Abstract
STUDY QUESTION
Does adjudin disrupt chloride ion (Cl−) ion transport function in human sperm and impede sperm capacitation and fertilizing ability in vitro?
SUMMARY ANSWER
In this study the results indicate that adjudin is a potent blocker of Cl− channels: disrupting Cl− ion transport function results in a decline in sperm capacitation and fertilizing ability in humans in vitro.
WHAT IS KNOWN ALREADY
Although our previous studies have demonstrated that adjudin exerts its effect by disrupting sertoli-germ cell adhesion junctions, most notably apical ectoplasmic specialization by targeting testin and actin filament bundles that disrupts the actin-based cytoskeleton in sertoli cells, it remains unclear whether adjudin impedes Cl− ion transport function in the human sperm.
STUDY DESIGIN, SIZE AND DURATION
Semen samples were obtained from 45 fertile men (aged 25–32). Spermatozoa were isolated from the semen in the human tube fluid (HTF) medium by centrifugation through a discontinuous Percoll gradient, and incubated with adjudin at 10 nM–10 µM and/or other reagents under capacitating conditions for 0–5 h.
PARTICIPANTS/MATERIALS, SETTING, METHODS
We evaluated the effect of adjudin and different reagents on sperm functions with which they were incubated at 37°C. Sperm motility and hyperactivation were analyzed by a computer-assisted sperm analysis (CASA) system. Sperm capacitation and the acrosome reaction were assessed by chlortetracycline fluorescence staining. Sperm fertilizing ability was evaluated by sperm penetration of zona-free hamster egg assay, and cellular cAMP levels in spermatozoa were quantified by the EIA kit. The proteins tyrosine, serine and threonine phosphorylation in the presence or absence of adjudin were analyzed by means of a immunodetection of spermatozoa, especially, compared the effect of adjudin on sperm hyperactivation and capacitation in the complete HTF medium with the Cl−-deficient HTF medium as well as the various Cl− channel blockers.
MAIN RESULTS AND THE ROLE OF CHANCE
Adjudin significantly inhibited sperm hyperactivation but not sperm motility. Adjudin-induced inhibition of sperm capacitation was reversible, and it was found to block the rhuZP3β- and progesterone-induced acrosome reaction in a dose-dependent manner. Adjudin also blocked sperm penetration of zona-free hamster eggs, and significantly inhibited both forskolin-activated transmembrane adenylyl cyclase and soluble adenylyl cyclase activities leading to a significant decline in the cellular cAMP levels in human spermatozoa. Adjudin failed to reduce sperm protein tyrosine phosphorylation but it did prevent sperm serine and threonine protein phosphorylation. Interestingly, adjudin was found to exert its inhibitory effects on sperm capacitation and capacitation-associated events only in the complete Cl−-HTF medium but not Cl−-deficient medium, illustrating the likely involvement of Cl−. Adjudin inhibits the fertility capacity of human sperm is mediated by disrupting chloride ion and its transport function.
LIMITATIONS, REASONS FOR CAUTION
This study has examined the effect of adjudin only on human sperm capacitation and fertilizing ability in vitro and thus has some limitations. Further investigations in vivo are needed to confirm adjudin is a potent male contraceptive.
WIDER IMPLICATIONS OF THE FINDINGS
Our studies demonstrated that adjudin inhibition of capacitation is reversible and its toxicity is low, opening the door for the examination of adjudin as a mediator of male fertility control. Adjudin may be a safe, efficient and reversible male antifertility agent and applicable to initial clinical trials of adjudin as a male antifertility agent in humans.
STUDING FUNDING/COMPETING INTEREST(S)
This work was supported by the National Basic Research Program of China (2006CB504002), the Nature Science Foundation of China (Nos. 81000244 and 81170554), Zhejiang Project of Science and Technology (2011C23046), the Nature Science Fund of Zhejiang province (Nos.Y2100058 and Y2090236), the key Science and Technology Innovation Team of Zhejiang Province (No.2012R10048-07) and the National Institutes of Health (NICHD U54 HD029990 project 5), USA. The authors declare no conflict of interest.
Keywords: adjudin, human sperm, fertilizing capacity, chloride
Introduction
Earlier studies have shown that adjudin [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide, formerly called AF-2364], an analog of lonidamine [1-(2,4-dichlorobenzyl) 1H-indazole-3-caboxylic acid], is a potential male contraceptive (Cheng et al., 2011). Adjudin exerts its effect by disrupting Sertoli-germ cell adhesion junctions, most notably apical ectoplasmic specialization (apical ES, a testis-specific anchoring junction type), by targeting testin and actin filament bundles that disrupt the actin-based cytoskeleton in Sertoli cells (Mruk and Cheng, 2011), causing the release of spermatids prematurely from the seminiferous epithelium, thereby resulting in rat infertility (Cheng et al., 2001). However, the effects of adjudin on sperm fertilizing ability in vitro, in particular humans have yet to be evaluated.
It is known that ejaculated human spermatozoa must spend a period of time in the female genital tract or in appropriate artificial medium in vitro in order to gain the capacity to fertilize an egg. This phenomenon is termed capacitation (Yanagimachi, 1994; Visconti et al., 1998, 2011; Florman and Ducibella, 2006). Capacitated spermatozoa acquire the ability to bind specifically to the zona pellucida (ZP) of the ovum and undergo the acrosome reaction (Liu and Baker, 1994). The acrosome reaction is an exocytosis event induced by progesterone or the ZP (Roldan et al., 1994; Yanagimachi, 1994). The acrosome reaction is crucial for successful sperm binding and penetration of the ZP, and is followed by plasma membrane fusion with the egg (Florman and Storey, 1982; Wassarman, 1987). The development of in vitro procedures for assessing the fertilizing capacity of human spermatozoa has been greatly enhanced due to wide-spread application of IVF for therapy of infertility (Yanagimachi et al., 1976; Steptoe and Edwards, 1978; Aitken et al., 1983; Trounson and Webb, 1984).
It was reported that AF-2785 [1-(2,4-dichlorobenzyl)-1H-indazole-3-acrylic acid], another analog of lonidamine, and a potent blocker of CFTR (cystic fibrosis transmembrane conductance regulator), plays an important role in male reproduction by impeding the secretion of chloride ions from the epithelial cells in the rat epididymis (Gong et al., 2000, 2002; Gong and Wong, 2000). It remains to be determined if adjudin inhibits the CFTR function leading to the reduction in sperm-fertilizing ability. CFTR acts as a cAMP-activated chloride (Cl−) channel, which plays an important role in male reproductive function (Wong, 1998). Besides CFTR Cl− channels in the sperm, four structural Cl− channel families have been identified to date: (i) Cl−-selective ion channels (Estevez et al., 2003); (ii) the GABAA receptor/Cl− channels (Shi and Roldan, 1995); (iii) Ca2+-activated Cl− channels (Orta et al., 2012) and (iv) Na+/K+/Cl− co-transporter channels (Wertheimer et al., 2008). As with other Cl− channels in the sperm, CFTR is regulated by pharmacological agents, such as forskolin and bicarbonate, which may activate separately the transmembrane adenylyl cyclases (tmACs) and soluble adenylyl cyclase (sAC) activities, leading to an increase in the intracellular cAMP levels and subsequent sperm capacitation and the acrosome reaction (Leclerc and Kopf, 1995; Scholich et al., 1997; Buck et al., 1999; Chen et al., 2000; Baxendale and Fraser, 2003). There is evidence for tmAC activity in the head region of capacitated mouse sperm in bicarbonate free-human tube fluid (HTF) medium (Liguori et al., 2004). Recent studies have shown that CFTRinh-172, a specific CFTR channel protein blocker (Muanprasat et al., 2004), completely blocked sperm capacitation and male fertility (Xu et al., 2007; Chen et al., 2009; Li et al., 2010). Since adjudin is an analog of lonidamine, the question arises whether adjudin inhibits human sperm fertilizing capacity in vitro, which is mediated via changes in Cl− transport. We thus sought to assess the effects of adjudin on human sperm fertilizing ability at a non-toxic dose, and to delineate its possible mechanism of action in vitro.
Materials and Methods
Reagents
Adjudin was provided by Prof. C. Yan Cheng. CFTRinh-172 was purchased from Calbiochem, Inc. (La Jolla, CA, USA; catalog no. 219670). Chlortetracycline (CTC), l-cysteine, BSA (Fraction V, fatty acid free), Hoechst 33258, sodium pyruvate, sodium l-lactate, progesterone, forskolin, pentoxifylline, dibutyryl cyclic AMP (dbcAMP), paraformaldehyde, hyaluronidase, DPC (diphenylamine-2-carboxylate), DIDS (4′4,diisothiocyano-stilbene-2′,2,-disulfonic acid), gluconate, ethylene glycol tetraacetic acid, ATP, 3-isobutyl-1-methyl xanthine, bicuculline and pronase were purchased from Sigma-Aldrich (St Louis, MO, USA). Dimethyl sulfoxide was obtained from Merck (Darmstadt, Germany). PercollTM was purchased from GE Healthcare BioSciences AB (Uppsala, Sweden). Anti-phosphotyrosine (PY) monoclonal antibody, rabbit anti-phosphoserine (PS) antibody and rabbit anti-phosphothreonine (PT) antibody were ordered from Invitrogen (Camarillo, USA). β-tubulin antibody (ab6046) was obtained from Abcam, Inc. (MA, USA). Recombinant human ZP3β (rhuZP3β) was a gift from Prof. Xu, Wan Xiang, Shanghai Institute of Planned Parenthood Research. Chemical reagents of analytical grade were purchased from Shanghai Chemical Reagent Co. (Shanghai, China). The cAMP enzyme immunoassay (EIA) kit was purchased from GE Healthcare (Little Chalfont, Buckinghamshire, UK, Catalog no. RPN 225).
