Highlights
► We investigate the osmotic potential of four polar green algal strains. ► We examine effects of osmotic stress on ultrastructure and photosynthesis. ► One investigated strain belongs to a different phylogenetic lineage. ► Arctic strains show higher osmotic values (more negative osmotic potentials). ► Physiological performance and ultrastructure are affected by osmotic stress.
Keywords: Electron microscopy, Green algae, Photosynthesis, Phylogeny, rbcL gene, Streptophyta, Zygnema
Abstract
The osmotic potential and effects of plasmolysis on photosynthetic oxygen evolution and chlorophyll fluorescence were studied in two Arctic Zygnema sp. (strain B, strain G) and two Antarctic Zygnema sp. (strain E, strain D). Antarctic strain D was newly characterized by rbcL sequence analysis in the present study. The two Antarctic strains, D and E, are most closely related and may represent different isolates of the same species, in contrast, strain B and G are separate lineages. Incipient plasmolysis in the cells was determined by light microscopy after incubating cells in sorbitol solutions ranging between 200 mM and 1000 mM sorbitol for 3, 6 and 24 h. In Zygnema strain B and G incipient plasmolysis occurred at ∼600 mM sorbitol solution (720 mOsmol kg−1, ψ = −1.67 MPa) and in strains D and E at ∼300 mM (318 mOsmol kg−1, ψ = −0.8 MPa) sorbitol solution. Hechtian strands were visualized in all plasmolysed cells, which is particularly interesting, as these cells lack pores or plasmodesmata. Ultrastructural changes upon osmotic stress were a retraction of the condensed cytoplasm from the cell walls, damages to chloroplast and mitochondrial membranes, increasing numbers of plastoglobules in the chloroplasts and membrane enclosed particles in the extraplasmatic space. Maximum photosynthetic rates (Pmax) in light saturated range were between 145.5 μmol O2 h−1 mg−1 Chl a in Zygnema G and 752.9 μmol O2 h−1 mg−1 Chl a in Zygnema E. After incubation in 800 mM sorbitol for 3 h Pmax decreased to the following percentage of the initial values: B: 16.3%, D: 16.8%, E: 26.1% and G: 35.0%. Osmotic stress (800 mM sorbitol) decreased maximum photochemical quantum yield of photosystem II (Fv/Fm) when compared to controls. Maximum values of relative electron transport rates of photosystem II (rETRmax) decreased after incubation in 400 mM sorbitol in Zygnema D and E, while they decreased in Zygnema B and G only after incubation in 800 mM sorbitol. The kinetics of the rETR curves were similar for the Arctic strains Zygnema B and G, but distinct from the Antarctic strains Zygnema D and E, which were similar when compared with each other. This suggests that the investigated Arctic Zygnema sp. strains might be better adapted to tolerate osmotic water stress than the investigated strains from the Antarctic.
1. Introduction
The streptophycean green alga Zygnema grows commonly in freshwater (Hawes, 1989, 1990) as well as semi-terrestrial Arctic (Kim et al., 2008, 2011; Holzinger et al., 2009) and Antarctic (Davey, 1991a,b) habitats. The semi-terrestrial polar habitats are rinsed by water, but occasionally fall dry, exposing the epipelic mats of algae to potentially stressful environmental conditions including high irradiation and UV (Holzinger et al., 2009; Pichrtová et al., 2012), and water loss by desiccation. Damage to the cells of these freshwater green algae could be expected as they experience occasional but severe desiccation.
The goal of the present study was to determine the osmotic potential of four strains of Zygnema sp. (Zygnematales, Streptophyta) obtained from semi-aquatic Arctic (Svalbard) and Antarctic (King George Island) sites in order to gain information on the water holding capacities of these algae. Increasing the water holding capacity is an important way to reduce water loss in algal cells exposed to periodically dry habitats. There are not many ways to experimentally study the water potential in green algae. Therefore we have chosen an experimental setup, previously used to detect incipient plasmolysis (Willmer and Beattie, 1978; Oparka, 1994). Moreover, determination of osmotic potentials by incubating e.g. Klebsormidium sp. in increasing sorbitol solutions was achieved (Kaplan et al., 2012). There, astonishingly high osmotic values were obtained, which likely contribute to the ability of these algae to tolerate an aeroterrestrial lifestyle in alpine habitats. Additionally, Gustavs et al. (2010) report on osmotic resistance of various aeroterrestrial green algae (Trebouxiophyceae), some of the investigated species even grew under full marine conditions.
Early observation of plasmolysis with sodium carbonate were made in Zygnema sp. by Höfler (1951). It is described that by the use of 0.5 N Na2CO3 (representing 0.25 M Na2CO3) a fast (within 2–3 min) convex plasmolysis was achieved in algae obtained from a bog area (Ramsauer Torfmoos) in Austria. The protoplast is retracted from the cross walls, but the cells did not survive this treatment for a prolonged period, and after 2–3 days, all cells were dead.
Information on osmotic values of Zygnema sp. has been generated by protoplast production procedures (Berliner, 1981). Ohiwa (1978) measured the internal osmotic pressure and determined 0.27 M sucrose-equivalent in Zygnema and 0.38 M sucrose-equivalent in Spirogyra, when attempting to generate fusion products of Spirogyra and Zygnema protoplasts. In a more recent study, turgor pressure was reduced by incubation in 0.3 M mannitol or sorbitol solution in the zygnematophycean green alga Spirogyra sp. (Iwata et al., 2001). Therefore it will be interesting to evaluate if cultured Zygnema sp. originating from extreme habitats have similar osmotic values or have the ability to generate a higher osmotic pressure.
In Zygnema the formation of akinetes, individual cells that contain high amounts of lipids and starch grains, has been described (McLean and Pessoney, 1971). In four week old cultured material chloroplasts appeared less distinct, at five weeks the material appeared exceedingly granular due to accumulation of storage material, and at six weeks individual akinetes were formed by separation from the parent filament (McLean and Pessoney, 1971).
There might be a direct correlation between osmotic potential and desiccation tolerance of aeroterrestrial green algae, as demonstrated for Klebsormidium sp. (Kaplan et al., 2012). Amongst streptophyte green algae, members of Klebsormidiales (Karsten et al., 2010, Karsten and Holzinger, 2012), Zygnematales (Holzinger et al., 2010) and Coleochaetales (Graham et al., 2012) have been shown to tolerate desiccation at different levels (for an overview and additional literature see Büdel, 2011). However, information is still limited regarding the osmotic potential in streptophyte green algae (e.g. Höfler, 1951; Ohiwa, 1978). Polar species of Zygnema (e.g. Davey, 1991a,b; Kim et al., 2008, 2011) have been demonstrated to be suitable test organisms for various stress scenarios (e.g. Holzinger et al., 2009; Pichrtová et al., 2012). Therefore we characterized these polar green algae for the first time under osmotic stress. In the present study, four strains of Zygnema sp. were investigated in order to better understand reaction of water-loss stressed cells. This is important to be investigated, as in nature these algae are periodically exposed to water-loss (e.g. by desiccation) during their normal life-cycle. We focused on documenting changes that occur at the structural and ultrastructural levels, as well as gathering important indicators of physiological performance such as photosynthetic oxygen production, photosystem II efficiency (Fv/Fm) and relative electron transport rates (rETR).
2. Materials and methods
2.1. Algal material, cultivation and molecular phylogenetics
Zygnema sp. Arctic strain B (CCALA 976), strain G (CCALA 977) and Antarctic strain E (CCCryo 278-06) were used for the experiments. For these strains sequence data (rbcL) were already available (Pichrtová et al., 2012). It is important to state that strains B and G were collected at a similar habitat, interconnected shallow seepage pools in Petunia Bay, Svalbard. There was only about 5 m distance between the two sampling sites and the Zygnema samples appeared different already in the field (M. Pichrtová, personal communication). While the final taxonomic determination of the species was not resolved, the strains were named Zygnema B, Zygnema G, Zygnema E in this publication. In addition, an Antarctic Zygnema sp. strain D (CCALA 982, courtesy of M. Pichrtová, original strain isolated by J. Elster and J. Šnokhousová, James Ross Island, Antarctica) was used, all algae were cultivated in liquid Bold's Basal Medium (BBM, Coleman, 1983) in a light-dark regime of 16:8 h in an Intellus environmental controller (Percival Scientific, Perry, USA) at 20 °C and 30–35 μmol photons m2 s−1 in the light period and with a reduced temperature to 13 °C in the dark. For all experiments, 3–5 weeks old cultures were used. In the case of physiological measurements, where higher amounts of biomass were necessary, occasionally older cultures (up to ∼10 weeks) were used.
DNA from strain D was extracted, and the rbcL gene PCR amplified and sequenced following the exact methods detailed in Pichrtová et al. (2012). The new rbcL sequence was compared against the NCBI sequence database using BLAST searches, and the closest matches, as well as a sampling of strains presented in Stancheva et al. (2012) were compiled manually into an alignment. Phylogenetic analysis was performed in PAUP* (Swofford, 2002) under the maximum likelihood (ML) criterion, using a GTR + I + gamma model, with parameter values estimated during the ML heuristic search. Bootstrap analysis was performed under the same model, except that parameter values were set based on the ML tree.
2.2. Plasmolysis and water potential
The four strains of Zygnema, B, G, D and E, were incubated in sorbitol (d-sorbitol, S-0900, Sigma–Aldrich, Steinheim, Germany) solutions ranging from 200 mM to 1000 mM sorbitol for 3, 6 and 24 h. Untreated cells from the above mentioned culture in BBM (2.1) were regarded as controls. Incipient plasmolysis, defined as the value at which at least 50% of the cells were visibly plasmolysed, was determined by light microscopy. Plasmolysed cells were allowed to recover in BBM for 0.5–1 h. The water potential of sorbitol solutions and culture medium was detected by a PSYPRO water potential datalogger, equipped with C-52 sample chambers (Wescor, South Loga, UT, USA) according to Kaplan et al. (2012). The osmolarity of the respective solutions was measured with a cryoscopic osmometer (OSMOMAT 030, Gonotec, Berlin, Germany).
2.3. Light- and confocal laser scanning microscopy
Light microscopy was performed with a Zeiss Axiovert 200 M microscope, equipped with a 63 × 1.4 NA objective lens. Images were captured with an Axiocam MRc5 camera and Zeiss Axiovision software.
Confocal laser scanning microscopy of 10 μM FM 1–43 (green biofilm cell stain, Invitrogen Ltd., Paisley, UK) stained Zygnema sp. control cells (in BBM) and 3 h sorbitol incubated Zygnema sp. cells (for method see Holzinger et al., 2011) were performed with a Zeiss Pascal confocal laser scanning microscope (CLSM), samples were excited at 488 nm, emission was collected in two separate channels at 505–550 nm and long-pass 560 nm. The first channel was false coloured green, the second channel false coloured red and final mages were merged.
2.4. Transmission electron microscopy
Zygnema sp. control cells and cells incubated for 3 h in 400 mM or 800 mM sorbitol were chemically fixed as previously described (Holzinger et al., 2009) with modifications. Briefly, cells were fixed in 2.5% glutaraldehyde in 50 mM cacodylate buffer (pH = 6.8) for 1 h, postfixed with 1% OsO4 at 4 °C for 12 h, rinsed and dehydrated in increasing ethanol concentrations and embedded in modified Spurr's resin (Ellis, 2006). Ultrathin sections were prepared with a Reichert Ultracut, counterstained with uranyl acetate and Reynold's lead citrate and investigated at an LIBRA 120 transmission electron microscope (TEM) at 80 kV.
