Abstract
The early social environment has a profound impact on developmental trajectories. Although an impoverished early environment can undermine the acquisition of appropriate social skills, the specific role played by the different components of an individual’s early environment in building social competencies has not been fully elucidated. Here we setup an asynchronous communal nesting paradigm in mice to disentangle the influence of maternal care and early peer interactions on adult social behavior and neural systems reportedly involved in the regulation of social interactions. The asynchronous communal nesting consists of three mothers giving birth three days apart, generating three groups of pups -- the Old, the Middle and the Young – all raised in a single nest from birth to weaning. We scored the amount of maternal and peer interactions received by these mice and by a fourth group reared under standard conditions. At adulthood, the four experimental groups have been investigated for social behavior in a social interaction test, i.e. facing an unfamiliar conspecific during five 20-min daily encounters, and for oxytocin receptor and BDNF levels. Results show that only individuals exposed to high levels of both maternal and peer interactions demonstrated elaborate adult agonistic competencies, i.e. the ability to promptly display a social status, and high BDNF levels in the hippocampus, frontal cortex and hypothalamus. By contrast, only individuals exposed to high levels of peer interactions showed enhanced adult affiliative behavior and enhanced oxytocin receptor levels in selected nuclei of the amygdala. Overall these findings indicate that early interactions with mother and peers independently shape specific facets of adult social behavior and neural systems involved in social interaction.
Keywords: early experiences, social, peer, mother, BDNF, oxytocin, mouse, environment
Introduction
The mother-child interaction is considered as the cornerstone of emotional development and mother-infant attachment has been conceptualized as an emotional bond that impacts behavior “from the cradle to the grave” (Bowlby, 1969). This perspective has had a fundamental influence upon studies on the development of behavior, with an individual’s social interactions, including those with peers, being assumed to be dependent upon the quality of these early interactions with the mother. However, this view has been challenged and it has been proposed that early peer interactions comprise a separate system of meaningful relationships that also acts as a predictor of developmental outcomes (Hartup, 1979; Parke et al., 2002). Indeed, peer aggression, social withdrawal or lack of friendship in childhood lead to long-term adjustment problems, including delinquency, addiction, academic difficulties, and vulnerability to anxiety and depression (Schneider, 2000). In addition, children’s interactions and play with peers have consequences on adult social competencies (Howes and Phillipsen, 1998; Hughes and Dunn, 1998). Finally, children who lack friendship are at risk of later poor emotional regulation (Walden et al., 1999) and show increased adult vulnerability to behavioral problems, including social dysfunction (Rutter et al., 2001; Fries et al., 2005). More recently, an even more radical view has been proposed, which argues that parents may play only a relatively minor role in determining individual differences in behavior and that these are actually mainly shaped by interactions with peers (Harris, 1995). Despite increasing empirical investigation of these issues, the relative contributions and interactive nature of the mother-infant relationship and of peer relations to the development of the individual is still highly debated.
The use of animal models has advanced our understanding of how variations in the quality of the early social environment can alter developmental trajectories. Seminal work by Harlow, Levine, Meaney and others showed that disruption of and individual differences in mother-infant interactions has consequences for the physiology and social behavior of adult offspring (Levine, 1957; Harlow, 1958; Liu et al., 1997; Champagne and Curley, 2009; Bale et al., 2010). However, the majority of research performed with animal models has focused on maternal care and has overlooked the contribution of the early peer interactions.
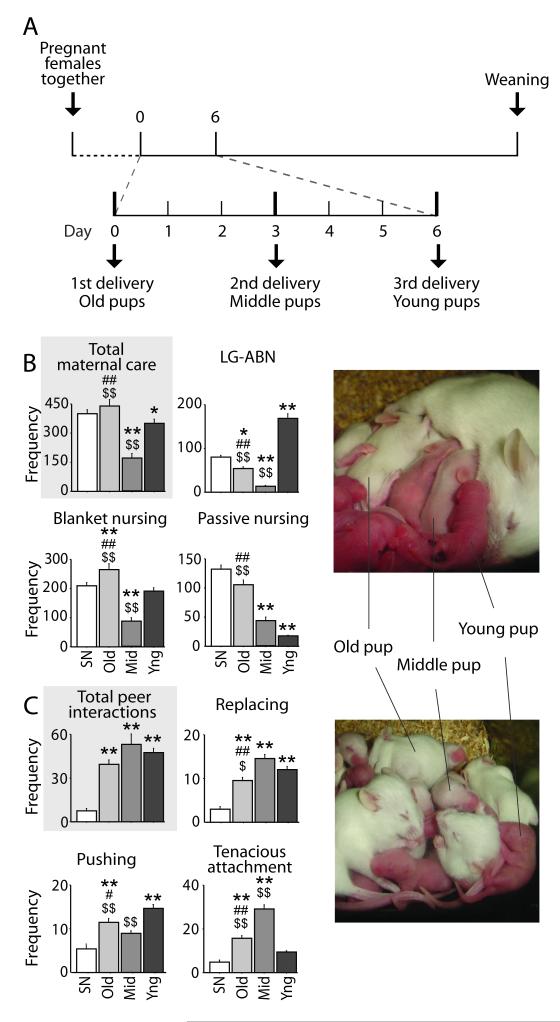
We have developed an experimental approach which allows us to uniquely characterize the relative contribution of mother-offspring and peer interactions in shaping adult social behavior through the use of a modified version of the communal nest (CN) paradigm (Branchi et al., 2006; Branchi, 2009). The CN paradigm, which consists of three lactating females that place their litters together in a single nest and engage in shared care-giving from birth to weaning, is an ethologically meaningful rearing strategy which induces variation in the amount of both maternal and peer social interactions. Although housing a single dam with her pups is typical in the laboratory, in the wild over 90% of female mice rear their offspring communally (Crowcroft and Rowe, 1963). This paradigm strongly affects adult endocrine and neurobehavioral profiles (Curley et al., 2009; Branchi et al., 2010; Branchi et al., 2011b), and especially social behavior (Branchi et al., 2006). The paradigm used in the present study is an asynchronous CN paradigm in which each female of the trio gives birth every three days (Fig. 1A). This procedure generates three experimental groups consisting of pups reared in the same nest but differing by age: pups in the Old group are three days older than the Middle group; pups in the Middle group are three days older than the Young group; while pups in the Young group are the last to be born. A fourth experimental group is comprised of mice reared under standard conditions (SN; one dam rearing her own litter).