Media
The media used throughout this study were HTF medium and BWW (Biggers–Whitten–Whittingham) medium. HTF (100 ml) medium consisted of 90.0 mM NaCl, 4.96 mM KCl, 1.80 mM CaCl2 2H2O, 6.98 mM MgCl2, 25.05 mM NaHCO3, 10.0 mM glucose, 10.0 mM sodium lactate, 0.27 mM sodium pyruvate, 1.17 mM KH2PO4, 2.0 mM HEPES, 400 mg BSA (fraction V, fatty acid-free) and 6 mg penicillin G (hereafter referred to as complete medium). For the HEPES-free HTF medium, an additional 58.5 mg NaCl was used per 100 ml medium in order to obtain a final osmolality of 300–305 mOsm/kg and pH 7.5 at room temperature (25°C). The complete Cl−-HTF medium contained 110.2 ± 5.5 mM Cl−; it was quantified by using a chloride-selective electrode (Roche Co., USA). All the Cl−, including NaCl, KCl, MgCl2 and CaCl2, in the HTF medium was replaced with gluconic salt to yield Cl−-deficient HTF medium (Cl−-DF HTF). Cl−-DF HTF medium contained 26.0 ± 0.4 µM Cl− as measured previously described by Chen et al. (2009). Preparation of the BWW medium was according to Biggers et al. (1971).
Collection and preparation of spermatozoa
Semen samples were obtained from 45 fertile men (aged 25–32), who were examined according to the World Health Organization (1999) standard andrology criteria, with the approval of the Human Medical Ethics Committee of the Zhejiang Academy of Medical Sciences. Only samples with motility ≥85%, viability ≥90% and sperm concentration ≥40 × 106 cells/ml were used. The sperm were isolated from the semen in the HTF medium by centrifugation at 600g for 15 min through a discontinuous Percoll gradient (45–90%) containing 4 mg/ml BSA. The sperm were washed and preincubated in the HTF medium with 2.5 µM progesterone and various concentrations of adjudin (10 nM–10 µM) except the negative control. Sperm were incubated for 5 h in a humidified CO2 incubator at 37°C under 5% CO2 /95% air (V/V). Sperm concentration was adjusted to 20–30 × 106 cells/ml. Sperm viability at this stage was 90–95%, as estimated using a trypan blue exclusion test.
Analysis of sperm motility and hyperactivation by CASA
Sperm motility and hyperactivation were analyzed by a CASA system, namely the Hamilton Throrne Research Motility Analyzer (HTM-IVOS with software Version 12.3N, Beverly, MA, USA), as previously described by Mortimer et al. (1998); Mortimer (2000) and Li et al. (2010). After incubation for 1 , 3 and 5 , a 5 μl aliquot of sperm suspension of each sample was pipetted into a microcell slide chamber with a depth of 20 μm, prewarmed to 37°C (Leja Products B.V., GN Nieuw Vennep, The Netherlands). The slide was then placed onto a warm stage (37°C) attached to an Olympus BH-2 compound microscope (Olympus Optical Co. Ltd, Tokyo, Japan) and analyzed under pseudo-dark-field illumination. For each sample, 10 randomly selected fields containing >200 moving sperm cell tracks were examined at 60 Hz. Curvilinear velocity (VCL), straight-line velocity (VSL), average path velocity (VAP), amplitude of lateral head displacement (ALH), linearity % (LIN, VSL/VCL multiplied by 100) and the percentage of motility were recorded. Spermatozoa were designated as hyperactive motility if they had a VCL ≥150 μM/s, ALH ≥7.0 μM and LIN ≤50%.
Evaluation of capacitation and the acrosome reaction monitored by CTC fluorescent staining
Sperm capacitation and the acrosome reaction in human spermatozoa were assessed by CTC fluorescence staining as previously described by DasGupta et al. (1993), Fraser et al. (1995), Shi et al. (1997) and Li et al. (2010). This conventional method for assessing sperm capacitation and the acrosome reaction is based on the alteration in staining patterns of the sperm head during capacitation process. To examine the effect of adjudin on human sperm capacitation, washed sperm were pre-incubated for 5 h with or without various concentrations of adjudin (10 nM–10 μM) and 2.5 μM progesterone, except the negative control, under capacitating conditions. Sperm suspensions were washed with the fresh HTF medium and then stimulated with 15 μM P4, except the negative control, for 15 min before the evaluation of sperm acrosomal status. The degree of spermatozoa capacitation was evaluated by the incidence of including ‘B’ pattern or ‘B’ plus ‘AR’ patterns by the spontaneous or the inducers with progesterone and/or pentoxifylline. According to DasGupta et al. (1993), Rathi et al. (2001), Shi et al. (1997) and Li et al. (2010), the sum of ‘B’ pattern or ‘AR’ patterns or the sum of ‘B’+’AR’ patterns is taken as an indicator of sperm capacitation in vitro. To assess the effect of adjudin on human acrosome reaction, sperm were preincubated for 5 h in the adjudin-free HTF medium under capacitating conditions and then washed with the fresh HTF medium. The washed spermatozoa were exposed to adjudin at 10 nM–10 μM for 15 min and then stimulated for 15 min with 20 μg/ml rhuZP3β or 15 μM progesterone, except the negative control, before the assessment of the acrosome reaction (Ni et al., 2007; Li et al., 2010). Samples were then immediately fixed by adding an equivalent amount of 8% glutaraldehyde and mixed gently for 5 min at 37°C. Sperm were stained with CTC for 10 min at 37°C and then stained with a vital dye, Hoechst 33258 (final concentration at 1 μg/ml), for 2 min in the dark at 37°C to discriminate between the living (negative staining) and dead cells (positive staining). Only the samples with Hoechst 33258-negative staining, i.e. living spermatozoa, were counted to assess capacitation and the acrosome reaction by acrosomal staining status. Samples were immediately examined at ×1000 using a fluorescence and phase-contrast microscope. An excitation beam that passed through a 380 nm and DM 420 nm fluorescence emission (Nikon 80i, Tokyo, Japan) was used in each sample. A total of 200 cells were assessed by two individuals.
Evaluation of human sperm penetration of zona-free hamster egg assay (SPA)
Hamster oocytes were obtained by superovulation as previously described (Shi et al., 1991; Li et al., 2010). Sperm fertilizing ability was evaluated by SPA as earlier described (Yanagimachi et al., 1976; Aitken et al., 1983; Shi et al., 1989, 1991). To determine whether adjudin prevents the fusion of sperm with zona-free hamster egg, washed spermatozoa were preincubated with or without adjudin 10 μM in the HEPES-free HTF medium for 5 h under capacitating conditions. Zona-free hamster eggs in the HEPES-free HTF medium were introduced separately into sperm suspensions (50:50 μl, eggs: spermatozoa ≥1.5–2.0:1000) and co-incubated for 3 h. After co-incubation, eggs were rinsed three times with HEPES-free HTF medium, transferred to a slide and compressed carefully under a coverglass supported at its corners with drops of paraffin wax–vaseline mixture (1:9, V/V). To rule out a possible direct role of adjudin on zona-free hamster egg, these eggs were preincubated for 0.5 h with or without 10 μM adjudin under capacitating conditions. Thereafter the eggs were washed two times to remove adjudin and then co-incubated for 3 h with capacitated spermatozoa that had been preincubated in the adjudin-free HTF medium for 5 h under capacitating conditions. The eggs were examined by phase-contrast microscopy (×400) for the presence of a decondensed sperm head with its tail within the ooplasm as evidence of fertilization. The penetration rate of eggs by spermatozoa is taken as the sperm fertilization capacity (Shi et al., 1989; Li et al., 2010).
Extraction of spermatozoa and measurement of cAMP levels
Cellular cAMP levels in spermatozoa were measured by the EIA kit as previously described (Xu et al., 2007; Chen et al., 2009). Briefly, spermatozoa were separately incubated in the HTF medium for 0 and 5 h with or without 10 µM forskolin in the presence or absence of 10 µM adjudin in the complete HTF medium under capacitating conditions. At the end of the incubation, sperm were centrifuged for 20 min (150g) at room temperature. Sperm suspensions (50 µl containing ∼2 × 106 cells) were separately added to an equal volume of the same medium. Incubations were terminated by the addition of five volumes of ice-cold 100 mM HCl in 100% ethanol. Samples were kept on ice for 30 min and then were lyophilized and assayed for cAMP levels.
Immunodetection of protein tyrosine, serine and threonine phosphorylation
Spermatozoa were incubated separately at 5 min and 5 h under capacitating conditions, washed twice with PBS and protein extracted in SDS lysis buffer. The proteins were separated by SDS-PAGE (10% acrylamide gel) and subsequently transferred to Immobilon-P (Millipore Corporation, Bedford, MA, USA) membrane. The membrane was blocked with 3% BSA. Immunodetection of phosphorylated proteins were carried out using PY, PS or PT antibodies as described previously (Visconti et al., 1995a,b; Naz, 1999; Chen et al., 2009). Proteins were detected using an enhanced chemiluminescence kit (Pierce Biotechnology, Rockford, USA) according to the manufacturer's instructions. After the phosphorylated proteins were detected, the same membrane was stripped. β-tubulin antibody (1 μg/ml) was used to re-immunoblot. The molecular weights of the detected proteins were deduced in comparison with a prestained protein ladder (Fermentas, Thermo Fisher Scientific, Inc., Burlington, USA). The gray intensity was quantified by Image J software (http://imagej.nih.gov/ij).
Effect of adjudin on capacitation elicited by forskolin, pentoxifylline or progesterone
Forskolin is an activator of transmembrane adenylate cyclases (tmACs) and directly stimulates the enzyme activity, and in turn increases intracellular cAMP levels leading to sperm capacitation (Leclerc and Kopf, 1995; Scholich et al., 1997; Fraser et al., 2003; Insel and Ostrom, 2003). Pentoxifylline is an inhibitor of phosphodiesterase, which suppresses cAMP degradation thereby increasing cAMP production that leads to sperm capacitation (Dasgupta et al., 1993). To examine whether adjudin inhibits forskolin- and pentoxifylline- or progesterone-elicited sperm capacitation, washed sperm were preincubated with or without 10 µM forskolin and 3.6 μM pentoxifylline or 2.5 μM P4 and/or 10 µM adjudin, 1 μM CFTRinh-172 or 10 µM bicuculline (an antagonist of GABAA receptor/Cl− channel) for 5 h under capacitating conditions and sperm capacitation was evaluated using CTC fluorescent staining.
