Abstract
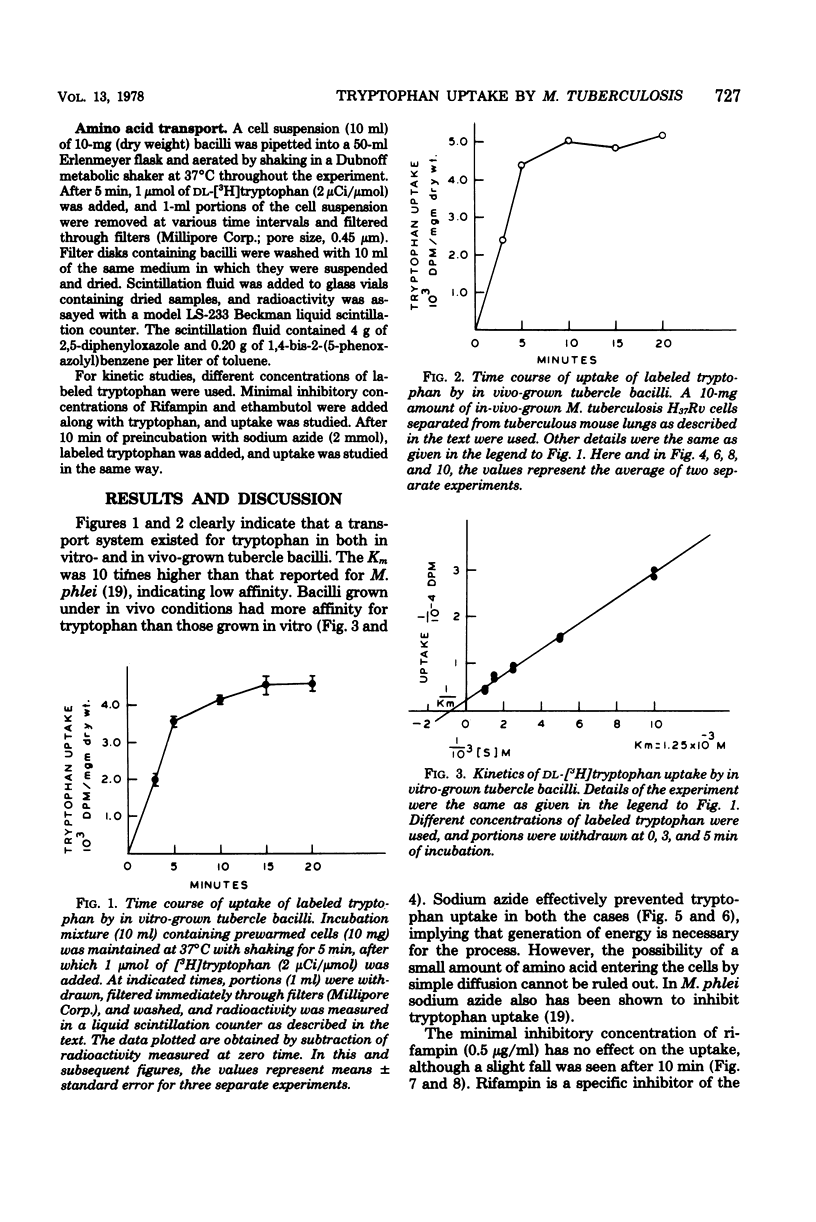
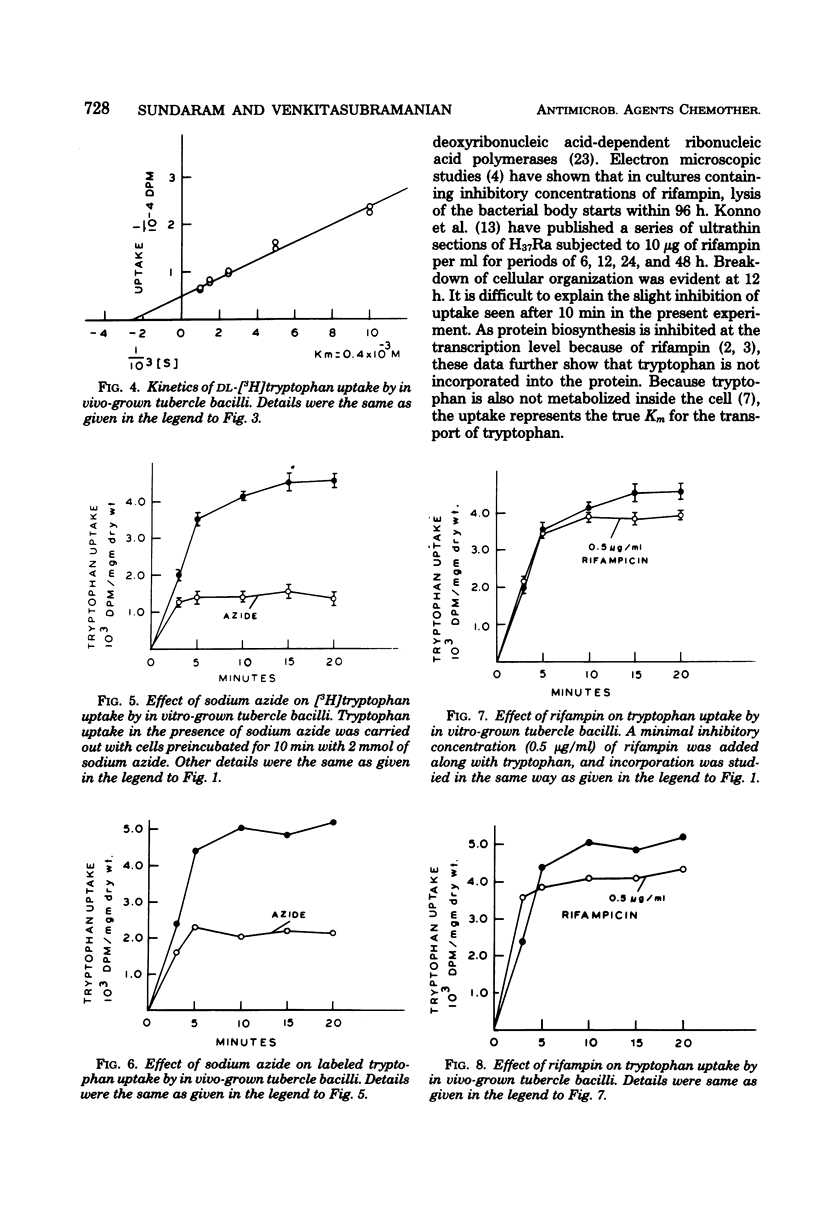
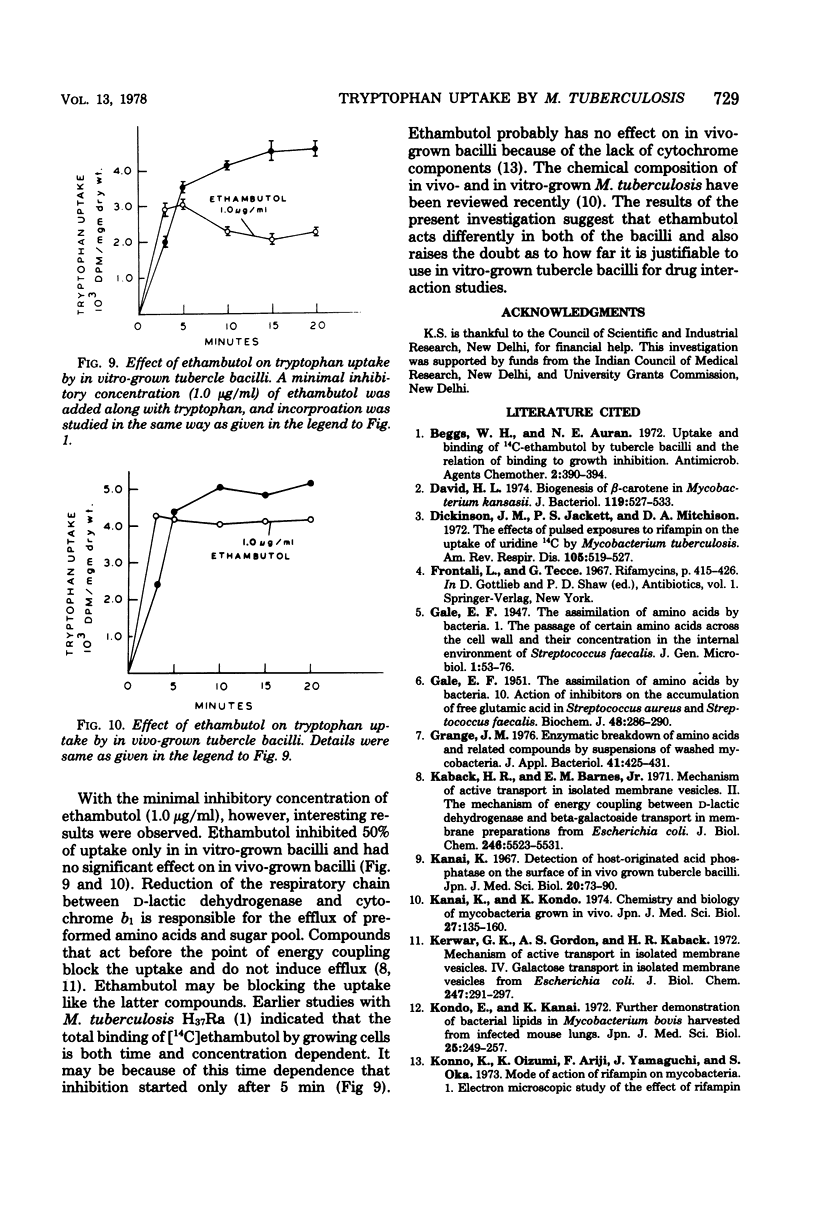
Uptake of radioactive tryptophan by Mycobacterium tuberculosis H37Rv grown in vitro and in vivo was investigated. Km values indicated low affinity, and sodium azide inhibited uptake. Rifampin at the minimal inhibitory concentration had no effect, whereas ethambutol inhibited uptake only in the bacilli grown under in vitro conditions. The significance of these results is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beggs W. H., Auran N. E. Uptake and binding of 14C-ethambutol by tubercle bacilli and the relation of binding to growth inhibition. Antimicrob Agents Chemother. 1972 Nov;2(5):390–394. doi: 10.1128/aac.2.5.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David H. L. Biogenesis of beta-carotene in Mycobacterium kansasii. J Bacteriol. 1974 Aug;119(2):527–533. doi: 10.1128/jb.119.2.527-533.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson J. M., Jackett P. S., Mitchison D. A. The effect of pulsed exposures to rifampin on the uptake of uridine- 14 C by Mycobacterium tuberculosis. Am Rev Respir Dis. 1972 Apr;105(4):519–527. doi: 10.1164/arrd.1972.105.4.519. [DOI] [PubMed] [Google Scholar]
