Abstract
Indoles: A joint effort of light and air We have developed a mild aerobic oxidation protocol using visible light photocatalysis to synthesize structurally diverse N-arylindoles. The procedure employs 4 mol% [Ru(bpz)3](PF6)2, 18W LED light, and is performed open to the atmosphere. Readily prepared o-stryryl anilines are converted to a variety of indoles via a cascade sequence composed of oxidation of anilines, C-N bond formation, and aromatization. A 1,2-carbon shift can be also incorporated into this cascade event to further extend the substrate scope of the method. bpz = 2, 2′-Bipyrazine
Keywords: cascade, indole, photocatalysis, ruthenium, visible light
Indoles are a common heterocyclic motif embedded in a large number of bioactive natural products and pharmaceuticals.[1] As such, the search for better, sustainable, and more efficient methods of indole synthesis has been a topic of constant interest.[2,3] Amination of vinyl C-H bonds of stryryl anilines provides a direct and potentially more efficient entry to indoles, particularly since stryryl anilines are readily prepared by the Buchwald-Hartwig amination[4] of 2-bromostyrene with an amine. This approach was first established with the assistance of a Pd complex at high temperature by the pioneering work from Hegedus.[5a,b] However, its use in indole synthesis has been limited. One notable example was a recent work by Buchwald on Pd-catalyzed cyclization of 2-chloro-N-(2-vinyl)aniline.[5c]
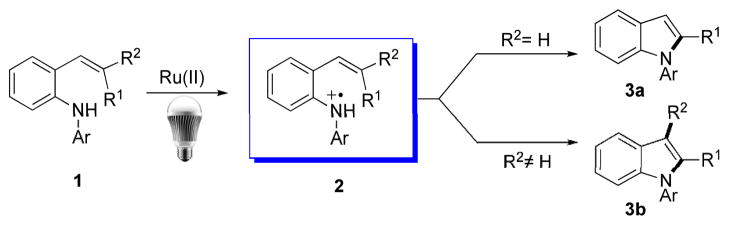
Recently, visible light photocatalysis has become a focus of research in organic chemistry and sparked a flurry of activity.[6] In parallel with other groups’ efforts in this field,[7–11] we[12] have been engaged in exploring new reactivities of nitrogen radical cations generated via direct oxidation of the corresponding amines by photoexcited ruthenium polypyridyl complexes[13]. Although this type of oxidation was first discovered in the late 1970s,[14] its potential in organic synthesis has not been extensively explored until recently. Under visible light photoredox conditions, the fate of nitrogen radical cations has been shown to follow one of three pathways: conversion to an iminium ion with concomitant release of a hydrogen radical,[15] conversion to an alpha amino radical by deprotonation,[16] or coupling with an irreversible ring opening process to form a carbon radical distal to the nitrogen atom.[12b] We speculated that other reaction pathways known to nitrogen radical cations, including electrophilic addition to olefins, might be amenable to visible light photocatalysis under similar conditions. Herein we report that nitrogen radical cation 2, generated from stryryl aniline 1, can undergo electrophilic addition to the tethered olefin, triggering a cascade event with aromatization (when R2=H in 2) or C-C bond migration followed by aromatization (when R2 ≠ H) to form indoles 3a–b (Scheme 1). This new photocatalytic entry to indoles is especially attractive, since mild aerobic oxidation conditions (visible light, open to air, and room temperature) are employed.
Scheme 1.
Visible light mediated indole synthesis.
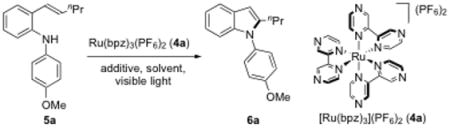
Stryryl aniline 5a was chosen as the model substrate for initial investigation (Table 1). Ru(bpz)3·(PF6)2 4a[17] was employed as the photocatalyst and an 18W LED white light was used as the source of visible light. Using 2 mol% of 4a in CH3CN and open to air, we were pleased to find that the desired indole product 6a was formed in 31% yield, although the reaction did not proceed to completion after 24 h (entry 1). Use of O2 in the place of ambient atmosphere (entry 2) or the use of TFE[18] as the solvent (entry 3) did not improve the yield. Addition of silica gel[18] to the reaction mixture significantly accelerated the reaction, which was complete after 12 h and provided indole 6a in 68% yield (entry 4). Doubling the catalyst loading further shortened the reaction time to 5 h and increased the yield of indole 6a to 88% (entry 5). Ru(bpy)3·(PF6)2 4b[17b] was found to be inferior to 4a.[19] Control studies showed that the catalyst, light, and air were all essential for this transformation (entries 7–9). It is noteworthy that a p-alkoxyphenyl group is also critical for the reaction.[18] When the p-methoxyphenyl group of 5bwas replaced by a phenyl group (N-phenyl-2-vinylaniline, 5b′), no reaction was observed under the same conditions. We are currently investigating the role of the p-alkoxyphenyl group in the reaction.[20]
Table 1.
