Abstract
Background
Ethanol induces similar behavioral responses in mammals and the fruit fly, Drosophila melanogaster. By coupling assays for ethanol-related behavior to the genetic tools available in flies, a number of genes have been identified that influence physiological responses to ethanol. To enhance the utility of the Drosophila model for investigating genes involved in ethanol-related behavior, we explored the value of an assay that measures the sedative effects of ethanol on negative geotaxis, an evoked locomotor response.
Methods
We established eRING (ethanol Rapid Iterative Negative Geotaxis) as an assay for quantitating the sedative effects of ethanol on negative geotaxis (i.e. startle-induced climbing). We validated the assay by assessing acute sensitivity to ethanol and rapid ethanol tolerance in several different control strains and in flies with mutations known to disrupt these behaviors. We also used eRING in a candidate screen to identify mutants with altered ethanol-related behaviors.
Results
Negative geotaxis measured in eRING assays was dose-dependently impaired by ethanol exposure. Flies developed tolerance to the intoxicating effects of ethanol when tested during a second exposure. Ethanol sensitivity and rapid ethanol tolerance varied across four control strains, but internal ethanol concentrations were indistinguishable in the four strains during a first and second challenge with ethanol. Ethanol sensitivity and rapid ethanol tolerance, respectively, were altered in flies with mutations in amnesiac and hangover, genes known to influence these traits. Additionally, mutations in the β integrin gene myospheroid and the α integrin gene scab increased the initial sensitivity to ethanol and enhanced the development of rapid ethanol tolerance without altering internal ethanol concentrations.
Conclusions
The eRING assay is suitable for investigating genetic mechanisms that influence ethanol sensitivity and rapid ethanol tolerance. Ethanol sensitivity and rapid ethanol tolerance depend on the function of α and β integrins in flies.
Keywords: behavior, alcohol, genetics
Introduction
Abuse of alcohol is a serious problem in most societies. The costs associated with the consequences of alcohol abuse extend into the hundreds of billions of dollars annually in the United States alone (DHHS, 2000). The identification of genes that influence the effects of alcohol is important for understanding the molecular mechanisms involved in alcohol action, assessing an individual’s potential for abusing alcohol, and developing novel pharmacological strategies for treating patients that abuse alcohol. Accordingly, a number of laboratories have explored mechanisms related to the effects of alcohol in genetically tractable organisms, principally mice, the fruit fly Drosophila melanogaster and the nematode Caenorhabditis elegans.
The intoxicating effects of ethanol in mammals and invertebrates are remarkably similar. Low doses of ethanol tend to cause psychomotor excitation whereas higher doses cause sedation. Additionally, prolonged or repeated exposure to ethanol causes tolerance to the drug (Davies et al., 2004; Goddeeris et al., 2003), defined as an decreased sensitivity to the intoxicating effects of ethanol (Tabakoff et al., 1986). Interestingly, many critical genetic pathways that influence ethanol-related behavior in mammals and invertebrates are functionally conserved (Cowmeadow et al., 2005; Davies et al., 2004; Davies et al., 2003; Kapfhamer et al., 2008; Wen et al., 2005). Mice, flies and worms are therefore promising models for identifying core mechanisms that underlie the effects of ethanol on behavior.
A number of assays have been used to examine the effects of ethanol on behavior in Drosophila. While most studies in flies have measured ethanol-induced loss of postural control (Moore et al., 1998; Urizar et al., 2007; Wen et al., 2005), other studies have examined the effect of ethanol on fly behavior by assessing spontaneous (Parr et al., 2001; Wolf et al., 2002) or evoked (Berger et al., 2004; Ramazani et al., 2007) locomotion. Studies using these methods have identified a number of genes that influence ethanol-responsive behaviors and have demonstrated that different genetic pathways affect different behaviors. For example, mutations in amnesiac (amn), a gene involved in cAMP signaling, increase the initial sensitivity to ethanol (Moore et al., 1998) and mutation of hangover (hang), a gene that encodes a zinc-finger transcription factor, blunts the development of rapid ethanol tolerance (Scholz et al., 2005). We reasoned that additional behavioral paradigms should offer complementary advantages for investigating the genetic basis of ethanol-related behavior in flies.
