Abstract
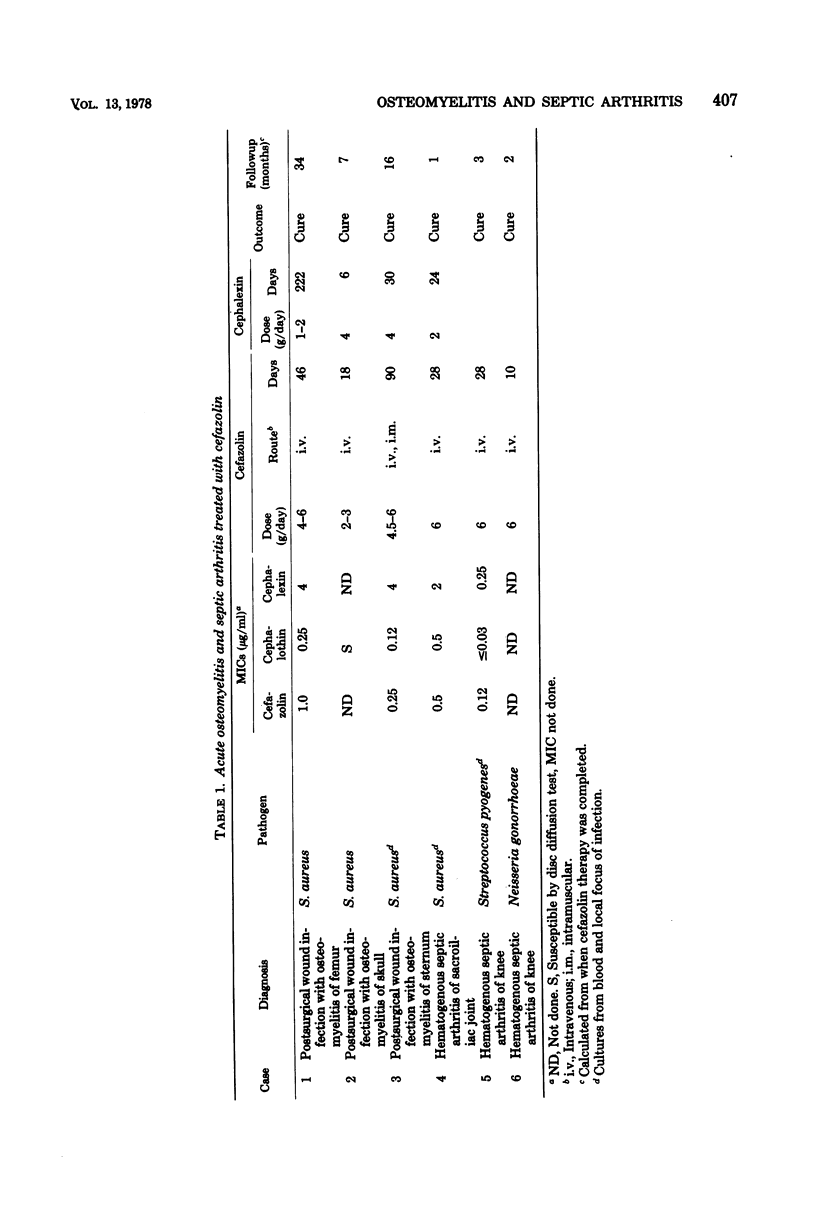
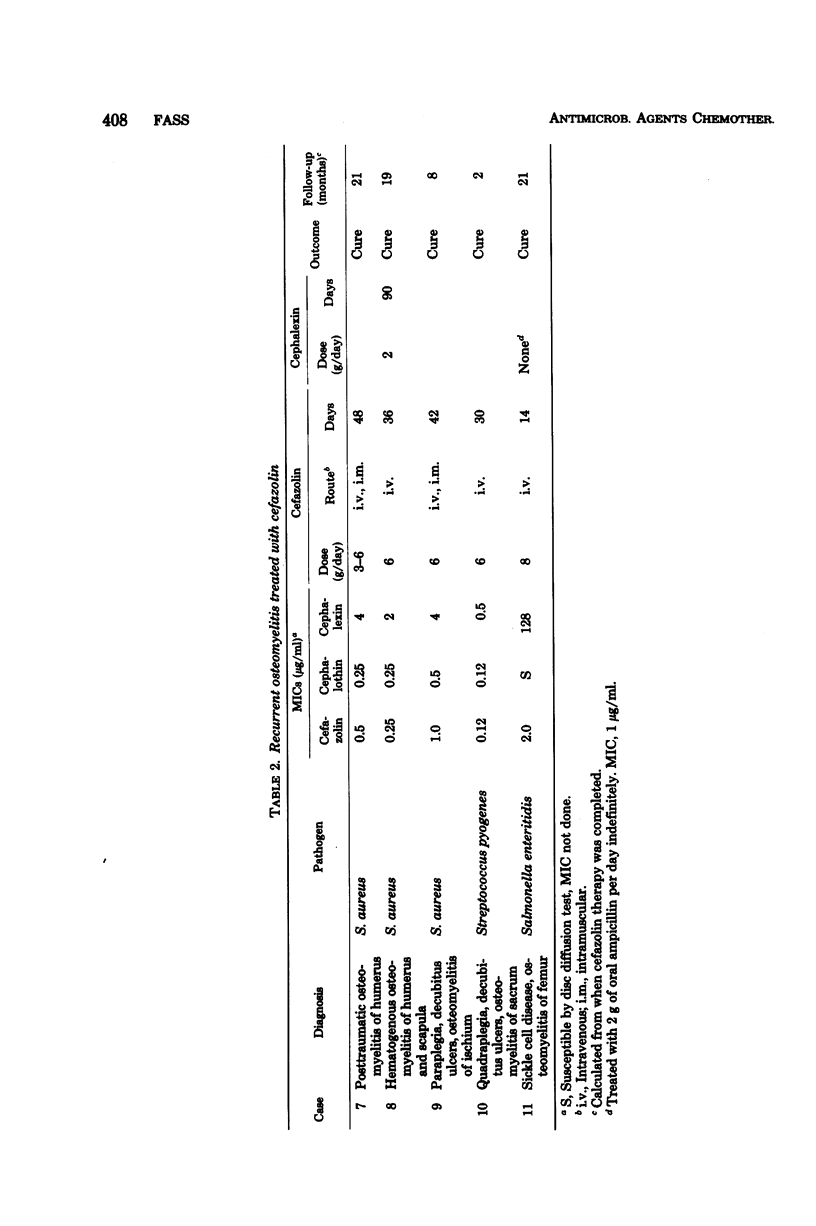
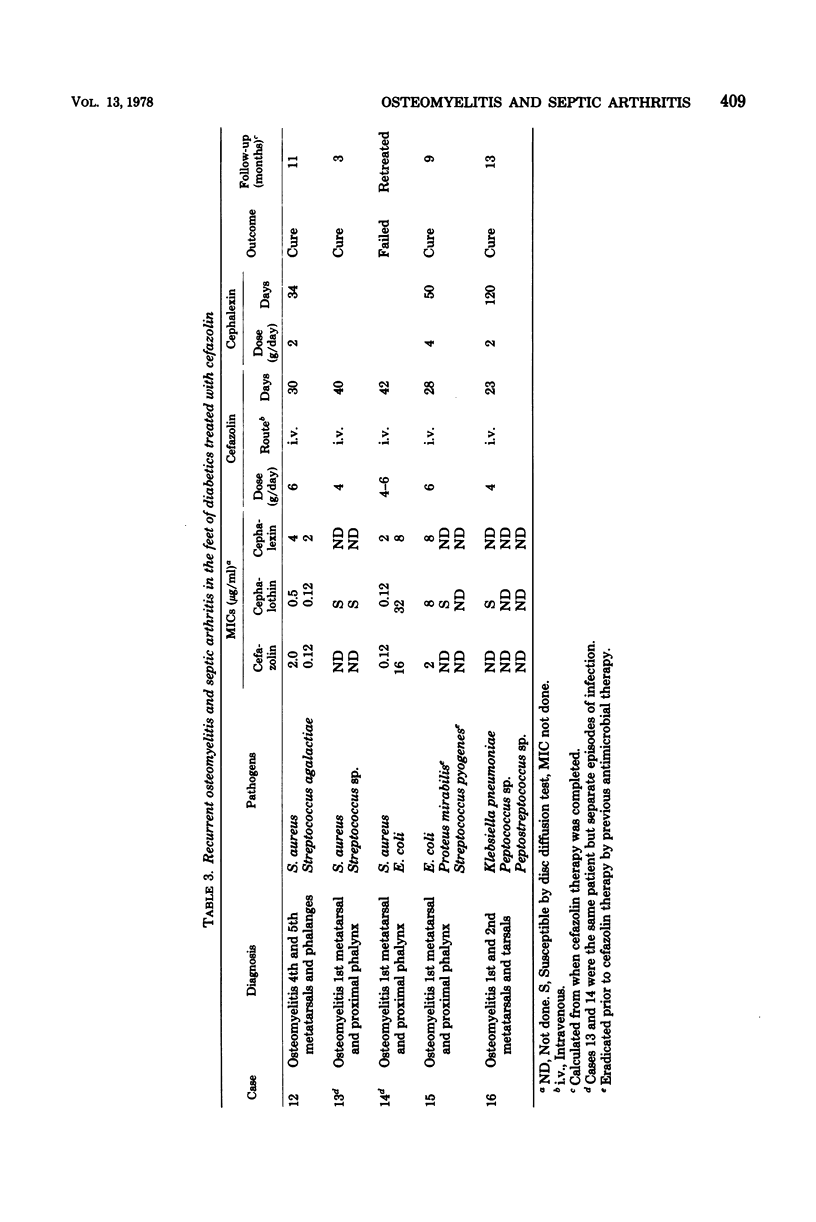
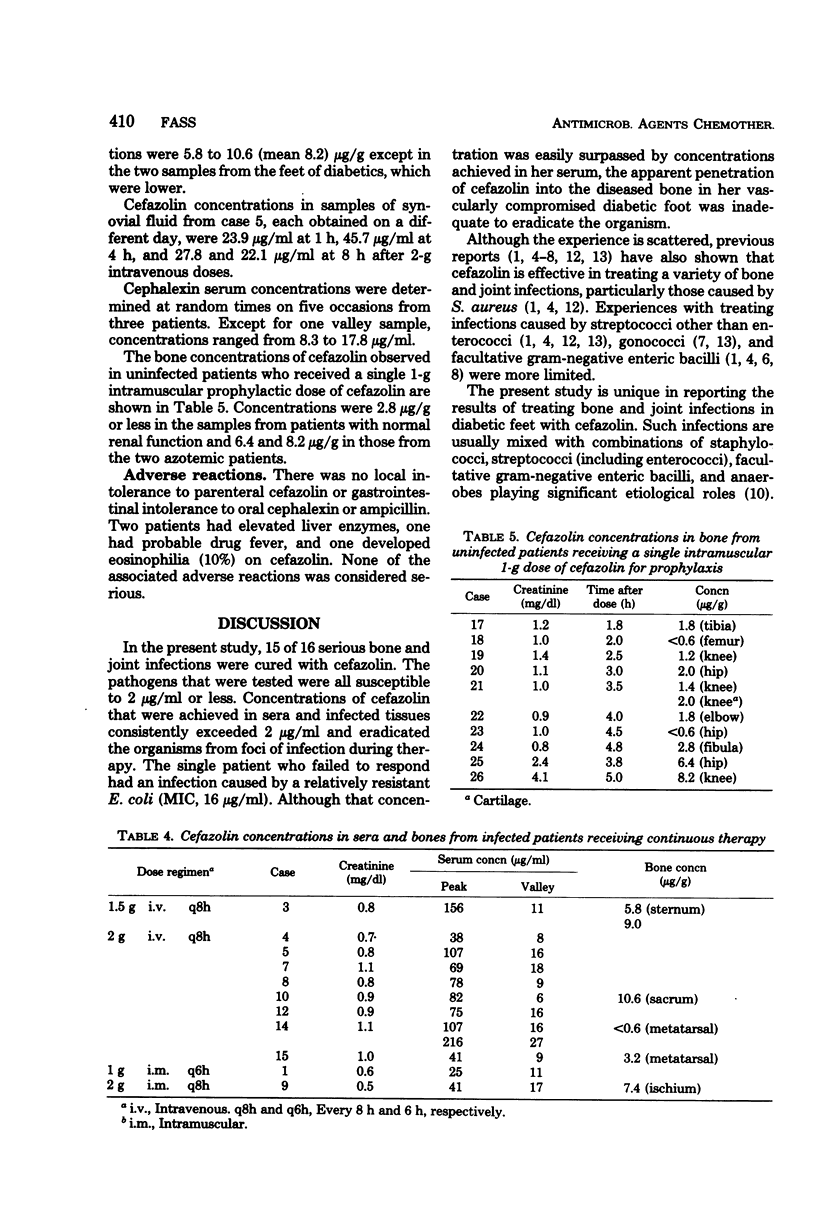
Sixteen cases of severe osteomyelitis and septic arthritis caused by staphylococci, streptococci, gonococci, and a variety of gram-negative bacilli were treated with 4 to 8 g of parenteral cefazolin per day; nine received subsequent therapy with oral cephalexin or ampicillin. Of 16 infections, 15 were apparently cured. Cefazolin concentrations in those patients were: serum (peak), 25 to 216 micrograms/ml; synovial fluid, 24 to 46 micrograms/ml; and bone, 3.2 to 10.6 micrograms/g. Bacterial pathogens had minimal inhibitory concentrations of cefazolin of 2 micrograms or less per ml and seemed to be eradicated from foci of infection during therapy. One infection in a diabetic patient did not respond; despite high concentrations of cefazolin in serum, no detectable antibiotic was present in her infected metatarsal, and the infecting Escherichia coli (minimal inhibitory concentration, 16 micrograms/ml) was not eradicated during therapy. Concentrations of cefazolin in bone in 10 uninfected patients