2.5. Photosynthetic oxygen evolution
Photosynthetic oxygen evolution rates under increasing photon fluence densities were measured with a Presens Fibox 3 oxygen optode (Presens, Nürnberg, Germany). According to Remias et al. (2009), a 3 ml thermostatic acrylic chamber DW1 (Hansatech Instruments, Norfolk, UK), tempered to 20 °C was combined with a magnetic stirrer. To ensure sufficient carbon supply during the measurement, 3 ml of algal filaments in BBM or sorbitol solution was enriched with HCO3− to a final concentration of about 2 mM. Nine light levels of photosynthetic active radiation (PAR) ranging from 0 to 1000 μmol photons m−2 s−1 were generated by a halogen lamp, adjusted with Hansatech A5 neutral glass filters and calibrated with a Hansatech QRT1 PAR sensor inside the cuvette. Incubation time at the different light intensities was between 2 and 6 min. During that time O2 concentration was detected. After each measurement, the cell suspension was filtered onto a Whatman GF/F glass fibre filter (Whatman, Dassel, Germany) to extract chlorophyll in dimethylformamide (DMF). Chlorophyll a (Chl a) was quantified according to Porra et al. (1989).
Three independent measurements (n = 3) were carried out in each of the four strains of Zygnema sp. both under control conditions in BBM and under osmotic stress in 800 mM sorbitol after an incubation period of 3 h. Data were fitted in Fig.P 2.7 software (Fig.P software corporation, Hamilton, ON, Canada, www.figpsoft.com) according to the hyperbolic tangent light saturation curve model of Jassby and Platt (1976): P = Pmax tanh (α·EPAR·Pmax−1), where P is the gross primary production per unit chlorophyll a (μmol O2 h−1 mg−1 Chl a), Pmax the maximal photosynthesis at optimal irradiance, α the initial slope of the P–E curve at low light levels and EPAR the irradiance (μmol photons m−2 s−1). The initial light saturation point Ek is the quotient of Pmax/α. The light compensation point Ec is calculated using the equation Ec = Ek tanh−1 (−R Pmax−1) (Chalker et al., 1983).
2.6. Measurements of photosystem II efficiency and relative electron transport rates
The maximum photochemical quantum yield of photosystem II (Fv/Fm) and light response curves (rETR as a function of PAR) were generated in Zygnema B, G, D and E in BBM and after incubation in 400 and 800 mM sorbitol solution with a PAM 2500 (Heinz Walz GmbH, Effeltrich, Germany). Samples treated with sorbitol were measured after 3, 6 and 24 h as a time series, samples in BBM were measured independently. Measurements were carried out in vivo in a suspension cuvette KS-2500. After a dark adapting period of 15 min, F0 and Fm were determined to calculate Fv/Fm value (Fv/Fm = (Fm − F0)/Fm). Then cells were exposed to 10 light steps (duration 30 s) ranging from 5 up to 2973 μmol photons m−2 s−1, actinic light was provided by red LEDs with a maximum emission at 630 nm. rETR values were detected after each illumination step with saturation pulsed analysis (red LEDs, 630 nm). The light response curve data were fitted with the formula rETR = I/(a·I2 + b·I + c) by Fig.P 2.7 software according to the model of Eilers and Peeters (1988). Via the parameters a, b and c values of rETRmax, α and Ek were calculated with following formulas: α = 1/c, rETRmax = 1/(b + 2·√a·c), Ek = c/(b + 2·√ a·c) (Macedo et al., 1998; Behrenfeld et al., 2004).
2.7. Statistical evaluation of the data
Data of P–E curves (oxygen evolution versus irradiance) were fitted according to Jassby and Platt (1976), P–E curves (rETR versus irradiation) were fitted according to Eilers and Peeters (1988).
Fv/Fm data were analysed by one-way ANOVA combined with Tukey's post hoc test (confidence interval = 0.001) to find, if there are significant differences between control and treated samples and between the two treatments. Repeated measure ANOVA (confidence interval = 0.001) was used to analyse the influence of time on the values in a time series. Analyses were carried out in SPSS 18.0 for windows (IBM Corporation, Somer, NY, USA).
3. Results
3.1. Molecular phylogenetic characterization
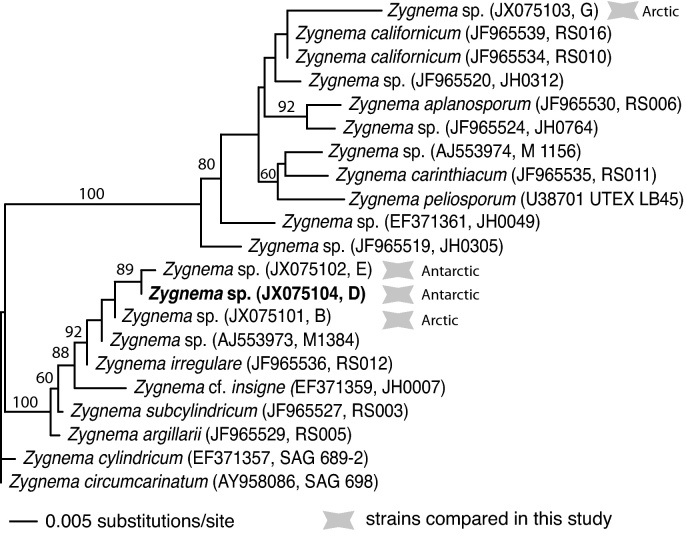
The sequence of the rbcL gene of Zygnema strains B, G, E were previously obtained (Pichrtová et al., 2012), and strain D was determined in this study (GenBank accession JX075104). The final rbcL alignment included 1384 unambiguously aligned nucleotides, with 1238 constant sites and 106 parsimony informative sites. The resulting phylogenetic analysis (Fig. 1) indicated that the rbcL sequences of the four polar strains do not closely group with those of known Zygnema species. A close relationship of the two Antarctic strains (D and E) was found, whereas the two Arctic strains (B and G) are not closely related and instead fall into two distinct lineages.
Fig. 1.
Unrooted maximum likelihood phylogenetic tree (score of −ln L = 3230.418) showing the placement of the four polar strains of Zygnema that were the focus of physiological investigations in this study (grey concave octagons). The tree was obtained using the GTR + I + gamma model of substitution, with parameter values as follows: RA-C = 1.3326107, RA-G = 6.209026, RA-T = 1.6984892, RC-G = 0.7738240, RC-T = 10.188825, RG-T = 1.0, gamma shape = 2.980115, I = 0.809959. Number associated with branches indicate the proportion of bootstrap replicates possessing that branch, based on 200 pseudo-replicate searches. Information from strain D is new to this study (boldface font).
3.2. Plasmolysis effects examined by light and confocal microscopy
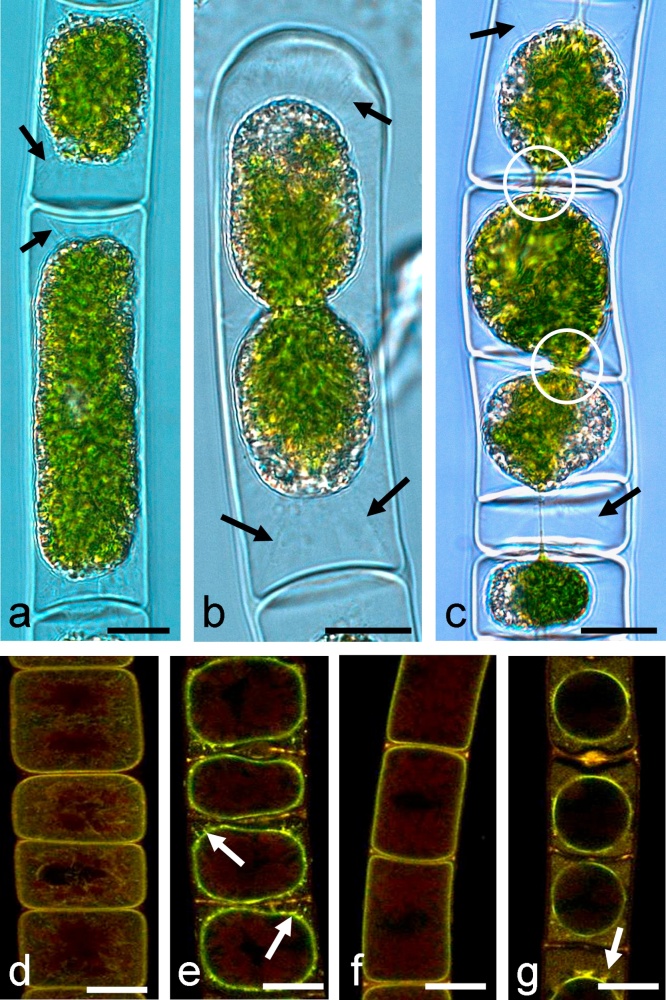
Control cells of 3–5 weeks old cultures of Zygnema strain B, G, D and E were shown in Fig. 2(a)–(d), respectively. It is important to state that within a culture of a certain age (e.g. 4 weeks after subculturing), different individual cell ages were found. Young cells can be distinguished from older cells by the degree of vacuolization (Fig. 2a, cell on left hand side) and the density of their cytoplasmic contents (Fig. 2a cells on right hand side, marked by asterisk). The latter cells were regarded as ‘pre-akinetes’ according to McLean and Pessoney (1971), who illustrated single celled ‘akinetes’ in six-week-old cultures.
Fig. 2.
Light microscopic images of Zygnema sp. control cells (a–d), cells treated with 600 mM sorbitol (e and f), 300 mM sorbitol (g and h), or 800 mM sorbitol (i–l) for 3 h. (a) Zygnema sp. B, (b) Zygnema sp. G, (c) Zygnema sp. D, (d) Zygnema sp. E, (e) Zygnema sp. B, (f) Zygnema sp. G, (g) Zygnema sp. D, (h) Zygnema sp. E, (i) Zygnema sp. B, (j) Zygnema sp. G, (k) Zygnema sp. D, (l) Zygnema sp. E. Plasmolysed cells are marked with arrows, pre-akinete cells are marked with asterisks. Bar 10 μm.
Two multi-lobed chloroplasts with pyrenoides were in each cell. Some cells contained large vacuoles and were considered to be younger, vegetative cells. In, older, pre-akinete cells, the cytoplasm was more dense and there were few and smaller vacuoles, that might appear brownish. Incipient plasmolysis occurred in Zygnema B (Fig. 2e) after 3 h in 600 mM sorbitol solution (water potential ψ = −1.67 MPa, compare Table 1). In Zygnema G, incipient palsmolysis was also found in 600 mM sorbitol (Fig. 2f), however due to the fact that many pre-akinete cells were present in these cultures it was hard to detect. In contrast, after 3 h in 800 mM sorbitol plasmolysis was easily detectable (Fig. 2j). In Zygnema D (Fig. 2g) and E (Fig. 2h) incipient plasmolysis was found in 300 mM sorbitol solution, 3 h, indicating a water potential ψ = −0.80 MPa (Table 1). Cells exposed to incipient plasmolysis recovered fully after incubation to BBM for 0.5 h (data not shown). Incubation in 800 mM sorbitol (equivalent to ψ = −2.09 MPa) for 3 h induced convex plasmolysis in the four strains (Fig. 2i–l). The plasmolytic retraction of the cytoplasm increased when higher concentrations of sorbitol were used (900, 1000 mM, Fig. 3a and b) and when the incubation time in the respective sorbitol solution was increased (24 h, Fig. 3c). Viewed under DIC optics, Hechtian strands were visible, protruding from the retracted cytoplasm to the cell periphery (Fig. 3a–c). Occasionally individual cells had connected protoplasts, likely due to defects in cross wall formation (Fig. 3c).