To this end we developed ethanol Rapid Iterative Negative Geotaxis (eRING) as an assay for evaluating the sedative effects of ethanol on evoked locomotor behavior in flies. The eRING assay is adapted from our previously described RING behavioral paradigm that measures negative geotaxis (startle-induced vertical climbing) (Gargano et al., 2005; Rhodenizer et al., 2008). We found that negative geotaxis measured in eRING assays was progressively impaired as the internal concentration of ethanol increased. In addition, we found that flies developed a rapid pharmacodynamic tolerance to the intoxicating effects of ethanol. We also found that the effects of mutations in amn and hang on ethanol sensitivity and rapid ethanol tolerance in eRING assays were largely consistent with the results of published studies using the inebriometer (Moore et al., 1998; Scholz et al., 2005). Additionally, by using the eRING assay in a screen of candidate mutants we found that mutations in myospheroid (mys) and scab (scb), genes that encode β and α integrins, respectively, increase the initial sensitivity to ethanol and enhance the development of rapid ethanol tolerance. Our studies indicate that integrins influence the pharmacodynamic properties of ethanol and that the eRING assay is suitable for investigating the genetics of ethanol-related behavior in Drosophila.
Methods
Fly stocks and husbandry
Flies were reared on standard food medium (10% sucrose, 2% yeast, 3.3% cornmeal, 1% agar) at 25°C/60% relative humidity under a 12h day/night cycle. Canton-S (CS), Samarkand (SAM), Lausanne-S (LUS) and Oregon-R (OR) stocks were obtained from the Drosophila Stock Center (Bloomington, IN). The w[CS] control stock contains the w1118 allele in a Canton-S background (Goddeeris et al., 2003). All mys alleles (kindly provided by N. Brown, Cambridge University, and D. Brower, University of Arizona) were backcrossed to w[CS] control strain for six generations and then maintained as homozygous stocks (mysts1 and mysts2) or as a balanced stock (mysXG/FM6) (Goddeeris et al., 2003). The scb alleles (Grotewiel et al., 1998) were also backcrossed to w[CS] for six generations and then maintained as homozygous stocks (scbVol2) and or as a balanced stock (scbVol1/CyO). The amnesiacChpd and ry506 control flies were provided by Ulrike Heberlein (University of California, San Francisco). The hangoverAE10 mutants and pZ[+];ry506 controls were provided by Henrike Scholz (University of Wurzburg).
eRING assays, ethanol sensitivity and rapid ethanol tolerance
All flies used in individual experiments were grown, collected and handled in parallel. For all studies, adult flies (2- to 4-days-old) were anaesthetized briefly with CO2, separated by sex, transferred to fresh food vials at 25 flies per vial, and allowed to recover for at least 16 hours at 25°C/60% relative humidity prior to behavioral tests. We adapted a Rapid Iterative Negative Geotaxis (RING) assay (Gargano et al., 2005) to assess the effects of ethanol on negative geotaxis (startle-induced climbing, (Rhodenizer et al., 2008)). The modified procedure was designated as ethanol RING (eRING). In eRING assays, 25 flies were transferred to each of 5 vertical tubes in the eRING apparatus. Each tube contained a 1 cm cotton plug (flyStuff.com) at the top with 500 µl of water or 500 µl of ethanol at 25, 50 or 75% in water. Flies were sharply rapped to the bottom of the vials to initiate negative geotaxis (climbing the vial walls). The distance the flies climbed during each test was documented in a digital photograph taken 4 s after initiating the behavior. Data (distances climbed) were extracted via computer-aided data analysis using Scion Image (PC version of NIH Image, Scion Corporation, Frederick, MD, USA). Flies that became sedated and remained at the bottom of the eRING tubes in each test were scored as zero. The temperature and relative humidity were maintained at 24–26°C and 55–65%, respectively, during eRING tests.
Ethanol sensitivity was assessed by measuring negative geotaxis in eRING assays during a single continuous exposure of flies to ethanol vapor from 25, 50 or 75% ethanol solutions. Flies were tested iteratively with 1 minute rest periods between each test to produce time-course plots. Sensitivity to ethanol was calculated from these time-course data as T50 values (time required for ethanol to impair negative geotaxis by 50% of its value in the absence of ethanol) derived from linear regression, the simplest model that fit all data sets. T50 values were determined for each vial of 25 flies such that each datum represents the average performance of 25 flies in a single vial tested at multiple time-points. Increased sensitivity to ethanol is reflected by decreased T50 values.
To assess the development of rapid ethanol tolerance, we measured negative geotaxis in eRING assays during a first (E) and second (EE) exposure of flies to vapor from 50% ethanol separated by 4 hours of recovery at 25°C/60% relative humidity in vials with food but no ethanol. The first exposure to ethanol was for 30 minutes. The second exposure was continued until all flies were fully sedated. Control-treated flies were tested in eRING assays during exposure to water alone (W) or water then ethanol (WE). Flies were fully sedated after the first exposure to ethanol and regained their normal negative geotaxis behavior within the first 2 hours of the recovery period. We performed eRING assays during the first and second ethanol exposures until the flies were sedated, typically at least 10 minutes. Thereafter, flies were left undisturbed in the presence of ethanol for the remainder of each 30 minutes exposure. The development of rapid ethanol tolerance was calculated as the percent change in T50 values between the first and second ethanol exposures [(T50EE/T50E – 1) × 100%].