Optimization of the catalytic system.
| ||||
|---|---|---|---|---|
| Entry | Conditions[a] | t [h] | Conv. of 5a [%][b] | Yield of 6a [%][b] |
| 1 | 4a (2 mol%), CH3CN | 24 | 44 | 31 |
| 2 | 4a (2 mol%), CH3CN, O2 | 24 | 49 | 33 |
| 3 | 4a (2 mol%), TFE | 24 | 42 | 21 |
| 4 | 4a (2 mol%), silica gel, CH3CN | 12 | 100 | 68 |
| 5 | 4a (4 mol%), silica gel, CH3CN | 5 | 100 | 88 |
| 6 | 4b (2 mol%), silica gel, CH3CN | 24 | 100 | 19 |
| 7 | Silica gel, CH3CN, no 4a, light | 24 | 8 | 4 |
| 8 | 4a (2 mol%), silica gel, CH3CN, no light | 24 | 4 | 2 |
| 9 | 4a (2 mol%), silica gel, CH3CN, degassed | 24 | 8 | 0 |
Conditions: 5a (0.2 mmol), solvent, open air, irradiation with a white LED light at rt.
Measured by GC using dodecane as an internal standard.
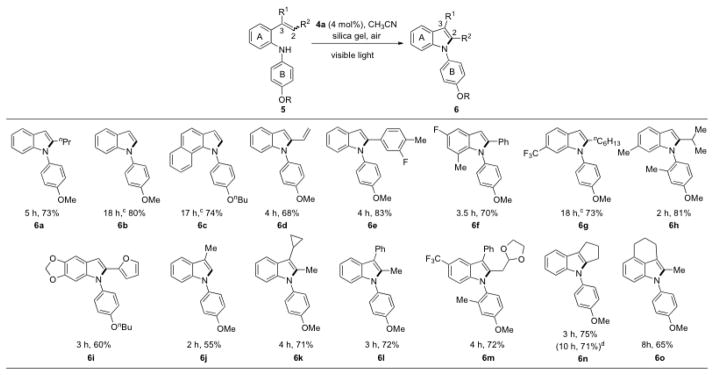
To examine the substrate scope of this method, a series of stryryl anilines 5 were prepared using the Buchwald-Hartwig amination of a 2-bromostyrene derivative with anilines. Under the optimized conditions (entry 7, Table 1), stryryl anilines 5 were converted to the desired indole products 6 in moderate to good yields (Table 2). Electron-donating and electron-withdrawing groups were tolerated on the aryl ring A. A number of alkenes substituted with various functional groups such as alkanes, alkenes, arenes, and furan were effective for this method as long as C2 was monosubstituted. Disubstituted (2,3- and 3,3-) and trisubstituted (2,3,3-) alkenes were all converted uneventfully to 2-substituted, 3-substituted, and 2,3-disubstituted indoles, respectively. Since a general solution to regioselectively introduce substituents at C2 and C3 of indoles is lacking,[2b,e,f] this method provides an appealing solution to address this unmet need in indole synthesis. Steric hindrance surrounding the alkene is well tolerated as shown by the example of 6h. When the alkene was part of a carbocycle, fused indoles (6n, 6o) were obtained as the product. The yield of 6n did not suffer albeit taking a longer time to go to completion when it was scaled up to 1 g.
Table 2.
|
Conditions, unless otherwise noted: 4 mol% Ru(bpz)3(PF6)2, silica gel, air, CH3CN, irradiation with a 18 W LED white light. The newly formed C-N bonds are shown in bold.
Isolated yields after column chromatography.
Using 6 mol% Ru(bpz)3(PF6)2.
Gram scale reaction.