Figure 1. Maternal care and peer interactions during the first two postnatal weeks.
(A) Scheme representing the communal nesting , detailing the relative time interval between deliveries of the three mothers. (B) Maternal care. The Middle (Mid) group (n=10) received less maternal care overall compared to standard nesting (SN) (n=11), Old (n=10) and Young (Yng) (n=10) group. In particular, the Middle group received less licking/grooming-arched back nursing (LG-ABN) and blanket nursing compared to the other groups (p < 0.01). (C) Early peer interactions during the first two postnatal weeks. SN pups (n=6 litters) showed lower levels of peer interactions particularly for those behaviors involving a direct competition (replacing and pushing) compared to the other groups (n=6 litters in each group) (at least p < 0.05). High levels of tenacious attachment, in the Middle group (p <0.01 vs. all the others) could compensate for the low levels of maternal care received (data shown in B). Gray boxes highlight total amount of maternal behavior and peer interactions. The cage was considered as the statistical unit. **, ## and $$ = p < 0.01 respectively vs. SN, Middle and Young; * and $ = p < 0.05 respectively vs. SN and Young. Data shown are means ± SEMs.
Methods and materials
Animals and Breeding Procedure
Forty male and eighty female mice of an outbred CD-1 Swiss-derived strain (ICR) were purchased from a commercial breeder (Harlan, 20050 Correzzana, MI, Italy). Animals were housed at 21±1 °C, relative humidity 60±10%, with lights on from 06.00 h to 18.00 h. Males and females were housed in same-sex groups of 8 individuals in 42 × 27 × 14 cm Plexiglas boxes with a metal top and sawdust as bedding, and with pellet food (Enriched standard diet purchased from Mucedola, Settimo Milanese, Italy) and tap water ad libitum.. All animal handling and experimental procedures were performed according to European Communities Council Directive 86/609 and the Italian Decreto L.vo 116/92.
At three months of age, breeding groups, made up of 1 male and 2 females, were formed. Vaginal plugs were checked twice a day (at 09.00 h and 19.00 h). Males were removed around gestational day 12 and females were assigned to one of the two experimental groups: standard nesting (SN) or communal nesting (CN). In the SN condition, one female was housed in a 33 × 13 × 14 cm Plexiglas cage. In the CN condition, females were housed in trios in 42 × 27 × 14 cm Plexiglas cages, five days before the first delivery. The three CN mothers gave birth with an interval between two consecutive deliveries of 3 days. Thus, each CN nest was formed by 3 groups of mouse pups of different age: Old, Middle and Young pups. Each litter was culled on the day after birth, postnatal day (PND) 1 (birth = PND 0) to four males and four females for the SN group, and to 12 males and 12 females for the CN group. Pups were weaned at PND 25 and males of each litter were housed in groups of 4 in 42 × 17 × 14 cm Plexiglas cages. Before weaning, the cage for the CN condition was about 2-3 times larger than the cage for the SN condition. Thus, the average amount of space for pup in the two conditions was comparable.
Maternal care observations
Eleven SN and ten CN cages were observed from PND 1 to PND 14. Sample size was: SN, n=11; Young, Middle, Old group; n=10 each. Maternal behavior was scored during three sessions each day. Data were collected in each session for each cage, with one-zero sampling, over 20 10-sec observations that were 180 sec apart (60 observation per day for each dam). The observer recorded whether the behavior was present or not during the 10- sec observation; more than one behavior could be present, and thus recorded. The sessions started at, 11.00 h, 15.00 h, 19.00 h. The last session took place during the active phase of the 12:12 cycle, and was performed under dim red light illumination. Maternal behavior has been analyzed according to previous work (Branchi et al., 2006). In particular, the following behaviors were scored: arched-back nursing: the dam is immobile and in a high upright dorsal arch posture supported by rigid fore- and hind limbs, the head is depressed, the trunk and limbs are bilaterally symmetrical, and pups are attached to the nipples; blanket nursing: the dam is over the pups, relatively immobile, bilaterally symmetrical, with the head not depressed, and in a low dorsal arch posture supported by rigid fore- and hind limbs or in a low dorsal arch posture supported by rigid fore limbs or rigid hind limbs or lies flat on top of the pups with little or no limb support; passive nursing: the dam body is lying down on her side with more than one pup usually attached to the nipples; total nursing is the sum of the three nursing positions; licking/grooming: licking and grooming of the pup body; ano-genital Licking: licking concentrated on the ano-genital region of the pup; licking/grooming and arched-back nursing (LG-ABN): the sum of arched back nursing and licking/grooming; retrieval: the dam picks up the pup gently with the incisors by its dorsal skin and carries it to the nest; dig: digging in the sawdust, moving it around using the snout and/or both the forepaws and hindpaws, mostly moving around the cage and sometimes changing the arrangement of the substrate material; rearing: the animal stands on its hind limbs, often sniffing; moving: the animal moves around the cage, actively exploring; eating: nibbling food pellets held in its forepaws or held in the food-containing compartment of the cage; drinking: drinking from the water bottle; self-grooming: the animal licks, combs, scratches any part of own body; resting: the animal lies still in a sleeping mode; out of nest: moving about the cage but not carrying pups or nesting material. Scoring of maternal behavior in the CN group occurred across 20 days (with observations for the Old group occurring across days 1-14, for the Middle group across days 4-17 and for the Young group across days 7-20).
In each cage, the behavior of all mothers was scored. Each mother was univocally identified through marks made with non-toxic hair dye.
Pup social interaction observations
Six SN and six CN cages were observed from PND 1 to PND 14. Sample size was: n=6 in each group. Cages used for scoring pup social interactions were a subset of those used for maternal observations. Sibling social interaction behavior was scored during three sessions each day using the same sampling protocol used for maternal care observations. In each cage, the behavior of all pups was scored. The sessions started at 12.00 h, 16.00 h, 20.00 h. The last session was during the dark phase of the 12:12 cycle, and was performed under dim red light illumination. Behavioral categories scored were [adapted from (Mendl and Paul, 1990)]: 1) pup nutritive behavior: eat; drink, 2) pup competitive behavior: tenacious attachment (pup clings to the nipple); pushing (pup pushing another individual to gain access to a resource); replacing (pup displaces another individual from the resource), 3) pup social behavior: allogroom (pup is licking/grooming another individual); allosniff (pup is sniffing another individual), 4) general activity: active (any locomotor activity); rest (pup lying still); out of nest. Scoring of pup social interactions in the CN groups occurred across 20 days (with observations for the Old group occurring across days 1-14, for the Middle group across days 4-17 and for the Young group across days 7-20).