- GALE E. F. The assimilation of amino-acids by bacteria; action of inhibitors on the accumulation of free glutamic acid in Staphylococcus aureus and Streptococcus faecalis. Biochem J. 1951 Mar;48(3):286–290. doi: 10.1042/bj0480286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange J. M. Enzymic breakdown of amino acids and related compounds by suspensions of washed mycobacteria. J Appl Bacteriol. 1976 Dec;41(3):425–431. doi: 10.1111/j.1365-2672.1976.tb00655.x. [DOI] [PubMed] [Google Scholar]
- KUSAKA T., SATO R., SHOJI K. COMPARISON OF CYTOCHROMES IN MYCOBACTERIA GROWN IN VITRO AND IN VIVO. J Bacteriol. 1964 Jun;87:1383–1388. doi: 10.1128/jb.87.6.1383-1388.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R., Barnes E. M., Jr Mechanisms of active transport in isolated membrane vesicles. II. The mechanism of energy coupling between D-lactic dehydrogenase and beta-galactoside transport in membrane preparations from Escherichia coli. J Biol Chem. 1971 Sep 10;246(17):5523–5531. [PubMed] [Google Scholar]
- Kanai K. Detection of host-originated acid phosphatase on the surface of "in vivo grown tubercle bacilli". Jpn J Med Sci Biol. 1967 Feb;20(1):73–90. [PubMed] [Google Scholar]
