Abstract
Translational research is needed to discover pharmacological targets and treatments for the diagnostic behavioral domains of autism spectrum disorders. Animal models with phenotypic relevance to diagnostic criteria offer clear experimental strategies to test the efficacy and safety of novel treatments. Antagonists of mGluR5 receptors are in clinical trials for Fragile X syndrome and under investigation for the treatment of autism spectrum disorders. However, in preclinical studies of mGluR5 compounds tested in our laboratory and others, increased locomotion following mGluR5 modulation has been observed. Understanding the influence of general activity on sociability and repetitive behaviors will increase the accuracy of interpretations of positive outcomes measured from pharmacological treatment that produces locomotor activating or sedating effects. In the present studies, dose-response curves for d-amphetamine (AMPH)-induced hyperlocomotion were similar in standard B6 mice and in the BTBR mouse model of autism. AMPH produced significant, robust reductions in the high level of repetitive self-grooming that characterizes BTBR, and also reduced the low baseline grooming in B6, indicating that AMPH-induced hyperlocomotion competes with time spent engaged in self-grooming. We then tested AMPH in B6 and BTBR on the 3-chambered social approach task. One component of sociability, the time spent in the chamber with the novel mouse, in B6 mice was reduced, while the sniffing time component of sociability in BTBR mice was enhanced. This finding replicated across multiple cohorts treated with AMPH and saline vehicle. In-depth analysis revealed that AMPH increased the number and decreased the duration of sniffing bouts in BTBR, suggesting BTBR treated with AMPH mostly engaged in brief sniffs rather than true social interactions with the novel mouse during the social approach task. Our data suggest that compounds with stimulant properties may have some direct benefits on reducing repetitive behaviors in autism spectrum disorders, particularly in the subset of autistic individuals with hyperactivity.
1. Introduction
Autism spectrum disorders (ASD) are defined by three diagnostic symptom domains: 1) qualitative impairments in social interaction, 2) deficits in communication, and 3) stereotyped repetitive behaviors with restricted interests (American Psychiatric Association., 1994; Dawson et al., 2010; Krasny et al., 2003; Landa, 2008; Lord et al., 2000; Zwaigenbaum et al., 2009). Recent genetic association investigations have identified a large number of autism susceptibility genes and copy number variants (Abrahams and Geschwind, 2008, 2010; Anney et al., 2010; Bucan et al., 2009; Happe and Ronald, 2008). At present, behavioral intervention is the only effective form of treatment, with many positive outcome measures reported (Dawson, 2008; Rogers and Vismara, 2008; Williams White et al., 2007). The only approved pharmacological treatments for autism are Risperidone (Risperdal®) and Aripiprazole (Abilify®), which target the associated symptoms of irritability that include self-injury, tantrums and aggression (Marcus et al., 2011; McCracken et al., 2002; McDougle et al., 2000; Varni et al., 2012). Translational research is needed to discover pharmacological targets for the diagnostic domains (McPheeters et al., 2011; Veenstra-VanderWeele and Blakely, 2012; Wink et al., 2010). Pursuing the discovery of effective pharmacological interventions requires appropriate preclinical screens. Animal models based on hypothesized causes of autism spectrum disorders, and/or with robust phenotypes of high relevance to diagnostic symptoms, offer innovative experimental strategies to test the efficacy and safety of proposed treatments.
Abnormal reciprocal social interactions include reduced interest in peers and difficulty maintaining social interaction, failure to use eye gaze and an absence of facial expressions. Communication deficits present as language delays, failure to respond to voices and lack of prosody or intonation. Repetitive behaviors with restricted interests include motor stereotypies (i.e. hand flapping or toe walking), repetitive use of the same objects, and insistence on sameness American Psychiatric Association., 1994; DiCicco-Bloom et al., 2006; Landa, 2008; Lord et al., 2000). Several inbred mouse strains display impaired social affiliative behaviors despite normal levels of aggressive, reproductive, and maternal behaviors (Bolivar et al., 2007; Brodkin, 2007; Brodkin et al., 2004; Defensor et al., 2011; Moy et al., 2008; Panksepp et al., 2007; Pobbe et al., 2010; Ryan et al., 2010). BTBR T+tf/J (BTBR) is a commercially available inbred strain of mice that displays multiple behavioral phenotypes relevant to all three diagnostic symptoms of autism. Both male and female BTBR engage in marked low levels of reciprocal social interactions at juvenile and adult ages and lack species-typical sociability in the 3-chambered social approach task (Bolivar et al., 2007; Defensor et al., 2011; Pobbe et al., 2011; Pobbe et al., 2010; Silverman et al., 2010a; Yang et al., 2012a; Yang et al., 2009; Yang et al., 2007a; Yang et al., 2007b). BTBR emit significantly fewer ultrasonic vocalizations in response to social olfactory cues and during reciprocal social interactions, as compared to other standard inbred strains such as C57BL/6J (B6) (Scattoni et al., 2008; Scattoni et al., 2011; Wohr et al., 2011). BTBR also produce fewer scent marks in response to social olfactory pheromones, consistent with an interpretation of impaired communication (Roullet et al., 2011). BTBR display significantly higher levels of repetitive self-grooming throughout their lifespan as compared to control strains, replicated in multiple cohorts and across several laboratory environments (McFarlane et al., 2008; Pearson et al., 2011; Silverman et al., 2012; Silverman et al., 2010a; Yang et al., 2007a; Yang et al., 2007b). Normal scores on measures of general health, motor functions, stress reactivity, acoustic startle reflex, prepulse inhibition and olfactory abilities (McFarlane et al., 2008; Silverman et al., 2010c; Yang et al., 2012a) support an interpretation of remarkably specific autism-relevant abnormalities in BTBR. Investigation into the background genes responsible for autism-relevant behavioral traits in inbred strains of mice is ongoing (Bolivar et al., 2011; Bothe et al., 2011; Jones-Davis et al., 2011; McFarlane et al., 2008). This genetically homogenous, commercially available strain provides a useful model system for assessing pharmacological therapeutics, with particular relevance to those individuals with autism whose genotypic variant is unknown. It is important to note, however, that BTBR differs from the conventional models of autism that are based on targeted mutations of candidate genes for autism. Despite their unknown genetics, BTBR is among the best animal models of autism in terms of face validity to core symptomatology, robustness and replicability of phenotypes.
We and others have employed experimental interventions to evaluate genetic reversal and pharmacological rescue in mouse models of neurodevelopmental disorders (Cobb et al., 2010; Dolen et al., 2007; Ehninger et al., 2008a; Ehninger et al., 2008b; Guy et al., 2007; Hayashi et al., 2007; Meikle et al., 2008; Ogier et al., 2007; Penagarikano et al., 2011; Silverman et al., 2012; Silverman et al., 2010a; Yan et al., 2005; Zhou et al., 2009). Discovery of elevated mGluR5-mediated signaling and protein synthesis in Fragile X knockout mice provided the rationale for testing mGluR5 antagonists in Fragile X clinical trials (Bear et al., 2004; Dolen et al., 2007; Jacquemont et al., 2011; Krueger and Bear, 2011). We recently reported beneficial actions of mGluR5 antagonists on reducing repetitive self-grooming in BTBR (Silverman et al., 2012; Silverman et al., 2010a). Improvements in some parameters of sociability were detected in BTBR mice treated with an mGluR5 negative allosteric modulator, GRN-529 (Silverman et al., 2012). However, a potential confound was noted. Increased entries in social approach accompanied the improved sociability in the automated 3-chambered apparatus. Similarly, in a novel open field test conducted with the same BTBR and B6 mice, increased distance traversed after treatment with mGluR5 antagonists was seen, consistent with other reports of mild hyperactivity after mGluR5 antagonist treatments (Mehta et al., 2011; Montana et al., 2009; Thomas et al., 2012).
Understanding the influence of general activity levels on sociability and repetitive behaviors will enhance the accurate interpretation of positive outcomes measured from any test compound that produces locomotor activating or sedating effects. In the present study, we tested the hypothesis that endogenous and drug-induced hyperactivity have direct effects on social and repetitive behaviors in the BTBR mouse model of autism. Specifically, doses of AMPH that increased open field locomotion were administered to BTBR and B6 mice in the repetitive self-grooming assay, and in our automated 3-chambered social approach assay, to evaluate the possibility that higher general exploration contributes to reduced repetitive and enhanced social behaviors.
2. Materials and Methods
2.1. Mice
C57BL/6J (B6) and BTBR T+ tf/J (BTBR) mice were the offspring of breeding pairs purchased from The Jackson Laboratory (JAX, Bar Harbor, ME). All mice were housed and bred in a conventional mouse vivarium at the National Institute of Mental Health (NIMH), Bethesda, Maryland, USA, using harem breeding trios. After two weeks with a male, females were separated into individual cages (Tecniplast, USA) before parturition. Pups were kept with the dam until weaning at postnatal day 21. After weaning, juveniles were group housed by sex and strain in standard plastic cages in groups not exceeding four per cage. Cages were maintained in ventilated racks in a temperature (20°C) and humidity (~55%) controlled vivarium on a 12 hour circadian cycle, lights on from 0700 to 1900 hr. Standard rodent chow and tap water were available ad libitum. In addition to standard bedding, a Nestlet square, shredded brown paper and a cardboard tube were provided in each cage. Light levels measured approximately 325 lux during the light phase. Background noise measured approximately 50–60 dB. All procedures were approved by the National Institute of Mental Health Animal Care and Use Committee.
2.2. Drug Treatment
d-amphetamine sulfate salt (AMPH; 1.0, 2.0 and 3.0 mg/kg, Sigma Aldrich, St. Louis, MO) was dissolved in saline (0.9% NaCl). Adult male and female B6 and BTBR mice weighing 25–40 grams received an intraperitoneal (i.p.) injection of saline vehicle or AMPH 30 minutes before the start of behavioral test sessions for the social approach, self-grooming, and open field behavioral tasks. Dose response curves and post treatment interval in open field locomotion were determined using previously published literature (Kelley et al., 1986; Mueller et al., 1989; Papaleo et al., 2008; Sills et al., 1998; Stromberg and Svensson, 1975; Yates et al., 2007).
2.3. Experimental Design for Behavioral Assays
Testing was conducted in dedicated behavioral testing rooms during the standard light phase, usually between 0900 and 1600 hr. Prior to each behavioral test, mice were acclimatized to the behavioral testing area for at least 60 continuous minutes. Testing began at ages 6–8 weeks. Treatment groups consisted of 10–16 mice per strain for each dose of drug or vehicle. Previous studies in our laboratory documented no sex differences on either sociability or self-grooming in BTBR or B6 (Silverman et al., 2010a; Yang et al., 2009; Yang et al., 2007a; Yang et al., 2007b). Therefore, male and female mice were used in all studies in approximately equal proportions. A single cohort (Cohort 1) was utilized to collect AMPH dose response curve data in the open field locomotion task. Each additional cohort used a between treatment factor design with a one week washout period, such that each mouse received an acute dose of AMPH or vehicle, and was tested in a behavioral task, one task per week. For cohorts 2 and 3, each mouse was used for all three behavioral tests, and received a total of three injections randomized across AMPH and vehicle. The behavioral task order was social approach (week 1), self-groom (week 2) and open field (week 3). For cohort 4, each mouse was used for two behavioral tests (social approach and self-grooming), and received a total of two injections randomized across AMPH and vehicle. The task order was social approach (week 1) followed by self-groom (week 2). Drug doses, toe tattoo patterns, and digital videotapes were coded to ensure that the raters were blind to the treatment condition. All procedures were conducted in strict compliance with the NIH Guidelines for the Care and Use of Laboratory Animals and approved by the Animal Care and Use Committees of the National Institute of Mental Health.