Developmental marks (e.g., size of the animal, fur density, eye opening, etc.) allowed the experimenter to identify which group -- Old, Middle or Young -- in each cage was receiving maternal care or peer interactions without any artificial mark. In all the analyses, PND indicated the actual age of the group under focal examination. As a consequence, the same PND for the Old, Middle or Young groups does not temporally overlap, as they were born three days apart.
Social interaction test
Social behavior was assessed using the protocol described previously (Branchi et al., 2006). From PND 90 to PND 95, after 2 weeks of isolation aimed at facilitating agonistic interactions (Alleva, 1993), mice (n = 20 for each experimental group) underwent a social interaction test consisting of a 20-min encounter each day, for five consecutive days. Experimental subjects were placed in a neutral cage with a standard opponent. The same experimental animal was paired with the same standard opponent, an adult CD-1 male of the same weight and age. During the agonistic interaction, behavior was video-recorded and behavioral analysis was conducted using commercial software (Observer 3.0 Wageningen, The Netherlands). The behavioral categories and elements scored were previously described and validated (Alleva, 1993). The behavioral categories and elements scored were:
- aggressive/offensive behaviors: latency to the first attack, the time from the beginning of the test session to the first biting attack; attack, fighting episode when a mouse belonging to the experimental group approaches the opponent and bites it; aggressive grooming, violent grooming of the animal on the back of the partner; chase, the animal goes after the opponent; offensive upright posture, the animal stands on its hindlimbs facing the opponent aggressively; tail rattling, fast tail vibration often observed in a distance ambivalence situation.
- defensive/subordinate behaviors: defensive upright posture, the animal stands on its hindlimbs and pushes the aggressive opponent with its forepaws; crouched posture, the animal lies on its ventrum, the head flat on the cage floor (this posture is often observed as an answer to aggressive grooming performed by the partner); submissive upright posture, the animal stands on its hindlimbs, the head pulled far back and its body rigid; freezing, animal reacts to the physical movements of the partner by remaining motionless; fleeing, animal moves very rapidly away from the partner, often associated with screaming;
- affiliative behaviors: anogenital sniff, sniffing the anogenital area of the partner; nose sniff, sniffing the head and the snout region of the partner; allogrooming, grooming the partner; body sniff, sniffing any other area of the body of the partner.
- non-social activities: wall rearing, animal stands on its hindlimbs and touches the walls of the cage with the forelimbs; bar holding, animal grasps the metal top of the cage holding itself above the level of the ground.; self-grooming, animal licks and mouths its own fur, sometimes helping itself with its forepaws; digging, animal digs the sawdust with the forelimbs, often kicking it away with the hindlimbs.
During the last fighting session, the social status of each experimental subject was identified:
- dominant, the mouse displays a consistent amount of attacks without being attacked
- subordinate, the mouse is continuously attacked and defeated without showing any counterattack.
Since not all animals established a clear hierarchy, the final sample size of SN, Old, Middle and Young was, for dominants, respectively n= 6, 12, 10 and 13, and for subordinates, respectively n= 13, 8, 8 and 5.
BDNF determination
Brain Derived Neurotrophic factor (BDNF) protein evaluation was carried out in the hippocampus, frontal cortex and hypothalamus by an ELISA kit “BDNF Emax ImmunoAssay System number G6891” by Promega, (Madison, WI, USA) according to the instructions of the manufacturer. Sample size was n=5-6 in each group. Tissues were homogenated in the kit calibration buffer and centrifuged. The brain tissues were homogenized with ultrasonication in extraction buffer 0.2% triton. Briefly, 96-well immunoplates were coated with 100 μl per well of monoclonal anti-mouse-BDNF antibody. After an overnight incubation at 4°C, the plates were washed three times with wash buffer and the samples were incubated in the coated wells (100 μl each) for 2 h at room temperature with shaking. After an additional five washes the immobilized antigen was incubated with an anti-human BDNF antibody for 2 h at room temperature with shaking. The plates were washed again with wash buffer, and then incubated with an anti-IgY HRP for 1 h at room temperature. After another wash the plates were incubated with a TMB/Peroxidase substrate solution for 15 min and then phosphoric acid 1M (100 μl/well) was added to the wells. The colorimetric reaction product was measured at 450 nm using a microplate reader (Dynatech MR 5000, PBI International, USA). BDNF concentrations were determined, from the regression line for the BDNF standard (ranging from 7.8 to 500 pg/ml purified mouse BDNF) incubated under similar conditions in each assay. The sensitivity of the assay was about 15 pg/g of BDNF and cross-reactivity with other related neurotrophic factors (NGF, Neurotrophin-3 and Neurotrophin-4) was less than 3%. All assays were performed in duplicate.
Oxytocin receptor (OTR) autoradiography
Brains for OTR autoradiography were different from those used for BDNF protein levels analysis. Mice (sample size: SN, Old, Middle and Young mice respectively, n = 9, 10, 9 and 10 per group) were sacrificed through rapid decapitation and brains extracted, placed briefly in isopentane, and stored at −80°C. Brains were sectioned in the coronal plane at 16 μm, and sections thaw mounted onto poly-L-lysine coated slides that were stored at −80°C until the assay was performed. Slide-mounted coronal brain sections were processed for Oxytocin (OT) receptor autoradiography using 125I-d(CH2)5[Tyr-Me)2,Tyr-NH29] OVT (New England Nuclear, Boston, MA, USA) (New England Nuclear, Boston, MA, USA) as previously described (Champagne et al., 2001; Curley et al., 2009). All autoradiograms were analyzed using an image-analysis system (MC1D-4, Interfocus Imaging, Cambridge, UK). Between three and six sections were analyzed bilaterally for each brain region. OT receptor binding was analyzed between Bregma -1.06 and -1.46 mm in the anterior cortical nucleus and the central nucleus of the amygdala, and between Bregma -1.58 and -1.82 mm in the posterior dorsal medial, lateral and basolateral nuclei of the amygdala. Sections that did not contain the region of interest and brains that had too few sections of sufficient quality were excluded from the analysis. The actual number of brains analyzed for ORT binding in the SN, Old, Middle and Young groups was, respectively, 8, 9, 8 and 7. For each animal, total and non-specific binding was measured for each region using adjacent brain slices and the difference taken to yield specific binding which was converted to fmol/mg using microscales (GE Healthcare). The statistical analysis was performed on the mean of these values for each animal by brain region according to the mouse brain atlas (Paxinos and Franklin, 2003). Brains for BDNF protein levels and for oxytocin receptor (OTR) autoradiography were collected one month after the social interaction test. Though, according to the literature, 1 month is a standard wash-out period, the influence of social interaction on BDNF protein levels and OTR binding cannot be completely ruled out.