The role of adjudin or different Cl− channel blockers on sperm capacitation
One of the upstream targets of cAMP is the CFTR Cl− which acts as a cAMP-activated Cl− channel and is present both in mature mouse and human sperm (Hernandez-Gonzalez et al., 2007; Xu et al., 2007; Li et al., 2010). Thus, the question arises as to whether adjudin inhibition of human sperm capacitation disrupts the major conductance of CFTR Cl−, which may also play a role leading to the reduction in sperm capacitation. Therefore, (i) to investigate whether the Cl− ion and its channels are involved in adjudin inhibition of sperm capacitation, washed sperm were preincubated for 5 h in the complete Cl−-HTF or Cl−-DF HTF medium in the presence of 10 μM adjudin and/or 2.5 μM progesterone, except the negative control, under capacitating conditions and the percentage of sperm capacitation was evaluated as mentioned above. (ii) To further explore whether the requirement for Cl− is mediated by cAMP when sperm capacitation was inhibited by adjudin, sperm were incubated for 1–5 h under capacitating conditions in the HTF medium with or without Cl− and in the presence or absence of cAMP agonist, dbcAMP (1 mM) or/and adjudin (10 μM) and then evaluated for sperm capacitation as described above. (iii) To further determine whether adjudin and various structural Cl− channels blockers exert their effects on sperm capacitation with different sensitivities, sperm were incubated separately for 5 h with or without the various transport blockers of Cl− channels and adjudin in the complete HTF medium. Among them, CFTR-172 (1 µM), adjudin (10 µM), DPC (10 µM), DIDS (10 µM) and bicuculline (10 μM) were tested to understand if sperm capacitation is affected differently by various Cl− transport blockers.
Statistical analysis
Results are expressed as the means ± SEM. For statistical analysis, two-tail Student's t-tests were used. For three or more groups, data were analyzed by one-way ANOVA and Dunnett's post-hoc test. A probability of P < 0.05 was considered to be statistically significant.
Results
Effects of adjudin on human sperm motility and hyperactivation
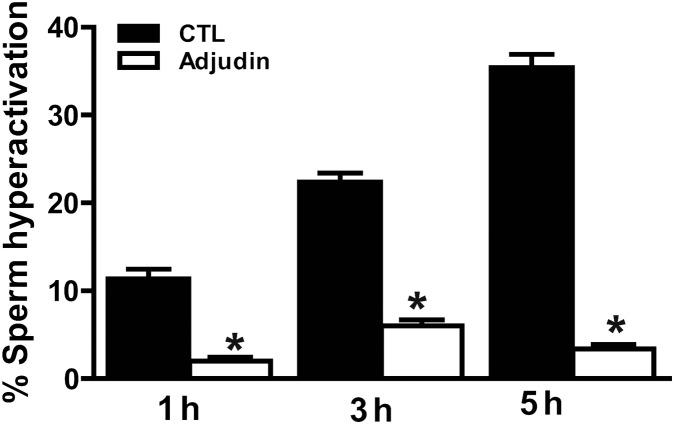
CASA analysis revealed that the percentages of sperm hyperactivation were increased significantly after incubation of 3 and 5 h versus 1 h (P < 0.01). ALH, and VCL were, in particular, greatly increased (P < 0.05), whereas LIN significantly decreased (P < 0.01), indicating that spermatozoa express strongly hyperactivated motility. In contrast, when sperm were incubated in the HTF medium with 10 µM adjudin for 3 and 5 h under the same conditions, the percentages of sperm hyperactivation were decreased markedly compared with the adjudin-free samples, P < 0.01 (Fig. 1), but it did not affect sperm motility (Table I), indicating that adjudin has no cytotoxicity at concentrations up to 10 µM, consistent with a recent report in rat (Su et al., 2011).
Figure 1.
Adjudin inhibits human spermatozoa hyperactivated motility. Sperm were incubated separately in the HTF medium for 1, 3 and 5 h with or without adjudin under capacitating conditions. Sperm motility and hyperactivation were assessed by using CASA. The results are expressed as the mean ± SEM (n = 8). *P < 0.001, when compared with control (adjudin-free). CTL, control; CASA, computer assisted sperm analysis.
Table I.
Effect of adjudin on human sperm motility and hyperactivated motion parameters in the HTF medium.
| Treatment | Motility (%) | VCL (µm/s) | VSL (µm/s) | ALH (µm) | LIN (%) | VAP (µm/s) |
|---|---|---|---|---|---|---|
| Control | 75.0 ± 7.8 | 154.9 ± 14.1 | 50.3 ± 14.3 | 7.8 ± 0.2 | 32.5 ± 4.5 | 75.1 ± 11.1 |
| Adjudin (10 µM) | 72.0 ± 10.5 | 116.9 ± 14.2* | 52.8 ± 12.6 | 4.8 ± 1.2* | 52.4 ± 4.4* | 60.0 ± 10.4* |
Sperm were incubated in the HTF medium for 5 h with or without 10 µM adjudin under capacitating conditions and then washed with the fresh HTF. Sperm hyperactivated motility motion and motility were analyzed by CASA. The results are expressed as the mean ± SEM of eight different experiments (n = 8). CASA, computer-assisted sperm analyzer; VCL, curvilinear velocity; VSL, straight line velocity; ALH, amplitude of lateral head displacement; VAP, average path velocity; LIN, linearity (VSL/VCL by multiple 100).
*When compared with control, P < 0.01.
Inhibition of progesterone-induced sperm capacitation by adjudin
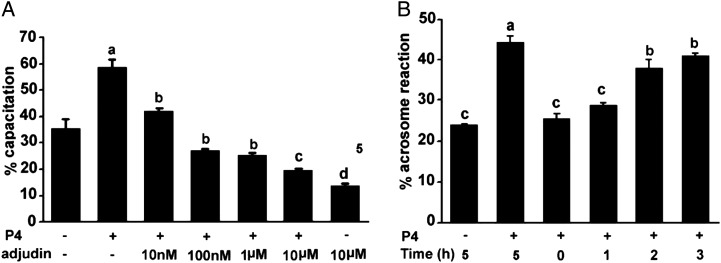
The three sequential and distinct patterns of CTC fluorescence staining on human spermatozoa were observed (shown in the Supplementary data, Fig. S1) as earlier reported (Li et al., 2010). Sperm capacitation was increased significantly in the HTF medium with 2.5 μM progesterone for incubation up to 5 h, which promoted the transition from F pattern to B pattern (‘B’ pattern from 2.3 ± 1.2% at 0 h up to 15.6 ± 2.5% at 5 h), followed by an increase in ‘AR’ patterns. The sum of ‘B’ + ‘AR’ patterns were increased from 21.3 ± 3% up to 35.0 ± 6% (Fig. 2A). These results showed that progesterone facilitated the ‘F’ pattern to ‘B’ pattern transition, followed by an increase in the ‘AR’ patterns, reaching a maximum value (the sum of ‘B + AR’ patterns: 58.5%) when compared with control (P < 0.001) after 5 h of incubation. These results demonstrated that progesterone significantly facilitated human sperm capacitation. However, this stimulatory effect was abolished by adjudin in a dose-dependent manner with concentrations of 10 nM–10 µM versus control (P < 0.01–P < 0.001), showing that adjudin significantly inhibits spontaneous- (Fig. 2a) and progesterone-induced sperm capacitation.
Figure 2.
(A). Inhibition of progesterone-elicited capacitation in human sperm by adjudin. Sperm were incubated with adjudin alone and/or in combination with progesterone (P4) 2.5 µM and different concentrations of adjudin (10 nM–10 μM) in the HTF medium for 5 h at 37°C. The percentages of sperm capacitation (‘B’+‘AR’ patterns) are expressed as the mean ± SEM (n = 3). a, compared with control (progesterone- and adjudin-free), P < 0.001; b versus a, c versus b and d versus c, P <0.05 to <0.001. (B) Adjudin inhibition of capacitation in human sperm is reversible. Sperm were preincubated for 5 h in HTF with 10 µM adjudin under capacitating conditions, and then spermatozoa were washed twice with the fresh HTF medium to remove adjudin and re-incubated thereafter in adjudin-free HTF for 1–3 h. The results (‘AR’ pattern alone) are expressed as the mean ± SEM (n = 3). Identical letters indicate that there is no significant difference. b when compared with a (P4 alone), P > 0.05. a versus c, P < 0.01; b versus c, P < 0.01.
Inhibition of human sperm capacitation by adjudin is reversible
Capacitation appears to be a reversible event (Shi and Friend, 1983; Yanagimachi,1994; Ni et al., 2009). Thus, a question arises as to whether the adjudin-induced inhibition of capacitation is reversible. To address the question, sperm were preincubated with 10 µM adjudin for 5 h in the HTF medium under capacitating conditions and washed twice with the fresh HTF medium to remove adjudin. Sperm were re-incubated for 1 to 3 h in the adjudin-free HTF medium under the same conditions and the cells were then challenged with 15 µM progesterone for 15 min prior to assessing the sperm acrosomal status. The percentages of ‘AR’ patterns progressively increased with the extension of incubation time (from 1 to 3 h) and were comparable with those of progesterone alone (positive control, P > 0.05) after an incubation period of 3 h. These results illustrated that capacitation was recovered in these spermatozoa, because only capacitated spermatozoa are able to undergo the acrosome reaction, indicating that the adjudin-inhibited capacitation is reversible (Fig. 2B) and it is unlikely that its capacitation-blocking effect is the result of cytotoxicity.