2.4. Social Approach
Social approach was tested in an automated 3-chambered apparatus from a design originally developed by our group (Nadler et al., 2004), using improved methods as recently described (Brielmaier et al., 2012; Papaleo et al., 2011; Silverman et al., 2012; Silverman et al., 2010a; Silverman et al., 2011; Silverman et al., 2010b; Yang et al., 2012b; Yang et al., 2011a; Yang et al., 2011b). Briefly, the apparatus was a rectangular, 3-chambered box made from clear polycarbonate. Retractable doorways within the two dividing walls allowed access to the side chambers. Number of entries and time spent in the chambers were automatically recorded from photocells embedded in the doorways. A top mounted CCTV camera (Security Cameras Direct, Luling, TX, USA) placed over the boxes recorded the session for subsequent scoring. The apparatus was cleaned with 70% ethanol and water between subjects. At least five minutes elapsed between cleaning and the start of the next test session, to allow for ethanol evaporation and clearance of ethanol vapor odors. To increase throughput, four mice were run simultaneously, in four adjacent chambers. Mice used as the novel stimulus target were 129Sv/ImJ, aged 12–20 weeks old, bred and maintained in the NIMH vivarium from breeding pairs originally purchased from JAX, and matched to the subject mice by sex and age. A different target mouse was used for each subject. Target mice are re-used throughout other experiments on different days, but each target mouse is used only once per day. Time spent in each chamber and number of entries into each chamber were calculated by the automated software, based on the movements of the subject mouse in sequentially breaking and unbreaking a series of photocell beams embedded in the openings between chambers for a 10 minute habituation session, followed by the 10 minute sociability session (Yang et al., 2011). Number of entries served as a within-task control for levels of general exploratory locomotion. An observer blind to the drug treatments scored the videos with a stopwatch for cumulative time in which the subject mouse sniffed the novel target mouse and the novel object. For further in-depth analysis of bouts, a highly trained observer blind to the drug treatments manually scored the videos obtained with the Noldus Observer 8.0XT software (Noldus Information Technology, Leesburg, VA). A bout of sniffing was defined as a state event measured by duration and occurrence. Number of bouts of sniffing the novel mouse, novel object and the summed total of these events were scored. Videos for these samples were selected blindly and at random, by choosing four videos from Cohort 2, three videos from Cohort 3, and three videos from Cohort 4, for each strain. At the end of each testing day, test chambers were thoroughly cleansed with Alconox detergent (Alconox, White Plains, NY, USA) diluted with warm water, followed by extensive rinsing with hot water and air drying.
2.5. Self-grooming assay
Mice were scored for spontaneous repetitive self-grooming behavior as previously described (Silverman et al., 2012; Silverman et al., 2010b). Briefly, each mouse was placed individually into a standard mouse cage, (46 cm length × 23.5 cm wide × 20 cm high) for a 10 minute habituation period, followed by 10 minutes of behavioral recording. Cages were empty, to eliminate digging in the bedding, a potentially competing behavior. The room was illuminated at ~15 lux. A trained observer uninformed of the drug treatment scored the videos. Cumulative time spent self-grooming was scored from the videos using a high-accuracy Traceable© stopwatch (Thomas Scientific, Swedesboro, NJ) with the auditory component silenced.
2.6. Open field locomotion
General exploratory locomotion in a novel open field environment was assayed as previously described (Bailey et al., 2007; Chadman et al., 2008; McFarlane et al., 2008; Silverman et al., 2010a; Silverman et al., 2011; Silverman et al., 2010b). Individual mice were placed in a standard Accuscan open field (AccuScan Instruments, Columbus, OH, USA) for 30 minutes. Illumination in the testing room measured ~ 15 lux. Test chambers consisted of clear Plexiglas sides and floor, approximately 40 × 40 × 30.5 cm. Mice were placed in the center of the open field at the initiation of the testing session. Photocells at standard heights for recording activity were aligned 8 to a side, dividing the chamber into 64 equal squares. Total distance, horizontal activity, vertical activity, and center time were automatically collected using the Versamax activity monitor and analyzer software system. Test chambers were cleaned with 70% ethanol between test subjects. At least five minutes between cleaning and the start of the next session was allowed for ethanol evaporation and odor dissipation.
2.7. Statistical analysis
Open field dose response effects of AMPH in Cohort 1 were analyzed with a Repeated Measures ANOVA using a between groups factor of drug within strain, and a within group factor of time course, for the parameters of total distance, horizontal activity, vertical activity or center time. Dose response experiments were followed by a Dunnett’s post hoc analysis, using SigmaPlot version 11.0 (Systat Inc., San Jose, CA) that compared individual means in cases where the ANOVA was significant at p < 0.05. AMPH effects on open field locomotion in Cohorts 2 and 3 were analyzed with a Repeated Measures ANOVA using a between groups factor of drug within strain, and a within group factor of time course, for the parameters of total distance, horizontal activity, vertical activity or center time (Supplementary Materials). Self-grooming was analyzed for drug effect using a within strain unpaired Student’s t-test for treatment, using StatView statistical software (Citewise.com, Acton, MA, USA). For social approach, a Repeated Measures ANOVA was conducted within each drug dose group and for the vehicle group, for each strain. Since times spent in each of the three chambers added to 10 minutes, and therefore were not independent, the test condition factor compared time spent only in the right versus left chambers. Center chamber times are shown in the graphs for illustrative purposes. Time spent sniffing the novel object versus the novel mouse was similarly analyzed within each dose for each strain. For number of entries during social approach, drug effects were compared within each strain by a separate between groups drug by entries Repeated Measures ANOVA. In cases where the overall ANOVA for entries was significant, the treatment factor for each strain was further analyzed with a Dunnett’s posthoc test to compare each drug dose group to its vehicle control group. For number of bouts and mean average duration of a sniffing bout, a within strain unpaired Student’s t-test for treatment was employed, using StatView statistical software.
3. Results
3.1. Amphetamine-induced hyperlocomotion in the open field in BTBR and B6 mice, Cohort 1
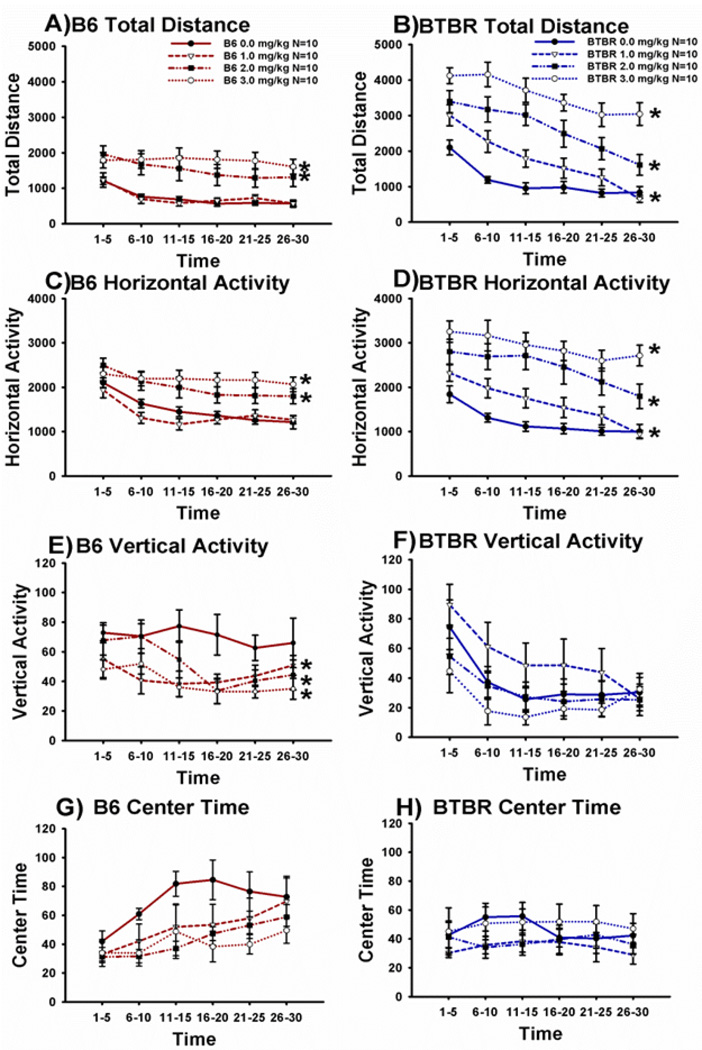
Figure 1 illustrates the dose-response curves on four parameters assessed in the Accuscan open field arena for exploratory locomotion in B6 and BTBR, beginning 30 minutes after AMPH (1.0 mg/kg, 2.0 mg/kg or 3.0 mg/kg) or saline vehicle injection. Across the 30 minute session, the time course for total distance traversed by both B6 and BTBR declined as expected, representing habituation to the novel open field (Main effect of time: Panel A, B6: F(5,150) = 15.2, p < 0.0001; Panel B, BTBR: F(5,145) = 47.8, p < 0.0001). Total distance scores were increased by AMPH in both strains as expected (Main effect of dose: Panel A, B6: F(3,30) = 9.17, p < 0.0002, Dunnett’s p < 0.05 for doses of 2mg/kg and 3mg/kg as compared to vehicle; Panel B, BTBR: F(3,29) = 23.8, p < 0.0001, Dunnett’s p < 0.05 for doses of 2mg/kg and 3mg/kg as compared to vehicle). A significant interaction between dose and distance traveled over time for both strains revealed that only the saline and 1mg/kg groups decreased total distance traveled over time (Panel A, B6: F(15,150) = 1.981, p =.020; Panel B, BTBR: F(15,150) = 2.468, p =.003). A two factor ANOVA, with strain and dose as the between subject factors, was conducted to determine if B6 and BTBR differentially responded to AMPH treatment. BTBR traversed a greater distance than B6 over the course of the 30-minute session (F(1,59) = 58.244, p < 0.0001). A significant strain by dose interaction (F(3,59) = 4.097, p < 0.05) revealed that BTBR traveled significantly greater distances than B6 following vehicle and each AMPH dose tested (vehicle: F(1,14) = 12.718, p < 0.05; AMPH 1mg/kg: F(1,14) = 19.544, p < 0.001; AMPH 2mg/kg: F(1,14) = 7.616, p < 0.05; AMPH 3mg/kg: F(1,14) = 34.272, p < 0.0001).
Figure 1. Cohort 1. Initial dose-response curves were conducted to determine the optimal dose of amphetamine for inducing hyperactivity in BTBR and B6 in a novel open field. Amphetamine elevated parameters of exploratory locomotion in both strains.
Parameters of total distance traversed, horizontal activity, vertical activity and time spent in the center of the arena were measured across a 30 minute test session in an Accuscan open field in BTBR and B6, following an intraperitoneal injection of amphetamine (AMPH) at doses of 1.0 mg/kg, 2.0 mg/kg, 3.0 mg/kg, or saline vehicle (0.0). Data are shown in 5 minute time bins. B6 displayed significant increases in (A) total distance traversed and (C) horizontal activity following AMPH administration at doses of 2.0 and 3.0 mg/kg, as compared to saline vehicle. (E) Vertical activity in B6 was reduced by AMPH at each dose tested. BTBR displayed significant increases in (B) total distance traversed and (D) horizontal activity following AMPH administration at each dose. (F) Vertical activity in BTBR was not significantly affected by AMPH administration. Time spent in the center of the arena did not differ in B6 (G) or BTBR (H) treated with any dose of AMPH compared to vehicle. For all figures, data are shown as mean ± standard error of the mean (SEM). *p < 0.05 as compared to vehicle. N=10 per strain per dose in Cohort 1. See Supplementary Material for the similar open field results in Cohorts 2 and 3.