Statistical analysis
All data were analyzed with ANOVAs, considering rearing condition (standard vs. communal) and birth order (Old, Middle and Young) as between-subject variables and subject as a random factor nested within rearing condition; time (day) as repeated measures within subjects. Post hoc comparisons were performed using the Tukey’s HSD test. Latency data were analyzed with a Mann-Whitney non-parametric test (statistical software Statview II, Abacus Concepts, CA, USA).
In all the analyses, including those concerning BDNF protein levels and OTR binding, the cage was considered as the statistical unit in order to take into account litter effects. When more individuals (e.g., pups) from the same cage and experimental group were considered, the average value was used in the analysis.
Results
Maternal care and pup social interaction observations
When considering maternal behavior, a main effect of rearing condition was found for the total amount of arched-back nursing with licking and grooming [F (3,481) = 137.21, p < 0.0001], blanket [F(3, 481) = 72.76, p < 0.0001 ], passive [F(3, 481) = 27.24, p <0.0001] and total nursing [F(3,39) = 125.29, p < 0.0001]. In particular, the Middle group received very low levels of maternal care compared with the other two ge groups (Fig. 1B and Fig. S1). This was particularly evident for licking/grooming and arched-back nursing (LG-ABN; p < 0.01), considered to be the two maternal behaviors with the most profound developmental influence on the adult offspring neurobehavioral profile of rodents (Meaney, 2001; Champagne and Curley, 2009), as well as blanket nursing (p < 0.01) . Moreover, in the CN group, mothers interacted with all pups in the nest and did not show a preference for their own litter (Fig. S2; for a picture see Fig. 1B). Rearing condition significantly affected early social interaction among peers, modifying all behaviors investigated: replacing [F(3,440) = 42.06, p < 0.0001], pushing [F(3, 440) = 15.72, p < 0.0001], and tenacious attachment behavior [F(3, 440) = 48.08, p < 0.0001]. Individuals reared in the SN had fewer peer interactions compared to the Old, Middle and Young groups (p < 0.01; Fig. 1C and S3). Thus, individuals from the Old and Young groups received high levels of both maternal and peer interactions, the SN group had high levels of maternal behavior but low levels of peer interaction and the Middle group was characterized by low levels of maternal behavior but high levels of peer interaction. With regard to peer interactions in the CN group, pups were most likely to initiate social interaction with same-age or younger pups, and rarely with older pups (Fig. S4). It is worth noticing that these differences in pup social interactions displayed by the experimental communal groups may be in part due to differences in mobility and body size and weight associated to age differences.
Social interaction test
Agonistic behavior
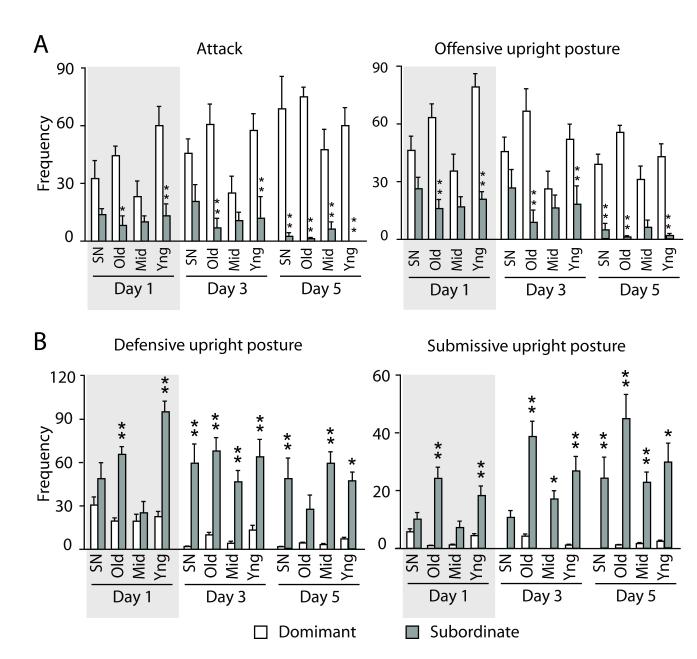
A significant interaction between rearing condition and social status was found for frequency of attack [F(3,67) = 4.165, p = 0.0091], offensive upright posture [F(3,67) = 4.210, p = 0.0087], defensive upright posture [F(3,67) = 2.558, p = 0.0625] and submissive upright posture [F(2,134) = 5.001, p = 0.0035]. While SN and Middle mice needed five social encounters to fully establish their specific social role (i.e. show the behavioral profile typical of a dominant or subordinate male), the Old and Young mice achieved a defined social role during the first social encounter demonstrating elaborate agonistic competencies. Social competencies are defined as the ability of an individual to modify the expression of its social behavior as a function of the available social information (Oliveira, 2009; Taborsky et al., 2012). In particular, dominants vs. subordinates in the Old and Young groups showed behavioral differences (at least, p < 0.05) already on day 1 of testing. It is important to note that the ability to promptly achieve a social status in the Old and Young group concerned not only aggressive/offensive behaviors, such as attacking, offensive upright postures and tail rattling (Fig. 2A and Fig. S5), but also defensive upright and submissive upright postures (Fig. 2B), demonstrating that the modification in social competencies cannot be simply attributed to an overall change in aggression levels. Overall, only those individuals that received both high levels of maternal stimulation and peer interactions showed elaborate agonistic social competencies at adulthood, indicating that both types of early social interactions are necessary for development of effective social skills. No difference in the number or proportion of mice displaying either the dominant or the subordinate role was found among the experimental groups.
Figure 2. Agonistic behavior in adulthood.