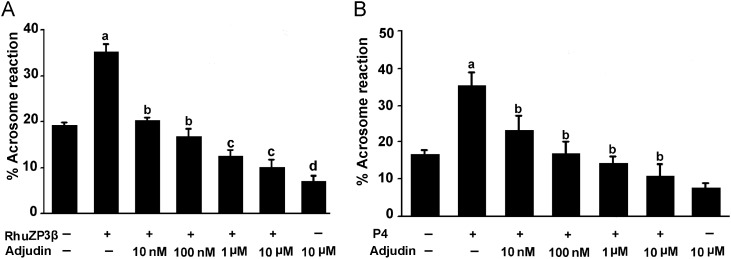
Adjudin inhibits sperm acrosome reaction induced by rhuZP3β or progesterone
To explore whether adjudin has an effect on capacitated spermatozoa, we examined the effect of adjudin on sperm acrosome reaction induced by rhuZP3β or progesterone. The results illustrated that sperm acrosome reactions induced by rhuZP3β (35.5 ± 3.2%) (Fig. 3A) and by progesterone (35.2 ± 3.8%) (Fig. 3B) were increased markedly in the adjudin-free HTF medium versus negative control (P < 0.01). However, this stimulatory effect on the acrosome reaction induced by rhuZP3β or progesterone was blocked completely by adjudin in a dose-dependent manner at 10 nM–10 µM, versus positive control (adjudin-free, P < 0.05–P < 0.001). The acrosome reactions induced by rhZP3β (12.1 ± 1.8%) and by progesterone (12.8 ± 2.2%) were inhibited completely by adjudin at 10 µM versus positive control (rhZP3 β- or progesterone-induced acrosome reaction) (P < 0.001).
Figure 3.
(A) Inhibition of rhuZP3β-triggered the acrosome reaction in human sperm by adjudin. Sperm were capacitated in the HTF medium for 5 h, washed, resuspended in the fresh HTF medium and exposed to different concentrations of adjudin (10 nM–10 µM) for 15 min and then the acrosome reactions were triggered with rhuZP3β (20 µg/ml) for 15 min. Results are expressed as the mean ± SEM (n = 3). a when compared with control, P < 0.001; b compared with a (rhuZP3β alone), P < 0.01 to <0.001. c versus b, P < 0.05; d versus c, P < 0.05. (B) Inhibition of progesterone-induced acrosome reaction in human sperm by adjudin. Sperm were incubated in the HTF medium under capacitating conditions for 5 h to induce capacitation, washed, resuspended in the HTF containing different concentrations of adjudin for 15 min and then elicited with 15 μM P4 for 15 min before evaluation of the acrosome reaction. Results are expressed as the mean ± SEM (n = 4). a when compared with control, P < 0.001; b when compared with progesterone, P < 0.01 to <0.001.
Inhibition of sperm–egg fusion in zona-free hamster egg by adjudin
The percentage of human spermatozoa penetration of zona-free hamster eggs in the presence of 10 µM adjudin was reduced significantly (27.9%, versus the control 70.8%, P < 0.001; Table II). Despite their repeated contact, only a few spermatozoa in the presence of adjudin attached to the ova surface when compared with control (shown in Supplementary data, Fig. S2). To evaluate if adjudin directly affects the fertilizing ability of zona-free hamster eggs, we pre-incubated the eggs for 0.5 h in the BWW medium containing 10 µM adjudin, which is the time consistent with the duration of fertilization in vivo. Thereafter eggs were washed twice with the fresh BWW to remove adjudin. Similarly, when spermatozoa were preincubated for 5 h in the HTF medium without adjudin, and then mixed with the ova and further co-incubated for 3 h under the same conditions, the fertilizing capacity of the zona-free hamster eggs was not affected significantly which was similar to control (P > 0.05, data not shown), indicating that adjudin did not directly affect the fertilizing ability of zona-free hamster eggs.
Table II.
Inhibition of human sperm penetration of zona-free hamster eggs by adjudin.
| Treatment | Number of eggs examined | Number of eggs penetrated | Penetration index | Percentage of penetration |
|---|---|---|---|---|
| Control | 65 | 46 | 2.8 | 70.8 |
| Adjudin (10µM) | 86 | 24 | 1.2 | 27.9* |
| Progesterone (P4, 15 µM) | 44 | 36 | 3.0 | 81.0 |
Sperm were preincubated with or without adjudin 10 µM in HTF for 5 h, washed, added separately to BWW medium containing 15–20 zone-free hamster oocytes and then co-incubated for 3 h at 37°C under 5% CO2 in air. Results are expressed as mean ± SEM. These data were represented in five different experiments (n = 5); adjudin significantly different from controls used. Paired samples test (*P < 0.001).
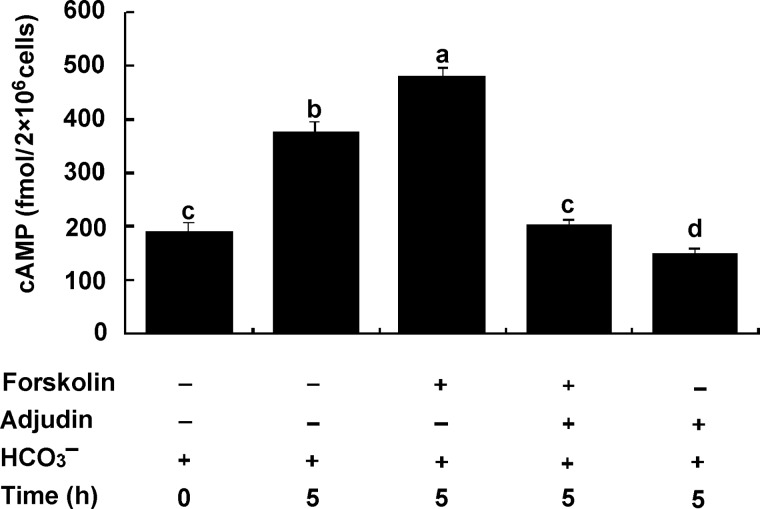
Inhibition of intracellular cAMP levels in spermatozoa by adjudin
To further examine whether adjudin inhibition of capacitation is mediated by intervention upstream of the cAMP signaling pathway, sperm were incubated for 5 h with or without 10 μM adjudin in the complete Cl−-HTF medium under capacitating conditions. The results showed that the mean levels of intracellular cAMP [cAMP]i in spermatozoa incubated in the HTF medium for 5 h were increased significantly. Forskolin prominently elevated the [cAMP]i levels (increased by 30%) in spermatozoa versus control at 5 h (P < 0.01). However, this stimulating effect on [cAMP]i levels was abolished completely (decreased by 60%) when sperm were incubated in the presence of 10 µM adjudin for the same period of time (Fig. 4) versus forskolin alone (P < 0.001). These results suggest that adjudin inhibits the forskolin-induced the increase in [cAMP]i levels that is through the activation of tmACs activity. However, the possibility that HCO3− in the medium may directly activate the sperm sAC activity leading to the increase in [cAMP]i levels should not be ruled out. To examine if adjudin inhibits sAC activity, the [cAMP]i levels of spermatozoa in the presence of HCO3− were measured. The results showed that adjudin also significantly inhibited [cAMP]i levels activated by HCO3− (Fig. 4, d versus b, P < 0.001). Thus, adjudin inhibited [cAMP]i levels, which was through both tmAC and sAC signaling pathways.
Figure 4.
Inhibition of forskolin- and HCO3− stimulated increase in the intracellular cAMP levels of human spermatozoa by adjudin. Sperm were separately incubated for 0 and 5 h in the HTF medium (containing 25 mM HCO3− ) with or without 3.6 µM forskolin and/or 10 μM adjudin. The results are expressed as the means ± SEM (fmol/2 × 106 cells; n = 5). The forskolin treatment (a) when compared with controls (b), (c) and (d) (HCO3− plus adjudin) after incubation of 0 h and 5 h. Adjudin significantly inhibits the increase in [cAMP]i level activated by both forskolin and HCO3− (a versus c, P < 0.001; a versus b, P < 0.05; b versus c, P < 0.01) or only HCO3− (d versus b, P < 0.01).
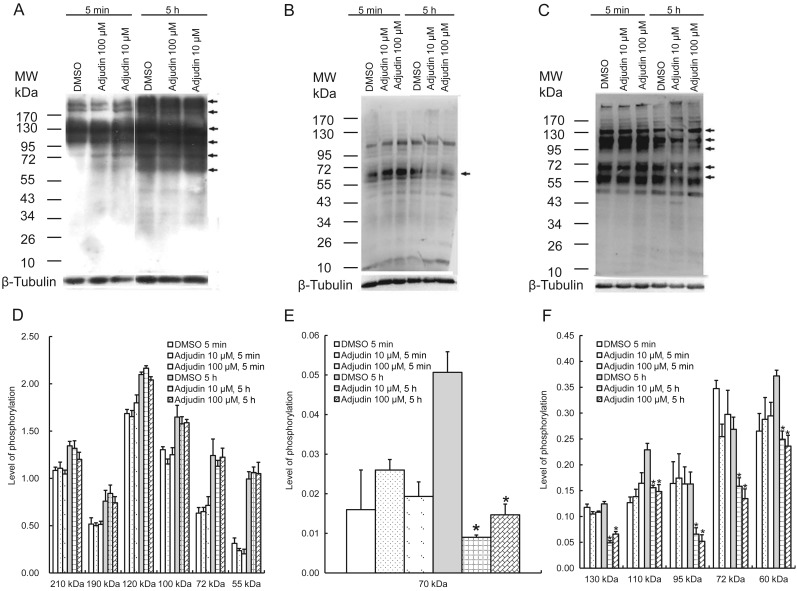
Effect of adjudin on protein tyrosine, serine and threonine phosphorylation
To further investigate whether adjudin affects protein tyrosine, serine and threonine phosphorylation during human sperm capacitation, protein tyrosine, serine and threonine phosphorylation and their signal intensities were analyzed quantitatively by western blotting. The results illustrated that protein tyrosine was phosphorylated increasingly during capacitation at multiple molecular regions, including 55, 72, 100, 120, 190 and 210 kDa. However, adjudin at 10 and 100 µM was not capable of inhibiting this increase in capacitation-dependent protein tyrosine phosphorylation, which was similar to that of control as shown in Fig. 5A and D (P > 0.05). However, adjudin at 10 and 100 µM was capable of inhibiting the increase in serine phosphorylation at 70 kDa protein (Fig. 5B and E, P < 0.05), and in threonine phosphorylation of 60, 72, 95, 110 and 130 kDa proteins (in Fig. 5C and F), compared with the control, P < 0.05. The results imply adjudin inhibits capacitation via the signal pathway of serine and threonine phosphorylation, but not tyrosine phosphorylation.