Possible dehydration during eRING testing and fly water content
Groups of 25 flies were weighed before and after eRING testing to determine whether behavioral testing caused dehydration. The water content of flies (i.e. the volume of distribution for ethanol) was determined by subtracting the dry weight from the wet weight of groups of 25 flies. Flies were desiccated by incubating at 55°C for 18–24 hours.
Internal ethanol concentrations
Flies were exposed to vapor from 50% ethanol for 0, 5, 15 and 30 minutes in eRING assays and then frozen immediately at −80°C. Internal ethanol concentrations were determined essentially as described (Moore et al., 1998) except that we used the water content of flies to estimate the volume of distribution for ethanol. Frozen flies were homogenized in 200 µl of sterile distilled water. The homogenate was centrifuged at 16,000 × g for 25 minutes at 4°C. The internal ethanol concentration was determined in the resulting supernatant according to the manufacturer’s instructions using the Alcohol Reagent Set (Pointe Scientific, Inc) and the empirically determined water content for each genotype.
Statistical analyses
All data are presented as mean ± S.E.M. One-, two- and three-way ANOVAs, Bonferroni’s multiple comparisons, one- and two-sample t tests, and Kruskal-Wallis one-way ANOVA and Dunn’s multiple comparisons were performed with Prism (GraphPad Software, San Diego, CA, USA) or JMP (SAS Institute, Cary, NC, USA). P values ≤0.05 were considered significant.
Results
Development of eRING, an assay for ethanol sensitivity and rapid ethanol tolerance in Drosophila
We reasoned that negative geotaxis measured with RING assays in the presence of ethanol (eRING) would show time- and dose-dependent effects of the drug. As predicted, negative geotaxis became increasingly impaired in control flies as the duration of ethanol exposure and concentration of ethanol used was increased (Figure 1). Evaluation of several time-course experiments confirmed that negative geotaxis was progressively