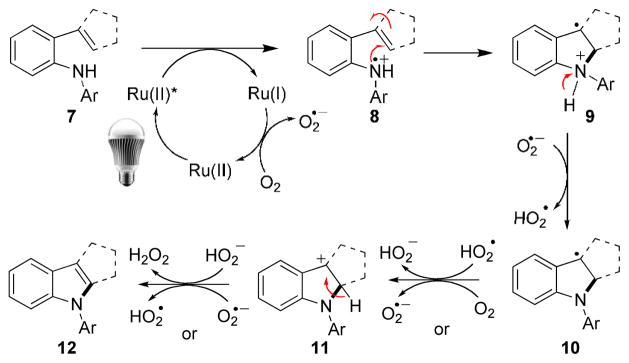
As a tentative working model, we propose the following catalytic cycle (Scheme 2). The key steps include (i) oxidation of amine 7 by a photoexcited Ru(bpz)3· (PF6)2 4a to nitrogen radical cation 8, (ii) electrophilic addition of nitrogen radical cation 8 to a tethered alkene to generate benzylic radical 9,[21] (iii) oxidation of benzylic radical 10 to its corresponding benzylic cation 11, and finally (iv) aromatization via deprotonation to form indole 12. A control study using TPP[22] in place of 4a did not lead to the desired indole. This result strongly indicated that the reaction was not mediated by singlet oxygen (see the SI for details).
Scheme 2.
Proposed Mechanistic Model.
We suspect that silica gel might play several roles in the reaction, including adsorbing oxygen, providing protons to ensure that the nitrogen radical cation 8 is protonated and/or facilitating the oxidation via a proton-coupled electron transfer process.[23] It alone did not catalyze the reaction (entry 9, Table 1). Replacing silica gel by HCl or TsOH led to the decomposition of the starting amine. Use of AcOH or PPTS in place of silica gel gave the desired indole, although they were not as effective as silica gel (see SI for the details of these studies). More studies are needed to understand the role(s) of silica gel.
Since a benzylic carbocation was proposed as an intermediate, substrates lacking a C2 C-H bond might participate in a 1,2 carbon shift, where a new C-C bond is formed (eq 1). The Driver group has extensively explored the synthetic utility of this shift in their studies of Rh-catalyzed decomposition of stryryl azides, presumably through similar benzylic carbocations.[24] To test this hypothesis, gem-diphenyl-substituted stryryl aniline 13a was prepared and subjected to the optimized visible light photocatalytic conditions (eq 1). To our delight, the expected 2,3-diphenyl indole 15a was isolated in 60 % yield. This lent further credence to the possible involvement of benzylic carbocation 14 in the visible-light-mediated reaction.
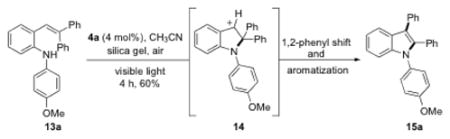 |
(1) |
To further explore the synthetic potential of this 1,2-carbon shift in indole synthesis, a series of gem-disubstituted stryryl anilines were synthesized and tested under the optimized conditions (Table 3). Whether they were independent (13b), part of a carbocycle (13c and 13d), or an oxocycle (13e), aryl groups preferentially migrated over alkyl groups. However, the 1,2-carbon shift is not limited to the migration of aryl groups only. When C2 was substituted by a cyclopentane ring, the desired ring-expansion product 15f was formed, although its efficiency was not as high as the aryl shift (entry 5). However, when C2 was substituted by two methyl groups, the desired methyl migration product was not formed. It is noteworthy that, when a tetralene product was formed, further oxidation to a fully aromatized product was observed (entry 2).
Table 3.