Table 1.
Osmolarity (n = 3) and water potential (n = between 4 and 11) of the culture medium and the media used to generate osmotic stress detected by an OSMOMAT 030 osmometer and a PSYPRO water potential data logger. Standard error added (±).
| Solution | Osmolarity/mOsm kg−1 | Water potential ψ/MPa |
|---|---|---|
| BBM | 12 ± 1.2 | −0.11 ± 0.07 |
| 200 mM sorbitol | 207 ± 4.0 | −0.58 ± 0.13 |
| 300 mM sorbitol | 318 ± 0.6 | −0.80 ± 0.21 |
| 400 mM sorbitol | 428 ± 0.0 | −1.01 ± 0.16 |
| 600 mM sorbitol | 673 ± 2.5 | −1.67 ± 0.13 |
| 800 mM sorbitol | 932 ± 2.9 | −2.09 ± 0.93 |
| 1000 mM sorbitol | 1235 ± 6.1 | −2.54 ± 0.07 |
Fig. 3.
Plasmolysis effects on the plasma membrane and visualization of Hechtian strands (marked with arrows): differential interference contrast (DIC) images (a–c), and plasma membrane staining with FM 1–43 (d–g). (a) Zygnema sp. G cell incubated in 1000 mM sorbitol for 3 h, (b) Zygnema sp. E terminal cell, incubated in 900 mM sorbitol for 3 h, (c) Zygnema sp. E incubated in 900 mM sorbitol for 24 h, occasionally the protoplasts of adjacent cells remain in contact, likely due to defects in the cross walls (circles), (d) Zygnema sp. B control cells, (e) Zygnema sp. B, 900 mM sorbitol, 3 h, (f) Zygnema sp. D control cells, (g) Zygnema sp. D, 800 mM sorbitol, 3 h. Bars10 μm.
When cells were stained by the membrane stain FM 1–43 the plasma membrane could be observed directly under the cell wall in control cells of Zygnema B and D (Fig. 3d and f) and Zygnema G and E (not shown). In 700–900 mM sorbitol (3 h), the retraction of the stained plasma membrane from the cell wall was clearly visible, as well as thin filaments protruding towards the cell wall (Fig. 3e and g).
3.3. Transmission electron microscopy
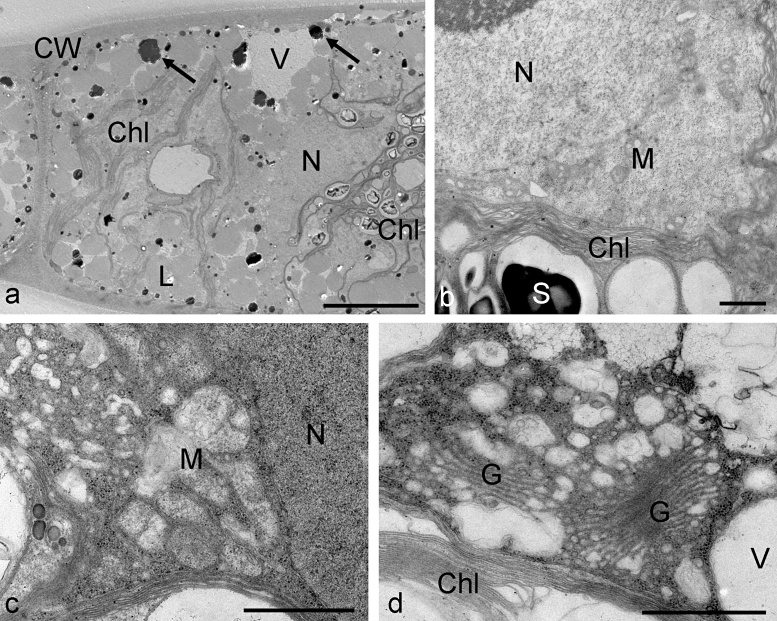
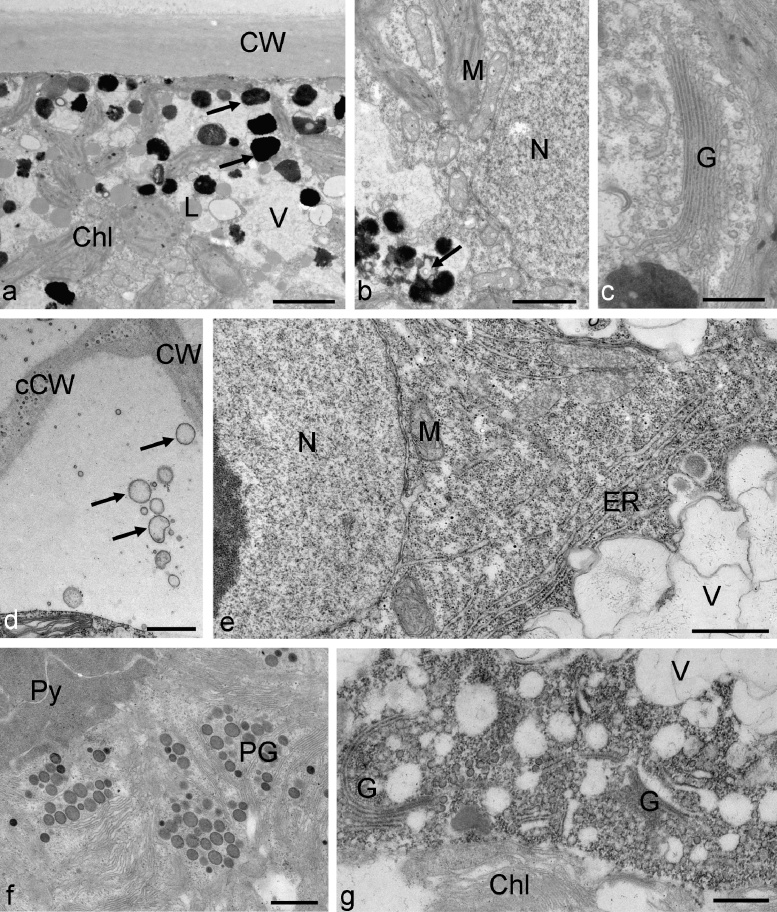
Arctic Zygnema B contained two chloroplasts. The illustrated cell contained electron dense particles with a diameter of up to 1 μm and numerous lipid bodies and was therefore considered a pre-akinete cell (Fig. 4a). A detail view showed the chloroplast structure with starch grains, mitochondria with cristae and the central nucleus (Fig. 4b). Zygnema B cells treated for 3 h with 800 mM sorbitol had an extremely condensed cytoplasm with densely arranged ribosomes (Fig. 4c), the lumen of the mitochondria were less electron dense. Golgi bodies appeared not changed within the dense cytoplasm, that still contained vacuoles (Fig. 4d).
Fig. 4.
Electron micrographs of Zygnema sp. B control cells (a and b) and cells treated for 3 h with 800 mM sorbitol (c and d). (a) Overview of a pre-akinete cell with chloroplasts, vacuoles and lipid droplets, electron dense particles are marked with arrows, (b) detail in the cell centre with nucleus, mitochondria and chloroplast, (c) condensed cytoplasm in the cell centre with altered mitochondria, (d) detail view on golgi bodies in condensed cytoplasm. Abbreviations: Chl, chloroplast; CW, cell wall; G, golgi body; L, lipid droplets; M, mitochondrion; N, nucleus; V, vacuole. Bars a 10 μm; b–d 1 μm.
Zygnema G cells had two chloroplasts, each containing one pyrenoid, vacuoles and a nucleus in the centre (Fig. 5a–c). We have chosen to illustrate here a pre-akinete cell, that still has a certain degree of vacuolization and lacks massive amounts of lipids (Fig. 5a). In these cells, mitochondria and golgi bodies were clearly visible and intact (Fig. 5b and c). When Zygnema G cells were exposed to 800 mM sorbitol for 3 h, mitochondria with damaged cristae were observed (Fig. 5d), and accumulations of plastoglobules appeared in partially destroyed chloroplasts (Fig. 5e).
Fig. 5.
Electron micrographs of Zygnema sp. G control cells (a–c) and cells treated for 3 h with 800 mM sorbitol (d–e). (a) overview of a pre-akinete cell that still shows vacuolization, nucleus, chloroplast and pyrenoids are clearly visible, (b) cell centre with mitochondria and ER, (c) golgi body, (d) cell centre with nucleus and partially disrupted mitochondria (arrows), (e) chloroplast with massive plastoglobules, golgi bodies with reduced cisternae. Abbreviations: Chl, chloroplast, CW, cell wall, ER, endoplasmic reticulum, G, golgi body, M, mitochondrion, N, nucleus, PG, plastoglobule, Py, pyrenoid, V, vacuole. Bars a 10 μm, b and c 1 μm, d and e 500 nm.