(A) and (B) The prompt ability to become either dominant or subordinate in a dyadic interaction is considered as an index of agonistic competencies. Such ability emerged at different time points in the different experimental groups both for offensive and defensive behaviors. The Old and the Young (Yng) group showed a significant difference in behavior between dominants and subordinates on the first day of agonistic interaction, while in the other groups this difference became significant only on the fifth encounter. Gray boxes highlight the first day of social interactions. (A) Offensive behaviors. (B) Defensive behaviors. Standard nesting (SN), Old, Middle (Mid) and Young dominants, respectively n= 6, 12, 10 and 13; subordinates, respectively n= 13, 8, 8 and 5. The cage was considered as the statistical unit.* and ** = respectively p < 0.05 and < 0.01 subordinate vs. dominant. Data shown are means ± SEMs.
Affiliative behavior
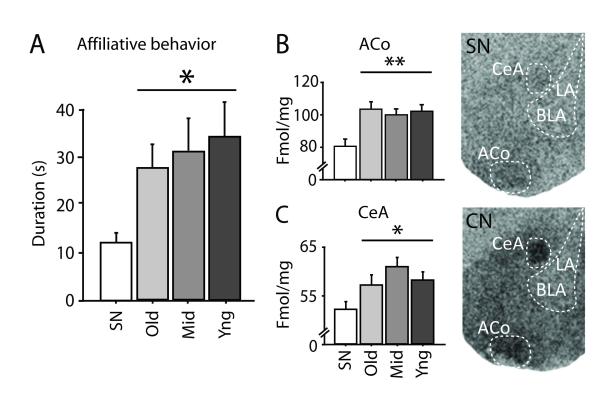
During the last encounter of the social interaction test when the social hierarchy was established, individuals from the different experimental groups showed different levels of reconciliatory affiliative behavior [F(3,146) = 3.211, p = 0.0279]. In particular, mice reared in a CN – the Old, Middle and Young groups – showed significantly higher levels of affiliative behavior towards a standard opponent male compared to SN individuals (p < 0.05; Fig. 4A). This result is in line with previous studies showing that, compared to mice reared in a SN, CN mice show more aggressive behavior when establishing a social hierarchy but engage in more affiliative behaviors when the hierarchy is already established (D’Andrea et al., 2007).
Figure 4. Being reared in a CN, independent of birth order, increased adult affiliative behavior and OTR binding, compared to the SN condition.
(A) Affiliative behavior measured on the fifth agonistic encounter when the social hierarchy is established. Old, Middle (Mid) and Young (Yng) groups (respectively, n =20, 19 and 20) showed a higher propensity to interact with a conspecific compared to standard nesting (SN) mice (n =18). (B) OTR binding. Old, Middle and Young mice (respectively, n =10, 9 and 10) showed higher levels of oxytocin receptor binding in the ACo and the CeA, compared to SN mice (n= 9). ACo, anterior cortical nucleus of the amygdala; CeA, central amygdala; LA, lateral amygdala; BLA, basolateral amygdala. The cage was considered as the statistical unit.* and ** = respectively p < 0.05 and < 0.01 vs. SN mice. Data shown are means ± SEMs.
BDNF
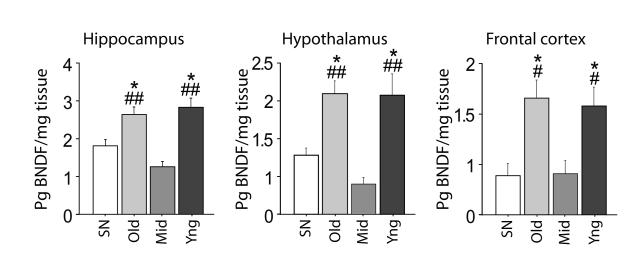
Congruent with this hypothesis, we found that the rearing condition altered adult BDNF levels in the hippocampus, frontal cortex and hypothalamus [respectively: F(3, 20) = 11.1622, p = 0.0002; F(3, 20) = 7.034, p = 0.0021; F(3, 26) = 6.529, p = 0.0019] In particular, the individuals from the Old and Young groups, which show more elaborate social competencies, have significantly higher levels of BDNF protein in the hippocampus, frontal cortex and hypothalamus compared to individuals from the Middle groups and SN reared mice (Fig. 3). These data show that being exposed during the early developmental period to both high levels of maternal and peer interactions leads to higher brain BDNF levels in adulthood compared to subjects exposed to only one form of early social stimulation.
Figure 3. Brain BDNF protein levels are modulated by the rearing condition.
Old and Young (Yng) groups showed higher BDNF levels in the hippocampus, hypothalamus and frontal cortex compared to both standard nesting (SN) and Middle (Mid) group (n= 5-6 per group). The cage was considered as the statistical unit. * = p < 0.05 vs. SN mice; # and ##= respectively, p < 0.05 and p < 0.01 vs. Middle group. Data shown are means ± SEMs.
OTR binding
We found that all of these groups have significantly higher levels of OTR binding in selected brain areas involved in social regulation, such as the anterior cortical nucleus of the amygdala (ACo; F(1, 32) = 10.570, p < 0.01; Fig. 4B), central amygdala (CeA; F(1, 32) = 4.931 p < 0.05; Fig. 4C) and dorsal posterior medial amygdala (dpMA; F(1, 30) = 5.472 p < 0.05; Fig. S6), compared to SN mice. No significant differences in oxytocin receptor binding were found in the lateral or basolateral nuclei of the amygdala. This result suggests that oxytocin functioning in various nuclei of the amygdala that are known to be critical mediators of social affiliation, recognition and aggression (Ferguson et al., 2002; Keverne and Curley, 2004) is strongly affected by enhanced early peer interactions, but is not affected by differences in maternal care between CN groups and SN reared individuals.
Discussion
The major finding of the present study is that early interactions with mother and peers independently shape social skills, brain BDNF expression and OTR binding in the amygdala at adulthood. In particular, high levels of interactions with mother and peers went along with elaborate social/agonistic competencies and with elevated brain BDNF levels. By contrast, high levels of peer interactions in the nest were selectively associated with high affiliative behavior and with increased OTR binding in different nuclei of the amygdala (for a summary of the main results see Table 1). Compared to previous data, these findings suggest that in addition to mother-infant interactions, peer interactions modify developmental trajectories, supporting the idea that the development of the central nervous system is molded by a complex interaction among early experiential systems (Parke et al., 2002; Glassman and Buettner, 2005).
Table 1.