Figure 5.
Effect of adjudin on human sperm protein phosphorylation during capacitation. The western blotting and the quantitative analysis of signal intensity of protein phosphorylation were performed as described in the section Materials and methods. (A) tyrosine, (B) serine and (C) threonine phosphorylation. Each experiment was repeated at least three times. The bands indicated by arrows were further analyzed by densitometry. Their phosphorylated levels (D–F) were expressed by the ratio of the gray intensity of bands compared with loading controls, β-tubulin (mean ± SEM, n = 3). The results indicated that 10 and 100 µM adjudin were not capable of inhibiting the increase in protein tyrosine phosphorylation compared with the control, P > 0.05, but did inhibit serine phosphorylation at 70 kDa protein, and threonine phosphorylation at 60, 72, 95, 110 and 130 kDa proteins, when compared with the control, *P < 0.05.
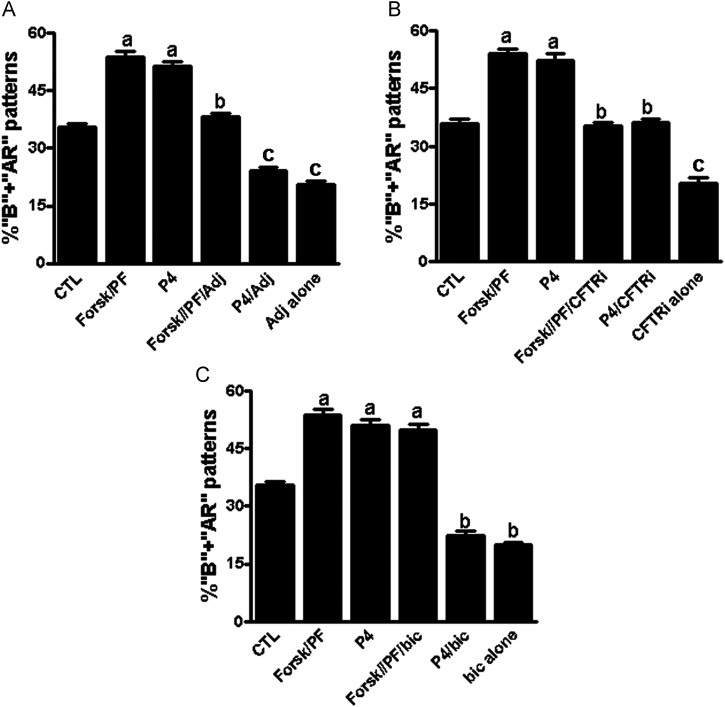
Forskolin and pentoxifylline-induced capacitation is inhibited by adjudin
Forskolin and pentoxifylline or progesterone alone were found to stimulate significantly sperm capacitation (‘B’ + ‘AR’ patterns) versus control (P < 0.001). However, this stimulatory effect was abolished entirely by adjudin (Fig. 6A) or by CFTRinh-172 (Fig. 6B) versus control (P < 0.001). Bicuculline was found to prohibit progesterone-induced capacitation, but it did not inhibit forskolin and pentoxifylline-induced capacitation (Fig. 6C). These findings suggest that adjudin inhibition of forskolin and pentoxifylline-induced capacitation may be mediated by disrupting the cAMP-activated Cl− channel (i.e. CFTR Cl− Channel), while its inhibition of progesterone-induced capacitation may be via the GABAA receptor/Cl− channel, respectively.
Figure 6.
Inhibition by adjudin (A), CFTRinh-172 (B) and bicuculline (C) on a combination of forskolin- and pentoxifylline- or progesterone-induced capacitation in human sperm. Sperm were preincubated with or without a combination of forskolin (10 μM) and pentoxifylline (3.6 µM) or progesterone (15 μM) alone for 5 h in the presence of or in the absence of 10 μM adjudin (A), 1 μM CFTRinh-172 (B) or 10 μM bicuculline (C) in the HTF medium under capacitating conditions, samples were washed and then incubated for 30 min before evaluation of sperm capacitation (‘B’ + ‘AR’ patterns). The results are expressed as the mean ± SEM of five different experiments (n = 5). For each group, letters above bars indicate significant differences. a, when compared with CTL, P < 0.001; b versus a, P < 0.01; c versus b, P < 0.01; a versus c, P < 0.001. CTL, control; CFTR, cystic fibrosis transmembrane conductance regulator; P4, progesterone.
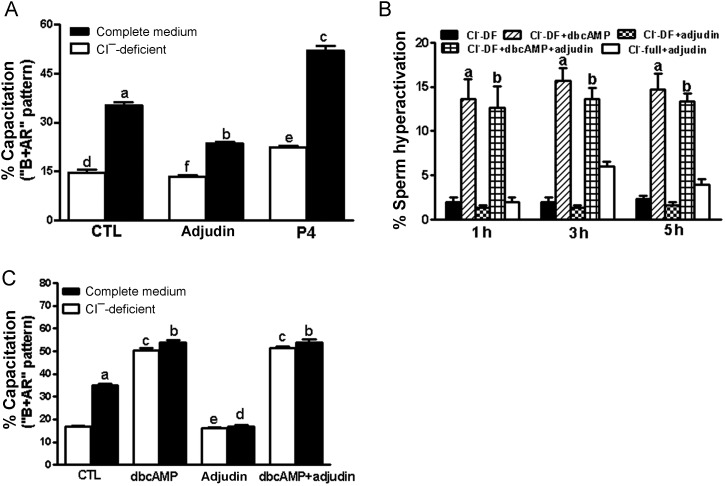
Inhibition of capacitation-associated Cl− channels transport by adjudin
CFTR acts as a upstream regulator of the intracellular cAMP production in sperm, which plays a role in modulating the exchanger for the Cl−/HCO3− (Chen et al., 2009) and Na+/HCO3− co-transporter (Demarco et al., 2003). The results showed that adjudin significantly blocked sperm capacitation in the complete Cl−-HTF medium versus control (P < 0.001), but it did not exert its inhibiting effect to sperm capacitation in the Cl−-DF HTF medium versus control in the Cl−-DF HTF medium (P > 0.05) (Fig. 7A), suggesting that adjudin inhibits sperm capacitation via Cl− and its channels. Addition of 1 mM dbcAMP to a sperm suspension in Cl−-DF HTF medium completely overcame adjudin-induced inhibition of sperm hyperactivation (Fig. 7B) and capacitation (Fig. 7C) in the absence of Cl−. These results suggest that adjudin inhibits sperm hyperactivation and capacitation through the prevention of Cl− and its channels, which exert their effects upstream of the cAMP signaling pathway.
Figure 7.
(A) Effect of adjudin on human sperm capacitation in the complete Cl−-HTF medium or the Cl−-DF HTF medium. Sperm were separately incubated for 5 h in the complete Cl−-HTF or Cl−-DF HTF medium with or without 10 μM adjudin under capacitating conditions. The results are expressed as the mean ± SEM (n = 5). a compared with Cl−-DF HTF medium (d), P < 0.001; b versus f, P < 0.01; c versus e, P < 0.001. e versus d, P > 0.05. (B) dbcAMP rescues the inhibition of human sperm hyperactivation induced by adjudin. Sperm were separately incubated for 1–5 h in the complete Cl−-HTF- and/or Cl−-DF HTF medium without or with adjudin (10 μM) in the presence of or the absence of 1 mM dbcAMP. Sperm hyperactivation was evaluated by CASA. The results are expressed as the mean ± SEM (n = 5). a versus b, P > 0.05; a/b when compared with Cl−-DF HTF or Cl−-full HTF alone or plus adjudin, P < 0.001. (C) dbcAMP rescues the inhibition of human sperm capacitation induced by adjudin. Sperm were separately incubated for 5 h in the complete Cl−-HTF medium with or without 10 μM adjudin or in the presence or absence of 1 mM dbcAMP. Sperm capacitation (‘B’ + ‘AR’ patterns) was evaluated by CTC staining. The results are expressed as the mean ± SEM (n = 5). There is no difference in sperm capacitation when treated with dbcAMP in the full HTF or DF-Cl HTF medium in the presence or absence of adjudin. b versus c, P > 0.05; e versus d, P > 0.05, except for a versus CTL, P < 0.001. CTL, control; dbcAMP, dibutyryl cAMP; P4, progesterone.
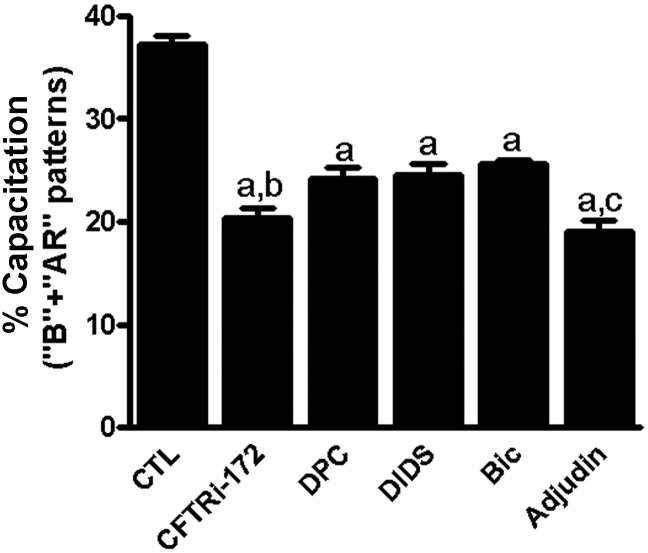
To further determine whether adjudin and various structural Cl− channel blockers exert their effects on sperm capacitation with different sensitivities, sperm were incubated with various structural blockers of Cl− channels versus adjudin in the complete HTF medium. The results showed that these Cl− transport blockers significantly prevented sperm capacitation, suggesting different Cl− transport channels are present in the sperm. Among them, CFTRinh-172 seems to be the most potent, followed by adjudin. DPC (CFTR antagonist) and DIDS (Cl− selective transport inhibitor) displayed lower sensitivity to inhibition of sperm capacitation: the sensitivity of the former was higher than that of the later. In addition, bicuculline markedly inhibited capacitation caused by progesterone, but it did not prevent capacitation elicited by forskolin/pentoxifylline, suggesting that three structural Cl− channel families present in sperm cells are mediated via different Cl− transporters (Fig. 8). The order of sperm capacitation with sensitivity to different Cl− channel transport inhibitors was CFTRinh-172 > adjudin > DPC > DIDS > bicuculline. The results suggest that adjudin inhibition of sperm hyperactivation and capacitation may be mediated by disrupting different Cl− channels per se and/or Cl− transport across the channels.