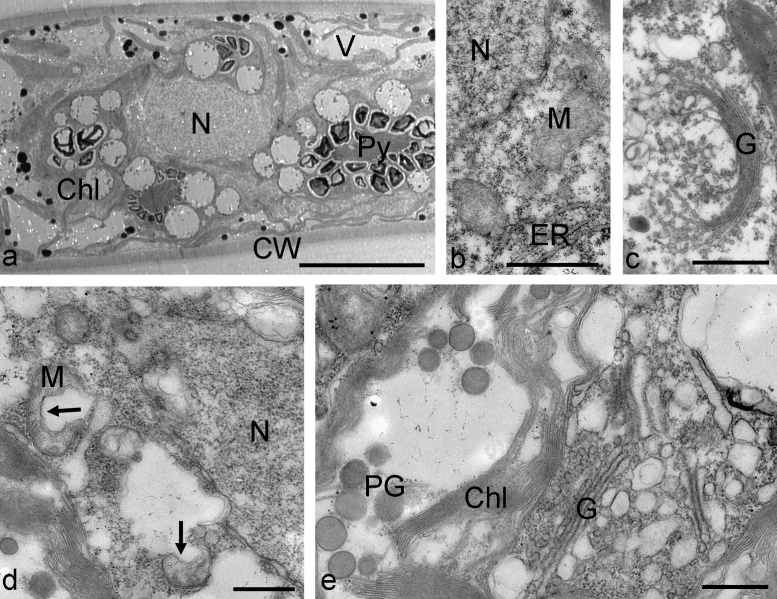
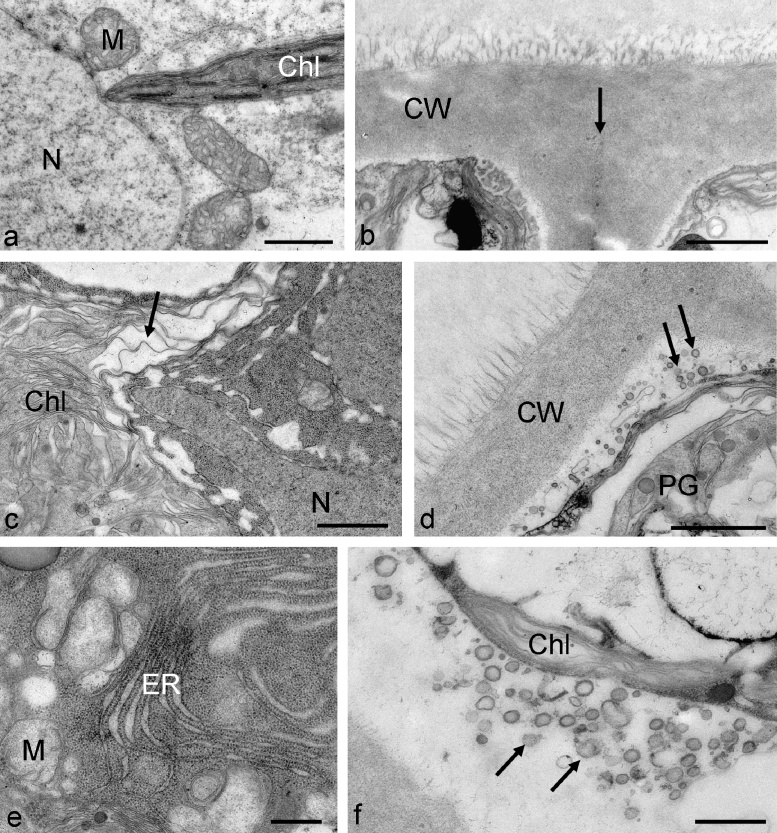
Antarctic Zygnema D cells showed characteristic multilobed chloroplasts, lipid bodies and electron dense particles with a diameter of about 500 nm in the cell cortex (Fig. 6a), the cell centre contained the nucleus and numerous mitochondria surrounded by chloroplast lobes (Fig. 6b) and golgi bodies (Fig. 6c). When Zygnema D was exposed to 400 mM sorbitol, a strong contraction of the protoplast from the cell wall was visible (Fig. 6d). In the periplasmatic space, numerous membrane enclosed particles were found with diameters of 280–450 nm (Fig. 6d). These structures were seen also close to the cell walls, but no direct attachment could be observed. The cross cell walls occasionally contained globular electron dense inclusions (Fig. 6d). The central cytoplasm appeared dense with numerous ER cisternae in vicinity of the nucleus, but still vacuoles were present (Fig. 6e). Zygnema D cells exposed to 800 mM sorbitol for 3 h showed an enhanced number of plastoglobules in the chloroplasts (Fig. 6f) and the number of cisternae of the golgi bodies appeared reduced within the dense cytoplasm (Fig. 6g).
Fig. 6.
Electron micrographs of Zygnema sp. D control cells (a–c) and cells treated for 3 h with 400 mM sorbitol (d and e) or 800 mM sorbitol (f and g). (a) Overview of the cell cortex with chloroplast lobes, lipid bodies and electron dense particles (arrows), (b) central area with nucleus and mitochondria, also electron dense structures were seen occasionally, (c) golgi body, (d) periplasmatic space in the edges of a cell between outer cell wall and cross wall, notice numerous membrane enclosed vesicular structures, (e) central area with dense accumulations of ER and mitochondria, (f) detail of the chloroplast with massive accumulations of plastoglobules, (g) golgi bodies with reduced number of cisternae in condensed cytoplasm. Abbreviations: Chl, chloroplast, CW, cell wall, cCW, cross cell wall, ER, endoplasmic reticulum, G, golgi body, L, lipid body, M, mitochondrion, N, nucleus, PG, plastoglobules, Py, pyrenoid, V, vacuole. Bars a and b, d and e 1 μm, c, f and g 500 nm.
Zygnema E control cells had a normal appearance with nucleus, mitochondria with numerous cristae and chloroplast lobes (Fig. 7a), the plasma membrane was next to the cell wall, a division line between individual cells in a filament was visible (Fig. 7b). Zygnema E cells treated with 400 mM sorbitol for 3 h showed dilatations of the thylakoid membranes in their chloroplasts (Fig. 7c), the cytoplasm appeared condensed and the plasma membrane was retracted from the cell wall (Fig. 7d). In the periplasmatic area numerous membrane enclosed vesicles with diameters of approx. 100 nm were observed. 800 mM sorbitol increased the effects, dense accumulations of ER were observed (Fig. 7e), the mitochondria appeared ‘empty’ by destruction of cristae. Again the extraplasmatic space was filled with numerous membrane enclosed particles (Fig. 7f).
Fig. 7.
Electron micrographs of Zygnema sp. E control cells (a and b) and cells treated for 3 h with 400 mM sorbitol (c and d) or 800 mM sorbitol (e and f). (a) central part of the cell with nucleus, mitochondria and chloroplast lobe, (b) cell wall area between two adjacent cells in a filament, arrow marks the division line, (c) chloroplast with dilated thylakoid membranes, condensed cytoplasm, (d) cortical area with vesicular structures in the periplasmatic space (arrows), chloroplast with plastoglobules, (e) dense accumulation of ER, mitochondria appear ‘empty’, (f) periplasmatic space filled with vesicles. Abbreviations: Chl, chloroplast, CW, cell wall, ER, endoplasmic reticulum, M, mitochondrion, N, nucleus, PG, plastoglobules. Bars a–d 1 μm, e and f 500 nm.
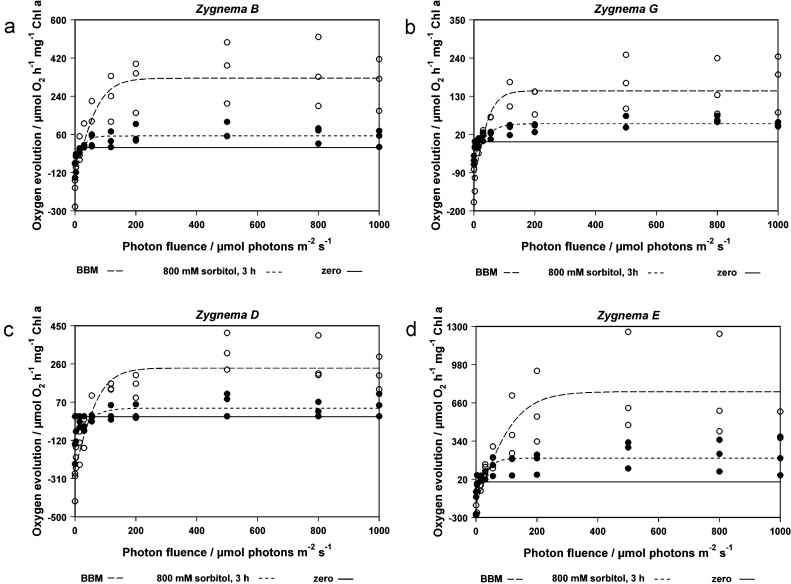
3.4. Photosynthetic oxygen production
Results of photosynthetic oxygen evolution under increasing light intensities (3–1000 μmol photons m−2 s−1) and dark respiration (control in BBM and after incubation in 800 mM sorbitol for 3 h) are illustrated in Fig. 8a–d. Based on the curves fitted according to Jassby and Platt (1976), values for Pmax (maximal net photosynthesis), Ec (light compensation value), Ek (initial light saturation point) and α (photosynthetic efficiency in the light-limited range) were calculated and are presented in Table 2.
Fig. 8.
Photosynthetic oxygen evolution as function of increasing photon fluence density (P–E curve) in Zygnema sp. B (a, n = 3), Zygnema sp. G (b, n = 3), Zygnema sp. D (c, n = 3) and Zygnema sp. E (d, n = 3) under control conditions in culture medium (white circles) and under osmotic stress condition (black dots, measurements carried out in 800 mM sorbitol solution after an incubation period about 3 h in sorbitol). Curves were fitted according to the hyperbolic tangent light-saturation model of Jassby and Platt (1976).
Table 2.
Characteristic values of P–E curves, oxygen evolution versus irradiance, calculated by the fit according to Jassby and Platt (1976) in four Zygnema sp. strains in BBM and after osmotic stress (800 mM sorbitol, 3 h).
| Sample | Pmax | Respiration | Ec | Ek | α | |
|---|---|---|---|---|---|---|
| Zygnema sp. B | Control | 324.6 ± 44.4 | −133.4 ± 36.9 | 25.1 | 83.8 | 5.5 ± 1.5 |
| Osm. stress | 53.0 ± 16.4 | −84.1 ± 14.5 | 26.1 | 36.5 | 3.8 ± 3.8 | |
| Zygnema sp. G | Control | 145.5 ± 27.0 | −116.9 ± 23.2 | 26.7 | 55.8 | 4.7 ± 1.3 |
| Osm. stress | 51.0 ± 7.8 | −30.67 ± 6.7 | 25.1 | 63.4 | 1.3 ± 0.3 | |
| Zygnema sp. D | Control | 239.7 ± 37.1 | −262.6 ± 30.7 | 50.5 | 87.1 | 5.8 ± 1.2 |
| Osm. stress | 40.2 ± 24.3 | −87.3 ± 20.4 | 62.7 | 74.8 | 1.7 ± 0.9 | |
| Zygnema sp. E | Control | 752.9 ± 115.1 | −181.7 ± 88.2 | 26.2 | 133.3 | 7.0 ± 2.4 |
| Osm. stress | 196.3 ± 44.5 | −83.7 ± 38.7 | 15.0 | 48.6 | 5.8 ± 2.5 |
Pmax – maximal rate of photosynthesis at optimal irradiance (μmol O2 h−1 mg−1 Chl a), respiration – respiration in the dark (μmol O2 h−1 mg−1 Chl a), Ec – light compensation value (μmol photons m−2 s−1), Ek – initial light saturation point (μmol O2 h−1 mg−1 Chl a). Ec and Ek values were calculated with the help of the three parameters Pmax, the respiration and α emanated from the fit. Standard error could not calculate for these values. α – initial slope at low light levels (μmol O2 h−1 mg−1 Chl a (μmol photons m−2 s−1)−1).
The maximal net photosynthesis Pmax differed in the four Zygnema sp. strains. The highest value under control conditions (BBM) was found in Zygnema E (Pmax was 752.9 μmol O2 h−1 mg−1 Chl a, Table 2), the lowest Pmax value was measured in Zygnema G (145.5 μmol O2 h−1 mg−1 Chl a Table 2). Zygnema D (Fig. 8c) and B (Fig. 8a) ranged between these values.
In cells of all strains incubated in 800 mM sorbitol for 3 h, Pmax values decreased. In Zygnema B, Pmax decreased under osmotic stress to 16.3% of the initial value in BBM, in Zygnema G to 35.0%, in Zygnema D to 16.8% and in Zygnema E to 26.1%. Additional Zygnema D and E were incubated in 400 mM sorbitol solution for 3 h because the cells plasmolysed even at a sorbitol concentration of 300 mM. But there was no marked difference in reduction when compared to 800 mM sorbitol. In Zygnema D, Pmax decreased to 23.8% and in E to 15.5% (data not shown).