who received 1-g intramuscular doses prophylactically before surgery were also measured. Concentrations in bones from those who had normal renal function ranged from less than 0.6 to 2.8 micrograms/g.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arango J. L., Trujillo H., Worren D., Uribe A., Agudelo N. H., de Vidal E. L. Effectiveness of two new cephalosporins, cephazolin and cephapirin, administered intermittently in acute and chronic osteomyelitis in children. J Int Med Res. 1976;4(3):183–194. doi: 10.1177/030006057600400307. [DOI] [PubMed] [Google Scholar]
- Ernst E. C., Berger S., Barza M., Jacobus N. V., Tally F. P. Activity of cefamandole and other cephalosporins against aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 1976 May;9(5):852–855. doi: 10.1128/aac.9.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass R. J., Rotilie C. A., Prior R. B. Interaction of clindamycin and gentamicin in vitro. Antimicrob Agents Chemother. 1974 Nov;6(5):582–587. doi: 10.1128/aac.6.5.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold J. A., McKee J. J., Ziv D. S. Experience with cefazolin: an overall summary of pharmacologic and clinical trials in man. J Infect Dis. 1973 Oct;128(Suppl):S415–S412. doi: 10.1093/infdis/128.supplement_2.s415. [DOI] [PubMed] [Google Scholar]