Figure 8.
Inhibition of human sperm capacitation by different blockers of Cl− channels. Sperm were preincubated with or without different blockers of Cl− channels, CFTRinh-172 (1 μM), adjudin (10 μM), DPC (10 μM), DIDs (10 μM) or/and bicuculline (Bic, 10 µM) for 5 h in the complete HTF medium under capacitating conditions. The results of capacitation (‘B’ + ‘AR’ patterns) are expressed as the mean ± SEM (n = 5). a compared with control, P < 0.01; b compared with a, P > 0.05; c compared with a, P < 0.05. Bic, bicuculline; CFTR, cystic fibrosis transmembrane conductance regulator; DID, 4′4,diisothiocyanostilbene-2,2-disulfonic acid; DPC, diphenylamine-2-carboxylate.
Discussion
Herein, we demonstrate for the first time that adjudin significantly inhibits human sperm hyperactivation, capacitation, the acrosome reaction, intracellular cAMP levels and protein serine/threonine residues phosphorylation; the net result of these effects blocks the sperm–egg fusion. The inhibitory effects of adjudin on sperm capacitation and the acrosome reaction were found to be dose dependent. More importantly, adjudin significantly inhibited sperm hyperactivation and fertilizing capacity, however, it did not affect sperm motility at a concentration of 10 µM (Table I). Since sperm cells are sensitive to various toxic compounds (Jin et al., 2008), sperm motility is a reliable indicator for evaluating toxic compounds. It is more important that adjudin did not affect spermatozoa motility at a concentration of 10 µM (Table I). The result of this study is in agreement with Mruk et al. (2006) who reported that the toxicity of adjudin is low in rats. Furthermore, the cytotoxicity of adjudin is far lower than gossypol, which is an extensively studied potential male contraceptive (Shi et al., 1987). Earlier studies have shown that sperm capacitation is also associated with sperm hyperactivated motility, with both events occurring simultaneously and dependent on exogenous calcium influx (Yanagimachi, 1994; Suarez and Pacey, 2006). Inhibition of sperm hyperactivation expression suggests that adjudin is also linked to capacitation. However, capacitation is a reversible event, but the acrosome reaction is not (Shi and Friend, 1983; Yanagimachi, 1994), and these two events are sequential but separate processes (Florman et al., 1992; Yanagimachi, 1994). We have shown herein that the inhibition of adjudin on capacitation is reversible. It seems to be similar to what Grima et al reported , who proposed adjudin inhibition of spermatogenesis in rat was reversible (Grima et al., 2001). It is noted that only capacitated spermatozoa are capable of undergoing an acrosome reaction in response to progesterone and ZP (Roldan et al., 1994), achieving sperm–egg fusion to complete fertilization. Our observations illustrated that adjudin not only significantly inhibited the acrosome reaction induced by progesterone, but also markedly prevented the acrosome reaction triggered by rhuZP3β, thereby blocking the sperm–oocyte fusion. In short, adjudin is capable of blocking sperm hyperactivated motility, capacitation, the acrosome reaction, [cAMP]i increase, protein serine and threonine phosphorylation and sperm–ovum fusion.
It is known that multiple steps in the fertilization process are controlled by sperm capacitation (Yanagimachi, 1994; Fraser et al., 2003; Florman et al., 2007; Visconti et al, 2011). Meanwhile, sperm capacitation is known to associate with ion channels and a cAMP/PKA-dependent phosphorylation increase (Wertheimer et al., 2008; Visconti et al., 2011). However, the mechanism by which adjudin prevents sperm capacitation is unknown. Previous studies have demonstrated that AF-2785, an analog of lonidamine, is a potent blocker of CFTR (Gong and Wong, 2000; Gong et al., 2002). Since adjudin shares similar structural features with AF-2785, it is possible that it blocks the function of CFTR Cl− transport in sperm, thereby leading to a failure in sperm capacitation. It is noted that CFTR is a cAMP-activated Cl− channel whose primary role is involved in the transport of HCO3− and Cl− across the plasma membrane (Reddy et al., 1999; Wang et al., 2003). Furthermore, it has been demonstrated that CFTR Cl− is present in the equatorial segment of the mouse, guinea pig and human sperm head (Xu et al., 2007; Chen et al., 2009; Li et al., 2010) and in the middle piece of mouse sperm tails (Hernandez-Gonzalez et al., 2007; Wertheimer et al., 2008; Chavez et al., 2012). Previous studies have shown that both Cl− and HCO3− are essential for sperm capacitation in the mouse, guinea pig, rat and human (DasGupta et al., 1993; Okamura et al., 1993; Chen et al., 2004, 2009; Wertheimer et al., 2008; Jin et al., 2009). We demonstrated herein that adjudin significantly blocked sperm hyperactivation and capacitation in the complete Cl−-HTF medium but not in Cl−-DF HTF medium, suggesting adjudin inhibits sperm hyperactivation and capacitation via CFTR Cl− and other Cl− transporters, perhaps by blockade of Cl−/HCO3− exchanger through Cl− channels (Chen et al., 2009), Na+/HCO3− co-transporter (Demarco et al., 2003), Na+/K+/Cl− co-trasporters (Wertheimer et al., 2008). This postulate is based on findings in which the addition of dbcAMP to spermatozoa incubated in the Cl−-free medium was shown to rescue sperm hyperactivation and capacitation (Fig. 7B and C), indicating that Cl− is necessary for sperm capacitation and its function is upstream of the cAMP/PKA signaling pathway. It seems to be very similar to what Wertheimer et al. reported in mouse sperm (Wertheimer et al., 2008). CFTRinh-172 and DPC are not able to inhibit the increase in tyrosine phosphorylation (Hernandez-Gonzalez et al., 2007; Wertheimer et al., 2008). Here we show the same lack of effect on capacitation-dependent tyrosine phosphorylation. However, our previous observations and the present studies have demonstrated that CFTRinh-172 and DPC are capable of inhibiting sperm capacitation and fertilization in mouse and human sperm in vitro (Xu et al., 2007; Chen et al., 2009; Li et al., 2010) which are different from the results reported by Hernandez-Gonzalez et al. (2007) and Wertheimer et al. (2008). Their results indicated that CfTRinh-172 and DPC are not able to inhibit sperm hyperactivation and fertilization in vitro. At present the reason for these different results is still unknown. It is possible that the source of reagents used is different.
It is established that cAMP is downstream of the Cl− effect in capacitation and the cAMP/PKA signaling pathway is necessary for sperm capacitation (Florman et al., 2007; Wertheimer et al., 2008; Chen et al., 2009). The dbcAMP is able to rescue sperm hyperactivation and capacitation in the Cl−-DF medium indicating that cAMP is involved in the Cl− requirement for sperm hyperactivation and Cl− exerts its effects upstream of the cAMP/PKA signaling pathway (Wertheimer et al., 2008; Chen et al., 2009).
Numerous proteins undergo phosphorylation at tyrosine, serine and threonine residuals by the activation of cAMP/PKA, and protein phosphorylation depends upon the balance action of protein kinases and protein phosphatase during capacitation (Signorelli et al., 2012). In the present study, adjudin is not capable of inhibiting the increase in tyrosine phosphorylation, but it is able to block the increase in serine/threonine phosphorylation. It suggests that there may be two signaling pathways that regulate protein phosphorylation at tyrosine and serine/threonine residues in human sperm during capacitation (Naz, 1999; Hess et al., 2005; Visconti et al., 2011). The reasonable understanding may be that adjudin affects the PKA pathway, which phosphorylates several proteins at serine and threonine residues, but not activate or balance the kinases and phosphatases downstream of its signal pathway, leading to the phosphorylation of tyrosine residues (Signorelli et al., 2012).
Another possible mechanism is that adjudin interferes upstream of signaling pathways by mediating the effects of cAMP. It is known that the G protein-regulated tmACs are unaffected by HCO3−. Even when sub-maximally stimulated by HCO3−, the tmACs activity is insensitive to HCO3− (Baxendale and Fraser, 2003; Fraser et al.,2003). Although a purified, engineered tmACs V type (tmACV), a soluble form of mACs, is fully responsive to forskolin (Scholich, et al., 1997; Insel and Ostrom, 2003), it is completely insensitive to HCO3−. Therefore, HCO3− stimulation is not a general feature of all ACs, mammalian cells and spermatozoa possesses two independent regulated cAMP signal transduction systems: G protein-tmACs and bicarbonate- and calcium-regulated sACs (Leclerc and Kopf, 1995; Scholich et al., 1997; Baxendale and Fraser, 2003; Liguori et al., 2004). In this study our results showed that forskolin significantly promoted the increase in [cAMP]i levels, which may be acting on tmACs activity and subsequent increasing in [cAMP]i levels leading to capacitation. HCO−3 present in the HTF medium directly activates sAC activity to induce an increase in [cAMP]i levels, accompanying capacitation. There is evidence that CFTR is present in the sperm which plays a critical role for regulation of the Cl−/HCO3− exchanger and CFTR interaction, leading to the entry of HCO3− into sperm (Hernandez-Gonzalez et al., 2007; Xu et al., 2007; Chen et al., 2009; Chavez et al., 2012) and in this sense, HCO3− may directly activate sperm sAC activity. Therefore, there are two signaling pathways that regulate ACs activity leading to capacitation.
