gynecol. 2005;192:1488. doi: 10.1016/j.ajog.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 19.Jelovsek J.E. Maher C. Barber M.D. Pelvic organ prolapse. Lancet. 2007;369:1027. doi: 10.1016/S0140-6736(07)60462-0. [DOI] [PubMed] [Google Scholar]
- 20.Nygaard I. Barber M.D. Burgio K.L. Kenton K. Meikle S. Schaffer J. Spino C. Whitehead W.E. Wu J. Brody D.J. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300:1311. doi: 10.1001/jama.300.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deprest J. Zheng F. Konstantinovic M. Spelzini F. Claerhout F. Steensma A. Ozog Y. De R.D. The biology behind fascial defects and the use of implants in pelvic organ prolapse repair. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(Suppl 1):S16. doi: 10.1007/s00192-006-0101-2. [DOI] [PubMed] [Google Scholar]
- 22.Smith F.J. Holman C.D. Moorin R.E. Tsokos N. Lifetime risk of undergoing surgery for pelvic organ prolapse. Obstet Gynecol. 2010;116:1096. doi: 10.1097/AOG.0b013e3181f73729. [DOI] [PubMed] [Google Scholar]
- 23.Gargett C.E. Ye L. Endometrial reconstruction from stem cells. Fertil Steril. 2012;98:11. doi: 10.1016/j.fertnstert.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Filshie R.J.A. Zannettino A.W.C. Makrynikola V. Gronthos S. Henniker A.J. Bendall L.J. Gottlieb D.J. Simmons P.J. Bradstock K.F. MUC18, a member of the immunoglobulin superfamily, is expressed on bone marrow fibroblasts and a subset of hematological malignancies. Leukemia. 1998;12:414. doi: 10.1038/sj.leu.2400922. [DOI] [PubMed] [Google Scholar]
- 25.Masuda H. Anwar S.S. Buhring H.J. Rao J.R. Gargett C.E. A novel marker of human endometrial mesenchymal stem-like cells. Cell Transplant. 2012 doi: 10.3727/096368911X637362. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Atienza J.M. Zhu J. Wang X. Xu X. Abassi Y. Dynamic monitoring of cell adhesion and spreading on microelectronic sensor arrays. J Biomol Screen. 2005;10:795. doi: 10.1177/1087057105279635. [DOI] [PubMed] [Google Scholar]
- 27.Gargett C.E. Bucak K. Rogers P.A.W. Isolation, characterization and long-term culture of human myometrial microvascular endothelial cells. Hum Reprod. 2000;15:293. doi: 10.1093/humrep/15.2.293. [DOI] [PubMed] [Google Scholar]
- 28.Werkmeister J.A. Adhikari R. White J.F. Tebb T.A. Le T.P. Taing H.C. Mayadunne R. Gunatillake P. A. Danon S.J. Ramshaw J.A. Biodegradable and injectable cure-on-demand polyurethane scaffolds for regeneration of articular cartilage. Acta Biomater. 2010;6:3471. doi: 10.1016/j.actbio.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 29.Glattauer V. White J.F. Tsai W.B. Tsai C.C. Tebb T.A. Danon S.J. Werkmeister J.A. Ramshaw J.A. Preparation of resorbable collagen-based beads for direct use in tissue engineering and cell therapy applications. J Biomed Mater Res A. 2010;92:1301. doi: 10.1002/jbm.a.32468. [DOI] [PubMed] [Google Scholar]
- 30.Tebb T.A. Tsai S.W. Glattauer V. White J.F. Ramshaw J.A. Werkmeister J.A. Development of porous collagen beads for chondrocyte culture. Cytotechnol. 2006;52:99. doi: 10.1007/s10616-006-9034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwab K.E. Chan R.W. Gargett C.E. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril. 2005;84(Suppl 2):1124. doi: 10.1016/j.fertnstert.2005.02.056. [DOI] [PubMed] [Google Scholar]
- 32.Diemert S. Dolga A.M. Tobaben S. Grohm J. Pfeifer S. Oexler E. Culmsee C. Impedance measurement for real time detection of neuronal cell death. J Neurosci Methods. 2012;203:69. doi: 10.1016/j.jneumeth.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Gimble J.M. Katz A.J. Bunnell B.A. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindner U. Kramer J. Behrends J. Driller B. Wendler N.O. Boehrnsen F. Rohwedel J. Schlenke P. Improved proliferation and differentiation capacity of human mesenchymal stromal cells cultured with basement-membrane extracellular matrix proteins. Cytotherapy. 2010;12:992. doi: 10.3109/14653249.2010.510503. [DOI] [PubMed] [Google Scholar]