The time course showing declining horizontal activity by both B6 and BTBR also represented normal habituation (Main effect of time: Panel C, B6: F(5,150) = 29.6, p < 0.0001; Panel D, BTBR: F(5,145) = 30.8, p < 0.0001). Horizontal activity was significantly increased by AMPH treatment in both strains of mice (Main effect of dose: Panel C, B6: F(3,30) = 8.78, p < 0.0002, Dunnett’s p < 0.05 for doses of 2mg/kg and 3mg/kg as compared to vehicle; Panel D, BTBR: F(3,29) = 13.6, p < 0.0001, Dunnett’s p < 0.05 for doses of 2mg/kg and 3mg/kg as compared to vehicle). A significant strain by dose interaction revealed that horizontal activity reduced over time in the saline and 1mg/kg groups (Panel C, B6: F(15,150) = 2.717, p =.001; Panel D, BTBR: F(15,150) = 1.876, p =.030) and that similar to distance scores BTBR exhibited higher horizontal activity counts (F(1,59) = 5.454, p < 0.05).
Vertical activity over the 30 minute test period declined as expected in both strains (Main effect of time: Panel E, B6: F(5,150) = 5.24, p < 0.0002; Panel F, BTBR: F(5,145) = 16.2, p < 0.0001). Vertical activity was lower in B6 treated with AMPH as compared to saline vehicle (Main effect of dose: Panel E, F(3, 30) = 3.23, p < 0.05, Dunnett’s p < 0.05 for each dose 1 mg/kg, 2mg/kg and 3mg/kg as compared to vehicle). Vertical activity did not differ across BTBR groups treated with AMPH, at any dose (F(3,29) = 1.6, p > 0.05). There was no significant dose by vertical activity interaction for either strain (B6: F(15,150) = 1.620, p = 0.07; BTBR: F(15,150) = 1.335, p = 0.19). B6 spent more time vertically active as compared to BTBR during the 30-minute assessment (F(1,59) = 6.02, p < 0.05). No significant strain by dose interaction was observed (F(3,59) = 1.984, p > 0.05).
Time spent in the center of the test arena increased, representing acclimation to the arena, over the time course in B6 (Main effect of time: Panel G, B6: F(5,150) = 9.34, p < 0.0001) but did not differ in BTBR (Panel H, BTBR: F(5,145) = 0.58, p > 0.05). Time in the center of the arena did not differ between vehicle and any dose of AMPH tested in B6 (Dose: F(3,30) = 2.06, p > 0.05) or BTBR (Dose: F(3,29) = 0.9, p > 0.05). There was no significant dose by center time interaction for either strain (B6: F(15,150) = 1.155, p > 0.05; BTBR: F(15,150) = .448, p > 0.05). No significant strain difference was observed on the amount of time spent in the center of the apparatus (F(1,59) = 2.411, p = 0.100), nor was there a significant strain by dose interaction for center time in the open field (F(3,59) = 1.531, p > 0.05). Open field results for Cohorts 2 and 3 appear in Supplementary Materials.
3.2. Effects of amphetamine on social approach in B6 and BTBR mice, Cohort 2
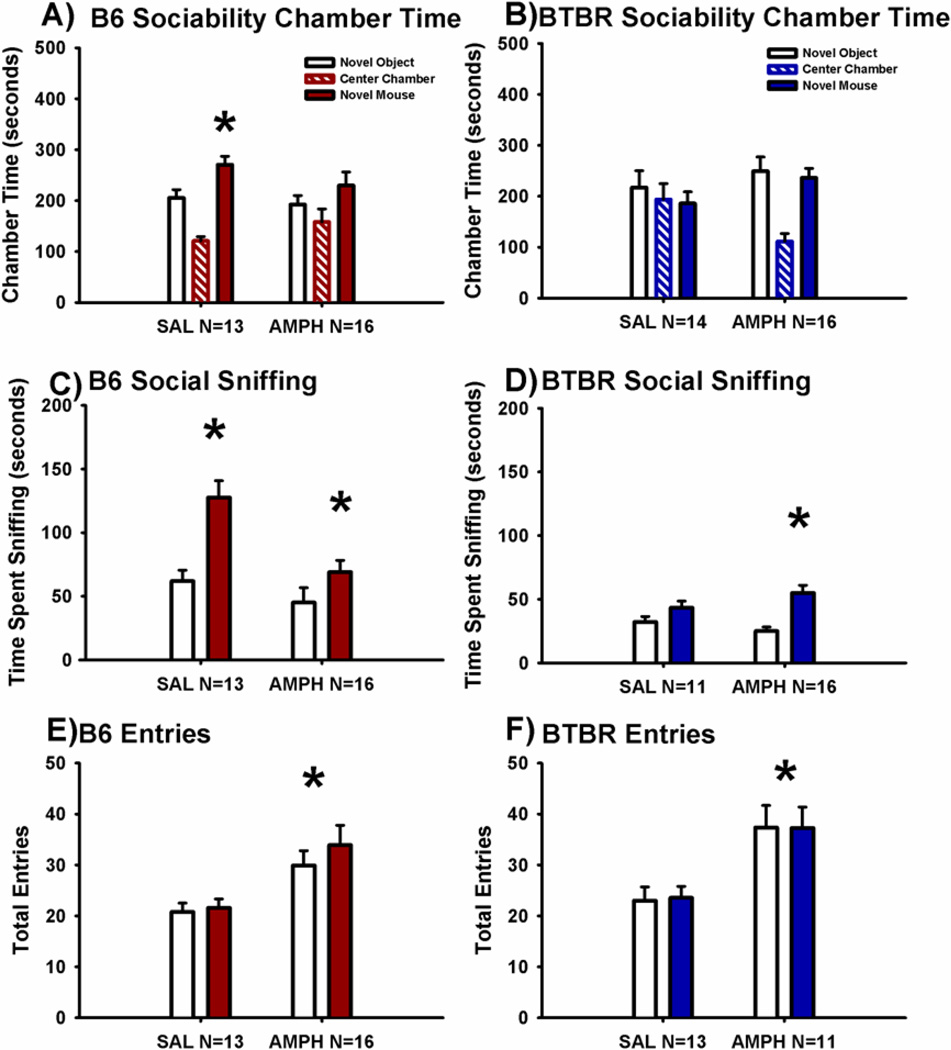
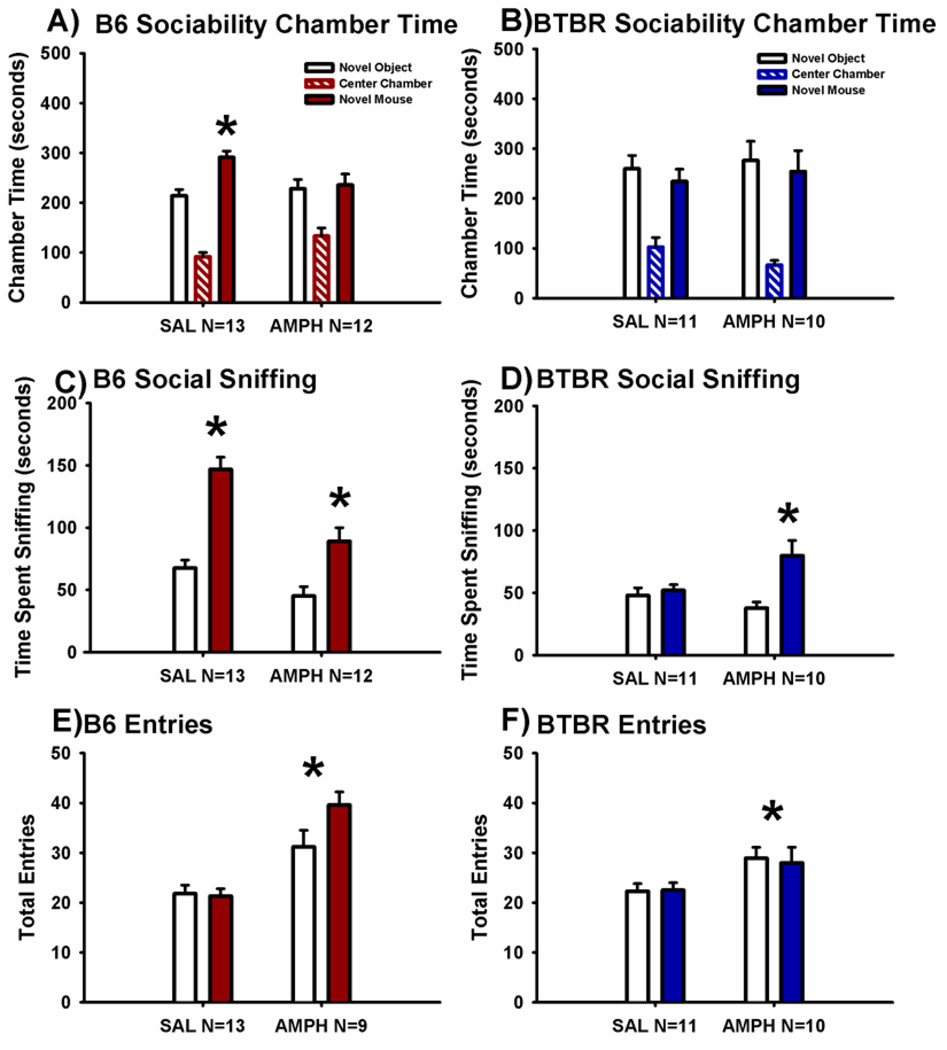
Figure 2 illustrates the sociability scores from the automated 3-chambered social approach task following a single acute intraperitoneal injection of 2.0 mg/kg AMPH as compared to saline vehicle in B6 and BTBR. Sociability, defined as spending more time in the chamber with the novel mouse than in the chamber with the novel object, was significant in the saline treated group of B6 mice, as expected (Panel A, F(1,12) = 10.8, p < 0.01). However, time spent in the chamber with the novel mouse did not differ from time spent in the chamber with the novel object in AMPH treated B6 mice (F(1,15) = 0.41, p > 0.05). In contrast, time spent sniffing the novel mouse, a more direct, sensitive measure of sociability (Fairless et al., 2011; Yang et al., 2011b) remained greater than time spent sniffing the novel object in both the saline treated (Panel C, F(1,12) = 44.8, p < 0.001) and AMPH treated B6 mice (F(1,15) = 11.9, p < 0.01). Entries into the side chambers were increased by AMPH in B6 mice, indicating a direct effect on exploratory locomotion during the social approach task (Panel E, F(1,27) = 7.3, p < 0.01).
Figure 2. Cohort 2. Amphetamine impaired sociability on chamber time in control B6 mice and increased social sniffing in BTBR.
Social approach was assayed in an automated photocell-equipped 3-chambered arena, along with observer scoring of direct sniffing interactions from videotapes of the social approach session. (A) The B6 control strain displayed normal sociability, defined as spending more time in the chamber with the novel mouse than in the chamber with the novel object, in the saline vehicle treated group. AMPH impaired B6 sociability on the chamber time parameter. (B) BTBR exhibited its characteristic lack of sociability, i.e. did not spend more time in the novel mouse chamber than in the novel object chamber, after treatment with either saline or AMPH. (C) B6 displayed normal sociability on the more sensitive parameter, time spent sniffing the novel mouse as compared to time spent sniffing the novel object, after treatment with both saline and AMPH 2.0 mg/kg. (D) BTBR exhibited its characteristic lack of sociability on the sniff time parameter following saline vehicle administration. However, BTBR treated with AMPH exhibited significant sociability on the more sensitive sniff time parameter. *p < 0.05, novel mouse versus novel object. (E) B6 and (F) BTBR displayed a greater number of entries into the side chambers after treatment with AMPH, indicating a general increase in exploratory locomotion during the social approach task. *p < 0.05 Amphetamine 2.0 mg/kg i.p. (AMPH) versus saline vehicle (SAL). N = 13–16 per dose for each strain in Cohort 2.