In control measurements, the initial slope of the PE-curve α was between 4.7 μmol O2 h−1 mg−1 Chl a (μmol photons m−2 s−1)−1 in Zygnema G and 7.0 μmol O2 h−1 mg−1 Chl a (μmol photons m−2 s−1)−1 in Zygnema E. After incubation in 800 mM sorbitol solution for 3 h, α value decreased in each of the four investigated Zygnema strains. In Zygnema G and D the α value decreased when compared to the initial value to 27.7% and 29.3%, respectively in Zygnema B and E to 69.1% and 82.9% of the initial α value.
Under control conditions Ec values ranged between 25.1 and 26.7 μmol photonen m−2 s−1, just the Ec value in Zygnema D was with 50.5 μmol photons m−2 s−1 higher (Table 2). Under osmotic stress Ec values behaved differently: it increased or decreased. The Ek values under control conditions were between 55.8 μmol O2 h−1 mg−1 Chl a in Zygnema G and 133.3 μmol O2 h−1 mg−1 Chl a in Zygnema E (Table 2). Apart from Zygnema G, the Ek values decreased under osmotic stress (Table 2).
3.5. Photosystem II efficiency and relative electron transport rates
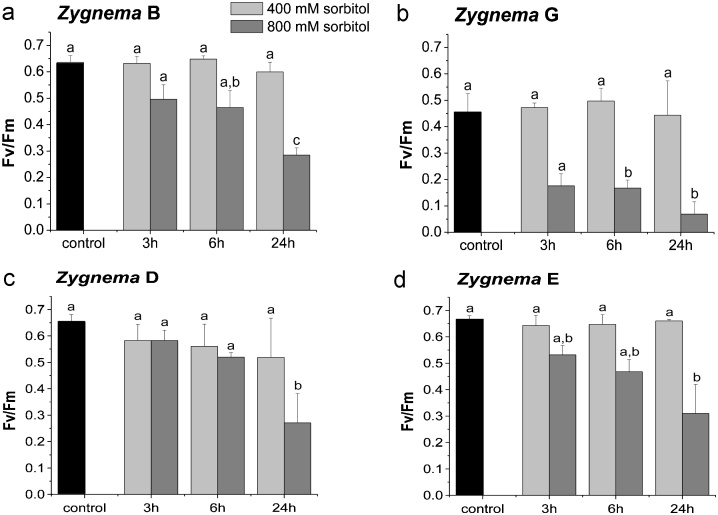
These parameters were measured under control conditions in BBM and under osmotic stress, after incubation in 400 mM and 800 mM sorbitol for 3, 6 and 24 h. Cells were dark adapted for 15 min. Maximum photochemical quantum yield of photosystem II (Fv/Fm) of control cells in BBM was similar in strains B, D and E but lower in stain G (Fig. 9). Incubation in 400 mM sorbitol solution for 3, 6 or 24 h did not induce significant inhibition of the Fv/Fm value in all four investigated Zygnema strains. In contrast, incubation in 800 mM sorbitol solution affected in Zygnema G already after 6 h a significant (p = 0.001) reduction of the Fv/Fm value compared to the control value. In Zygnema strains B and E (p < 0.001) and in strain D (p = 0.001), a significant reduction was found only after 24 h in 800 mM sorbitol solution (Fig. 9). In Zygnema B, after 6 h of osmotic stress there was a significant (p = 0.001) difference between cells treated with 400 mM and 800 mM sorbitol (Fig. 9a). Repeated measure ANOVA demonstrated that there is no significant influence of time in the dependent measurements of sorbitol treated cells.
Fig. 9.
Photosystem II efficiency Fv/Fm. (a) Zygnema sp. B (n = 3), (b) Zygnema sp. G (culture medium, 800 mM n = 3, 400 mM n = 2), (c) Zygnema sp. D (n = 3) and (d) Zygnema sp. E (n = 3) in culture medium (control) and after incubation in 400 mM and 800 mM sorbitol for 3, 6 and 24 h. Lower case letter indicate p ≤ 0.001. In Zygnema sp. B (a), the Fv/Fm value after incubation for 6 h in 800 mM sorbitol (lower case letter a, b) is significantly different to the Fv/Fm value after incubation in 400 mM sorbitol for 6 h (lower case letter a).
rETR as a function of increasing irradiance up to 2973 μmol photon m−2 s−1 was detected under control conditions and after treatment with 400 mM and 800 mM sorbitol solution for 3, 6 and 24 h. Curves were fitted according to Eilers and Peeters (1988). The characterising parameters rETRmax, Ek and α of the fitted curves are summarised in Table 3.
Table 3.
Characterising parameters of the P–E curves, relative electron transport rate as a function of irradiation, fitted according to Eilers and Peeters (1988). P–E curves were determined in the Zygnema sp. strains B, G, D and E in BBM, in 400 mM and 800 mM sorbitol solution after 3, 6 and 24 h incubation.
| BBM | 400 Sorbitol |
800 Sorbitol |
|||||
|---|---|---|---|---|---|---|---|
| 3 h | 6 h | 24 h | 3 h | 6 h | 24 h | ||
| Zygnema sp. B | |||||||
| α | 0.10 | 0.12 | 0.09 | 0.15 | 0.07 | 0.05 | 0.07 |
| rETRmax | 24.3 | 21.0 | 21.6 | 25.4 | 14.5 | 9.1 | 4.4 |
| Ek | 242.6 | 176.8 | 244.9 | 165.7 | 204.3 | 180.0 | 59.3 |
| Zygnema sp. G | |||||||
| α | 0.07 | 0.07 | 0.05 | 0.07 | 0.01 | 0.03 | 0.52 |
| rETRmax | 10.3 | 9.6 | 9.6 | 7.9 | 3.3 | 2.6 | 0.9 |
| Ek | 154.6 | 147.1 | 193.1 | 109.2 | 228.2 | 87.9 | 1.8 |
| Zygnema sp. D | |||||||
| α | 0.23 | 0.20 | 0.17 | 0.14 | 0.13 | 0.07 | 0.03 |
| rETRmax | 38.0 | 21.3 | 17.7 | 19.7 | 15.8 | 9.8 | 5.9 |
| Ek | 165.1 | 105.6 | 107.3 | 145.5 | 122.4 | 146.2 | 223.0 |
| Zygnema sp. E | |||||||
| α | 0.19 | 0.13 | 0.12 | 0.20 | 0.12 | 0.08 | 0.08 |
| rETRmax | 31.4 | 26.4 | 25.2 | 23.0 | 19.3 | 14.5 | 12.2 |
| Ek | 164.8 | 197.8 | 207.6 | 116.1 | 162.7 | 189.8 | 161.9 |
α – initial slope at low light levels (electron/photon), rETRmax – maximal relative electron transport rate (μmol electrons m−2 s−1), Ek – initial light saturation point (μmol photons m−2 s−1). These values were calculated using the results for a, b and c from the fit rETR = I/(a·I2 + b·I + c) (see Section 2).
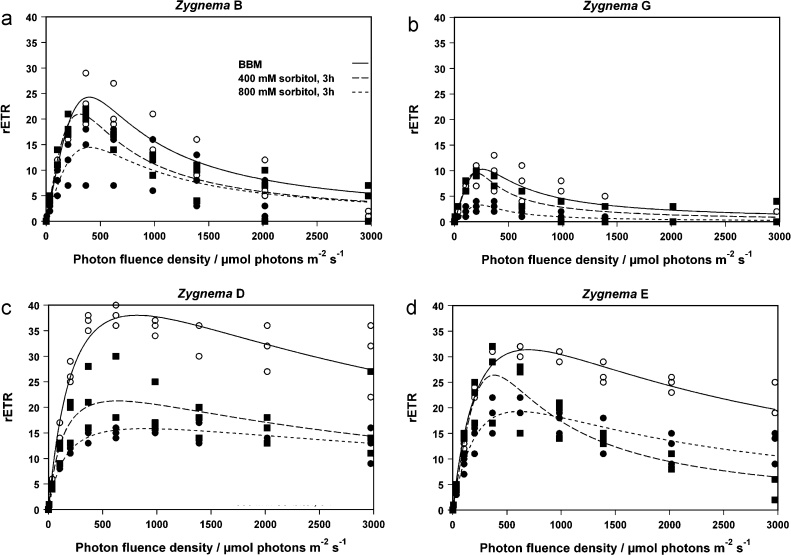
In Fig. 10, differences between the control samples (solid lines) of the four investigated Zygnema strains were illustrated. The rETRmax value (at 366 μmol photons m−2 s−1) of strain G was found to be significantly different (p < 0.005) from all other investigated strains. In Zygnema B and G (Fig. 10a and b) the rETRmax curves had peaks under optimal irradiance and then rETR decreased quickly, but not to zero. In Zygnema D (Fig. 10c) and E (Fig. 10d), rETR decreased slowly after rETRmax was reached. Under osmotic stress rETRmax decreased stronger when compared to control conditions, incubation in 800 mM sorbitol for 3 h decreased rETRmax stronger than 400 mM sorbitol (Fig. 10a–c). Only in Zygnema E (Fig. 10d) under high photon fluence density above 900 μmol photons rETR was higher after incubation in 800 mM sorbitol than in 400 mM sorbitol. The curves reached rETRmax under control conditions at light intensities of approximately 270 μmol photons m−2 s−1 in Zygnema G, 400 μmol photons m−2 s−1 in Zygnema B, 650 μmol photons m−2 s−1 in Zygnema E and 700 μmol photons m−2 s−1 in Zygnema D (Fig. 10a–d).
Fig. 10.
Relative electron transport rate (rETR/μmol electrons m−2 s−1) under increasing light intensity up to 2973 μmol photons m−2 s−1 in Zygnema B sp. (a, n = 3), Zygnema sp. G (b, BBM, 800 mM, n = 3; 400 mM, n = 2), Zygnema sp. D (c, n = 3) and Zygnema sp. E (d, n = 3). Measurements were carried out in BBM (solid line) and after incubation in 400 mM (dashed line) and 800 mM (smooth dashed line) sorbitol for 3 h. P–E curves were fitted according to Eilers and Peeters (1988).
For investigating the time factor under osmotic stress, rETR was measured after an incubation time of 3, 6 and 24 h in 400 mM and 800 mM sorbitol solution. In Zygnema D an E (Table 3) rETRmax decreased over the time in both, 400 mM and 800 mM sorbitol. In Zygnema B and G (Table 3), rETRmax decreased only at 800 mM sorbitol over the time. In 400 mM sorbitol solution rETRmax even increased in Zygnema B, in Zygnema G it did not decreased until 24 h treatment.
4. Discussion
In the present study we investigated the reaction of Zygnema strains from Arctic habitats (Zygnema B, G) and Antarctic habitats (Zygnema D, E) to osmotic stress by incubating their cells in hypertonic sorbitol solutions, which allowed us to determine the osmotic potential of these green algae. We found the highest osmotic values in strains B and G at ∼600 mM sorbitol (ψ = −1.67 MPa), in Zygnema sp. strains D and E at ∼300 mM sorbitol (ψ = −0.80 MPa). For the determination of the osmotic potential, at least 50% of the cells had to be clearly plasmolysed. It has to be stated, that according to our observation, plasmolysis occurred in young and pre-akinete cells at similar osmolarities. However, the degree of the retraction of the protoplast is much stronger in younger cells, due to a higher vacuolization. In contrast, in pre-akinete cells the plasma is filled with storage material (McLean and Pessoney, 1971), which prevents a strong retraction of the cytoplasm from the cell wall and makes plasmolysis sometimes difficult to detect. It is remarkable that the streptophyte green alga Klebsormidium crenulatum has a higher osmotic potential (ψ = −2.09 MPa), there plasmolysis was found to occur in 800 mM sorbitol (Kaplan et al., 2012). This could be an indicator for the different environmental conditions of the aereoterrestrial Klebsormidium, with more frequent drought periods (Karsten et al., 2010), when compared to the habitats of Zygnema, which were frequently rinsed by water (Pichrtová et al., 2012). However, the aim of the present study was not so much on an ecophysiological performance, still we considered habitat conditions for our experimental design. For examples, all experiments were carried out at room temperature, and the cultures were grown with 20° day temperature. This might appear high for polar species, however it is know that this temperature cannot only be tolerated by Antarctic Zygnema sp. but was found to increase the growth rates drastically (Davey, 1991a).