- Kanai K., Kondo E. Chemistry and biology of mycobacteria grown in vivo. Jpn J Med Sci Biol. 1974 Jun;27(3):135–160. doi: 10.7883/yoken1952.27.135. [DOI] [PubMed] [Google Scholar]
- Kerwar G. K., Gordon A. S., Kaback H. R. Mechanisms of active transport in isolated membrane vesicles. IV. Galactose transport by isolated membrane vesicles from Escherichia coli. J Biol Chem. 1972 Jan 10;247(1):291–297. [PubMed] [Google Scholar]
- Kondo E., Kanai K. Separation of tuberculin-active proteins from the infected mouse lung tissue. Jpn J Med Sci Biol. 1972 Aug;25(4):249–257. doi: 10.7883/yoken1952.25.249. [DOI] [PubMed] [Google Scholar]
- Prasad R., Kalra V. K., Brodie A. F. Active transport of glutamine and glutamic acid in membrane vesicles from Mycobacterium phlei. Biochem Biophys Res Commun. 1975 Mar 3;63(1):50–56. doi: 10.1016/s0006-291x(75)80009-x. [DOI] [PubMed] [Google Scholar]
- Prasad R., Kalra V. K., Brodie A. F. Different mechanisms of energy coupling for transport of various amino acids in cells of Mycobacterium phlei. J Biol Chem. 1976 Apr 25;251(8):2493–2498. [PubMed] [Google Scholar]