As expected, BTBR did not exhibit significant sociability on the chamber time parameter, defined as no difference between time spent in the side chamber with the novel mouse as compared to time spent in the side chamber with the novel object in either the saline group (Panel B, F(1,13) = 0.29, p > 0.05) or the AMPH group (F(1,11) = 0.80, p > 0.05). Similarly, time spent sniffing the novel mouse versus the novel object was not significant in the BTBR for the saline vehicle treated group (Panel D, F(1,13) = 2.59, p > 0.05), consistent with earlier publications (Moy et al., 2007; Pobbe et al., 2011; Silverman et al., 2010a; Yang et al., 2012a). However, time spent sniffing the novel mouse was greater than time spent sniffing the novel object in the BTBR treated with AMPH (F(1,11) = 15.5, p < 0.01). Entries into the side chambers were increased by AMPH in BTBR mice, indicating a direct effect on exploratory locomotion during the social approach task (Panel F, F(1,21) = 4.19, p < 0.05).
No innate side preference for either the right or left side chamber was present in B6 (F(1,28) = 1.13, p > 0.05) or BTBR (F(1,22) = 1.32, p > 0.05), as shown by similar amounts of time in the left and right side chambers during the 10 minute habituation session before the start of social testing. No sex differences were exhibited for sociability chamber time or sniff time, respectively, in B6 (F(1,26) = 0.29, p > 0.05; F(1,26) = 0.85, p > 0.05) or BTBR (F(1, 24) = 1.47, p > 0.05; F(1,24) = 1.51, p > 0.05) in the social approach task at any drug dose treatment in Cohort 2.
3.3. Effects of amphetamine on social approach in B6 and BTBR mice, Cohort 3
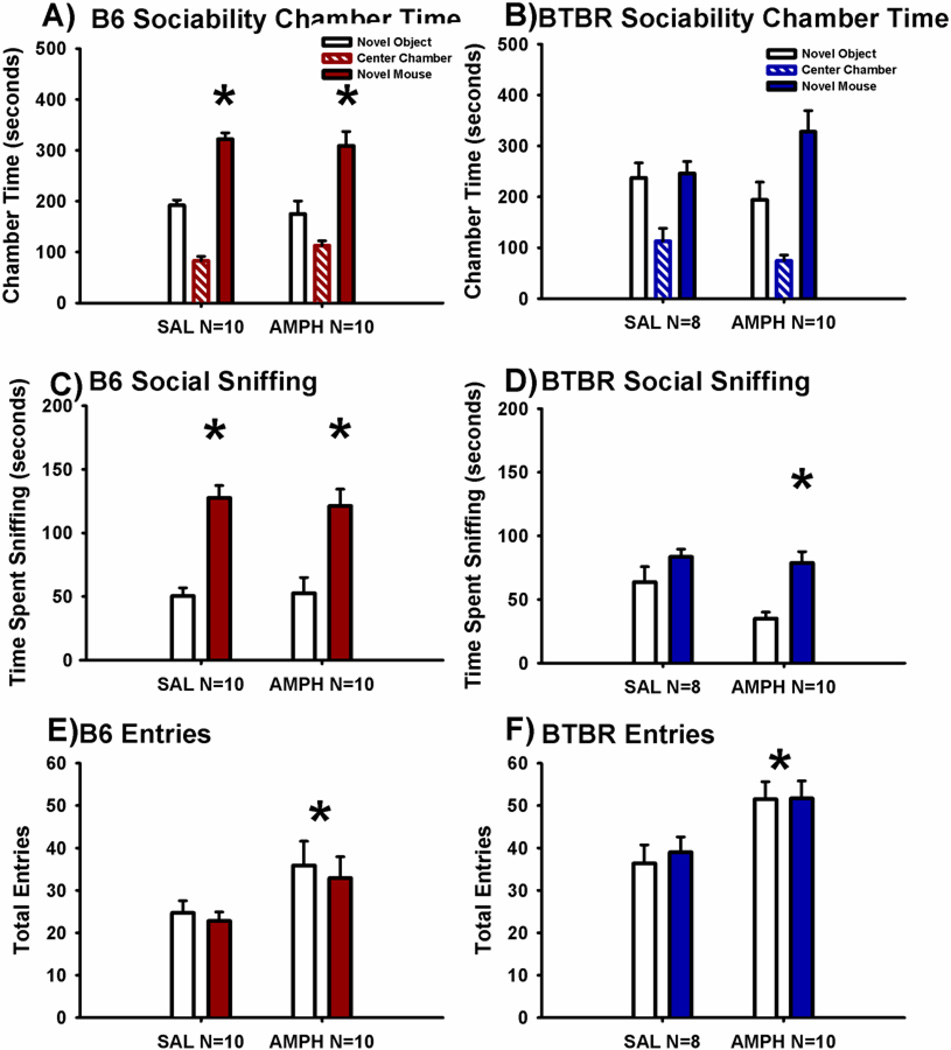
Figure 3 illustrates sociability scores in a second independent cohort treated with AMPH 2.0 mg/kg or saline vehicle, conducted by a second investigator. Sociability was significant in the saline treated B6 (Panel A, F(1,12) = 4.1, p < 0.05). However, as seen in Cohort 2, time spent in the chamber with the novel mouse did not differ from time spent in the chamber with the novel object in AMPH treated B6 mice (F(1,11) = 0.91, p > 0.05). The more sensitive parameter, time spent sniffing the novel mouse versus the novel object, again remained significantly higher than time spent sniffing the novel object in both saline treated (Panel C, F(1,12) = 12.3, p < 0.01) and AMPH treated B6 mice (F(1,11) = 5.65, p < 0.05). Entries into the side chambers were again increased by AMPH in B6 mice, indicating a significant and direct effect on exploratory locomotion during the social approach task (Panel E, F(1,22) = 4.0, p < 0.05).
Figure 3. Cohort 3. Amphetamine impaired sociability on chamber time in control B6 mice and increased social sniffing in BTBR.
Replication was confirmed in a second independent cohort, tested by a different investigator. (A) The B6 control strain displayed normal sociability after saline treatment, but again did not show significant sociability after AMPH. (B) BTBR exhibited its characteristic lack of sociability after treatment with saline or AMPH. (C) B6 displayed sociability on the more sensitive parameter, time spent sniffing the novel mouse as compared to time spent sniffing the novel object, after both saline and AMPH. (D) BTBR exhibited its characteristic lack of sociability on the sniff parameter following saline vehicle administration. BTBR treated with AMPH exhibited significant sociability on the more sensitive sniff time parameter. *p < 0.05, novel mouse versus novel object. (E) B6 and (F) BTBR displayed more entries into the side chambers after treatment with AMPH than saline during the social approach task. *p < 0.05 AMPH 2.0 mg/kg i.p. versus SAL. N = 10–13 per dose for each strain in Cohort 3.
BTBR did not exhibit chamber time sociability, as expected, showing no difference between time spent in the side chamber with the novel mouse as compared to time spent in the side chamber with the novel object in either the saline (Panel B, F(1,10) = 0.43, p > 0.05) or AMPH treated groups (F(1,11) = 0.68, p > 0.05). Similarly, time spent sniffing the novel mouse versus the novel object was not significant in BTBR treated with saline (Panel D, F(1,10) = 3.3, p > 0.05). However, time spent sniffing the novel mouse was again greater than time spent sniffing the novel object in BTBR treated with AMPH (F(1,11) = 20.0, p < 0.01). Entries into the side chambers were again increased by AMPH in BTBR mice, indicating a significant and direct effect on exploratory locomotion during the social approach task (Panel F; F(1,21) = 5.1, p < 0.05).
No innate side preference was present in B6 (F(1,24) = 2.1, p > 0.05) or BTBR (F(1,22) = 0.04, p > 0.05), as shown by similar amounts of time in the left and right side chambers during the 10 minute habituation session before the start of social testing in Cohort 3. No sex differences were exhibited for sociability chamber time or sniff time in B6 (F(1,24) = 0.07, p > 0.05; F(1,24) = 1.07, p > 0.05) or BTBR (F(1,20) = 0.29, p > 0.05; F(1,20) = 2.71, p > 0.05) in the social approach task at any drug dose treatment in Cohort 3.
3.4. Effects of amphetamine on social approach in B6 and BTBR, Cohort 4
Figure 4 illustrates a third independent cohort tested by a third investigator, on social approach in the automated 3-chambered task following a single dose of AMPH or saline in B6 and BTBR mice. In Cohort 4, sociability was significant for chamber time in B6 administered saline (Panel A, F(1,9) = 36.8, p < 0.0002) and AMPH (F(1,9) = 6.5, p < 0.05). As expected, time spent sniffing the novel mouse versus the novel object was significantly higher than the time spent sniffing the novel object in both the saline treated (Panel C, F(1,9) = 45.1, p < 0.0001) and AMPH treated B6 (F(1,9) = 22.3, p < 0.001). Entries into the side chambers were again increased by AMPH in B6 (Panel E, F(1,17) = 9.5, p < 0.01).
Figure 4. Cohort 4. Amphetamine increased social sniffing in BTBR.
(A) The B6 control strain displayed normal sociability after saline vehicle treatment. In Cohort 4, B6 treated with AMPH spent more time in the novel mouse chamber compared to the time spent in the novel object chamber. (B) BTBR exhibited its characteristic lack of sociability after both saline and AMPH. (C) B6 displayed sociability on the more sensitive parameter, time spent sniffing the novel mouse as compared to time spent sniffing the novel object, after both saline and AMPH. (D) BTBR exhibited its characteristic lack of sociability on the sniff parameter following saline vehicle administration. BTBR treated with AMPH exhibited significant sociability on the sensitive sniff time parameter. *p < 0.05, novel mouse versus novel object. (E) B6 and (F) BTBR displayed more entries into the side chambers after AMPH, indicating a general increase in exploratory activity during the social approach task. *p < 0.05 AMPH 2.0 mg/kg i.p. versus SAL. N = 8–10 per dose for each strain in Cohort 4.
As expected, BTBR did not exhibit sociability, showing no difference between time spent in the side chamber with the novel mouse as compared to time spent in the side chamber with the novel object in both the saline (Panel B, F(1,7) = 0.04, p > 0.05) and AMPH treated groups (F(1,9) = 3.2, p > 0.05). Similarly, time spent sniffing the novel mouse versus the novel object did not differ in the BTBR treated with saline (Panel D, F(1,7) = 2.1, p > 0.05). In contrast, time spent sniffing the novel mouse was again greater than time spent sniffing the novel object in BTBR treated with AMPH (F(1,9) = 22.9, p < 0.01). Entries into the side chambers were again increased by AMPH in BTBR mice (Panel F, F(1,16) = 7.4, p < 0.02).
No innate side preference was present in B6 (F(1,18) = 2.9, p > 0.05) or BTBR (F(1,17) = 0.22, p > 0.05), as shown by similar amounts of time in the left and right side chambers during the 10 minute habituation session before the start of social testing in cohort 4. No sex differences were exhibited for sociability chamber time or sniff time in B6 (F(1,18) = 0.49, p > 0.05; F(1,18) = 0.40, p > 0.05) or BTBR (F(1,20) = 0.54, p > 0.05; F(1,20) = 0.27, p > 0.05) in the social approach task at any drug dose treatment in Cohort 4.