Summary of the major effects of the different rearing conditions
| Experimental group |
Early experiences |
Outcome at adulthood |
||||
|---|---|---|---|---|---|---|
| Maternal care |
Peer interaction |
Agonistic behavior |
BDNF levels |
Affiliative behavior |
Oxytocin receptor |
|
| SN | + | − | − | − | − | − |
| Old | + | + | + | + | + | + |
| Middle | − | + | − | − | + | + |
| Young | + | + | + | + | + | + |
Here we show that, in an asynchronous CN mouse model, the amount of maternal care received changes according to birth order, with the Middle group receiving the least amount of care. In the mixed age communal nest group, Old pups successfully compete with the others to get access to lactating dams, while Young pups represent the most powerfully attractive stimulus for the mother and thus receive highest levels of active maternal care (i.e. LG-ABN). Though it is worth noticing that different factors are likely to shape this phenomenon in different species, interestingly it shares similarities with the condition observed in the middle child in humans, who often receives less maternal care than the elder or younger sibling. Our results also indicate that the amount of peer interactions is directly related to the number and age of nest-mates present.
Both maternal care and peer relationships have been shown to be relevant for the development of adult social behavior in rodents and non-human primates. For instance, decreased maternal behavior has been associated with reduced propensity to interact socially at adulthood (Macri and Laviola, 2004; Mintz et al., 2005) and early peer interactions shape individual differences in adult social behavior (Laviola and Alleva, 1995; Terranova and Laviola, 1995; Hudson, 2005; Suomi, 2005; Bautista et al., 2008; Yang et al., 2011). We thus investigated whether differential levels of maternal care and peer interactions might affect adult social behavior in two distinct domains: agonistic behavior (i.e., the ability to manage social interactions, playing either the dominant or the subordinate role) and affiliative behavior (i.e., the display of behavioral patterns aimed at reducing aggressive behavior and increasing social tolerance). Both domains are critical for the establishment and maintenance of successful social interactions. While agonistic behavior is used in the early phases of group formation and enables individuals to identify their relative hierarchical status, affiliative behavior is exploited -- in a post-conflict reconciliatory fashion -- to attenuate future aggression (Pellegrini, 2008). Indeed, affiliative behavior is a natural aspect of animal interactions that has co-evolved with aggression to achieve group cohesion (Hartup et al., 1988; de Waal, 1989; Verbeek, 2008 ).
The present findings show for the first time that the combination of both components of the early social environment are critical for the development of the adult social agonistic competencies. In fact, once mice reached adulthood, only those having received a combination of both high levels of maternal care and peer interactions during infancy displayed increased competencies, which consist of both rapid social learning and social plasticity - i.e. the ability to assume either aggressive/offensive or submissive/defensive behaviors, according to the faced unfamiliar conspecific and the context. Significantly, the different experimental groups did not differ with respect to the proportion of individuals that become dominant or subordinate, suggesting that they showed overall similar levels of aggressive behavior.
The combination of high levels of maternal care and peer interactions during infancy has been found to be critical also in determining the adult levels of brain BDNF, a key player in brain development and plasticity (Thoenen, 1995; Castren, 2005; Cirulli et al., 2009). Indeed, the experimental groups exposed to high levels of both social components showed significantly higher hippocampal, frontal cortical and hypothalamic BDNF protein levels compared to the other two groups, which were exposed to high levels of only component. Thus, experimental groups showing increased BDNF levels in selected brain areas reported to be involved in regulation of social behavior (Tsankova et al., 2006; Holmes et al., 2007; Lagace et al., 2010) displayed also greater agonistic social competencies. We have previously shown that increased BDNF levels are associated with social plasticity and increased social competencies, consequent to being reared in a synchronous CN where all litters are born on the same day (Branchi et al., 2006). Such association is consistent also with previous studies by other authors illustrating a key role of BDNF in the regulation of social behavior. For instance, BDNF knock-out mice show hyper-aggressiveness associated mainly with reduced levels of this neurotrophin in the hippocampus and cortex (Lyons et al., 1999; Chan et al., 2006; Lang et al., 2009). Importantly, BDNF expression in the mesolimbic dopamine pathway has been associated with learning about the consequences of social interactions, such as experience-dependent social aversion (Berton et al., 2006). Previous studies suggest that modifications in adult BDNF levels induced by being reared in a socially enriched condition, such as that provided by the CN, could be determined by early changes in the BDNF epigenetic structure (Roth et al., 2009; Champagne, 2010; Branchi et al., 2011a).
In contrast to agonistic social competencies, enhanced affiliative behavior emerged only in those groups who displayed high levels of early peer interactions during infancy - and independently from the amount of maternal care - suggesting that different adult social domains are structured through diverse early social experiences. High levels of peer interactions led also to higher adult OTR binding in selected areas of the amygdala known to be involved in the regulation of social affiliation and recognition, such as the ACo, CeA and dpMA. Previous studies have described that affiliative behavior is associated with OTR levels (Rodrigues et al., 2009; Ross and Young, 2009) and the neuropeptide hormone oxytocin has been implicated in the regulation of a variety of social behaviors (Lee et al., 2009; Walum et al., 2012). In particular, enhanced oxytocin functioning has been shown to facilitate affiliative behavior and social recognition (Cho et al., 1999; Bielsky and Young, 2004), though not intermale aggression (DeVries et al., 1997). In addition, long-term changes in central levels of the OTR have been shown to be particularly affected by differential social experiences (Curley et al., 2011).
Overall, these results indicate that early interactions with mother and peers independently affect adult social behavior and the neural substrates involved in its regulation in mice. Though we are not arguing that there is strict isomorphism between animal and human affiliative behavior, these findings are particularly interesting for future studies aiming to investigate the neurobiological mechanisms underlying how pathological aggressive behavior can lead to an escalation of violence without inhibitory control associated to affiliative behavior and reconciliation (Patrick, 2008). On the other hand, in view of the reported impairments of the oxytocin system and affiliative behavior in human mental disorders characterized by social deficits, such as autistic spectrum disorder (Lerer et al., 2008), the present data suggest that early peer stimulation might be considered as a potential complementary behavioral strategy to those treatment programs that already focus upon empowering social competences through adult-infant interactions (Dawson, 2008; Vismara and Rogers, 2010).
Taking a life-long perspective, the foundation of a healthy life relies upon early experiences. When these are appropriate, physiological systems are typically healthy and adaptive. However, when early life relationships are impoverished, several systems, including those relevant for mental health, may be dysfunctional laying the ground for maladaptive behavior and disability. These data may have implications for health policies and prevention strategies as the identification of early controllable influences on mental health as well as social skills can guide the targeting of more effective policies and services for young children with emotional disabilities and their families.