4.1. Phylogenetic relationships
Stancheva et al. (2012) recently characterized several species of Zygnema using molecular and morphological data, but an understanding of the diversity of Zygnema is far from complete in this genus of over 120 species (Algaebase: http://www.algaebase.org/). The two Antarctic strains (D and E) are supported strongly as sister taxa in the phylogenetic analysis (Fig. 1), and given the small number of nucleotide differences between this sequence, may represent two isolates of the same species. The two Arctic isolates (B and G) are distributed phylogenetically into very distinct lineages. This could also be an explanation for distinct physiological reactions observed in strain G, when compared to the other strains (see below, Section 4.3). None of the polar Zygnema strains used in this study are closely related to other known and molecularly characterized species, so we are unable place these strains into defined species. Rather, these strains may represent novel, previously unsampled diversity within the genus, emphasizing the need for further taxonomic and diversity studies in Zygnema.
4.2. Structure and ultrastructure during plasmolysis
We had to face the problem, that our cultures contained young cells as well as pre-akinete cells, which lead to an inhomogeneous appearance of the plasmolysed cells. Obviously previous investigations had to face similar problems in filamentous green algae exposed to osmotic stress. For example in Spirogyra sp., various cells in a filament behaved differently to the same osmotic treatment: While about 31% of the cells survived, 69% were necrotic and died, and in another cultivated sample, the ratio was reversed and 59% of the cells survived, while 41% died (Höfler, 1951). This is likely due to the cell age or developmental stage.
Ultrastructural details of the investigated Zygnema strains were similar to previously described for cultured material (McLean and Pessoney, 1971; Bakker and Lokhorst, 1987) as well as for an Arctic strain collected in the field in Svalbard (Holzinger et al., 2009). Moreover, some of the strains analysed in this study (Zygnema strain B, G, E) were previously investigated by high pressure freeze fixation, and revealed the two star shaped chloroplasts, the central nucleus, vacuoles and electron dense particles in the cell cortex (Pichrtová et al., 2012). The related Zygogonium ericetorum (Zygnematales, Streptophyta), had a distinct ultrastructure, with larger vacuoles and only a thin cytoplasmic area surrounding the chloroplasts with a pillow-shaped central part and irregular radiating processes (Holzinger et al., 2010).
McLean and Pessoney (1971) described the formation of akinetes in older cultures of Zygnema sp. by filling the cells with storage material like lipids and starch. The cultures investigated in the present study were 3–5 weeks old, which was necessary to gain the needed filament density for the investigations. As demonstrated by light and electron microscopy, these cultures contained enough recently divided algal cells not densely filled with storage material, and still containing lager vacuoles.
The detected ultrastructural changes induced by incubation in sorbitol solution in the different Zygnema strains were similar to previous observations of sorbitol treated samples of other streptophyceaen green algae e.g. Klebsormidium (Kaplan et al., 2012) or Micrasterias (Affenzeller et al., 2009; Darehshouri and Lütz-Meindl, 2010). One key finding was a condensed cytoplasm, which was a logical consequence of water extraction by the hypertonic sorbitol treatment and also observed in osmotically stressed Klebsormidium sp. cells (Kaplan et al., 2012). Interestingly, vacuoles were still present in the osmotically stressed cells, but appeared small, like those reported earlier for mosses (Proctor et al., 2007; Pressel et al., 2009). Damaging effects particularly to membranes of chloroplasts and mitochondria might also be related to the difficulty in properly fixing strongly dehydrated plant tissue (e.g. Platt et al., 1997), which is even difficult to fix by high pressure freeze fixation (Wesley-Smith, 2001; Kaplan et al., 2012). Damage to mitochondria and chloroplasts was also reported in desiccated mosses (e.g. Proctor et al., 2007; Pressel et al., 2009; Pressel and Duckett, 2010).
In Zygnema sp., the occurrence of Hechtian strands was demonstrated for the first time in this study by DIC optics as well as by staining with the membrane dye FM 1–43, successfully used to visualize Hechtian strands in higher plants (Bolte et al., 2004; Volgger et al., 2010). At the ultrastructural level, the periplasmatic space between the cell walls was filled with membrane enclosed structures, particularly obvious in Zygnema D and Zygnema E. These particels were likely a consequence of the impossibility of a ‘lateral shrinkage’ of the plasma membrane, and possibly represent fragmented Hechtian strands. These observations go along with findings by Domozych et al., 2003, who demonstrated Hechtian strands in Closterium acerosum. While in Klebsormidium the evidence for Hechtian strands was rather poor, similar membrane enclosed particles were observed in the periplasmatic area (Kaplan et al., 2012). These structures could be helpful in reestablishing the cellular architecture upon replasmolysis, but little is known about the exact mechanism (Johnson-Flanagan and Singh, 1986).
In multicellular tissue Hechtian strands are expected to occur at plasmodesmata, which were totally missing in Zygnema. However, Pont-Lezica et al. (1993) report on many attachment loci independent from plasmodesmata also in onion bulb scale epidermis. These authors postulated that wall glycoproteins seem to be good candidates for the wall-to-membrane linkers. Hydroxyproline-rich glycoproteins were detected in a punctuate distribution on the cell walls in onion (Pont-Lezica et al., 1993), and glycoproteins were also present in cell walls of Zygnematales (e.g. Kim et al., 2007; Yoon et al., 2009). However, in the membrane category of cellular compartments, severe differences were found between e.g. Spirogyra sp. and Arabidopsis thaliana, interpreted by the need of more membrane proteins organizing the cell-to-cell communication in parenchymatous tissue when compared to a filamentous organism (Timme and Delwiche, 2010). This is particularly interesting when seen in the context that conjugating green algae might be sister to land plants (e.g. Wodniok et al., 2011).
To compensate for the volume loss in terms of a surface area regulation of the plasma membrane, also the formation of endocytotic extrusions is possible, as demonstrated for A. thaliana protoplasts by incubation in gradually increasing concentrations of sorbitol from 0.6 to 2.2 M (Yamazaki et al., 2008). Another interesting protection strategy is the occurrence of mechanosensitive channels, which control osmolyte transport in A. thaliana, and ensure compensation for abrupt changes in cytoplasmic osmolarity (Veley et al., 2012).
4.3. Physiological parameters during plasmolysis
To evaluate the physiological status of the cells under osmotic stress we measured the photosynthetic oxygen evolution, the maximum photochemical quantum yield Fv/Fm and the relative electron transport rate (rETR) of photosystem II in control cells and under osmotic stress. The oxygen evolution as well as the rETR decreased under osmotic stress in all strains. Also the Fv/Fm value decreased under osmotic stress but only at higher sorbitol concentrations. Under moderate osmotic influence the investigated Zygnema strains displayed restricted photosystem II efficiency. Zygnema strain G was remarkably distinct in all measured physiological reactions from the other investigated strains, by showing the lowest values of oxygen production, Fv/Fm as well as rETR, already in control cells. Such differences could be explained by a distinct phylogenetic position of strain G (see above, Section 4.1).
Compared to oxygen evolution values measured in Klebsormidium strains where oxygen evolution decreased to about 70% of the initial value in sorbitol (Kaplan et al., 2012), in the here investigated Zygnema strains the net photosynthesis under osmotic stress decreased to about 20–35% of the initial value. This observation agrees with the in general lower osmotic values of the Zygnema cells in comparison to the Klebsormidium cells (Kaplan et al., 2012). When considering, that higher osmotic values result in better water holding capacities, this and the physiological observations could lead to the suggestion that Zygnema is less desiccation tolerant than Klebsormidium.
The Pmax values of Zygnema sp. (between 145.5 and 752.9 μmol O2 mg−1 Chl a h−1) when compared to Pmax values (between 37.9 and 87.0 μmol O2 mg−1 Chl a h−1) of P–E curves in Klebsormidium (Karsten et al., 2010; Karsten and Holzinger, 2012) are much higher, indicating a good photosynthesis performance of the investigated Zygnema strains.
In another zygnematophyceaen green alga found at Svalbard on a glacier, Ancylonema nordenskiöldii, the oxygen evolution was determined (Remias et al., 2012). The maximal oxygen evolution measured under standard temperature of the habitat (1 °C) was approximately 390 μmol O2 mg−1 chl h−1. Remias et al. (2012) explained the good photosynthesis performance at higher irradiances (>400 μmol PAR m−2 s−1) by the presence of high-light-adapted photosystems or by protection of the chloroplast against effects of photoinhibition.
In Klebsormidium dissectum, the Fv/Fm value decreased to zero during a desiccation period of 3 h in the air (Karsten and Holzinger, 2012). Osmotic stress in liquid medium had not so strong effects in Zygnema, the Fv/Fm value did not reach zero in the measurement period of 24 h. Incubation in 400 mM sorbitol solution had no significant effect on the Fv/Fm values in the four Zygnema strains, but 800 mM sorbitol caused a significant reduction. Given that, it seems that 400 mM sorbitol had no influence on the PS II. But under increasing light intensities the rETR decreased even in 400 mM sorbitol solution, indicating that a moderate osmotic environment had a repressive effect in the cells.
Comparing the oxygen evolution (μmol O2 h−1mg−1 Chl a) – and the rETR (μmol electrons m−2s−1) – irradiance curves it seems clear that the rETR values are much higher than the oxygen evolution values (rETR s−1, oxygen evolution h−1). The oxygen evolution values increased fast under low photon fluence density (up to approximately 200 μmol photons m−2 s−1) and reached the Pmax value at higher irradiance. Pmax is reached in the investigated Zygnema strains approximately between a photon fluence density of 125 μmol photons m−2 s−1 (in Zygnema G) and 333 μmol photons m−2 s−1 (in Zygnema E). At this photon fluence densities, rETR is still increasing in all strains. At lower irradiance there is an increase of both oxygen evolution and rETR, at higher irradiance intensities there is still an increasing rETR without an increasing O2 evolution. This indicates increasing numbers of electrons passing through PS II for every O2 evolved (Longstaff et al., 2002). Several studies deal with marine algae where rETR measurements were compared with photosynthetic oxygen production. Longstaff et al. (2002) found in Ulva at low to medium irradiances (0–300 μmol photons m−2 s−1) a significant correlation between O2 evolution and rETR, but not at higher irradiances. Here rETR continued to increase, while O2 evolution tended towards an asymptote. At high irradiance (600–1200 μmol photons m−2 s−1), rETR was lowered like in this study. In two other studies similar results were described for different Ulva and Porphyra species (Figueroa et al., 2003; Franklin and Badger, 2001). A variable correlation between rETR and photosynthetic oxygen evolution in the course of a day in surface water blooms of cyanobacteria was reported by Masojídek et al. (2001).