3.5. In-depth analysis of sniffing events in B6 and BTBR mice
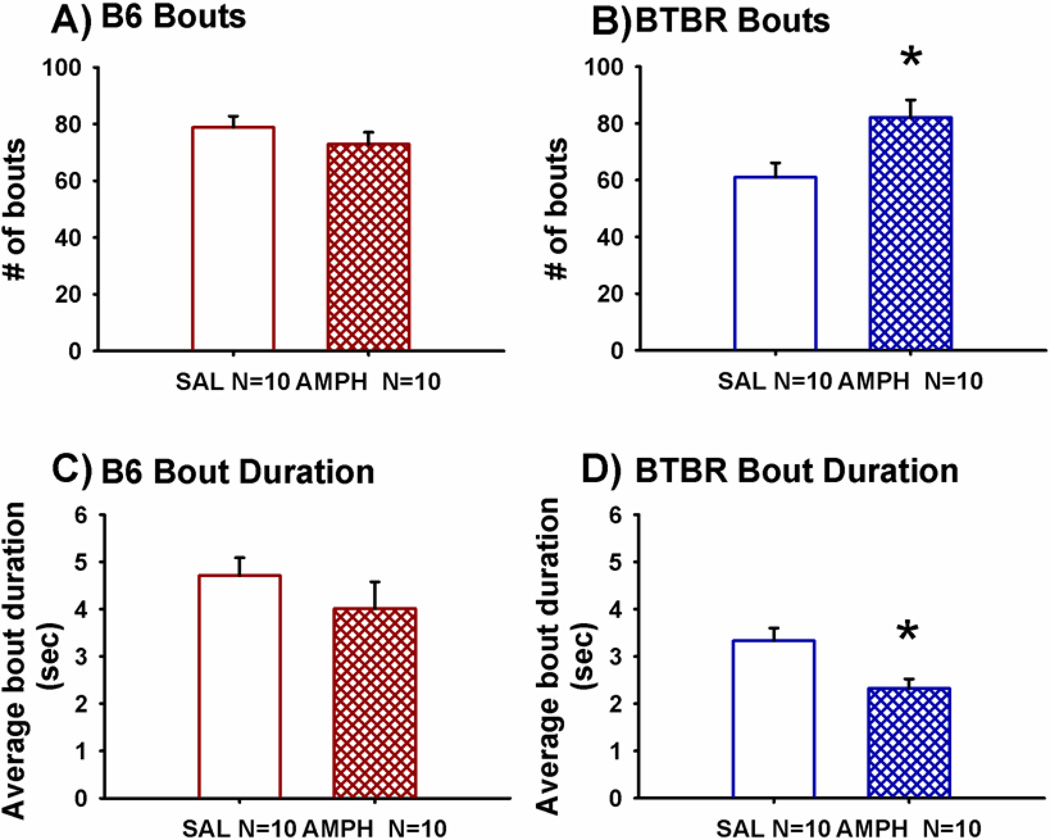
Figure 5 illustrates a representative cohort (using randomized sampling from each of the independent cohorts) that was scored in greater depth, to calculate the number and duration of bouts of sniffing the novel mouse and the novel object during the 3-chambered social approach task. In B6, AMPH, 2.0 mg/kg, had no effect on the total number of bouts of sniffing the novel mouse and novel object (Panel A, t(1,18) = 1.04, p > 0.05) nor the average duration of those bouts in B6 (Panel C, t(1,18) = 1.00, p > 0.05) as compared to saline treatment. In BTBR, AMPH, 2.0 mg/kg, produced a unique profile. AMPH significantly increased the total number of bouts of sniffing the novel mouse and novel object (Panel B, t(1,18) = −2.60, p < 0.02) and reduced the average duration of those bouts (Panel D, t(1,18) =2.81, p < 0.02) as compared to saline treatment in BTBR, indicative of more quick sniffs in passing, and fewer incidents of extended exploratory sniffs.
Figure 5. Amphetamine increased the number of sniffing bout events and reduced the duration of sniffing bouts in BTBR.
To quantitate the apparent qualitative difference in sniffing by BTBR treated with AMPH, which had been qualitatively apparent to observers, the number and duration of bout events of sniffing the novel mouse and novel object were scored over the 10 minute sociability session from videos of the social approach task. A representative sampling was created using sessions from the three independent cohorts. Videos were independently scored by three investigators, all uninformed of the drug treatment. For B6, (A) Number of discrete sniffing bouts, and (C) average duration of sniffing bouts did not differ in the AMPH versus saline treated groups. For BTBR, higher numbers of sniffing bouts occurred in mice treated with AMPH as compared to saline vehicle, and (D) the average duration of sniffing bouts was less in mice treated with AMPH compared to saline, indicative of many short sniffs in passing, rather than longer stationary exploratory sniffing of the novel mouse. *p < 0.02 AMPH 2.0 mg/kg i.p. as compared to SAL. N = 10 per dose for each strain.
Additional comparisons were performed to determine if a change in total number of bouts could be specifically attributed to bouts directed toward the novel mouse or novel object. AMPH, 2.0 mg/kg, did not have a differential effect on the number of bouts of sniffing the novel mouse (B6, t(1,18) = 1.26, p > 0.05; BTBR, t(1,18) = -1.69, p > 0.05) versus the novel object (B6, t(1,18) = −0.25, p > 0.05; BTBR, t(1,18) = -1.73, p > 0.05) in B6 and BTBR.
Comparisons were also performed to determine whether or not the sniffs following saline or amphetamine were directed toward the social stimulus in B6 and BTBR. Number of bouts of sniffing in B6 directed toward the novel mouse was greater than number of bouts directed toward the novel object in B6 administered saline (F(1,9) = 26.9, p < 0.001) but not B6 treated with AMPH (F(1,9) = 1.15, p > 0.05), paralleling the total time spent sniffing, as shown in Figures 2–3A. Number of bouts of sniffing in BTBR directed toward the novel mouse were similar to the number of bouts directed toward the novel object in BTBR administered saline (F(1,9) = 2.10, p > 0.05) or AMPH (F(1,9) = 0.43, p > 0.05), suggesting sniffing behavior that is less focused on the target.
3.6. Effects of amphetamine on repetitive self-grooming in B6 and BTBR, Cohorts 2, 3 and 4
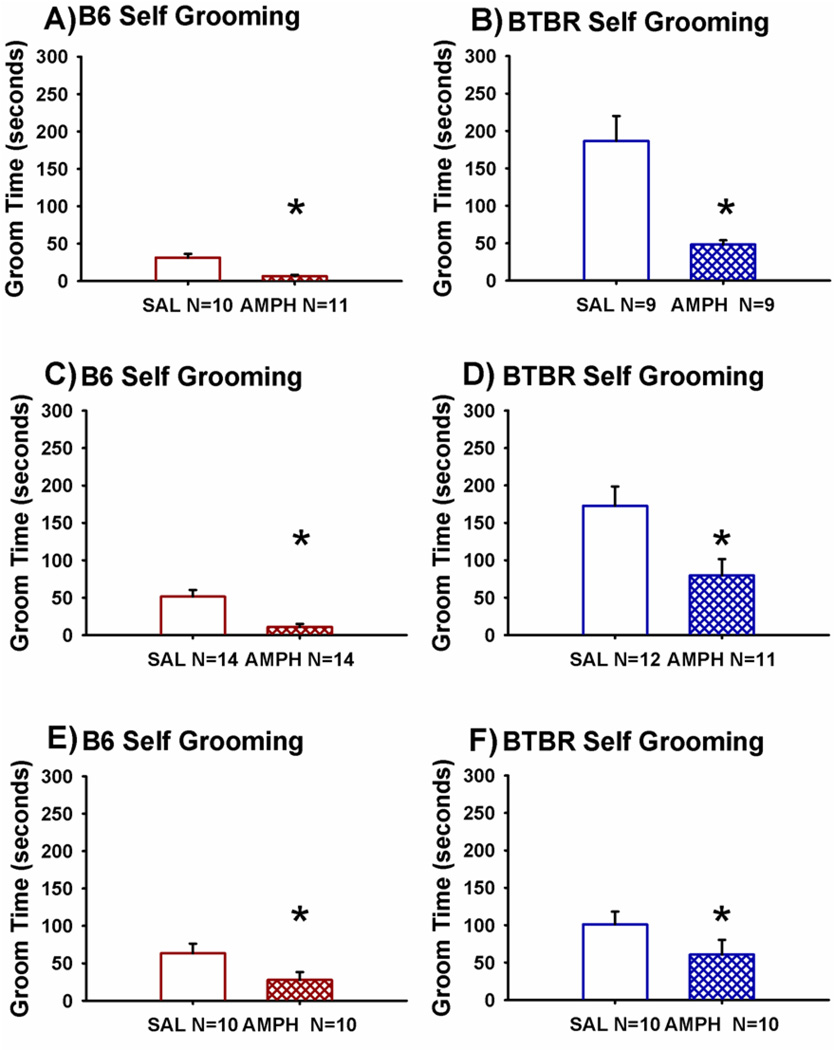
Figure 6 illustrates self-grooming scores for B6 and BTBR mice treated with saline vehicle or the moderate dose of AMPH, 2.0 mg/kg, in three independent cohorts run by three different investigators under similar laboratory conditions. AMPH significantly reduced repetitive self-grooming scores as compared to saline treatment in Cohort 1 B6 (Panel A, t(1,19) = 4.92, p < 0.0001) and BTBR (Panel B, t(1,16) = 4.13, p < 0.001), in Cohort 2 B6 (Panel C, t(1,26) = 4.16, p < 0.001) and BTBR (Panel D, t(1,21) =2.72, p < 0.01), and in Cohort 3 B6 (Panel E, t(1,18) = –2.13, p < 0.05) and BTBR (Panel F, t(1,16) = –2.7, p < 0.05).
Figure 6. Amphetamine reduced normal levels of self-grooming in B6 and reduced the characteristically high levels of repetitive self-grooming in BTBR.
Cumulative time spent engaged in self-grooming behavior was scored over a 10 minute session, in three independent cohorts scored by three investigators blind to drug treatment. Cohort 2: (A) Robust reductions in the normally low levels of self-grooming in B6 mice were detected after AMPH as compared to saline vehicle. (B) BTBR displayed significant reductions in their innately high levels of repetitive self-grooming after AMPH. Cohort 3: AMPH significantly reduced self-grooming in (C) B6 and (D) BTBR. Cohort 4: AMPH significantly reduced self-grooming in (E) B6 and (F) BTBR. *p < 0.05 AMPH 2.0 mg/kg i.p. as compared to SAL. N = 9–14 per dose for each strain.
4. Discussion
Effective pharmacological interventions in preclinical mouse model assays require a robust signal-to-noise phenotype, to provide an easily replicable baseline for the detection of a therapeutic response that has predictive validity specific to the human disorder. Model systems with high replicability in a variety of laboratory settings offer distinct advantages for discovering therapeutic benefits, and confirming their reliability. The inbred strain mouse model, BTBR, differs from the conventional models of autism that are based on targeted mutations of candidate genes for autism. Genes responsible for the autism-relevant behavioral abnormalities in BTBR have not yet been identified. BTBR therefore does not model a known genetic mutation associated with autism spectrum disorder. However, at present, BTBR is among the best animal models of autism in terms of robustness and replicability of phenotypes, and was therefore employed in the present pharmacological studies.
One promising target for treating symptoms of autism is the mGluR5 receptor. Antagonists and negative allosteric modulators effectively reversed several autism-relevant behavioral phenotypes in the Fmr1 mutant mouse, the valproic acid model of prenatal environmental insult, and the BTBR inbred strain mouse model of autism, including repetitive self-grooming and marble burying, seizures, and prepulse inhibition of acoustic startle, as well as mood disorder-like phenotypes in the elevated plus-maze, stress induced hyperthermia, forced swim task, conflict drinking test and four plate assessment (Bear et al., 2008; de Vrij et al., 2008; Dolen and Bear, 2008; Dolen et al., 2007; Hughes et al., 2012; Mehta et al., 2011; Nordquist et al., 2007; Pilc et al., 2002; Silverman et al., 2010a; Spooren et al., 2000; Tatarczynska et al., 2001; Yan et al., 2005). Recently we discovered that an mGluR5 negative allosteric modulator improved some components of social behaviors in BTBR. However, mGluR5 compounds appear to increase locomotor activity, (Mehta et al., 2011; Montana et al., 2009; Silverman et al., 2012; Silverman et al., 2010a; Thomas et al., 2012). In the present studies we addressed the extent to which hyperactivity induced by pharmacological agents may compete with, or contribute to social and repetitive behaviors in the BTBR mouse model of autism.