- 35.Cao W. Mah K. Carroll R.S. Slayden O.D. Brenner R.M. Progesterone withdrawal up-regulates fibronectin and integrins during menstruation and repair in the rhesus macaque endometrium. Hum Reprod. 2007;22:3223. doi: 10.1093/humrep/dem216. [DOI] [PubMed] [Google Scholar]
- 36.Veevers-Lowe J. Ball S.G. Shuttleworth A. Kielty C.M. Mesenchymal stem cell migration is regulated by fibronectin through α5β1-integrin-mediated activation of PDGFR-β and potentiation of growth factor signals. J Cell Sci. 2011;124:1288. doi: 10.1242/jcs.076935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Z. Kreiner M. Edrada-Ebel R. Cui Z. van der Walle C.F. Mardon H.J. Perfusion culture enhanced human endometrial stromal cell growth in alginate-multivalent integrin α5β1 ligand scaffolds. J Biomed Mater Res A. 2011;99A:211. doi: 10.1002/jbm.a.33177. [DOI] [PubMed] [Google Scholar]
- 38.Hartmann I. Hollweck T. Haffner S. Krebs M. Meiser B. Reichart B. Eißner G. Umbilical cord tissue-derived mesenchymal stem cells grow best under GMP-compliant culture conditions and maintain their phenotypic and functional properties. J Immunol Methods. 2010;363:80. doi: 10.1016/j.jim.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Cervello I. Gil-Sanchis C. Mas A. gado-Rosas F. Martinez-Conejero J.A. Galan A. Martinez-Romero A. Martinez S. Navarro I. Ferro J. Horcajadas J.A. Esteban F.J. O'Connor J.E. Pellicer A. Simon C. Human endometrial side population cells exhibit genotypic, phenotypic and functional features of somatic stem cells. PLoS One. 2010;5:e10964. doi: 10.1371/journal.pone.0010964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.dos Santos F. Andrade P.Z. Boura J.S. Abecasis M.M. da Silva C.L. Cabral J.M. Ex vivo expansion of human mesenchymal stem cells: a more effective cell proliferation kinetics and metabolism under hypoxia. J Cell Physiol. 2010;223:27. doi: 10.1002/jcp.21987. [DOI] [PubMed] [Google Scholar]
- 41.Fehrer C. Brunauer R. Laschober G. Unterluggauer H. Reitinger S. Kloss F. Gully C. Gassner R. Lepperdinger G. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007;6:745. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- 42.Grayson W.L. Zhao F. Izadpanah R. Bunnell B. Ma T. Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J Cell Physiol. 2006;207:331. doi: 10.1002/jcp.20571. [DOI] [PubMed] [Google Scholar]
- 43.Tamama K. Kawasaki H. Kerpedjieva S.S. Guan J. Ganju R.K. Sen C.K. Differential roles of hypoxia inducible factor subunits in multipotential stromal cells under hypoxic condition. J Cell Biochem. 2011;112:804. doi: 10.1002/jcb.22961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cukierman E. Pankov R. Stevens D.R. Yamada K.M. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 45.Frith J.E. Thomson B. Genever P.G. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng Part C Methods. 2010;16:735. doi: 10.1089/ten.TEC.2009.0432. [DOI] [PubMed] [Google Scholar]
- 46.Karlsen T.A. Mirtaheri P. Shahdadfar A. Floisand Y. Brinchmann J.E. Effect of three-dimensional culture and incubator gas concentration on phenotype and differentiation capability of human mesenchymal stem cells. J Cell Biochem. 2011;112:684. doi: 10.1002/jcb.22978. [DOI] [PubMed] [Google Scholar]
- 47.Brooke G. Cook M. Blair C. Han R. Heazlewood C. Jones B. Kambouris M. Kollar K. McTaggart S. Pelekanos R. Rice A. Rossetti T. Atkinson K. Therapeutic applications of mesenchymal stromal cells. Semin Cell Dev Biol. 2007;18:846. doi: 10.1016/j.semcdb.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 48.Silva G.V. Litovsky S. Assad J.A. Sousa A.L. Martin B.J. Vela D. Coulter S.C. Lin J. Ober J. Vaughn W.K. Branco R.V. Oliveira E.M. He R. Geng Y. J. Willerson J.T. Perin E.C. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 49.Lee K.D. Kuo T.K. Whang-Peng J. Chung Y.F. Lin C.T. Chou S.H. Chen J.R. Chen Y.P. Lee O.K. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatol. 2004;40:1275. doi: 10.1002/hep.20469. [DOI] [PubMed] [Google Scholar]