Possible explanations for these differences are alternative sinks for photosynthetic electron transport. Sinks could be an increased cycling around PS II or non-photochemical quenching in PS II, an increased Mehler ascorbate peroxidase pathway activity or an increased photorespiration (Longstaff et al., 2002).
In field collected Arctic Zygnema sp., Holzinger et al. (2009) described high Ek values ranging from 399 to 486 μmol photons m−2 s−1 implying a sun adaptation of the cells. The rETRmax values were between 50 and 60 electrons m−2 s−1. In the Arctic and Antarctic Zygnema strains used in this study the Ek values were lower (154.6–242.6 μmol photons m−2 s−1) just like the rETRmax values (between 10.3 and 38.0 μmol electrons m−2 s−1).
The high Ek values of P–E curves (rETR versus irradiance) in Zygnema sp. found by Holzinger et al. (2009) may have been caused by the multiple layers of filaments in the mats, causing signals measured from cells with different photo-acclimation by self-shading. This can be the reason for the differences between the Ek values and the rETRmax values in these two studies. It is possible that the same effect had an influence on the measurements in this study too, because in the measurement chamber the filaments may form similar self-shading phenomena. On the other hand, the chamber contained fewer cells than a mat. A second explanation is that the differences between the two studies can be explained by the use of cells from culture versus cells taken from their natural habitat.
In Chlamydomonas nivalis inhabiting snow surface with very high irradiation intensities, Stibal et al. (2007) found Ek values ranging between 523 and 826 μmol photons m−2 s−1 indicating a higher saturating irradiance. In contrast, Prasiola crispa, collected from a terrestrial growth site in Svalbard, had rather low Ek values of 23–24 μmol photons m−2 s−1 (Holzinger et al., 2006), which was also described for K. crenulatum (Ek = 58 μmol photons m−2 s−1, Karsten et al., 2010).
In the green macroalga Urospora, Roleda et al. (2009, 2010) measured Ek values of 82 μmol photons m−2 s−1 in an Arctic strain, and Ek of 252 μmol photons m−2 s−1 in an Antarctic strain. This was assumed to be caused by the different irradiance conditions between the two habitats, where these algae were collected. But in the investigated Zygnema sp. strains no difference between the Ek values from the two habitats was observed.
rETR irradiance curves decreased after reaching the rETRmax value, indicating photoinhibition under high irradiance. The kinetics of the rETR curves differed between the Arctic Zygnema strains B and G and in the Antarctic strains E and D. While in the Antarctic strains the rETRmax values were higher than in the Arctic strains and even under high photon fluence density (>600 μmol photons m−2 s−1) rETR increased slowly, in Arctic rETRmax reached a maximum of 270–400 μmol photons m−2 s−1 and then dropped suddenly. This indicates an adaptation to higher light intensities in the Antarctic Zygnema sp. strains and a lower photosynthetic capacity in the Arctic strains.
Also in this study the origin of the different Zygnema sp. strains could be a factor for the differences in rETR-irradiance kinetics. The Arctic strains were collected in Svalbard (78°55N–11°56E), the Antarctic in King George Island (62°11S–58°27W). The cartographic information can be used to conclude that in the Antarctic habitat plants are acclimated to higher irradiance than in the Arctic. The high latitude where Svalbard is located causes a low zenith angle and Hanelt (1998) reported that even on a sunny day in August 1995 only low rates of PAR, about ≈1100 μmol m−2 s−1 were measured in air. In contrast, on King George Island, Antarctic, Gomez et al. (1997) measured in the Antarctic spring and summer period (1993) irradiances of up to 1700 μmol photons m−2 s−1 on sunny days. Hoyer et al. (2001) measured a similar maximal irradiance of 1748 μmol photons m−2 s−1 in January 1998 on King George Island.
Another example of dependency of photosynthetic capacity and irradiance intensity was highlighted by Silva et al. (1998). They show in the marine red algae Gelidium sesquipedale that response curves (rETR – irradiance) had a different ETRmax value dependent on the depth of the collection side. They conclude that the photosynthetic capacity decreases with depth because of a ‘sun’and ‘shade’ acclimation pattern (Silva et al., 1998).
4.4. Summary
We found that the Zygnema sp. isolates from the Arctic Zygnema B and Zygnema G had the highest osmotic values (most negative osmotic potential), while the Antarctic isolates had lower osmotic values. The later were found in the range of other freshwater Zygnematales (e.g. Höfler, 1951; Ohiwa, 1978; Berliner, 1981). The overall lowest physiological performance was found in Zygnema G. In this strain the reduction of the photosynthetic oxygen production was reduced the least, when compared to the other strains. It is important to note, that by phylogenetic analysis strain G was found to be a distinct linage, which could explain its different physiological behaviour in comparison to the other strains.
We can conclude that particularly the Arctic Zygnema sp. isolates investigated in this study had higher osmotic values, which results in better water holding capacities, likely contributing to their survival in the natural habitat. However, to generalize these findings one would have to increase the number of investigated strains per habitat. It is likely, that the phylogenetically closely related strains D and E are more dependent on water availability in their natural growing site. This was the first study on the osmotic potentials of Arctic and Antarctic Zygnema strains and contributes to our understanding of the semi-terrestrial lifestyle of these algae.
Acknowledgements
We would like to thank Martina Pichrtová, Charles University Prague for providing the algal strains investigated in this study. Moreover, we are thankful to Lisa Huber, University of Innsbruck for preliminary data on osmotic stress of Zygnema and to Manuel Pramsohler, University of Innsbruck for help with the PSYPRO water potential measurements. The study was supported by U.S. NSF DEB-1036448 to L.L. and FWF project P24242 to A.H.
References
- Affenzeller M.J., Darehshouri A., Andosch A., Lütz C., Lütz-Meindl U. Salt stress induced cell death in the unicellular green alga Micrasterias denticulata. Journal of Experimental Botany. 2009;60:939–954. doi: 10.1093/jxb/ern348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker M.E., Lokhorst G.M. Ultrastructure of mitosis and cytokinesis in Zygnema sp. (Zygnematales, Chlorophyta) Protoplasma. 1987;138:105–118. [Google Scholar]
- Behrenfeld M.J., Prasil O., Babin M., Bruyant F. In search of a physiological basis for covariations in light-limited and light-saturated photosynthesis. Journal of Phycology. 2004;40:4–25. [Google Scholar]
- Berliner M.D. Protoplasts of eukaryotic algae. International Review of Cytology. 1981;73:1–20. [Google Scholar]
- Bolte S., Talbot C., Boutte Y., Catrice C., Read N.D., Satiat-Jeunemaitre B. FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. Journal of Microscopy – Oxford. 2004;214:159–173. doi: 10.1111/j.0022-2720.2004.01348.x. [DOI] [PubMed] [Google Scholar]
- Büdel B. Eucaryotic algae. In: Lüttge U., Beck U., Bartels E.D., editors. Plant Desiccation Tolerance. Ecological Studies 215. Springer; Heidelberg: 2011. pp. 45–63. [Google Scholar]
- Chalker B.E., Dunlap W.C., Oliver J.K. Bathymetric adaptations of reef-building corals at Davies Reef, Great Barrier Reef, Australia. II. Light saturation curves for photosynthesis and respiration. Journal of Experimental Marine Biology and Ecology. 1983;73:37–56. [Google Scholar]
- Coleman A.W. The role of resting spores and akinetes in chlorophyte survival. In: Fryxell G.A., editor. Survival Strategies of the Algae. Cambridge University Press; Cambridge, New York: 1983. pp. 1–21. [Google Scholar]
- Darehshouri A., Lütz-Meindl U. H2O2 localization in the green alga Micrasterias after salt and osmotic stress by TEM-coupled electron energy loss spectroscopy. Protoplasma. 2010;239:49–56. doi: 10.1007/s00709-009-0081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey M.C. Effects of physical factors on the survival and growth of Antarctic terrestrial algae. British Phycological Journal. 1991;26:315–325. [Google Scholar]
- Davey M.C. The seasonal periodicity of algae on Antarctic fellfield soils. Holarctic Ecology. 1991;14:112–120. [Google Scholar]
- Domozych D.S., Roberts R., Danyow C., Flitter R., Smith B., Providence K. Plasmolysis, hechtian strand formation, and localized membrane-wall adhesions in the desmid, Closterium acerosum (Chlorophyta) Journal of Phycology. 2003;39:1194–1206. [Google Scholar]
- Eilers P.H.C., Peeters J.C.H. A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecological Modelling. 1988;42:199–215. [Google Scholar]
- Ellis E.A. Solutions to the problem of substitution of ERL 4221 for vinyl cyclohexene dioxide in Spurr low viscosity embedding formulations. Microscopy Today. 2006;14:32–33. [Google Scholar]
- Figueroa F.L., Conde-Álvarez R., Gómez I. Relations between electron transport rates determined by pulse amplitude modulated chlorophyll fluorescence and oxygen evolution in macroalgae under different light conditions. Photosynthesis Research. 2003;75:259–275. doi: 10.1023/A:1023936313544. [DOI] [PubMed] [Google Scholar]
- Franklin L.A., Badger M.R. A comparison of photosynthetic electron transport rates in macroalgae measured by pulse amplitude modulated chlorophyll fluorometry and mass spectrometry. Journal of Phycology. 2001;37:756–767. [Google Scholar]
- Gomez I., Weykam G., Kloser H., Wiencke C. Photosynthetic light requirements, metabolic carbon balance and zonation of sublittoral macroalgae from King George Island (Antarctica) Marine Ecology Progress Series. 1997;148:281–293. [Google Scholar]
- Gustavs L., Eggert A., Michalik D., Karsten U. Physiological and biochemical responses of green microalgae from different habitats to osmotic and matric stress. Protoplasma. 2010;243:3–14. doi: 10.1007/s00709-009-0060-9. [DOI] [PubMed] [Google Scholar]
- Graham L.E., Arancibia-Avila P., Strother Taylor W.A., Cook P.K.M.E. Aeroterrestrial Coleochaete (Streptophyta, Coleochaetales) models early plant adaptation to land. American Journal of Botany. 2012;99:130–144. doi: 10.3732/ajb.1100245. [DOI] [PubMed] [Google Scholar]
- Hanelt D. Capability of dynamic photoinhibition in Arctic macroalgae is related to their depth distribution. Marine Biology. 1998;131:361–369. [Google Scholar]