To directly test the hypothesis that drug-induced hyperlocomotion influences sociability in BTBR mice, we selected a dose of AMPH that induces hyperactivity directly. AMPH administration increased open field locomotion by ~ 2.0 fold in B6, replicated across three cohorts, consistent with the extensive literature on amphetamine-induced hyperactivity in mice (Moisset and Welch, 1973; Mueller et al., 1989; Yates et al., 2007). We found that AMPH increased open field locomotion similarly in BTBR, ~ 2.0 fold. A moderate dose that induced hyperlocomotion, 2.0 mg/kg, was then administered before the social approach task. We found that AMPH abolished one component of sociability in B6 mice, and enhanced one component of sociability in BTBR mice, consistently across three cohorts.
Specifically, AMPH-treated B6 did not display the normal sociability parameter of more time in the chamber with the novel mouse than time in the chamber with the novel object, although sociability on the more sensitive measure of time spent sniffing the novel mouse versus the novel object remained intact. In BTBR, which usually do not display sociability on either parameter, AMPH did not affect chamber time, but increased sociability on the more sensitive sniffing parameter. The sniff time parameter is highly precise and susceptible to change. Thus, it could be used as a read-out measure for drug treatment assays (Fairless et al., 2011; Silverman et al., 2012; Silverman et al., 2010a; Yang et al., 2011b). However, without corroboration of the chamber time parameter, this dichotomous profile is difficult to interpret.
One potential interpretation involves hypothalamic-pituitary-adrenal axis neuroendocrine factors. Circulating corticosterone is elevated in BTBR, as reported by three independent laboratories (Benno et al., 2009; Frye and Llaneza, 2010; Silverman et al., 2010c), and increased glucocorticoid receptor mRNA was detected in the BTBR hippocampal regions (Silverman et al., 2010c). Repeated exposure to stressors, which increases circulating corticosterone, is known to enhance the behavioral responses to AMPH (Kalivas and Stewart, 1991). Further, AMPH-induced hyperactivity is reduced by adrenalectomy, and dose-dependently increased by chronic corticosterone administration, actions that are dependent on central glucocorticoid receptors (Cador et al., 1993; Cools, 1991; Rivet et al., 1989). The unusual basal neuroendocrine profile of BTBR may therefore underlie the enhanced response to AMPH on sniffing in the social approach task. A second potential contributing factor is the high baseline locomotor activity exhibited by BTBR in a novel non-social environment (McFarlane et al., 2008; Pobbe et al., 2011; Silverman et al., 2010a). An increase in the sensitive sniffing parameter in BTBR treated with AMPH may be the result of a stimulant-induced reduction in dopaminergic transmission in the striatum (Nicola et al., 1996). A third consideration is that d-AMPH and methylphenidate (Ritalin) are widely used for treating the symptoms of attention deficit hyperactivity disorder (ADHD). Hyperactivity and lack of cognitive control are symptoms that often appear in ASD and Fragile X syndrome. (Farzin et al., 2006; Kochhar et al., 2011; Leyfer et al., 2006; Sullivan et al., 2006). Further, d-AMPH was reported to improve some behavioral phenotypes in the Fmr1 mutant mouse model of Fragile X syndrome (Ventura et al., 2004).
A fourth potential explanation for the BTBR sniffing reversal following AMPH administration is that the effect is an artifact of stereotyped sniffing induced directly by AMPH. AMPH administration elicits elevated frequencies of sniffing events, similar to other stimulants, including cocaine and caffeine, as characterized by a large number of sniffing bouts of short durations (Antoniou et al., 1998). We investigated this possibility by measuring sniffing bouts in greater detail. Three independent raters watched the videos again and observed qualitative differences in the type of exploratory and social behaviors exhibited by B6 and BTBR in the 3-chambered apparatus after AMPH treatment. A subset of randomly sampled videos was then recoded and scored on quantitative parameters of the sniffing bouts. In B6 mice, AMPH did not affect the total number of sniffing bouts, or the average duration of sniffing bouts. However in BTBR, AMPH increased the number and decreased the duration of sniffing bouts. This quantitative analysis supported the qualitative impressions that BTBR engaged in brief, cursory sniffs, mainly in passing, rather than directed exploratory sniffing of the novel mouse and the novel object. Disruptions in the normal balance between general locomotion and focused exploratory locomotion have been described in AMPH-treated rodents (Robbins and Iverson 1973). The apparent increase in sociability on the sniffing parameter in BTBR mice treated with AMPH may therefore be caused by brief non-exploratory sniff movements during hyperlocomotion. This interpretation is supported by lack of improvement in BTBR sociability on the chamber time parameter, and the increased number of entries into the side chambers by BTBR during the social approach task, indicative of AMPH-induced hyperlocomotion.
It is intriguing to speculate on the relevance of the present findings to the treatment of repetitive behaviors in autism. Therapeutic benefits, including reductions in aberrant behaviors and improvements in social communication, have been reported following methylphenidate or amphetamine stimulants in children with autism or pervasive developmental disorder who display hyperactivity (Jahromi et al., 2009; Network, 2005; Nickels et al., 2008; Quintana et al., 1995). Our experiments with B6 and BTBR mice indicate that a dose of AMPH which increases locomotion, decreased normal grooming scores in B6 and decreased the high levels of repetitive self-grooming in BTBR. Our previous reports that mGluR5 antagonism reduced self-grooming in BTBR (Silverman et al., 2010, 2012) showed a profile with some similarities to AMPH treatment, however, the hyperlocomotion induced by mGluR5 modulators was milder, and reductions in self-grooming by mGluR5 modulators were specific to the high levels of repetitive self-grooming displayed by BTBR. It will be important to compare mGluR5 compounds with the present AMPH findings on social approach, using the detailed quantitative analysis of sniffing time bout properties in BTBR and B6. These future studies would serve to evaluate the possibility that mGluR5 receptors present a therapeutic target for treating symptoms of autism, in concert with, or independent of, their less prominent activating actions on general exploratory behaviors. Further, clinical studies with mGluR5 antagonists may be enhanced by stratifying subjects into subgroups with and without ADHD comorbidity, similar to earlier research on therapeutic effects of methylphenidate (Handen et al., 2000).
In summary, our results may inform the experimental design for evaluating pharmacological therapeutics for autism spectrum disorders, particularly in cases where the drug influences general activity levels. The present findings could conceivably support the use of stimulant compounds in a defined subset of autistic individuals with hyperactivity.
Supplementary Material
Research Highlights.
Amphetamine dose dependently induced hyperlocomotion in B6 and BTBR mice
Amphetamine impaired sociability on one parameter of sociability in control B6 mice
Amphetamine increased social sniffing in the BTBR mouse model of autism
Amphetamine reduced low self-grooming in B6 and repetitive self-grooming in BTBR
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahams BS, Geschwind DH. Connecting genes to brain in the autism spectrum disorders. Arch Neurol. 2010;67:395–399. doi: 10.1001/archneurol.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic criteria from DSM-IV. Washington, DC: The Association; 1994. [Google Scholar]
- Anney R, Klei L, Pinto D, Regan R, Conroy J, Magalhaes TR, Correia C, Abrahams BS, Sykes N, Pagnamenta AT, Almeida J, Bacchelli E, Bailey AJ, Baird G, Battaglia A, Berney T, Bolshakova N, Bolte S, Bolton PF, Bourgeron T, Brennan S, Brian J, Carson AR, Casallo G, Casey J, Chu SH, Cochrane L, Corsello C, Crawford EL, Crossett A, Dawson G, de Jonge M, Delorme R, Drmic I, Duketis E, Duque F, Estes A, Farrar P, Fernandez BA, Folstein SE, Fombonne E, Freitag CM, Gilbert J, Gillberg C, Glessner JT, Goldberg J, Green J, Guter SJ, Hakonarson H, Heron EA, Hill M, Holt R, Howe JL, Hughes G, Hus V, Igliozzi R, Kim C, Klauck SM, Kolevzon A, Korvatska O, Kustanovich V, Lajonchere CM, Lamb JA, Laskawiec M, Leboyer M, Le Couteur A, Leventhal BL, Lionel AC, Liu XQ, Lord C, Lotspeich L, Lund SC, Maestrini E, Mahoney W, Mantoulan C, Marshall CR, McConachie H, McDougle CJ, McGrath J, McMahon WM, Melhem NM, Merikangas A, Migita O, Minshew NJ, Mirza GK, Munson J, Nelson SF, Noakes C, Noor A, Nygren G, Oliveira G, Papanikolaou K, Parr JR, Parrini B, Paton T, Pickles A, Piven J, Posey DJ, Poustka A, Poustka F, Prasad A, Ragoussis J, Renshaw K, Rickaby J, Roberts W, Roeder K, Roge B, Rutter ML, Bierut LJ, Rice JP, Salt J, Sansom K, Sato D, Segurado R, Senman L, Shah N, Sheffield VC, Soorya L, Sousa I, Stoppioni V, Strawbridge C, Tancredi R, Tansey K, Thiruvahindrapduram B, Thompson AP, Thomson S, Tryfon A, Tsiantis J, Van Engeland H, Vincent JB, Volkmar F, Wallace S, Wang K, Wang Z, Wassink TH, Wing K, Wittemeyer K, Wood S, Yaspan BL, Zurawiecki D, Zwaigenbaum L, Betancur C, Buxbaum JD, Cantor RM, Cook EH, Coon H, Cuccaro ML, Gallagher L, Geschwind DH, Gill M, Haines JL, Miller J, Monaco AP, Nurnberger JI, Jr, Paterson AD, Pericak-Vance MA, Schellenberg GD, Scherer SW, Sutcliffe JS, Szatmari P, Vicente AM, Vieland VJ, Wijsman EM, Devlin B, Ennis S, Hallmayer J. A genome-wide scan for common alleles affecting risk for autism. Hum Mol Genet. 2010;19:4072–4082. doi: 10.1093/hmg/ddq307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou K, Kafetzopoulos E, Papadopoulou-Daifoti Z, Hyphantis T, Marselos M. D-amphetamine, cocaine and caffeine: a comparative study of acute effects on locomotor activity and behavioural patterns in rats. Neurosci Biobehav Rev. 1998;23:189–196. doi: 10.1016/s0149-7634(98)00020-7. [DOI] [PubMed] [Google Scholar]
- Bailey KR, Pavlova MN, Rohde AD, Hohmann JG, Crawley JN. Galanin receptor subtype 2 (GalR2) null mutant mice display an anxiogenic-like phenotype specific to the elevated plus-maze. Pharmacol Biochem Behav. 2007;86:8–20. doi: 10.1016/j.pbb.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Dolen G, Osterweil E, Nagarajan N. Fragile X: translation in action. Neuropsychopharmacology. 2008;33:84–87. doi: 10.1038/sj.npp.1301610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Benno R, Smirnova Y, Vera S, Liggett A, Schanz N. Exaggerated responses to stress in the BTBR T+tf/J mouse: an unusual behavioral phenotype. Behav Brain Res. 2009;197:462–465. doi: 10.1016/j.bbr.2008.09.041. [DOI] [PubMed] [Google Scholar]
- Bolivar V, Solanki M, Du W, Day S, Manley K. Society for Neuoscience. Washington DC: 2011. Autism-like behaviors in mice are modified by genetic background, sex and testing protocol. [Google Scholar]
- Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav Brain Res. 2007;176:21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothe G, Solanki M, Du W, Kusek G, Auerbach R, Manley K, Bolivar V. Genetic investigations of corpus callosum abnormalities in Btbr t+ tf/j mice Society for Neuroscience. Washington DC: 2011. [Google Scholar]