In short, it is likely that adjudin inhibits sperm capacitation and capacitation-associated events, such as hyperactivation, capacitation, the acrosome reaction and sperm–egg fusion via: (i) obstructing Cl− transport function in the sperm by impeding Cl− transporters and (ii) inhibiting tmACs and sAC activities, in turn, leading to a decline in [cAMP]i levels and of serine/threonine protein phosphorylation. Thus, besides serving as a potential male contraceptive, adjudin can also be used in females as a potential contraceptive by blocking the fertilizing capacity of sperm in humans.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
Q.X.S. and C.Y.C. were involved in substantial contributions to conception and design. K.L., Y.H., Y.N., W.Y.C. were involved in acquisition of data. Q.X.S., K.L., W.Y.C., J.X.L. were involved in analysis and interpretation of data. Q.X.S., K.L. were involved in drafting the article. C.Y.C., Q.X.S. and R.S.G. were involved in revising it critically for important intellectual content. C.Y.C., Q.X.S. were involved in final approval of the version to be published.
Funding
This work was supported by the National Basic Research Program of China (2006CB504002), the Nature Science Foundation of China (Nos. 81000244 and 81170554), Zhejiang Project of Science and Technology (2011C23046), the Nature Science Fund of Zhejiang province (Nos. Y2100058and Y2090236), the key Science and Technology Innovation Team of Zhejiang Province (No. 2012R10048-07) ) and the National Institutes of Health (NICHD U54 HD029990 project 5), USA.
Conflict of interest
None declared.
Supplementary Material
Acknowledgements
The authors wish to thank Prof. Xu, Wan Xiang, Shanghai Institute of Planned Parenthood Research for generous gift of Recombinant human ZP3β.
References
- Aitken RJ, Wang YF, Liu J, Best F, Richardson DW. The influence of medium composition, osmolarity and albumin content on the acrosome reaction and fertilizing capacity of human spermatozoa: development of an improved zona-free hamster egg penetration test. Int J Androl. 1983;6:180–193. doi: 10.1111/j.1365-2605.1983.tb00337.x. [DOI] [PubMed] [Google Scholar]
- Baxendale RW, Fraser LR. Evidence for multiple distinctly localized adenylyl cyclase isoforms in mammalian spermatozoa. Mol Reprod Dev. 2003;66:181–189. doi: 10.1002/mrd.10344. [DOI] [PubMed] [Google Scholar]
- Biggers JD, Whitten WK, Whittinghan DC. The culture of mouse embryos in vitro. In: Daniel JC, editor. Methods in Mammalian Embryology. San Francisco: Freeman Press; 1971. pp. 86–116. [Google Scholar]
- Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc Natl Acad Sci USA. 1999;96:79–84. doi: 10.1073/pnas.96.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez JC, Hernandez-Gonzalez EO, Wertheimer E, Visconti PE, Darszon A, Trevino CL. Participation of the Cl−/HCO3− exchangers SLC26A3 and SLC26A6, the Cl− channel CFTR, and the regulatory factor SLC9A3R1 in mouse sperm capacitation. Biol Reprod. 2012;86:1–14. doi: 10.1095/biolreprod.111.094037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- Chen WY, Yuan YY, Shi QX, Chen AJ, Zhou CX, Chan HC, Roldan ERS. Chloride ion is required for sperm capacitation and its channels in guinea pig. Biol Reprod. 2004;((special issue)):184. [Google Scholar]
- Chen WY, Xu WM, Chen ZH, Ni Y, Yuan YY, Zhou SC, Zhou WW, Tsang LL, Chung YW, Hoglund P, et al. Cl− is required for HCO3− entry necessary for sperm capacitation in guinea pig. Involvement of a Cl−/HCO3− exchanger (SLC26A3) and CFTR. Biol Reprod. 2009;80:115–123. doi: 10.1095/biolreprod.108.068528. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Silvestrini B, Grima J, Mo MY, Zhu LJ, Johansson E, Saso L, Leone MG, Palmery M, Mruk D. Two new male contraceptives exert their effects by depleting germ cells prematurely from the testis. Biol Reprod. 2001;65:449–461. doi: 10.1095/biolreprod65.2.449. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Lie PP, Wong EW, Mruk DD, Silvestrini B. Adjudin disrupts spermatogenesis via the action of some unlikely partners: Eps8, Arp2/3 complex, drebrin E, PAR6 and 14–3-3. Spermatogenesis. 2011;1:291–297. doi: 10.4161/spmg.1.4.18393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta S, Mills CL, Fraser LR. Ca(2+)-related changes in the capacitation state of human spermatozoa assessed by a chlortetracycline fluorescence assay. J Reprod Fertil. 1993;99:135–143. doi: 10.1530/jrf.0.0990135. [DOI] [PubMed] [Google Scholar]
- Demarco IA, Espinosa F, Edwards J, Sosnik J, De La Vega-Beltran JL, Hockensmith JW, Kopf GS, Darszon A, Visconti PE. Involvement of a Na+/HCO3− cotransporter in mouse sperm capacitation. J Biol Chem. 2003;278:7001–7009. doi: 10.1074/jbc.M206284200. [DOI] [PubMed] [Google Scholar]
- Estevez R, Schroeder BC, Accardi A, Jentsch TJ, Pusch M. Conservation of chloride channel structure revealed by an inhibitor binding site in ClC-1. Neuron. 2003;38:47–59. doi: 10.1016/s0896-6273(03)00168-5. [DOI] [PubMed] [Google Scholar]
- Florman HM, Ducibella T. Fertilization in mammals. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. San Diego, CA: Elsevier; 2006. pp. 55–111. [Google Scholar]
- Florman HM, Storey BT. Mouse gamete interactions: the zona pellucida is the site of the acrosome reaction leading to fertilization in vitro. Dev Biol. 1982;91:121–130. doi: 10.1016/0012-1606(82)90015-x. [DOI] [PubMed] [Google Scholar]
- Florman HM, Corron ME, Kim TD, Babcock DF. Activation of voltage-dependent calcium channels of mammalian sperm is required for zona pellucida-induced acrosomal exocytosis. Dev Biol. 1992;152:304–314. doi: 10.1016/0012-1606(92)90137-6. [DOI] [PubMed] [Google Scholar]
- Florman HM, Jungnickel MK, Sutton KA. What can we learn about fertilization from cystic fibrosis? Proc Natl Acad Sci USA. 2007;104:11123–11124. doi: 10.1073/pnas.0703626104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser LR, Abeydeera LR, Niwa K. Ca(2+)-regulating mechanisms that modulate bull sperm capacitation and acrosomal exocytosis as determined by chlortetracycline analysis. Mol Reprod Dev. 1995;40:233–241. doi: 10.1002/mrd.1080400213. [DOI] [PubMed] [Google Scholar]
- Fraser LR, Adeoya-Osiguwa SA, Baxendale RW. First messenger regulation of capacitation via G protein-coupled mechanisms: a tale of serendipity and discovery. Mol Hum Reprod. 2003;9:739–748. doi: 10.1093/molehr/gag097. [DOI] [PubMed] [Google Scholar]
- Gong XD, Wong PY. Characterization of Lonidamine and AF2785 blockade of the cyclic AMP-activated chloride current in rat epididymal cells. J Membr Biol. 2000;178:225–233. doi: 10.1007/s002320010030. [DOI] [PubMed] [Google Scholar]
- Gong XD, Wong YL, Leung GP, Cheng CY, Silvestrini B, Wong PY. Lonidamine and analogue AF2785 block the cyclic adenosine 3′, 5′-monophosphate-activated chloride current and chloride secretion in the rat epididymis. Biol Reprod. 2000;63:833–838. doi: 10.1095/biolreprod63.3.833. [DOI] [PubMed] [Google Scholar]
- Gong XD, Linsdell P, Cheung KH, Leung GP, Wong PY. Indazole inhibition of cystic fibrosis transmembrane conductance regulator Cl (−) channels in rat epididymal epithelial cells. Biol Reprod. 2002;67:1888–1896. doi: 10.1095/biolreprod.102.007450. [DOI] [PubMed] [Google Scholar]
- Grima J, Silvestrini B, Cheng CY. Reversible inhibition of spermatogenesis in rats using a new male contraceptive, 1-(2,4-dichlorobenzyl)-indazole-3-carbohydrazide. Biol Reprod. 2001;64:1500–1508. doi: 10.1095/biolreprod64.5.1500. [DOI] [PubMed] [Google Scholar]
- Hernandez-Gonzalez EO, Trevino CL, Castellano LE, de la Vega-Beltran JL, Ocampo AY, Wertheimer E, Visconti PE, Darszon A. Involvement of cystic fibrosis transmembrane conductance regulator in mouse sperm capacitation. J Biol Chem. 2007;282:24397–24406. doi: 10.1074/jbc.M701603200. [DOI] [PubMed] [Google Scholar]
- Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, Kamenetsky M, Miyamoto C, Zippin JH, Kopf GS, Suarez SS, et al. The soluble adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell. 2005;9:249–259. doi: 10.1016/j.devcel.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel PA, Ostrom RS. Forskolin as a tool for examining adenylyl cyclase expression, regulation, and G protein signaling. Cell Mol Neurobiol. 2003;23:305–314. doi: 10.1023/a:1023684503883. [DOI] [PubMed] [Google Scholar]
- Jin JY, Ni Y, Lu JX, Shi QX. Impact of organic environmental contaminants on male fertility. J Environ Occup Med. 2008;25:496–499. [Google Scholar]
- Jin JY, Chen WY, Zhou CX, Chen ZH, Yu-Ying Y, Ni Y, Chan HC, Shi QX. Activation of GABAA receptor/Cl− channel and capacitation in rat spermatozoa: HCO3− and Cl− are essential. Syst Biol Reprod Med. 2009;55:97–108. doi: 10.1080/19396360802626648. [DOI] [PubMed] [Google Scholar]