impaired with increasing exposure time and ethanol concentration in control females (Figure 2A) and males (Figure 2B). In the absence of ethanol, flies retained robust negative geotaxis when tested repeatedly (Figure 3A and 3B). As a static measure of ethanol sensitivity, we calculated T50 values (the time required for negative geotaxis to be reduced by 50%) from the time-course studies in Figure 2A and 2B. T50 values progressively decreased as the concentration of ethanol increased in Canton-S control females and males (Figure 2C), indicating that T50 is a suitable measure of ethanol sensitivity in eRING assays. Importantly, exposure to ethanol in eRING assays did not dehydrate flies (Figure S1A, provided as supporting information) and all flies recovered their normal negative geotaxis behavior within two hours after withdrawal of the ethanol (not shown). Together, these observations indicate that the impairment of negative geotaxis in the presence of ethanol is due to intoxication and that the eRING assay is suitable method for assessing ethanol sensitivity in flies.
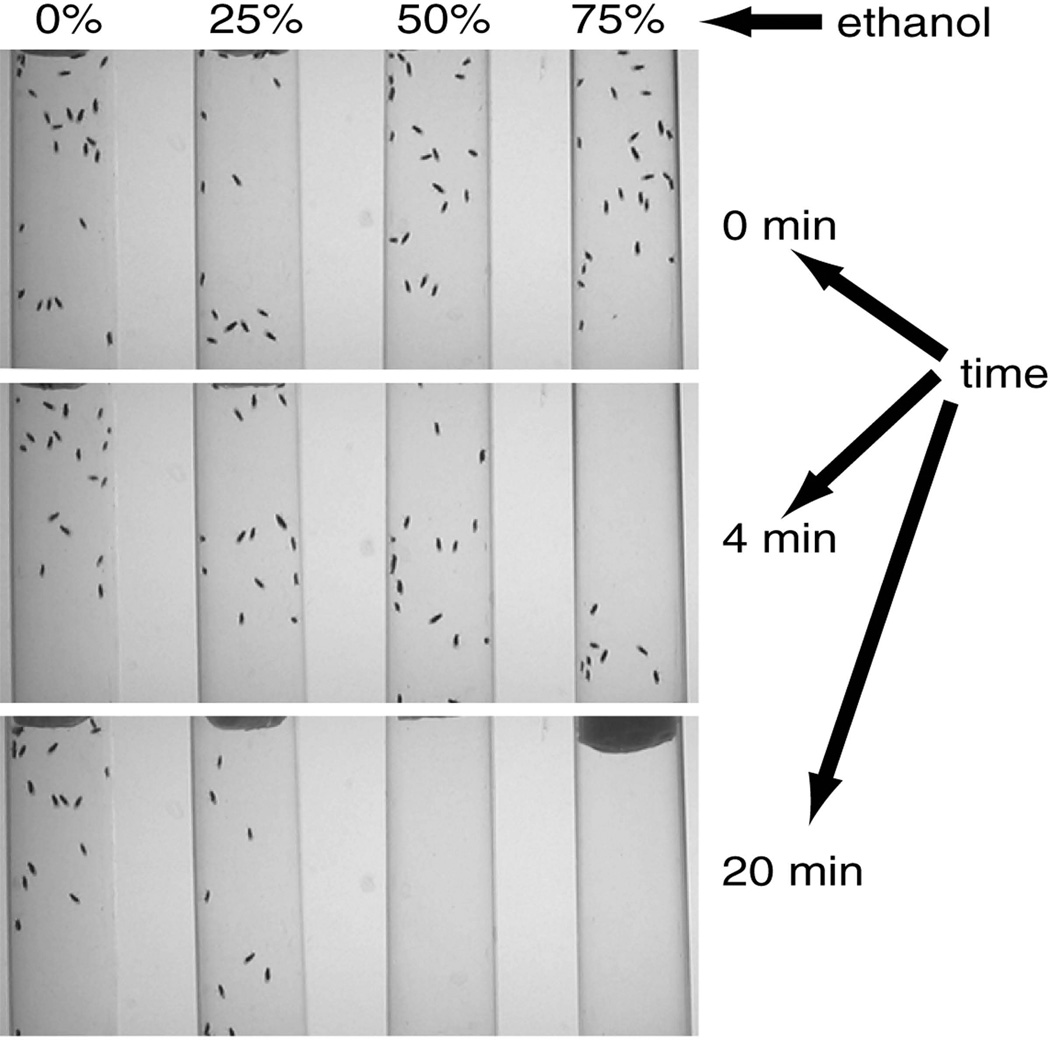
Figure 1. Negative geotaxis in control flies exposed to ethanol in eRING.
Images of w[CS] control flies (25 flies per tube) performing negative geotaxis in the presence of ethanol (500 µl of 0, 25, 50, or 75% in a cotton plug at the top of the vials) after 0, 4 or 20 minutes of exposure.
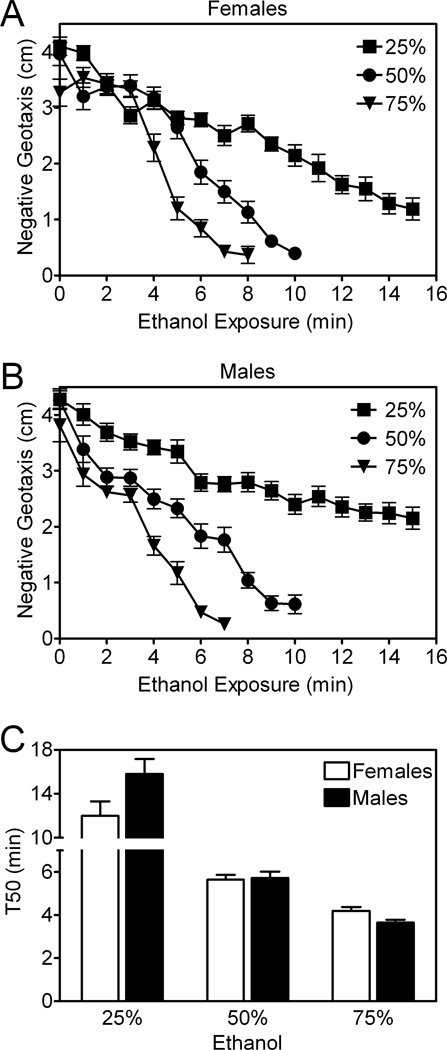
Figure 2. Time- and dose-dependent effects of ethanol on negative geotaxis in control flies.
Negative geotaxis measured in eRING assays was significantly affected by ethanol concentration and time of exposure in female (A) and male (B) Canton-S (CS) control flies (two- way ANOVA, p<0.0001). (C) T50 values derived from data in panels A and B decreased as the ethanol concentration was increased (two-way ANOVA, p<0.0001), but there was no effect of sex (two-way ANOVA, ns). Data (mean ± S.E.M.) are compiled for n=15 vials (25 flies/vial) from three independent experiments.