- Hawes I. Filamentous green algae in freshwater streams on Signy Island, Antarctica. Hydrobiologia. 1989;172:1–18. [Google Scholar]
- Hawes I. Effects of freezing and thawing on a species of Zygnema (Chlorophyta) from the Antarctic. Phycologia. 1990;29:326–331. [Google Scholar]
- Höfler K. Plasmolyse mit Natriumkarbonat. Protoplasma. 1951;40:426–460. [Google Scholar]
- Holzinger A., Karsten U., Lütz C., Wiencke C. Ultrastructure and photosynthesis in the supralittoral green macroalga Prasiola crispa (Lightfoot) Kützing from Spitsbergen (Norway) under UV exposure. Phycologia. 2006;45:168–177. [Google Scholar]
- Holzinger A., Lütz C., Karsten U. Desiccation stress causes structural and ultrastructural alterations in the aeroterrestrial green alga Klebsormidium crenulatum (Klebsormidiophyceae, Streptophyta) isolated from an alpine soil crust. Journal of Phycology. 2011;47:591–602. doi: 10.1111/j.1529-8817.2011.00980.x. [DOI] [PubMed] [Google Scholar]
- Holzinger A., Roleda M.Y., Lütz C. The vegetative arctic freshwater green alga Zygnema is insensitive to experimental UV exposure. Micron. 2009;40:831–838. doi: 10.1016/j.micron.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Holzinger A., Tschaikner A., Remias D. Cytoarchitecture of the desiccation-tolerant green alga Zygogonium ericetorum. Protoplasma. 2010;243:15–24. doi: 10.1007/s00709-009-0048-5. [DOI] [PubMed] [Google Scholar]
- Hoyer K., Karsten U., Sawall T., Wiencke C. Photoprotective substances in Antarctic macroalgae and their variation with respect to depth distribution, different tissues and developmental stages. Marine Ecology Progress Series. 2001;211:117–129. [Google Scholar]
- Iwata K., Tazawa M., Itho T. Turgor pressure regulation and the orientation of cortical microtubules in Spirogyra cells. Plant and Cell Physiology. 2001;42:594–598. doi: 10.1093/pcp/pce073. [DOI] [PubMed] [Google Scholar]
- Jassby A.D., Platt T. Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnology and Oceanography. 1976;21:540–547. [Google Scholar]
- Johnson-Flanagan A.M., Singh J. Membrane deletion during plasmolysis in hardened and nonhardened plant cells. Plant, Cell & Environment. 1986;9:299–305. [Google Scholar]
- Kaplan F., Lewis L.A., Wastian J., Holzinger A. Plasmolysis effects and osmotic potential of two phylogenetically distinct alpine strains of Klebsormidium (Streptophyta) Protoplasma. 2012;249:789–804. doi: 10.1007/s00709-011-0324-z. [DOI] [PubMed] [Google Scholar]
- Karsten U., Holzinger A. Light, temperature and desiccation effects on photosynthetic activity and drought-induced ultrastructural changes in the green alga Klebsormidium dissectum (Streptophyta) from a high alpine soil crust. Microbial Ecology. 2012;63:51–63. doi: 10.1007/s00248-011-9924-6. [DOI] [PubMed] [Google Scholar]
- Karsten U., Lütz C., Holzinger A. Ecophysiological performance of the aeroterrestrial green alga Klebsormidium crenulatum (Charophyceae, Streptophyta) isolated from an alpine soil crust with an emphasis on desiccation stress. Journal of Phycology. 2010;46:1187–1197. doi: 10.1111/j.1529-8817.2011.00980.x. [DOI] [PubMed] [Google Scholar]
- Kim G.H., Yoon M., West J.A., Klochkova T.A., Kim S.-H. Possible surface carbohydrates involved in signaling during conjugation process in Zygnema cruciatum monitored with fluorescin isothiocyanate-lectins (Zygnemataceae, Chlorophyta) Phycological Research. 2007;55:135–142. [Google Scholar]
- Kim G.H., Klochkova T.A., Kang S.H. Notes on freshwater and terrestrial algae from Ny-Alesund, Svalbard (high Arctic sea area) Journal of Environment Biology. 2008;29:485–491. [PubMed] [Google Scholar]
- Kim G.H., Klochkova T.A., Han J.W., Kang S.H., Choi H.G., Chung K.W., Kim S.J. Freshwater and terrestrial algae from Ny-Alesund and Blomstrandhalvoya island (Svalbard) Arctic. 2011;64:25–31. [PubMed] [Google Scholar]
- Longstaff B.J., Kildea T., Runcie J.W., Cheshire A., Dennison W.C., Hurd C., Kana T., Raven J.A., Larkum W.D. An in situ study of photosynthetic oxygen exchange and electron transport rate in marine macroalga Ulva lactuca (Chlorophyta) Photosynthesis Research. 2002;74:281–293. doi: 10.1023/A:1021279627409. [DOI] [PubMed] [Google Scholar]
- Macedo M.F., Ferreira J.G., Duarte P. Dynamic behaviour of photosynthesis-irradiance curves determined from oxygen production during variable incubation periods. Marine Ecology Progress Series. 1998;165:31–43. [Google Scholar]
- Masojídek J., Grobbelaar J.U., Pechar L., Koblízek M. Photosystem II electron transport rates and oxygen production in natural water blooms of freshwater cyanobacteria during a diel cycle. Journal of Plankton Research. 2001;23:57–66. [Google Scholar]
- McLean R.J., Pessoney G.F. Formation and resistance of akinetes of Zygnema. In: Parker B.C., Brown R.M. Jr., editors. Contributions in Phycology. Allen Press; Lawrence, KS: 1971. pp. 145–152. [Google Scholar]
- Ohiwa T. Behavior of cultured fusion products from Zygnema and Spirogyra protoplasts. Protoplasma. 1978;97:185–200. [Google Scholar]
- Oparka K.J. Plasmolysis: new insights into an old process. New Phytologist. 1994;126:571–591. [Google Scholar]
- Pichrtová, M., Remias, D., Lewis, L.A., Holzinger, A., 2012. Changes in phenolic compounds and cellular ultrastructure of arctic and Antarctic strains of Zygnema (Zygnematophyceae, Streptophyta) after exposure to experimentally enhanced UV to PAR ratio. Microbial Ecology, http://dx.doi.org/10.1007/s00248-012-0096-9. [DOI] [PMC free article] [PubMed]
- Platt K.A., Oliver M.J., Thomson W.W. Importance of the fixative for reliable ultrastructural preservation of poikilohydric plant tissues. Observations on dry, partially, and fully hydrated tissues of Selaginella lepidophylla. Annals of Botany. 1997;80:599–610. [Google Scholar]
- Pont-Lezica R.F., McNally J.G., Pickard B.G. Wall-to-membrane linkers in onion epidermis: some hypothesis. Plant, Cell & Environment. 1993;16:111–123. [Google Scholar]
- Porra R.J., Thompson W.A., Kriedmann P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochimica et Biophysica Acta. 1989;975:384–394. [Google Scholar]
- Pressel S., Duckett J.G. Cytological insights into the desiccation biology of a model system: moss protonemata. New Phytologist. 2010;185:944–963. doi: 10.1111/j.1469-8137.2009.03148.x. [DOI] [PubMed] [Google Scholar]
- Pressel S., Duckett J.G., Ligrone R., Proctor M.C.F. Effects of de- and rehydration in desiccation tolerant liverworts: a cytological and physiological study. International Journal of Plant Sciences. 2009;170:182–199. [Google Scholar]
- Proctor M.C.F., Ligrone R., Duckett J.G. Desiccation tolerance in the moss Polytrichum formosum: Physiological and fine-structural changes during desiccation and recovery. Annals of Botany – London. 2007;99:75–93. doi: 10.1093/aob/mcl246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remias D., Holzinger A., Aigner S., Lütz C. Ecophysiology and ultrastructure of Ancylonema nordenskiöldii (Zygnematales, Streptophyta), causing brown ice on glaciers in Svalbard (high arctic) Polar Biology. 2012;35:899–908. [Google Scholar]
- Remias D., Holzinger A., Lütz C. Ultrastructure and physiological characterization of the ice alga Mesotaenium berggrenii (Zygnemaphyceae, Chlorophyta) from glaciers in the European alps. Phycologia. 2009;48:302–312. [Google Scholar]
- Roleda M.Y., Campana G.L., Wiencke C., Hanelt D., Quartino M.L., Wulff A. Sensitivity of Antarctic Urospora penicilliformis (Ulotrichales, Chlorophyta) to ultraviolet radiation is life stage dependent. Journal of Phycology. 2009;45:600–609. doi: 10.1111/j.1529-8817.2009.00691.x. [DOI] [PubMed] [Google Scholar]
- Roleda M.Y., Lütz-Meindl U., Wiencke C., Lütz C. Physiological, biochemical, and ultrastructural responses of the green macroalga Urospora penicilliformis from Arctic Spitsbergen to UV radiation. Protoplasma. 2010;243:105–116. doi: 10.1007/s00709-009-0037-8. [DOI] [PubMed] [Google Scholar]
- Silva J., Santos R., Serodio J., Melo R.A. Light response curves for Gelidium sesquipedale from different depths, determined by two methods: O2 evolution and chlorophyll fluorescence. Journal of Applied Physiology. 1998;10:295–301. [Google Scholar]
- Stancheva R., Hall J.D., Sheath R.G. Systematics of the genus Zygnema (Zygnematophyceae, Charophyta) from Californian watersheds. Journal of Phycology. 2012 doi: 10.1111/j.1529-8817.2012.01127.x. [DOI] [PubMed] [Google Scholar]
- Stibal M., Elster J., Šabacká M., Kaštovská K. Seasonal and diel changes in photosynthetic activity of the snow alga Chlamydomonas nivalis (Chlorophyceae) from Svalbard determined by pulse amplitude modulation fluorometry. FEMS Microbiology Ecology. 2007;59:265–273. doi: 10.1111/j.1574-6941.2006.00264.x. [DOI] [PubMed] [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland, MA: 2002. PAUP* Ver.# 4b10. [Google Scholar]
- Timme R.E., Delwiche C.F. Uncovering the evolutionary origin of plant molecular processes: comparison of Coleochaete (Coleochaetales) and Spirogyra (Zygnematales) transcriptomes. BMC Plant Biology. 2010;10:96. doi: 10.1186/1471-2229-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veley K.M., Marshburn S., Clure C.E., Haswell E.S. Mechanosensitive channels protect plastids from hypoosmotic stress during normal plant growth. Current Biology. 2012;22:408–413. doi: 10.1016/j.cub.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgger M., Lang I., Ovecka M., Lichtscheidl I. Plasmolysis and cell wall deposition in wheat root hairs under osmotic stress. Protoplasma. 2010;243:51–62. doi: 10.1007/s00709-009-0055-6. [DOI] [PubMed] [Google Scholar]
- Wesley-Smith J. Freeze-substitution of dehydrated plant tissues: artefacts of aqueous fixation revisited. Protoplasma. 2001;218:154–167. doi: 10.1007/BF01306605. [DOI] [PubMed] [Google Scholar]
- Willmer C.M., Beattie L.N. Cellular osmotic phenomena during stomatal movements of Comelina communis I, limitiations of the incipient plasmolysis technique for determining osmotic pressures. Protoplasma. 1978;95:321–332. [Google Scholar]
- Wodniok S., Brinkmann H., Glöckner G., Heidel A.J., Philippe H., Melkonian M., Becker B. Origin of land plants: do conjugating green algae hold the key? BMC Evolutionary Biology. 2011;11:104. doi: 10.1186/1471-2148-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T., Kawamura Y., Uemura M. Crobehaviour of the plasma membrane in protoplasts isolated from cold-acclimated Arabidopsis leaves is related to surface area regulation. Plant and Cell Physiology. 2008;49:944–957. doi: 10.1093/pcp/pcn068. [DOI] [PubMed] [Google Scholar]
- Yoon M., Kim M.K., Kim G.H. Conjugation process in Spirogyra varians monitored with FITC-lectins (Zygnemataceae, Chlorophyta) Algae. 2009;24:39–45. [Google Scholar]