- Brielmaier J, Matteson PG, Silverman JL, Senerth JM, Kelly S, Genestine M, Millonig JH, DiCicco-Bloom E, Crawley JN. Autism-relevant social abnormalities and cognitive deficits in engrailed-2 knockout mice. PLoS One. 2012 doi: 10.1371/journal.pone.0040914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodkin ES. BALB/c mice: low sociability and other phenotypes that may be relevant to autism. Behav Brain Res. 2007;176:53–65. doi: 10.1016/j.bbr.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Brodkin ES, Hagemann A, Nemetski SM, Silver LM. Social approach-avoidance behavior of inbred mouse strains towards DBA/2 mice. Brain Res. 2004;1002:151–157. doi: 10.1016/j.brainres.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Bucan M, Abrahams BS, Wang K, Glessner JT, Herman EI, Sonnenblick LI, Alvarez Retuerto AI, Imielinski M, Hadley D, Bradfield JP, Kim C, Gidaya NB, Lindquist I, Hutman T, Sigman M, Kustanovich V, Lajonchere CM, Singleton A, Kim J, Wassink TH, McMahon WM, Owley T, Sweeney JA, Coon H, Nurnberger JI, Li M, Cantor RM, Minshew NJ, Sutcliffe JS, Cook EH, Dawson G, Buxbaum JD, Grant SF, Schellenberg GD, Geschwind DH, Hakonarson H. Genome-wide analyses of exonic copy number variants in a family-based study point to novel autism susceptibility genes. PLoS Genet. 2009;5:e1000536. doi: 10.1371/journal.pgen.1000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cador M, Dulluc J, Mormede P. Modulation of the locomotor response to amphetamine by corticosterone. Neuroscience. 1993;56:981–988. doi: 10.1016/0306-4522(93)90144-5. [DOI] [PubMed] [Google Scholar]
- Chadman KK, Gong S, Scattoni ML, Boltuck SE, Gandhy SU, Heintz N, Crawley JN. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 2008;1:147–158. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb S, Guy J, Bird A. Reversibility of functional deficits in experimental models of Rett syndrome. Biochem Soc Trans. 2010;38:498–506. doi: 10.1042/BST0380498. [DOI] [PubMed] [Google Scholar]
- Cools AR. Differential role of mineralocorticoid and glucocorticoid receptors in the genesis of dexamphetamine-induced sensitization of mesolimbic, alpha 1 adrenergic receptors in the ventral striatum. Neuroscience. 1991;43:419–428. doi: 10.1016/0306-4522(91)90304-7. [DOI] [PubMed] [Google Scholar]
- Dawson G. Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Dev Psychopathol. 2008;20:775–803. doi: 10.1017/S0954579408000370. [DOI] [PubMed] [Google Scholar]
- Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, Donaldson A, Varley J. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics. 2010;125:e17–e23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vrij FM, Levenga J, van der Linde HC, Koekkoek SK, De Zeeuw CI, Nelson DL, Oostra BA, Willemsen R. Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiol Dis. 2008;31:127–132. doi: 10.1016/j.nbd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defensor EB, Pearson BL, Pobbe RL, Bolivar VJ, Blanchard DC, Blanchard RJ. A novel social proximity test suggests patterns of social avoidance and gaze aversion-like behavior in BTBR T+ tf/J mice. Behav Brain Res. 2011;217:302–308. doi: 10.1016/j.bbr.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCicco-Bloom E, Lord C, Zwaigenbaum L, Courchesne E, Dager SR, Schmitz C, Schultz RT, Crawley J, Young LJ. The developmental neurobiology of autism spectrum disorder. J Neurosci. 2006;26:6897–6906. doi: 10.1523/JNEUROSCI.1712-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolen G, Bear MF. Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J Physiol. 2008;586:1503–1508. doi: 10.1113/jphysiol.2008.150722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, Ramesh V, Silva AJ. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med. 2008a;14:843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Li W, Fox K, Stryker MP, Silva AJ. Reversing neurodevelopmental disorders in adults. Neuron. 2008b;60:950–960. doi: 10.1016/j.neuron.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairless AH, Shah RY, Guthrie AJ, Li H, Brodkin ES. Deconstructing sociability, an autism-relevant phenotype, in mouse models. Anat Rec (Hoboken) 2011;294:1713–1725. doi: 10.1002/ar.21318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzin F, Perry H, Hessl D, Loesch D, Cohen J, Bacalman S, Gane L, Tassone F, Hagerman P, Hagerman R. Autism spectrum disorders and attentiondeficit/ hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr. 2006;27:S137–S144. doi: 10.1097/00004703-200604002-00012. [DOI] [PubMed] [Google Scholar]
- Frye CA, Llaneza DC. Corticosteroid and neurosteroid dysregulation in an animal model of autism, BTBR mice. Physiol Behav. 2010;100:264–267. doi: 10.1016/j.physbeh.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handen BL, Johnson CR, Lubetsky M. Efficacy of methylphenidate among children with autism and symptoms of attention-deficit hyperactivity disorder. J Autism Dev Disord. 2000;30:245–255. doi: 10.1023/a:1005548619694. [DOI] [PubMed] [Google Scholar]
- Happe F, Ronald A. The 'fractionable autism triad': a review of evidence from behavioural, genetic, cognitive and neural research. Neuropsychol Rev. 2008;18:287–304. doi: 10.1007/s11065-008-9076-8. [DOI] [PubMed] [Google Scholar]
- Hayashi ML, Rao BS, Seo JS, Choi HS, Dolan BM, Choi SY, Chattarji S, Tonegawa S. Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice. Proc Natl Acad Sci U S A. 2007;104:11489–11494. doi: 10.1073/pnas.0705003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ZA, Neal SJ, Smith DL, Sukoff Rizzo SJ, Pulicicchio CM, Lotarski S, Lu S, Dwyer JM, Brennan J, Olsen M, Bender CN, Kouranova E, Andree TH, Harrison JE, Whiteside GT, Springer D, O'Neill SV, Leonard SK, Schechter LE, Dunlop J, Rosenzweig-Lipson S, Ring RH. Negative allosteric modulation of metabolic glutamate receptor 5 results in broad spectrum activity relevant to treatment resistant depression. Neuropharmacology. 2012 Apr 21; doi: 10.1016/j.neuropharm.2012.04.007. Epub. [DOI] [PubMed] [Google Scholar]
- Jacquemont S, Curie A, des Portes V, Torrioli MG, Berry-Kravis E, Hagerman RJ, Ramos FJ, Cornish K, He Y, Paulding C, Neri G, Chen F, Hadjikhani N, Martinet D, Meyer J, Beckmann JS, Delange K, Brun A, Bussy G, Gasparini F, Hilse T, Floesser A, Branson J, Bilbe G, Johns D, Gomez-Mancilla B. Epigenetic modification of the FMR1 gene in fragile X syndrome is associated with differential response to the mGluR5 antagonist AFQ056. Sci Transl Med. 2011 Jan 5;3(64):64ra1. doi: 10.1126/scitranslmed.3001708. [DOI] [PubMed] [Google Scholar]
- Jahromi LB, Kasari CL, McCracken JT, Lee LS, Aman MG, McDougle CJ, Scahill L, Tierney E, Arnold LE, Vitiello B, Ritz L, Witwer A, Kustan E, Ghuman J, Posey DJ. Positive effects of methylphenidate on social communication and self-regulation in children with pervasive developmental disorders and hyperactivity. J Autism Dev Disord. 2009;39:395–404. doi: 10.1007/s10803-008-0636-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Davis D, Yang M, Rider E, Sen S, Crawley J, Sherr E. Society for Neuroscience. Washington DC: 2011. Identification of loci associated with autism-relevant behavioral traits in the BTBR strain of mouse. [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Winnock M, Stinus L. Amphetamine, apomorphine and investigatory behavior in the rat: analysis of the structure and pattern of responses. Psychopharmacology (Berl) 1986;88:66–74. doi: 10.1007/BF00310515. [DOI] [PubMed] [Google Scholar]
- Kochhar P, Batty MJ, Liddle EB, Groom MJ, Scerif G, Liddle PF, Hollis CP. Autistic spectrum disorder traits in children with attention deficit hyperactivity disorder. Child Care Health Dev. 2011;37:103–110. doi: 10.1111/j.1365-2214.2010.01123.x. [DOI] [PubMed] [Google Scholar]
- Krasny L, Williams BJ, Provencal S, Ozonoff S. Social skills interventions for the autism spectrum: essential ingredients and a model curriculum. Child Adolesc Psychiatr Clin N Am. 2003;12:107–122. doi: 10.1016/s1056-4993(02)00051-2. [DOI] [PubMed] [Google Scholar]
- Krueger DD, Bear MF. Toward fulfilling the promise of molecular medicine in fragile X syndrome. Annu Rev Med. 2011;62:411–429. doi: 10.1146/annurev-med-061109-134644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa RJ. Diagnosis of autism spectrum disorders in the first 3 years of life. Nat Clin Pract Neurol. 2008;4:138–147. doi: 10.1038/ncpneuro0731. [DOI] [PubMed] [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, Tager-Flusberg H, Lainhart JE. Comorbid psychiatric disorders in children with autism: interview development and rates of disorders. J Autism Dev Disord. 2006;36:849–861. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- Marcus RN, Owen R, Manos G, Mankoski R, Kamen L, McQuade RD, Carson WH, Corey-Lisle PK, Aman MG. Aripiprazole in the treatment of irritability in pediatric patients (aged 6–17 years) with autistic disorder: results from a 52-week, open-label study. J Child Adolesc Psychopharmacol. 2011;21:229–236. doi: 10.1089/cap.2009.0121. [DOI] [PubMed] [Google Scholar]
- McCracken JT, McGough J, Shah B, Cronin P, Hong D, Aman MG, Arnold LE, Lindsay R, Nash P, Hollway J, McDougle CJ, Posey D, Swiezy N, Kohn A, Scahill L, Martin A, Koenig K, Volkmar F, Carroll D, Lancor A, Tierney E, Ghuman J, Gonzalez NM, Grados M, Vitiello B, Ritz L, Davies M, Robinson J, McMahon D. Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002;347:314–321. doi: 10.1056/NEJMoa013171. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Scahill L, McCracken JT, Aman MG, Tierney E, Arnold LE, Freeman BJ, Martin A, McGough JJ, Cronin P, Posey DJ, Riddle MA, Ritz L, Swiezy NB, Vitiello B, Volkmar FR, Votolato NA, Walson P. Research Units on Pediatric Psychopharmacology (RUPP) Autism Network. Background and rationale for an initial controlled study of risperidone. Child Adolesc Psychiatr Clin N Am. 2000;9:201–224. [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- McPheeters ML, Warren Z, Sathe N, Bruzek JL, Krishnaswami S, Jerome RN, Veenstra-Vanderweele J. A systematic review of medical treatments for children with autism spectrum disorders. Pediatrics. 2011 May;127(5):e1312–e1211. doi: 10.1542/peds.2011-0427. [DOI] [PubMed] [Google Scholar]
- Mehta MV, Gandal MJ, Siegel SJ. mGluR5-antagonist mediated reversal of elevated stereotyped, repetitive behaviors in the VPA model of autism. PLoS One. 2011;6:e26077. doi: 10.1371/journal.pone.0026077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle L, Pollizzi K, Egnor A, Kramvis I, Lane H, Sahin M, Kwiatkowski DJ. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J Neurosci. 2008;28:5422–5432. doi: 10.1523/JNEUROSCI.0955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisset B, Welch BL. Effects of d-amphetamine upon open field behaviour in two inbred strains of mice. Experientia. 1973;29:625–626. doi: 10.1007/BF01926708. [DOI] [PubMed] [Google Scholar]
- Montana MC, Cavallone LF, Stubbert KK, Stefanescu AD, Kharasch ED, Gereau RWt. The metabotropic glutamate receptor subtype 5 antagonist fenobam is analgesic and has improved in vivo selectivity compared with the prototypical antagonist 2-methyl-6-(phenylethynyl)-pyridine. J Pharmacol Exp Ther. 2009;330:834–843. doi: 10.1124/jpet.109.154138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Segall SK, Andrade GM, Crawley JN, Magnuson TR. Social approach and repetitive behavior in eleven inbred mouse strains. Behav Brain Res. 2008;191:118–129. doi: 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller K, Kunko PM, Whiteside D, Haskett C. Time course of amphetamine-induced locomotor stereotypy in an open field. Psychopharmacology (Berl) 1989;99:501–507. doi: 10.1007/BF00589899. [DOI] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Network RU o. P. P. R. A. Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch Gen Psychiatry. 2005;62:1266–1274. doi: 10.1001/archpsyc.62.11.1266. [DOI] [PubMed] [Google Scholar]