Scope of Indoles via C-N Bond Formation/1,2-Carbon Shift/Aromatization.[a]
| Entry | Stryryl Anilines | Indole Product | t [h] | Yield [%][b] |
|---|---|---|---|---|
| 1 |
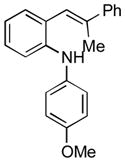 13b |
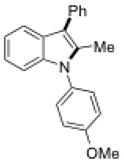 6l |
4.5 | 62 |
| 2 |
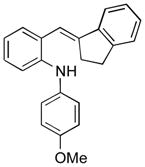 13c |
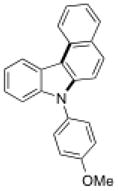 15c |
6 | 52 |
| 3 |
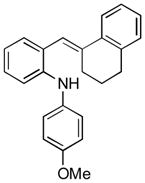 13d |
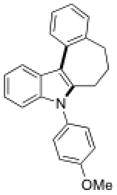 15d |
6 | 60 |
| 4 |
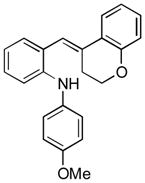 13e |
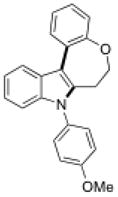 15e |
6 | 58 |
| 5 |
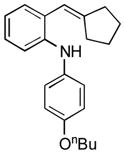 13f |
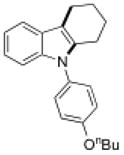 15f |
16 | 40 |
Conditions, unless otherwise noted: 4 mol% Ru(bpz)3(PF6)2, silica gel, air, CH3CN, irradiation with a 18 W LED white light. The newly formed C-N and C-C bonds are shown in bold.
Isolated yields after column chromatography.
Using 6 mol% Ru(bpz)3(PF6)2. Newly formed C-N and C-C bonds are shown in bolds.
In summary, we have developed a visible light mediated photocatalytic method for the synthesis of N-arylindoles. These reactions were conducted at ambient temperature with the aid of 4 mol% [Ru(bpz)3](PF6)2 4a, 18W LED white light, and open to air. The mild aerobic oxidation conditions were shown to be compatible with a variety of functional groups. More importantly, these studies revealed for the first time that arylamines could participate in C-N bond formation directly under visible light photoredox conditions. Studies leading to understand the roles of the p-alkoxyphenyl group and silica gel in these reactions, and application of this method to the synthesis of other types of indoles are ongoing in our laboratory.
Supplementary Material
Acknowledgments
We thank the University of Arkansas, the Arkansas Bioscience Institute, and the NIH NCRR COBRE grant (P30 RR031154 and P30GM103450-03) for generous support of this research. We also thank Prof. Bill Durham for insightful discussions on photochemistry.
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org.
References
- 1.For selected recent reviews on biological activities and synthesis of indoles, see: Cacchi S, Fabrizi G. Chem Rev. 2011;111:PR215. doi: 10.1021/cr100403z.Kochanowska-Karamyan AJ, Hamann MT. Chem Rev. 2010;110:4489. doi: 10.1021/cr900211p.Bandini M, Eichholzer A. Angew Chem. 2009;121:9786.Angew Chem Int Ed. 2009;48:9608.
- 2.For selected recent examples, see: Ackermann L, Lygin AV. Org Lett. 2012;14:764. doi: 10.1021/ol203309y.Inman M, Carbone A, Moody CJ. J Org Chem. 2012;77:1217. doi: 10.1021/jo201866c.Lu B, Luo Y, Liu L, Ye L, Wang Y, Zhang L. Angew Chem. 2011;123:8508. doi: 10.1002/anie.201103014.Angew Chem Int Ed. 2011;50:8358.Bonnamour J, Bolm C. Org Lett. 2011;13:2012. doi: 10.1021/ol2004066.Tan Y, Hartwig JF. J Am Chem Soc. 2010;132:3676. doi: 10.1021/ja100676r.Pathak TP, Gligorich KM, Welm BE, Sigman MS. J Am Chem Soc. 2010;132:7870. doi: 10.1021/ja103472a.Mei TS, Wang X, Yu JY. J Am Chem Soc. 2009;131:10806. doi: 10.1021/ja904709b.Shi Z, Zhang C, Li S, Pan D, Ding S, Cui Y, Jiao N. Angew Chem. 2009;121:4642. doi: 10.1002/anie.200901484.Angew Chem Int Ed. 2009;48:4572.Bernini R, Fabrizi G, Sferrazza A, Cacchi S. Angew Chem. 2009;121:8222. doi: 10.1002/anie.200902440.Angew Chem Int Ed. 2009;48:8078.Stuart DR, Bertrand-Laperle M, Burgess KMN, Fagnou K. J Am Chem Soc. 2008;130:16474. doi: 10.1021/ja806955s.Leogane O, Lebel H. Angew Chem. 2008;120:356. doi: 10.1002/anie.200703671.Angew Chem Int Ed. 2008;47:350.Würtz S, Rakshit S, Neumann JJ, Dröge T, Glorius F. Angew Chem. 2008;120:7340.Angew Chem, Int Ed. 2008;47:7230.Stokes BJ, Dong H, Leslie BE, Pumphrey AL, Driver TG. J Am Chem Soc. 2007;129:7500. doi: 10.1021/ja072219k.Tokuyama T, Yamashita T, Reding MT, Kaburagi Y, Fukuyama T. J Am Chem Soc. 1999;121:3791.Fukuyama T, Chen X, Peng G. J Am Chem Soc. 1994;116:3127.