- 50.Ortiz L.A. Gambelli F. McBride C. Gaupp D. Baddoo M. Kaminski N. Phinney D.G. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003;100:8407. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Le B.K. Frassoni F. Ball L. Locatelli F. Roelofs H. Lewis I. Lanino E. Sundberg B. Bernardo M.E. Remberger M. Dini G. Egeler R.M. Bacigalupo A. Fibbe W. Ringden O. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 52.Suva D. Garavaglia G. Menetrey J. Chapuis B. Hoffmeyer P. Bernheim L. Kindler V. Non-hematopoietic human bone marrow contains long-lasting, pluripotential mesenchymal stem cells. J Cell Physiol. 2004;198:110. doi: 10.1002/jcp.10396. [DOI] [PubMed] [Google Scholar]
- 53.Mareddy S. Crawford R. Brooke G. Xiao Y. Clonal isolation and characterization of bone marrow stromal cells from patients with osteoarthritis. Tissue Eng. 2007;13:819. doi: 10.1089/ten.2006.0180. [DOI] [PubMed] [Google Scholar]
- 54.Bonab M.M. Alimoghaddam K. Talebian F. Ghaffari S.H. Ghavamzadeh A. Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006;7:14. doi: 10.1186/1471-2121-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoshimura K. Shigeura T. Matsumoto D. Sato T. Takaki Y. iba-Kojima E. Sato K. Inoue K. Nagase T. Koshima I. Gonda K. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol. 2006;208:64. doi: 10.1002/jcp.20636. [DOI] [PubMed] [Google Scholar]
- 56.Gargett C.E. Masuda H. Adult stem cells in the endometrium. Mol Hum Reprod. 2010;16:818. doi: 10.1093/molehr/gaq061. [DOI] [PubMed] [Google Scholar]
- 57.Cui C.H. Uyama T. Miyado K. Terai M. Kyo S. Kiyono T. Umezawa A. Menstrual blood-derived cells confer human dystrophin expression in the murine model of duchenne muscular dystrophy via cell fusion and myogenic transdifferentiation. Mol Biol Cell. 2007;18:1586. doi: 10.1091/mbc.E06-09-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hida N. Nishiyama N. Miyoshi S. Kira S. Segawa K. Uyama T. Mori T. Miyado K. Ikegami Y. Cui C.H. Kiyono T. Kyo S. Shimizu T. Okano T. Sakamoto M. Ogawa S. Umezawa A. Novel cardiac precursor-like cells from human menstrual blood-derived mesenchymal cells. Stem Cells. 2008;26:1695. doi: 10.1634/stemcells.2007-0826. [DOI] [PubMed] [Google Scholar]
- 59.Patel A.N. Park E. Kuzman M. Benetti F. Silva F.J. Allickson J.G. Multipotent menstrual blood stromal stem cells: isolation, characterization, and differentiation. Cell Transplant. 2008;17:303. doi: 10.3727/096368908784153922. [DOI] [PubMed] [Google Scholar]
- 60.Wolff E.F. Wolff A.B. Du H. Taylor H.S. Demonstration of multipotent stem cells in the adult human endometrium by in vitro chondrogenesis. Reprod Sci. 2007;14:524. doi: 10.1177/1933719107306896. [DOI] [PubMed] [Google Scholar]
- 61.Schuring A.N. Schulte N. Kelsch R. Ropke A. Kiesel L. Gotte M. Characterization of endometrial mesenchymal stem-like cells obtained by endometrial biopsy during routine diagnostics. Fertil Steril. 2011;95:423. doi: 10.1016/j.fertnstert.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 62.Dominici M. Le B.K. Mueller I. Slaper-Cortenbach I. Marini F. Krause D. Deans R. Keating A. Prockop D. Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 63.Taylor H.S. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA. 2004;292:81. doi: 10.1001/jama.292.1.81. [DOI] [PubMed] [Google Scholar]
- 64.Aghajanova L. Horcajadas J.A. Esteban F.J. Giudice L.C. The bone marrow-derived human mesenchymal stem cell: potential progenitor of the endometrial stromal fibroblast. Biol Reprod. 2010;82:1076. doi: 10.1095/biolreprod.109.082867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.