- Leclerc P, Kopf GS. Mouse sperm adenylyl cyclase: general properties and regulation by the zona pellucida. Biol Reprod. 1995;52:1227–1233. doi: 10.1095/biolreprod52.6.1227. [DOI] [PubMed] [Google Scholar]
- Li CY, Jiang LY, Chen WY, Li K, Sheng HQ, Ni Y, Lu JX, Xu WX, Zhang SY, Shi QX. CFTR is essential for sperm fertilizing capacity and is correlated with sperm quality in humans. Hum Reprod. 2010;25:317–327. doi: 10.1093/humrep/dep406. [DOI] [PubMed] [Google Scholar]
- Liguori L, Rambotti MG, Bellezza I, Minelli A. Electron microscopic cytochemistry of adenylyl cyclase activity in mouse spermatozoa. J Histochem Cytochem. 2004;52:833–836. doi: 10.1369/jhc.3B6141.2004. [DOI] [PubMed] [Google Scholar]
- Liu DY, Baker HW. Disordered acrosome reaction of spermatozoa bound to the zona pellucida: a newly discovered sperm defect causing infertility with reduced sperm-zona pellucida penetration and reduced fertilization in vitro. Hum Reprod. 1994;9:1694–1700. doi: 10.1093/oxfordjournals.humrep.a138776. [DOI] [PubMed] [Google Scholar]
- Mortimer ST. CASA—practical aspects. J Androl. 2000;21:515–524. [PubMed] [Google Scholar]
- Mortimer ST, Swan MA, Mortimer D. Effect of seminal plasma on capacitation and hyperactivation in human spermatozoa. Hum Reprod. 1998;13:2139–2146. doi: 10.1093/humrep/13.8.2139. [DOI] [PubMed] [Google Scholar]
- Mruk D, Cheng CY. Testin and actin are key molecular targets of adjudin, an anti-spermatogenic agent, in the testis. Spermatogenesis. 2011;1:137–146. doi: 10.4161/spmg.1.2.16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk DD, Wong CH, Silvestrini B, Cheng CY. A male contraceptive targeting germ cell adhesion. Nat Med. 2006;12:1323–1328. doi: 10.1038/nm1420. [DOI] [PubMed] [Google Scholar]
- Muanprasat C, Sonawane ND, Salinas D, Taddei A, Galietta LJ, Verkman AS. Discovery of glycine hydrazide pore-occluding CFTR inhibitors: mechanism, structure-activity analysis, and in vivo efficacy. J Gen Physiol. 2004;124:125–137. doi: 10.1085/jgp.200409059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naz RK. Involvement of protein serine and threonine phosphorylation in human sperm capacitation. Biol Reprod. 1999;60:1402–1409. doi: 10.1095/biolreprod60.6.1402. [DOI] [PubMed] [Google Scholar]
- Ni Y, Li K, Xu W, Song L, Yao K, Zhang X, Huang H, Zhang Y, Shi QX. Acrosome reaction induced by recombinant human zona pellucida 3 peptides rhuZP3a22∼176 and rhuZP3b177∼348 and their mechanism. J Androl. 2007;28:381–388. doi: 10.2164/jandrol.106.001289. [DOI] [PubMed] [Google Scholar]
- Ni Y, Zhou Y, Chen WY, Zheng M, Yu J, Li C, Zhang Y, Shi QX. HongrES1, a cauda epididymis-specific protein, is involved in capacitation of guinea pig sperm. Mol Reprod Dev. 2009;76:984–993. doi: 10.1002/mrd.21063. [DOI] [PubMed] [Google Scholar]
- Okamura N, Tanba M, Fukuda A, Sugita Y, Nagai T. Forskolin stimulates porcine sperm capacitation by increasing calcium uptake. FEBS Lett. 1993;316:283–286. doi: 10.1016/0014-5793(93)81309-n. [DOI] [PubMed] [Google Scholar]
- Orta G, Ferreira G, Jose O, Trevino CL, Beltran C, Darszon A. Human spermatozoa possess a calcium-dependent chloride channel that may participate in the acrosomal reaction. J Physiol. 2012;590:2659–2675. doi: 10.1113/jphysiol.2011.224485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathi R, Colenbrander B, Bevers MM, Gadella BM. Evaluation of in vitro capacitation of stallion spermatozoa. Biol Reprod. 2001;65:462–470. doi: 10.1095/biolreprod65.2.462. [DOI] [PubMed] [Google Scholar]
- Reddy MM, Light MJ, Quinton PM. Activation of the epithelial Na+ channel (ENaC) requires CFTR Cl− channel function. Nature. 1999;402:301–304. doi: 10.1038/46297. [DOI] [PubMed] [Google Scholar]
- Roldan ER, Murase T, Shi QX. Exocytosis in spermatozoa in response to progesterone and zona pellucida. Science. 1994;266:1578–1581. doi: 10.1126/science.7985030. [DOI] [PubMed] [Google Scholar]
- Scholich K, Barbier AJ, Mullenix JB, Patel TB. Characterization of soluble forms of nonchimeric type V adenylyl cyclases. Proc Natl Acad Sci USA. 1997;94:2915–2920. doi: 10.1073/pnas.94.7.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi QX, Friend DS. Gossypol-induced inhibition of guinea pig sperm capacitation in vitro. Biol Reprod. 1983;29:1027–1032. doi: 10.1095/biolreprod29.4.1027. [DOI] [PubMed] [Google Scholar]
- Shi QX, Roldan ERS. Evidence that a GABAA-like receptor is involved in progesterone-induced acrosomal exocytosis in mouse spermatozoa. Biol Reprod. 1995;52:373–381. doi: 10.1095/biolreprod52.2.373. [DOI] [PubMed] [Google Scholar]
- Shi QX, Tso WW, Friend DS. Gossypol inhibition of sperrmatogenesis, sperm motility and metabolism. In: Mohri H, editor. New Horizons in Sperm Cell Research. New Nork, London, Paris, Montreux: Japan Sci Soc Press/Gordon and Breach Sci Publisher; 1987. pp. 389–408. [Google Scholar]
- Shi QX, Zhong CL, Ye Z, Liu LC. Spermine—an inhibitor of in vitro capacitation and fertilization in hamster sperm. Chin Sci Bull. 1989;34:1820–1825. [Google Scholar]
- Shi QX, Zhong CL, Ye Z, Yuan YY, Ren Y, Wang ZJ. Spermine inhibition of in vitro fertilizing ability of human spermatozoa and its possible mode of action. Acta Physiol Sin. 1991;43:480–488. [PubMed] [Google Scholar]
- Shi QX, Yuan YY, Roldan ER. Gamma-aminobutyric acid (GABA) induces the acrosome reaction in human spermatozoa. Mol Hum Reprod. 1997;3:677–683. doi: 10.1093/molehr/3.8.677. [DOI] [PubMed] [Google Scholar]
- Signorelli J, Diaz ES, Morales P. Kinases, phosphatases and proteases during sperm capacitation. Cell Tissue Res. 2012;349:765–782. doi: 10.1007/s00441-012-1370-3. [DOI] [PubMed] [Google Scholar]
- Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;2:366. doi: 10.1016/s0140-6736(78)92957-4. [DOI] [PubMed] [Google Scholar]
- Su L, Mruk DD, Cheng CY. Drug transporters, the blood-testis barrier, and spermatogenesis. J Endocrinol. 2011;208:207–223. doi: 10.1677/JOE-10-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez SS, Pacey AA. Sperm transport in the female reproductive tract. Hum Reprod Update. 2006;12:23–37. doi: 10.1093/humupd/dmi047. [DOI] [PubMed] [Google Scholar]
- Trounson A, Webb J. Fertilization of human oocytes following reinsemination in vitro. Fertil Steril. 1984;41:816–819. doi: 10.1016/s0015-0282(16)47891-x. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 1995a;121:1129–1137. doi: 10.1242/dev.121.4.1129. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Moore GD, Bailey JL, Leclerc P, Connors SA, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development. 1995b;121:1139–1150. doi: 10.1242/dev.121.4.1139. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Galantino-Homer H, Moore GD, Bailey JL, Ning X, Fornes M, Kopf GS. The molecular basis of sperm capacitation. J Androl. 1998;19:242–248. [PubMed] [Google Scholar]
- Visconti PE, Krapf D, de la Vega-Beltran JL, Acevedo JJ, Darszon A. Ion channels, phosphorylation and mammalian sperm capacitation. Asian J Androl. 2011;13:395–405. doi: 10.1038/aja.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XF, Zhou CX, Shi QX, Yuan YY, Yu MK, Ajonuma LC, Ho LS, Lo PS, Tsang LL, Liu Y, et al. Involvement of CFTR in uterine bicarbonate secretion and the fertilizing capacity of sperm. Nat Cell Biol. 2003;5:902–906. doi: 10.1038/ncb1047. [DOI] [PubMed] [Google Scholar]
- Wassarman PM. Early events in mammalian fertilization. Annu Rev Cell Biol. 1987;3:109–142. doi: 10.1146/annurev.cb.03.110187.000545. [DOI] [PubMed] [Google Scholar]
- Wertheimer EV, Salicioni AM, Liu W, Trevino CL, Chavez J, Hernandez-Gonzalez EO, Darszon A, Visconti PE. Chloride is essential for capacitation and for the capacitation-associated increase in tyrosine phosphorylation. J Biol Chem. 2008;283:35539–35550. doi: 10.1074/jbc.M804586200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PY. CFTR gene and male fertility. Mol Hum Reprod. 1998;4:107–110. doi: 10.1093/molehr/4.2.107. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. 4th edn. Cambridge, UK: Cambridge University Press; 1999. [Google Scholar]
- Xu WM, Shi QX, Chen WY, Zhou CX, Ni Y, Rowlands DK, Yi LG, Zhu H, Ma ZG, Wang XF, et al. Cystic fibrosis transmembrane conductance regulator is vital to sperm fertilizing capacity and male fertility. Proc Natl Acad Sci USA. 2007;104:9816–9821. doi: 10.1073/pnas.0609253104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill J, editors. The Physiology of Reproduction. New York: Raven Press; 1994. pp. 189–317. [Google Scholar]
- Yanagimachi R, Yanagimachi H, Rogers BJ. The use of zona-free animal ova as a test-system for the assessment of the fertilizing capacity of human spermatozoa. Biol Reprod. 1976;15:471–476. doi: 10.1095/biolreprod15.4.471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.