- 3.Jana S, Clements MD, Sharp BK, Zheng N. Org Lett. 2010;12:3736. doi: 10.1021/ol101130e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a) Surry DS, Buchwald SL. Chem Sci. 2011;2:27. doi: 10.1039/C0SC00331J. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hartwig JF. Acc Chem Res. 2008;41:1534. doi: 10.1021/ar800098p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Harrington PJ, Hegedus LS. J Org Chem. 1984;49:2657. [Google Scholar]; (b) Harrington PJ, Hegedus LS, McDaniel KF. J Am Chem Soc. 1987;109:4335. [Google Scholar]; (c) Tsvelikhovsky D, Buchwald SL. J Am Chem Soc. 2010;132:14048. doi: 10.1021/ja107511g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.For recent reviews on photoredox catalysis, see: Xuan J, Xiao W-J. Angew Chem. 2012;124:6934.Angew Chem Int Ed. 2012;51:6828.Tucker JW, Stephenson CRJ. J Org Chem. 2012;77:1617. doi: 10.1021/jo202538x.Narayanam JMR, Stephenson CRJ. Chem Soc Rev. 2011;40:102. doi: 10.1039/b913880n.Teplý F. Collect Czech Chem Commun. 2011;76:859.Yoon TP, Ischay MA, Du J. Nat Chem. 2010;2:527. doi: 10.1038/nchem.687.Zeitler K. Angew Chem. 2009;121:9969.Angew Chem Int Ed. 2009;48:9785.
- 7.a) Nagib DA, MacMillan DWC. Nature. 2011;480:224. doi: 10.1038/nature10647. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Pham PV, Nagib DA, MacMillan DWC. Angew Chem. 2011;123:6243. doi: 10.1002/anie.201101861. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:6119. [Google Scholar]; c) Shih HW, Wal MNV, Grange RL, MacMillan DWC. J Am Chem Soc. 2010;132:13600. doi: 10.1021/ja106593m. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Nagib DA, Scott ME, MacMillan DWC. J Am Chem Soc. 2009;131:10875. doi: 10.1021/ja9053338. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Nicewicz DA, MacMillan DWC. Science. 2008;322:77. doi: 10.1126/science.1161976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.a) Parrish JD, Ischay MA, Lu Z, Guo S, Peters NR, Yoon TP. Org Lett. 2012;14:1640. doi: 10.1021/ol300428q. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lin S, Ischay MA, Fry CG, Yoon TP. J Am Chem Soc. 2011;133:19350. doi: 10.1021/ja2093579. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Du J, Espelt LR, Guzei IA, Yoon TP. Chem Sci. 2011;2:2115. doi: 10.1039/C1SC00357G. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Lu Z, Shen M, Yoon TP. J Am Chem Soc. 2011;133:1162. doi: 10.1021/ja107849y. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Ischay MA, Lu Z, Yoon TP. J Am Chem Soc. 2010;132:8572. doi: 10.1021/ja103934y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a) Tucker JW, Stephenson CRJ. Org Lett. 2011;13:5468. doi: 10.1021/ol202178t. [DOI] [PubMed] [Google Scholar]; b) Furst L, Narayanam JMR, Stephenson CRJ. Angew Chem. 2011;123:9829. doi: 10.1002/anie.201103145. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:9655. [Google Scholar]; c) Nguyen JD, Tucker JW, Konieczynska MD, Stephenson CRJ. J Am Chem Soc. 2011;133:4160. doi: 10.1021/ja108560e. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Dai C, Narayanam JMR, Stephenson CRJ. Nat Chem. 2011;3:140. doi: 10.1038/nchem.949. [DOI] [PubMed] [Google Scholar]; e) Furst L, Matsuura BS, Narayanam JMR, Tucker JW, Stephenson CRJ. Org Lett. 2010;12:3104. doi: 10.1021/ol101146f. [DOI] [PubMed] [Google Scholar]; f) Narayanam JMR, Tucker JW, Stephenson CRJ. J Am Chem Soc. 2009;131:8756. doi: 10.1021/ja9033582. [DOI] [PubMed] [Google Scholar]