- Prasad R., Kalra V. K., Brodie A. F. Effect of phospholipase A on active transport of amino acids with membrane vesicles of Mycobacterium phlei. J Biol Chem. 1975 May 25;250(10):3699–3703. [PubMed] [Google Scholar]
- SEGAL W. COMPARATIVE STUDY OF MYCOBACTERIUM GROWN IN VIVO AND IN VITRO. V. DIFFERENCES IN STAINING PROPERTIES. Am Rev Respir Dis. 1965 Feb;91:285–287. doi: 10.1164/arrd.1965.91.2.285. [DOI] [PubMed] [Google Scholar]
- SEGAL W., MILLER W. T. COMPARATIVE STUDY OF IN VIVO AND IN VITRO GROWN MYCOBACTERIUM TUBERCULOSIS. 3. LIPID COMPOSITION. Proc Soc Exp Biol Med. 1965 Mar;118:613–616. doi: 10.3181/00379727-118-29919. [DOI] [PubMed] [Google Scholar]
- Wayne L. G., Diaz G. A. Autolysis and secondary growth of Mycobacterium tuberculosis in submerged culture. J Bacteriol. 1967 Apr;93(4):1374–1381. doi: 10.1128/jb.93.4.1374-1381.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. J., Lancini G. C., Silvestri L. G. Mechanism of action of rifampin on Mycobacterium smegmatis. J Bacteriol. 1971 Nov;108(2):737–741. doi: 10.1128/jb.108.2.737-741.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabu K. The uptake of D-glutamic acid by Mycobacterium avium. Biochim Biophys Acta. 1967 Feb 1;135(1):181–183. doi: 10.1016/0005-2736(67)90026-0. [DOI] [PubMed] [Google Scholar]