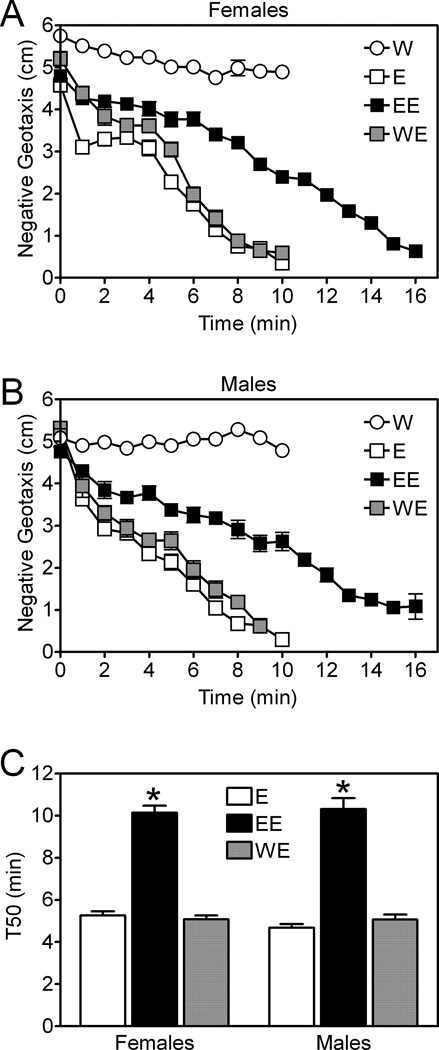
Figure 3. Development of rapid ethanol tolerance measured by eRING.
Negative geotaxis was measured in Canton-S (CS) female (A) and male (B) flies during a first exposure to ethanol vapor from a 50% solution (E), a second exposure to ethanol (EE), or during an exposure to water (W) and then ethanol (WE). EE and WE flies were allowed to recover for 4 hours between a first exposure to ethanol or water and a final exposure to ethanol. Time of ethanol exposure and ethanol treatment group significantly affected negative geotaxis in females and males (repeated measures two-way ANOVAs; time, p<0.0001; group, p<0.0001). (C) T50 values derived from the data in panels A and B were significantly higher in EE flies compared to E or WE flies in females and males (two-way ANOVA, p<0.0001, *Bonferroni’s multiple comparison test, p<0.001). E and WE flies performed indistinguishably (two-way ANOVA, n.s.) in both sexes. Data in all panels are mean ± S.E.M for n=15 vials (25 flies/vial) from three independent experiments. Data from females and males were analyzed separately.
Rapid ethanol tolerance in Drosophila is defined as a decrease in ethanol sensitivity during a second drug exposure (Berger et al., 2004). We assessed the utility of eRING for quantitating rapid ethanol tolerance by measuring negative geotaxis during two ethanol exposures separated by a 4 hour recovery period. In time-course studies, control Canton-S females (Figure 3A) and males (Figure 3B) were significantly less sensitive to ethanol during a second exposure to the drug (group EE) than during a first exposure (group E). Exposure of animals to water did not consistently impact negative geotaxis (group W) and exposure to water first had little or no obvious effect on negative geotaxis during a subsequent round of eRING tests with ethanol (group WE). Additionally, exposure to ethanol during a first eRING test did not impact negative geotaxis in the absence of ethanol during a second test (not shown). Quantitation of these effects by comparing T50 values derived from the time-course data in Figure 3A and 3B revealed that Canton-S control females and males were approximately two-fold less sensitive to ethanol during a second exposure (group EE) than a first exposure (group E) to ethanol (Figure 3C). Additionally, this quantitation demonstrated that exposure to water first had no discernable effect on negative geotaxis in the presence of ethanol (group WE). All of these data indicate that the decreased sensitivity observed during a second ethanol exposure reflects the development of rapid ethanol tolerance.
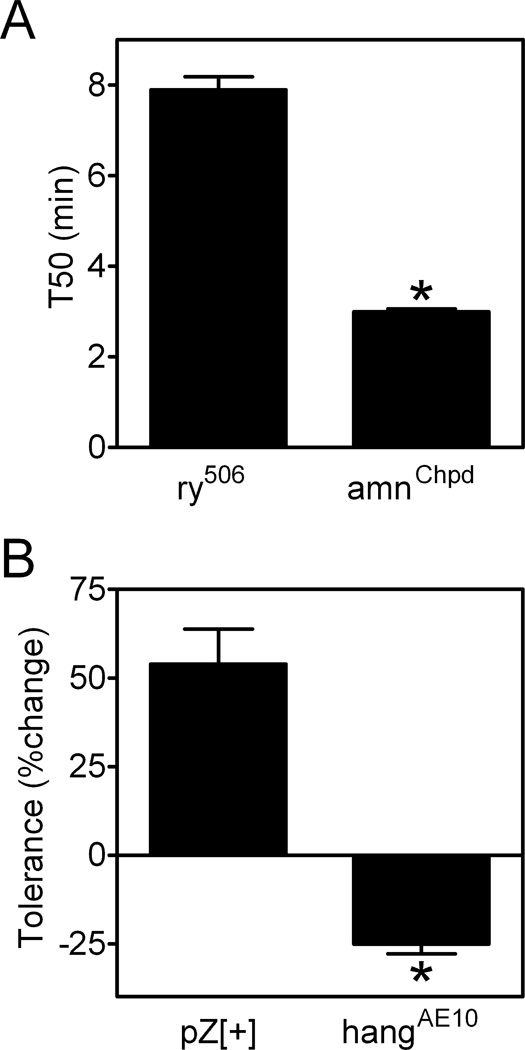
Disruption of the cAMP signaling pathway via mutations in genes such as amnesiac (amn, a neuropeptide gene (Feany and Quinn, 1995)) increases sensitivity to ethanol by ~25% as measured in the inebriometer, an assay based on postural control (Moore et al., 1998). Additionally, mutation of hangover (hang, a gene for a zinc-finger protein (Scholz et al., 2005)) blunts the development of rapid ethanol tolerance by 50–70% as measured in the inebriometer (Scholz et al., 2005). When measured in eRING assays, ethanol sensitivity was increased in amn mutants by ~60% (Figure 4A) and rapid ethanol tolerance was substantially altered in hang mutants (Figure 4B). Interestingly, hang mutant flies not only lacked rapid ethanol tolerance, but were actually more sensitive to ethanol during a second exposure to the drug (Figure 4B). These results indicate that ethanol sensitivity and rapid ethanol tolerance measured in inebriometer and eRING assays are dependent on overlapping genetic pathways. These data also raise the possibility that using eRING assays to evaluate mutants like amn and hang might uncover more severe or even additional ethanol-related phenotypes.