Supplementary Material
Acknowledgements
We thank Nadia Francia for technical support.
Role of funding source This study was supported by the Italian Ministry of Health, projects 11US/1 to EA and RF-2009-1498890 to FC. FAC and JPC are supported by Grant Number DP2OD001675 from the Office of the Director, National Institutes of Health. The mentioned granting agencies had no further role in study design, in the collection, analysis and interpretation of data, in the writing of the report and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors Igor Branchi: designed the experiment, setup the asynchronous communal nest model, wrote the paper
James P. Curley: performed the oxytocin receptor binding characterization, wrote the paper
Ivana D’Andrea: setup the asynchronous communal nest model, performed the behavioral characterization and BDNF analysis
Francesca Cirulli: helped in devising the experimental protocol, wrote the paper
Frances A. Champagne: helped in devising the experimental protocol, wrote the paper
Enrico Alleva: supervised and contributed to the experiments and manuscript writing
Conflict of interest All the authors declare that they have no conflicts of interest.
References
- Alleva E. Assessment of aggressive behaviour in rodents. In: Conn MP, editor. Methods in Neuroscience. Paradigms for the study of behavior. Academic press; New York: 1993. pp. 111–137. [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, Nemeroff CB, Reyes TM, Simerly RB, Susser ES, Nestler EJ. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68:314–319. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista A, Martínez-Gómez M, Hudson R, Alves PC, Ferrand N, Hackländer K. Mother-Young and Within-Litter Relations in the European Rabbit <i>Oryctolagus cuniculus</i> Lagomorph Biology. Springer; Berlin Heidelberg: 2008. pp. 211–223. [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25:1565–1574. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Attachment. Basic Books; New York: 1969. [Google Scholar]
- Branchi I. The mouse communal nest: investigating the epigenetic influences of the early social environment on brain and behavior development. Neurosci Biobehav Rev. 2009;33:551–559. doi: 10.1016/j.neubiorev.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Branchi I, D’Andrea I, Cirulli F, Lipp HP, Alleva E. Shaping brain development: mouse communal nesting blunts adult neuroendocrine and behavioral response to social stress and modifies chronic antidepressant treatment outcome. Psychoneuroendocrinology. 2010;35:743–751. doi: 10.1016/j.psyneuen.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Branchi I, Karpova NN, D’Andrea I, Castren E, Alleva E. Epigenetic modifications induced by early enrichment are associated with changes in timing of induction of BDNF expression. Neurosci Lett. 2011a;495:168–172. doi: 10.1016/j.neulet.2011.03.038. [DOI] [PubMed] [Google Scholar]
- Branchi I, D’Andrea I, Santarelli S, Bonsignore LT, Alleva E. The richness of social stimuli shapes developmental trajectories: Are laboratory mouse pups impoverished? Prog Neuropsychopharmacol Biol Psychiatry. 2011b doi: 10.1016/j.pnpbp.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Branchi I, D’Andrea I, Fiore M, Di Fausto V, Aloe L, Alleva E. Early social enrichment shapes social behavior and nerve growth factor and brain-derived neurotrophic factor levels in the adult mouse brain. Biol Psychiatry. 2006;60:690–696. doi: 10.1016/j.biopsych.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Castren E. Is mood chemistry? Nat Rev Neurosci. 2005;6:241–246. doi: 10.1038/nrn1629. [DOI] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci U S A. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA. Epigenetic influence of social experiences across the lifespan. Dev Psychobiol. 2010;52:299–311. doi: 10.1002/dev.20436. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Curley JP. Epigenetic mechanisms mediating the long-term effects of maternal care on development. Neurosci Biobehav Rev. 2009;33:593–600. doi: 10.1016/j.neubiorev.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Chan JP, Unger TJ, Byrnes J, Rios M. Examination of behavioral deficits triggered by targeting Bdnf in fetal or postnatal brains of mice. Neuroscience. 2006;142:49–58. doi: 10.1016/j.neuroscience.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav Neurosci. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Francia N, Berry A, Aloe L, Alleva E, Suomi SJ. Early life stress as a risk factor for mental health: role of neurotrophins from rodents to non-human primates. Neurosci Biobehav Rev. 2009;33:573–585. doi: 10.1016/j.neubiorev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowcroft P, Rowe FP. Social organization and. territorial behavior in the wild house mice. Proc Zool Soc Lond. 1963;140:517–531. [Google Scholar]
- Curley JP, Davidson S, Bateson P, Champagne FA. Social enrichment during postnatal development induces transgenerational effects on emotional and reproductive behavior in mice. Front Behav Neurosci. 2009;3:25. doi: 10.3389/neuro.08.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley JP, Jensen CL, Mashoodh R, Champagne FA. Social influences on neurobiology and behavior: Epigenetic effects during development. Psychoneuroendocrinology. 2011;36:352–371. doi: 10.1016/j.psyneuen.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Andrea I, Alleva E, Branchi I. Communal nesting, an early social enrichment, affects social competences but not learning and memory abilities at adulthood. Behav Brain Res. 2007;183:60–66. doi: 10.1016/j.bbr.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Dawson G. Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Dev Psychopathol. 2008;20:775–803. doi: 10.1017/S0954579408000370. [DOI] [PubMed] [Google Scholar]
- de Waal F. Peacemaking among primates. Harvard University Press; Cambridge, MA: 1989. [Google Scholar]
- DeVries AC, Young WS, 3rd, Nelson RJ. Reduced aggressive behaviour in mice with targeted disruption of the oxytocin gene. J Neuroendocrinol. 1997;9:363–368. doi: 10.1046/j.1365-2826.1997.t01-1-00589.x. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Insel TR. The neuroendocrine basis of social recognition. Front Neuroendocrinol. 2002;23:200–224. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- Fries AB, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proc Natl Acad Sci U S A. 2005;102:17237–17240. doi: 10.1073/pnas.0504767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassman M, Buettner CK. The role of trait affiliation in human community. Behav Brain Sci. 2005;28:353. [Google Scholar]