- Hammer G. S., Ribner B. S., Meyers B. R., Hirschman S. Z. Clinical studies with cefazolin: a new cephalosporin antibiotic. Mt Sinai J Med. 1975 Mar-Apr;42(2):142–149. [PubMed] [Google Scholar]
- Handsfield H. H., Wiesner P. J., Holmes K. K. Treatment of the gonococcal arthritis-dermatitis syndrome. Ann Intern Med. 1976 Jun;84(6):661–667. doi: 10.7326/0003-4819-84-6-661. [DOI] [PubMed] [Google Scholar]
- Kirby W. M., Regamey C. Pharmacokinetics of cefazolin compared with four other cephalosporins. J Infect Dis. 1973 Oct;128(Suppl):S341–S346. doi: 10.1093/infdis/128.supplement_2.s341. [DOI] [PubMed] [Google Scholar]
- Louie T. J., Bartlett J. G., Tally F. P., Gorbach S. L. Aerobic and anaerobic bacteria in diabetic foot ulcers. Ann Intern Med. 1976 Oct;85(4):461–463. doi: 10.7326/0003-4819-85-4-461. [DOI] [PubMed] [Google Scholar]
- Nightingale C. H., Greene D. S., Quintiliani R. Pharmacokinetics and clinical use of cephalosporin antibiotics. J Pharm Sci. 1975 Dec;64(12):1899–1926. doi: 10.1002/jps.2600641202. [DOI] [PubMed] [Google Scholar]