Figure 4. Ethanol sensitivity and rapid ethanol tolerance are altered in amnesiac and hangover mutants, respectively.
(A) T50 values from eRING assays with vapor from a 50% ethanol solution were lower in amnesiacChpd (amnChpd) mutants compared to ry506 control flies (*t-test, p<0.0001). (B) Development of rapid ethanol tolerance in eRING assays was significantly altered in hangoverAE10 (hangAE10) mutants compared to pZ[+] controls (*t-test, p<0.0001). Control pZ[+] flies exhibited significant rapid ethanol tolerance during a second ethanol exposure, while hangoverAE10 mutants were more sensitive to ethanol during a second exposure (individual one sample t tests, p<0.0001). Data in all panels are mean ± S.E.M for n=15 vials (25 flies/vial) from three independent experiments.
Impact of sex and genetic background on ethanol sensitivity and rapid ethanol tolerance
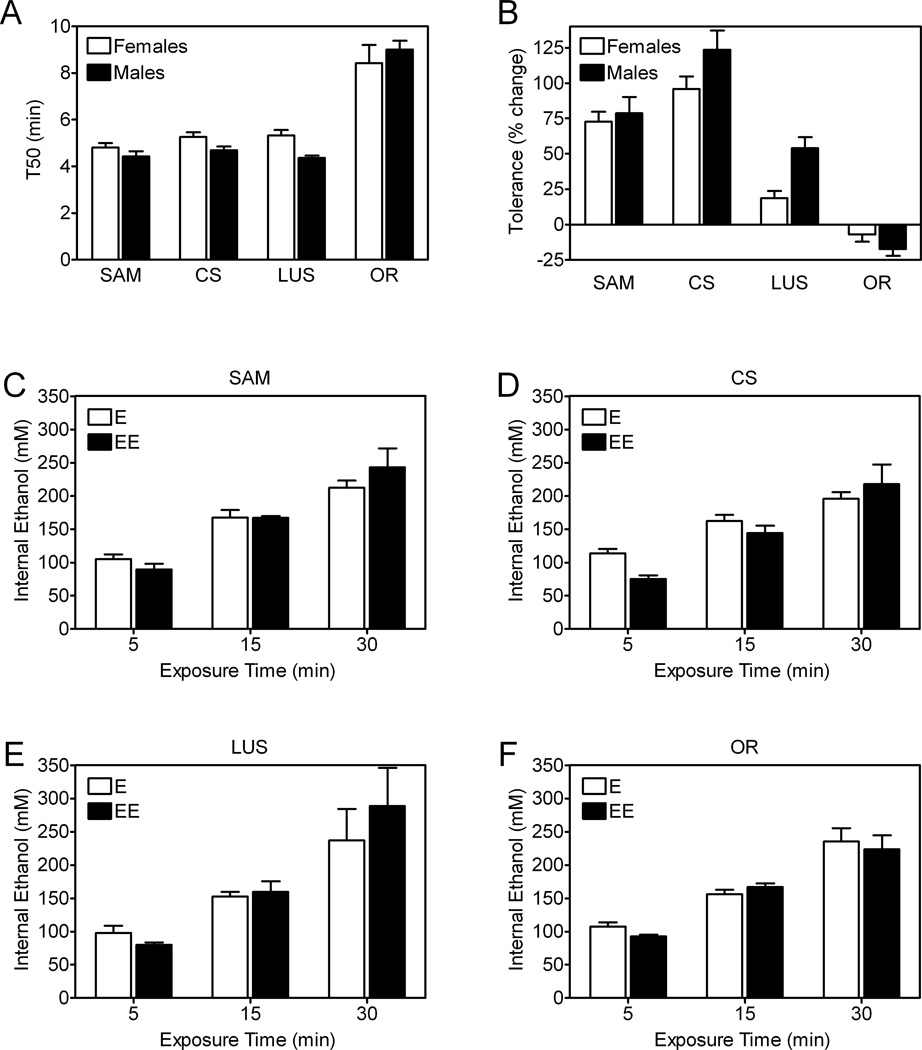
Genetic background impacts complex behaviors in Drosophila (Connolly and Tully, 1998). Additionally, sex influences the genetics of alcohol abuse in humans (Prescott et al., 1999) and ethanol-associated behaviors in mice (Abramov et al., 2006; Giancola and Zeichner, 1995; Meliska et al., 1995), although the effects of sex in mice are not universally observed (Eckhardt and Crane, 2008; Heath et al., 1997; Little et al., 1999). To determine if genetic background and sex influence ethanol-related behavior in eRING assays, we assessed initial ethanol sensitivity and rapid ethanol tolerance in males and females of four control strains: Samarkand (SAM), Canton-S (CS), Lausanne-S (LUS) and Oregon-R (OR). Overall, males and females performed indistinguishably in these experiments (Figure 5A and B), suggesting that sex has little or no discernable effect on ethanol sensitivity and rapid ethanol tolerance in eRING studies. These two measures, however, were sensitive to genetic background. Ethanol sensitivity was indistinguishable in SAM, CS and LUS flies, whereas OR flies were considerably less sensitive (Figure 5A). Rapid ethanol tolerance was greatest in CS flies, followed in decreasing order by SAM, LUS and OR (Figure 5B). Interestingly, OR flies exhibited no measurable rapid ethanol tolerance in eRING assays after exposure to 50% (Figure 5B) or higher concentrations of ethanol (not shown).
Figure 5. Effect of sex and genetic background on ethanol sensitivity and rapid ethanol tolerance in eRING assays.