- Harlow HF. The nature of love. Am Psychologist. 1958;13:537–685. [Google Scholar]
- Harris JR. Where is the child’s environment? A group socialization theory of development. Psychol Rev. 1995;102:458–489. [Google Scholar]
- Hartup WW. The social worlds of childhood. American Psychologist. 1979;34:944–950. [Google Scholar]
- Hartup WW, Laursen B, Stewart MI, Eastenson A. Conflict and the friendship relations of young children. Child Dev. 1988;59:1590–1600. doi: 10.1111/j.1467-8624.1988.tb03686.x. [DOI] [PubMed] [Google Scholar]
- Holmes MM, Rosen GJ, Jordan CL, de Vries GJ, Goldman BD, Forger NG. Social control of brain morphology in a eusocial mammal. Proc Natl Acad Sci U S A. 2007;104:10548–10552. doi: 10.1073/pnas.0610344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes C, Phillipsen L. Continuity in Children’s Relations with Peers. Social Development. 1998;7:340–349. [Google Scholar]
- Hudson R. The contribution of sibling relations to the emergence of individual behavioural phenotypes. International Conference of Ethology; Budapest. 2005. p. 6. [Google Scholar]
- Hughes C, Dunn J. Understanding mind and emotion: Longitudinal associations with mental-state talk between young friends. Dev Psychol. 1998;34:1026–1037. doi: 10.1037//0012-1649.34.5.1026. [DOI] [PubMed] [Google Scholar]
- Keverne EB, Curley JP. Vasopressin, oxytocin and social behaviour. Curr Opin Neurobiol. 2004;14:777–783. doi: 10.1016/j.conb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Lagace DC, Donovan MH, DeCarolis NA, Farnbauch LA, Malhotra S, Berton O, Nestler EJ, Krishnan V, Eisch AJ. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc Natl Acad Sci U S A. 2010;107:4436–4441. doi: 10.1073/pnas.0910072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang UE, Gunther L, Scheuch K, Klein J, Eckhart S, Hellweg R, Danker-Hopfe H, Oehler J. Higher BDNF concentrations in the hippocampus and cortex of an aggressive mouse strain. Behav Brain Res. 2009;197:246–249. doi: 10.1016/j.bbr.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Laviola G, Alleva E. Sibling effects on the behavior of infant mouse litters (Mus domesticus) J Comp Psychol. 1995;109:68–75. doi: 10.1037/0735-7036.109.1.68. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Macbeth AH, Pagani JH, Young WS., 3rd Oxytocin: the great facilitator of life. Prog Neurobiol. 2009;88:127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerer E, Levi S, Salomon S, Darvasi A, Yirmiya N, Ebstein RP. Association between the oxytocin receptor (OXTR) gene and autism: relationship to Vineland Adaptive Behavior Scales and cognition. Mol Psychiatry. 2008;13:980–988. doi: 10.1038/sj.mp.4002087. [DOI] [PubMed] [Google Scholar]
- Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126:405. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A. 1999;96:15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macri S, Laviola G. Single episode of maternal deprivation and adult depressive profile in mice: interaction with cannabinoid exposure during adolescence. Behav Brain Res. 2004;154:231–238. doi: 10.1016/j.bbr.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Mendl M, Paul ES. Parental care, sibling relationships, and the development of aggressive behaviour in two lines of wild house mice. Behaviour. 1990;116:11–41. [Google Scholar]
- Mintz M, Ruedi-Bettschen D, Feldon J, Pryce CR. Early social and physical deprivation leads to reduced social motivation in adulthood in Wistar rats. Behav Brain Res. 2005;156:311–320. doi: 10.1016/j.bbr.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Oliveira RF. Social behavior in context: Hormonal modulation of behavioral plasticity and social competence. Integrative and Comparative Biology. 2009;49:423–440. doi: 10.1093/icb/icp055. [DOI] [PubMed] [Google Scholar]
- Parke RD, Simpkins SD, McDowell DJ, Kim M, Killian C, Dennis JM, Flyr ML, Wild M, Rah Y. Relative contributions of families and peers to children’s social development. In: Smith PK, Hart CH, editors. Blackwell handbook of childhood social development. Blackwell Publishing; Malden: 2002. pp. 156–177. [Google Scholar]
- Patrick CJ. Psychophysiological correlates of aggression and violence: an integrative review. Philos Trans R Soc Lond B Biol Sci. 2008;363:2543–2555. doi: 10.1098/rstb.2008.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. 3rd Edition Elsevier Academic Press; San Diego: 2003. [Google Scholar]
- Pellegrini AD. The roles of aggressive and affiliative behaviors in resource control: A behavioral ecological perspective. Dev Rev. 2008;28:461–487. [Google Scholar]
- Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proc Natl Acad Sci U S A. 2009;106:21437–21441. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter ML, Kreppner JM, O’Connor TG. Specificity and heterogeneity in children’s responses to profound institutional privation. Br J Psychiatry. 2001;179:97–103. doi: 10.1192/bjp.179.2.97. [DOI] [PubMed] [Google Scholar]
- Schneider B. Friends and enemies: Peer relations in childhood. Arnold; London: 2000. [Google Scholar]
- Suomi SJ. Mother-infant attachment, peer relationships, and the development of social networks in rhesus monkeys. Hum Dev. 2005;48:67–79. [Google Scholar]
- Taborsky B, Arnold C, Junker J, Tschopp A. The early social environment affects social competence in a cooperative breeder. Animal Behaviour. 2012;83:1067–1074. doi: 10.1016/j.anbehav.2012.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova ML, Laviola G. Individual differences in mouse behavioural development: effects of precocious weaning and ongoing sexual segregation. Anim Behav. 1995;50:1261–1271. [Google Scholar]
- Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Verbeek P. Peace ethology. Behaviour. 2008;145:1497–1524. [Google Scholar]
- Vismara LA, Rogers SJ. Behavioral treatments in autism spectrum disorder: what do we know? Annu Rev Clin Psychol. 2010;6:447–468. doi: 10.1146/annurev.clinpsy.121208.131151. [DOI] [PubMed] [Google Scholar]
- Walden TA, Lemerise EA, Smith M. Friendship and popularity in preschool classrooms. Early Educ Dev. 1999;10:351–371. [Google Scholar]
- Walum H, Lichtenstein P, Neiderhiser JM, Reiss D, Ganiban JM, Spotts EL, Pedersen NL, Anckarsater H, Larsson H, Westberg L. Variation in the oxytocin receptor gene is associated with pair-bonding and social behavior. Biol Psychiatry. 2012;71:419–426. doi: 10.1016/j.biopsych.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Perry K, Weber MD, Katz AM, Crawley JN. Social peers rescue autism-relevant sociability deficits in adolescent mice. Autism Res. 2011;4:17–27. doi: 10.1002/aur.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.