- Pickering L. K., O'Connor D. M., Anderson D., Bairan A. C., Feigin R. D., Cherry J. D. Comparative evaluation of cefazolin and cephalothin in children. J Pediatr. 1974 Dec;85(6):842–847. doi: 10.1016/s0022-3476(74)80357-4. [DOI] [PubMed] [Google Scholar]
- Reller L. B., Karney W. W., Beaty H. N., Holmes K. K., Turck M. Evaluation of cefazolin, a new cephalosporin antibiotic. Antimicrob Agents Chemother. 1973 Apr;3(4):488–497. doi: 10.1128/aac.3.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabath L. D., Wilcox C., Garner C., Finland M. In vitro activity of cefazolin against recent clinical bacterial isolates. J Infect Dis. 1973 Oct;128(Suppl):S320–S326. doi: 10.1093/infdis/128.supplement_2.s320. [DOI] [PubMed] [Google Scholar]
- Simon H. J., Yin E. J. Microbioassay of antimicrobial agents. Appl Microbiol. 1970 Apr;19(4):573–579. doi: 10.1128/am.19.4.573-579.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilack J. D., Flittie W. H., Williams T. W., Jr Bone concentrations of antimicrobial agents after parenteral administration. Antimicrob Agents Chemother. 1976 Jan;9(1):169–171. doi: 10.1128/aac.9.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]