Males and females of four different genetic backgrounds (Samarkand (SAM), Canton-S (CS), Lausanne (LUS) and Oregon-R (OR)) were assessed in eRING assays for ethanol sensitivity (A), development of rapid ethanol tolerance (B), and internal ethanol concentrations (C-F) during exposure to vapor from a 50% ethanol solution. (A) Genetic background, but not sex, affected T50 values for ethanol sensitivity (two-way ANOVA: genetic background, p<0.0001; sex, n.s.). The T50 values for OR males and females were significantly higher than all other genetic backgrounds tested (Bonferroni’s multiple comparison test, p<0.05). (B) The development of rapid ethanol tolerance was significantly affected by genetic background, but not sex (two-way ANOVA: genetic background, p<0.0001; sex, n.s.). Internal ethanol concentrations in SAM (C), CS (D), LUS (E) and OR (F) males increased with the duration of ethanol exposure time, but were not affected by genetic background and were statistically indistinguishable during a first and second exposure (three-way ANOVA: time, p<0.0001; genetic background, n.s.; first vs. second exposure, n.s.). Data are mean ± S.E.M. in all panels. Data in panels A and B were compiled from three independent experiments for a total of 15 vials of 25 flies per genotype and sex. Data in panels C-F are from 18 vials total (25 flies/vial) for each strain.
To determine if the effects of genetic background on ethanol sensitivity and rapid ethanol tolerance could be explained by differences in internal ethanol concentrations, we measured whole body ethanol concentrations in males and females of the four control strains tested in eRING assays during a first and second exposure to the drug. We estimated the volume of distribution for ethanol in flies by determining their water content. Water content was not affected by genetic background, but was greater in females (0.79–0.84 µl/fly) than in males (0.53–0.57 µl/fly) as expected (Figure S1B). Internal ethanol concentrations increased with drug exposure time in both sexes in all four strains (Figure 5C–F). Overall, genetic background and sex had no effect on internal ethanol concentrations (Figure 5C–F), indicating that the effects of genetic background on performance in eRING assays is likely to be related to genetic differences in the response to ethanol instead of differences in ethanol absorption or metabolism. Additionally, internal ethanol concentrations were indistinguishable during a first and second drug exposure (Figure 5C–F). The decreased sensitivity to ethanol during a second drug exposure in eRING tests, therefore, reflects an adaptive pharmacodynamic change indicative of rapid ethanol tolerance rather than a pharmacokinetic change related to altered ethanol absorption or metabolism.