- 10.Koike T, Akita M. Chem Lett. 2009;38:166. [Google Scholar]
- 11.a) Hari DP, Schroll P, König B. J Am Chem Soc. 2012;134:2958. doi: 10.1021/ja212099r. [DOI] [PubMed] [Google Scholar]; b) Zou YQ, Chen JR, Liu XP, Lu LQ, Davis RL, Jørgensen KA, Xiao WJ. Angew Chem. 2012;124:808. [Google Scholar]; Angew Chem Int Ed. 2012;51:784. [Google Scholar]; c) Kalyani D, McMurtrey KB, Neufeldt SR, Sanford MS. J Am Chem Soc. 2011;133:18566. doi: 10.1021/ja208068w. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Larraufie M-H, Pellet R, Fensterbank L, Goddard J-P, Lacôte E, Malacria M, Ollivier C. Angew Chem. 2011;123:4555. doi: 10.1002/anie.201007571. [DOI] [PubMed] [Google Scholar]; Angew Chem, Int Ed. 2011;50:4463. [Google Scholar]; e) Neumann M, Fuldner S, König B, Zeitler K. Angew Chem. 2011;123:981. doi: 10.1002/anie.201002992. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:951. [Google Scholar]; f) Andrews RS, Becker JJ, Gagné MR. Org Lett. 2011;13:2406. doi: 10.1021/ol200644w. [DOI] [PubMed] [Google Scholar]; g) Su Y, Zhang L, Jiao N. Org Lett. 2011;13:2168. doi: 10.1021/ol2002013. [DOI] [PubMed] [Google Scholar]; h) Zlotorzynska M, Sammis GM. Org Lett. 2011;13:6264. doi: 10.1021/ol202740w. [DOI] [PubMed] [Google Scholar]; i) Maji T, Karmakar A, Reiser O. J Org Chem. 2011;76:736. doi: 10.1021/jo102239x. [DOI] [PubMed] [Google Scholar]; j) Chen Y, Kamlet AS, Steinman JB, Liu DR. Nat Chem. 2011;3:146. doi: 10.1038/nchem.932. [DOI] [PMC free article] [PubMed] [Google Scholar]; k) Liu Q, Li YN, Zhang HH, Chen B, Tung CH, Wu LZ. J Org Chem. 2011;76:1444. doi: 10.1021/jo102062u. [DOI] [PubMed] [Google Scholar]; l) Zhou L, Yang H, Wang PJ. Org Chem. 2011;76:5873. doi: 10.1021/jo200692c. [DOI] [PubMed] [Google Scholar]; m) Andrews RS, Becker JJ, Gagné MR. Angew Chem. 2010;122:7432. doi: 10.1002/anie.201004311. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2010;49:7274. [Google Scholar]; n) Borak JB, Falvey DE. J Org Chem. 2009;74:3894. doi: 10.1021/jo900182x. [DOI] [PubMed] [Google Scholar]
- 12.a) Zhu M, Zheng N. Synthesis. 2011:2223. doi: 10.1055/s-0030-1260082. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Maity S, Zhu M, Shinabery RS, Zheng N. Angew Chem. 2012;124:226. doi: 10.1002/anie.201106162. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2012;51:222. doi: 10.1002/anie.201106162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.For photophysical and redox properties of Ru(II): Inagaki A, Akita M. Coord Chem Rev. 2010;254:1220.Juris A, Balzani V, Barigelletti F, Campagna S, Belser P, Zelewsky AV. Coord Chem Rev. 1988;84:85.Kalyanasundaram K, Grätzel M. Coord Chem Rev. 1998;177:347.Kalyanasundaram K. Coord Chem Rev. 1982;46:159.
- 14.For selected early studies on photoreduction of Ru (II) polypyridyl complexes by amines: DeLaive PJ, Lee JT, Sprintschnik HW, Abruña H, Meyer TJ, Whitten DG. J Am Chem Soc. 1977;99:7094.DeLaive PJ, Foreman TK, Giannotti C, Whitten DG. J Am Chem Soc. 1980;102:5627.