- Nickels K, Katusic SK, Colligan RC, Weaver AL, Voigt RG, Barbaresi WJ. Stimulant medication treatment of target behaviors in children with autism: a population-based study. J Dev Behav Pediatr. 2008;29:75–81. doi: 10.1097/dbp.0b013e31815f24f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Kombian SB, Malenka RC. Psychostimulants depress excitatory synaptic transmission in the nucleus accumbens via presynaptic D1-like dopamine receptors. J Neurosci. 1996;16:1591–1604. doi: 10.1523/JNEUROSCI.16-05-01591.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordquist RE, Durkin S, Jaeschke G, Spooren W. Stress-induced hyperthermia: effects of acute and repeated dosing of MPEP. Eur J Pharmacol. 2007;568:199–202. doi: 10.1016/j.ejphar.2007.04.034. [DOI] [PubMed] [Google Scholar]
- Ogier M, Wang H, Hong E, Wang Q, Greenberg ME, Katz DM. Brain-derived neurotrophic factor expression and respiratory function improve after ampakine treatment in a mouse model of Rett syndrome. J Neurosci. 2007;27:10912–10917. doi: 10.1523/JNEUROSCI.1869-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, Jochman KA, Kim JU, Koy JJ, Wilson ED, Chen Q, Wilson CR, Lahvis GP. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS One. 2007;2:e351. doi: 10.1371/journal.pone.0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F, Crawley JN, Song J, Lipska BK, Pickel J, Weinberger DR, Chen J. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. J Neurosci. 2008;28:8709–8723. doi: 10.1523/JNEUROSCI.2077-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F, Silverman JL, Aney J, Tian Q, Barkan CL, Chadman KK, Crawley JN. Working memory deficits, increased anxiety-like traits, and seizure susceptibility in BDNF overexpressing mice. Learn Mem. 2011;18:534–544. doi: 10.1101/lm.2213711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson BL, Pobbe RL, Defensor EB, Oasay L, Bolivar VJ, Blanchard DC, Blanchard RJ. Motor and cognitive stereotypies in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011;10:228–235. doi: 10.1111/j.1601-183X.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, Sonnenblick LI, Gruver R, Almajano J, Bragin A, Golshani P, Trachtenberg JT, Peles E, Geschwind DH. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147:235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilc A, Klodzinska A, Branski P, Nowak G, Palucha A, Szewczyk B, Tatarczynska E, Chojnacka-Wojcik E, Wieronska JM. Multiple MPEP administrations evoke anxiolytic- and antidepressant-like effects in rats. Neuropharmacology. 2002;43:181–187. doi: 10.1016/s0028-3908(02)00082-5. [DOI] [PubMed] [Google Scholar]
- Pobbe RL, Defensor EB, Pearson BL, Bolivar VJ, Blanchard DC, Blanchard RJ. General and social anxiety in the BTBR T+ tf/J mouse strain. Behav Brain Res. 2011;216:446–451. doi: 10.1016/j.bbr.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobbe RL, Pearson BL, Defensor EB, Bolivar VJ, Blanchard DC, Blanchard RJ. Expression of social behaviors of C57BL/6J versus BTBR inbred mouse strains in the visible burrow system. Behav Brain Res. 2010;214:443–449. doi: 10.1016/j.bbr.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana H, Birmaher B, Stedge D, Lennon S, Freed J, Bridge J, Greenhill L. Use of methylphenidate in the treatment of children with autistic disorder. J Autism Dev Disord. 1995;25:283–294. doi: 10.1007/BF02179289. [DOI] [PubMed] [Google Scholar]
- Rivet JM, Stinus L, LeMoal M, Mormede P. Behavioral sensitization to amphetamine is dependent on corticosteroid receptor activation. Brain Res. 1989;498:149–153. doi: 10.1016/0006-8993(89)90411-3. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Vismara LA. Evidence-based comprehensive treatments for early autism. J Clin Child Adolesc Psychol. 2008;37:8–38. doi: 10.1080/15374410701817808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roullet FI, Wohr M, Crawley JN. Female urine-induced male mice ultrasonic vocalizations, but not scent-marking, is modulated by social experience. Behav Brain Res. 2011;216:19–28. doi: 10.1016/j.bbr.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan BC, Young NB, Crawley JN, Bodfish JW, Moy SS. Social deficits, stereotypy and early emergence of repetitive behavior in the C58/J inbred mouse strain. Behav Brain Res. 2010;208:178–188. doi: 10.1016/j.bbr.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One. 2008;3:e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes Brain Behav. 2011;10:44–56. doi: 10.1111/j.1601-183X.2010.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sills TL, Onalaja AO, Crawley JN. Mesolimbic dopaminergic mechanisms underlying individual differences in sugar consumption and amphetamine hyperlocomotion in Wistar rats. Eur J Neurosci. 1998;10:1895–1902. doi: 10.1046/j.1460-9568.1998.00201.x. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Smith DG, Sukoff Rizzo SJ, Karras MN, Turner SM, Tolu SS, Bryce DK, Smith DL, Fonseca K, Ring RH, Crawley JN. Negative allosteric modulation of the mGluR5 receptor reduces repetitive behaviors and rescues social deficits in mouse models of autism. Science Translational Medicine. 2012 Apr 25;4(131):131ra51. doi: 10.1126/scitranslmed.3003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010a;35:976–989. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Turner SM, Barkan CL, Tolu SS, Saxena R, Hung AY, Sheng M, Crawley JN. Sociability and motor functions in Shank1 mutant mice. Brain Res. 2011;1380:120–137. doi: 10.1016/j.brainres.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010b;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Turner SM, Katz AM, Bell DB, Koenig JI, Crawley JN. Low stress reactivity and neuroendocrine factors in the BTBR T+tf/J mouse model of autism. Neuroscience. 2010c;171:1197–1208. doi: 10.1016/j.neuroscience.2010.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooren WP, Vassout A, Neijt HC, Kuhn R, Gasparini F, Roux S, Porsolt RD, Gentsch C. Anxiolytic-like effects of the prototypical metabotropic glutamate receptor 5 antagonist 2-methyl-6-(phenylethynyl)pyridine in rodents. J Pharmacol Exp Ther. 2000;295:1267–1275. [PubMed] [Google Scholar]
- Stromberg U, Svensson TH. Differences between (+)- and (−)-amphetamine in effects on locomotor activity and L-dopa potentiating action in mice. Naunyn Schmiedebergs Arch Pharmacol. 1975;287:171–179. doi: 10.1007/BF00510448. [DOI] [PubMed] [Google Scholar]
- Sullivan K, Hatton D, Hammer J, Sideris J, Hooper S, Ornstein P, Bailey D., Jr ADHD symptoms in children with FXS. Am J Med Genet A. 2006;140:2275–2288. doi: 10.1002/ajmg.a.31388. [DOI] [PubMed] [Google Scholar]
- Tatarczynska E, Klodzinska A, Chojnacka-Wojcik E, Palucha A, Gasparini F, Kuhn R, Pilc A. Potential anxiolytic- and antidepressant-like effects of MPEP, a potent, selective and systemically active mGlu5 receptor antagonist. Br J Pharmacol. 2001;132:1423–1430. doi: 10.1038/sj.bjp.0703923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AM, Bui N, Perkins JR, Yuva-Paylor LA, Paylor R. Group I metabotropic glutamate receptor antagonists alter select behaviors in a mouse model for fragile X syndrome. Psychopharmacology (Berl) 2012;219:47–58. doi: 10.1007/s00213-011-2375-4. [DOI] [PubMed] [Google Scholar]
- Varni JW, Handen BL, Corey-Lisle PK, Guo Z, Manos G, Ammerman DK, Marcus RN, Owen R, McQuade RD, Carson WH, Mathew S, Mankoski R. Effect of Aripiprazole 2 to 15 mg/d on Health-Related Quality of Life in the Treatment of Irritability Associated with Autistic Disorder in Children: A Post Hoc Analysis of Two Controlled Trials. Clin Ther. 2012 doi: 10.1016/j.clinthera.2012.02.023. In press. [DOI] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Blakely RD. Networking in autism: leveraging genetic, biomarker and model system findings in the search for new treatments. Neuropsychopharmacology. 2012;37:196–212. doi: 10.1038/npp.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams White S, Keonig K, Scahill L. Social skills development in children with autism spectrum disorders: a review of the intervention research. J Autism Dev Disord. 2007;37:1858–1868. doi: 10.1007/s10803-006-0320-x. [DOI] [PubMed] [Google Scholar]
- Wink LK, Erickson CA, McDougle CJ. Pharmacologic treatment of behavioral symptoms associated with autism and other pervasive developmental disorders. Curr Treat Options Neurol. 2010;12:529–538. doi: 10.1007/s11940-010-0091-8. [DOI] [PubMed] [Google Scholar]
- Wohr M, Roullet FI, Crawley JN. Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011;10:35–43. doi: 10.1111/j.1601-183X.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Yang M, Abrams DN, Zhang JY, Weber MD, Katz AM, Clarke AM, Silverman JL, Crawley JN. Low sociability in BTBR T+tf/J mice is independent of partner strain. Physiol Behav. 2012a doi: 10.1016/j.physbeh.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Bozdagi O, Scattoni ML, Wöhr M, Roullet FI, Katz AM, Abrams DN, Kalikhman D, Simon H, Zhang JY, Harris MJ, Saxena R, Silverman JL, Buxbaum JD, Crawley JN. Reduced excitatory neurotransmission and mild autism-relevant phenotypes in adolescent shank3 null mutant mice. Journal of Neuroscience. 2012b May 9;32(19):6525–6541. doi: 10.1523/JNEUROSCI.6107-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Clarke AM, Crawley JN. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur J Neurosci. 2009;29:1663–1677. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Perry K, Weber MD, Katz AM, Crawley JN. Social peers rescue autism-relevant sociability deficits in adolescent mice. Autism Res. 2011a;4:17–27. doi: 10.1002/aur.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Scattoni ML, Zhodzishsky V, Chen T, Caldwell H, Young WS, McFarlane HG, Crawley JN. Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+ tf/J, C57BL/6J, and vasopressin receptor 1B mutant mice. Front Behav Neurosci. 2007a;1:1. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Silverman JL, Crawley JN. Automated three-chambered social approach task for mice. Chapter 8, Unit8. Curr Protoc Neurosci. 2011b;26 doi: 10.1002/0471142301.ns0826s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Dev Neurosci. 2007b;25:515–521. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates JW, Meij JT, Sullivan JR, Richtand NM, Yu L. Bimodal effect of amphetamine on motor behaviors in C57BL/6 mice. Neurosci Lett. 2007;427:66–70. doi: 10.1016/j.neulet.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Blundell J, Ogawa S, Kwon CH, Zhang W, Sinton C, Powell CM, Parada LF. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J Neurosci. 2009;29:1773–1783. doi: 10.1523/JNEUROSCI.5685-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Lord C, Rogers S, Carter A, Carver L, Chawarska K, Constantino J, Dawson G, Dobkins K, Fein D, Iverson J, Klin A, Landa R, Messinger D, Ozonoff S, Sigman M, Stone W, Tager-Flusberg H, Yirmiya N. Clinical assessment and management of toddlers with suspected autism spectrum disorder: insights from studies of high-risk infants. Pediatrics. 2009;123:1383–1391. doi: 10.1542/peds.2008-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.