- 15.a) Freeman DB, Furst L, Condie AG, Stephenson CRJ. Org Lett. 2012;14:94. doi: 10.1021/ol202883v. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zou Y-Q, Lu L-Q, Fu L, Chang N-J, Rong J, Chen J-R, Xiao W-J. Angew Chem. 2011;123:7309. doi: 10.1002/anie.201102306. [DOI] [PubMed] [Google Scholar]; Angew Chem, Int Ed. 2011;50:7171. [Google Scholar]; c) Wang C, Xie Z, deKrafft KE, Lin W. J Am Chem Soc. 2011;133:13445. doi: 10.1021/ja203564w. [DOI] [PubMed] [Google Scholar]; d) Hari DP, König B. Org Lett. 2011;13:3852. doi: 10.1021/ol201376v. [DOI] [PubMed] [Google Scholar]; e) Rueping M, Zhu S, Koenigs RM. Chem Commun. 2011;47:12709. doi: 10.1039/c1cc15643h. [DOI] [PubMed] [Google Scholar]; f) Rueping M, Leonori D, Poisson T. Chem Commun. 2011;47:9615. doi: 10.1039/c1cc13660g. [DOI] [PubMed] [Google Scholar]; g) Xuan J, Cheng Y, An J, Lu LQ, Zhang XX, Xiao WJ. Chem Commun. 2011;47:8337. doi: 10.1039/c1cc12203g. [DOI] [PubMed] [Google Scholar]; h) Condie AG, González-Gómez JC, Stephenson CRJ. J Am Chem Soc. 2010;132:1464. doi: 10.1021/ja909145y. [DOI] [PubMed] [Google Scholar]
- 16.a) Miyake Y, Nakajima K, Nishibayashi Y. J Am Chem Soc. 2012;134:3338. doi: 10.1021/ja211770y. [DOI] [PubMed] [Google Scholar]; b) Kohls P, Jadhav D, Pandey G, Reiser O. Org Lett. 2012;14:672. doi: 10.1021/ol202857t. [DOI] [PubMed] [Google Scholar]; c) MacNally A, Prier CK, MacMillan DWC. Science. 2011;334:1114. doi: 10.1126/science.1213920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.a) Crutchley RJ, Lever ABP. J Am Chem Soc. 1980;102:7128. [Google Scholar]; b) Rillema DP, Allen G, Meyer TJ, Conrad D. Inorg Chem. 1983;22:1617. [Google Scholar]
- 18.Fluorocarbon solvents and silica gel enhance the solubility of molecular oxygen (see: Lee HB, Sung MJ, Blackstock SC, Cha JK. J Am Chem Soc. 2001;123:11322. doi: 10.1021/ja017043f.
- 19.The effectiveness of the catalysts correlates with the redox potentials of Ru2+*/Ru1+ (vs. SCE): Ru(bpz)3, 1.3 V; Ru(bpy)3, 0.78 V. The values are taken from reference 17a.
- 20.Electron rich p-alkoxyphenyl groups lower the oxidation potential of anilines: see cyclic voltammogram of 5b and 5b′ in SI.
- 21.Formation of 3-cyclopropyl-substituted indole 6k in high yield does not rule out our proposed mechanism. Bullock reported that, because of the stabilization provided by the benzyl group, ring opening of the cyclopropylbenzyl radical is much slower (2.7×105) than that of the cyclopropylmethyl radical (2.1×108), which would allow for competitive oxidation of benzylic radical 10 to benzylic cation 11 (see: Masnovi J, Samsel EG, Bullock RM. J Chem Soc, Chem Commun. 1989:1044.
- 22.TPP as a photosensitizer to generate singlet oxygen, see: Jiang G, Chen J, Huang JS, Che CM. Org Lett. 2009;11:4568. doi: 10.1021/ol9018166.
- 23.For a review on proton-coupled electron transfer, see: Huynh MHV, Meyer TJ. Chem Rev. 2007;107:5004. doi: 10.1021/cr0500030.
- 24.a) Stokes BJ, Liu S, Driver TG. J Am Chem Soc. 2011;133:4702. doi: 10.1021/ja111060q. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Sun K, Liu S, Bec PM, Driver TG. Angew Chem. 2011;123:1740. doi: 10.1002/anie.201006917. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:1702. [Google Scholar]; c) Shen M, Leslie BE, Driver TG. Angew Chem. 2008;120:5134. doi: 10.1002/anie.200800689. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:5056. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.