Ethanol sensitivity and rapid ethanol tolerance are altered in integrin mutants
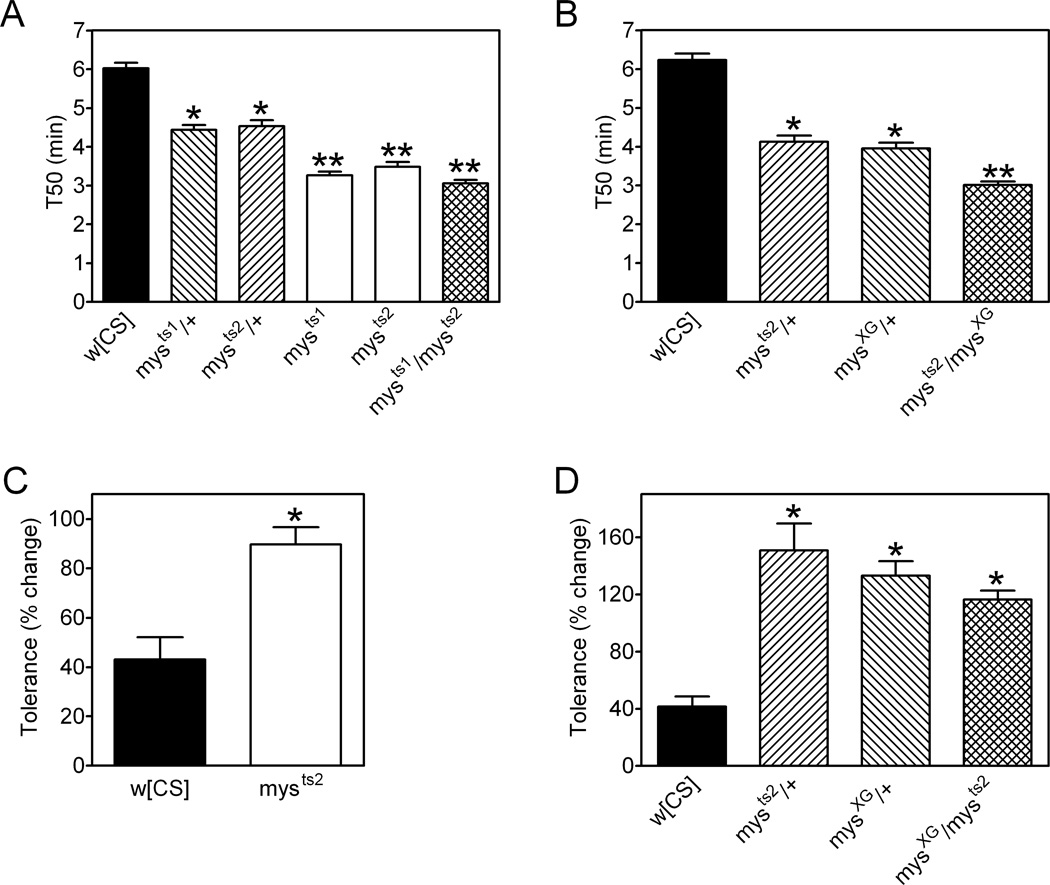
Toward identifying genes involved in ethanol-related behavior, we performed eRING assays on 46 strains with mutations in several genes previously implicated in nervous system function. As part of this candidate screen, we analyzed flies with loss of function alleles of myospheroid (mys), the gene that encodes the integrin βPS (MacKrell et al., 1988). Flies heterozygous for the partial loss of function mutations mysts1 and mysts2 (Bunch et al., 1992) were more sensitive to a first exposure to ethanol in eRING assays compared to control animals (Figure 6A). Flies homozygous for these alleles had a further increase in ethanol sensitivity (Figure 6A). Additionally, flies heterozygous for the null allele mysXG had increased sensitivity to ethanol compared to w[CS] flies (Figure 6B). To determine if the different mys alleles would complement for ethanol sensitivity, we assessed mysts1/mysts2 and mysXG/mysts2 flies in eRING tests. These two transheterozygous genotypes had increased ethanol sensitivity that was more severe than mysts1, mysts2 or mysXG simple heterozygotes (Figure 6A and B). Negative geotaxis in the absence of ethanol was not significantly different in w[CS] controls and mys mutant flies (Figure S2A, provided as supporting information). Thus, the enhanced ethanol-induced impairment of negative geotaxis in mys mutants was not secondary to defects in their baseline locomotor abilities.
Figure 6. Ethanol sensitivity and rapid ethanol tolerance in myospheroid β integrin mutant flies.
(A and B). Ethanol sensitivity in eRING assays with vapor from a 50% ethanol solution. Overall, genotype had a significant effect on T50 values (individual one-way ANOVAs for panels A and B, p<0.0001). (A) Flies heterozygous for mysts1 or mysts2 had T50 values lower than w[CS] controls (*Bonferroni’s multiple comparison test, p<0.05) and flies homozygous or transheterozygous for these two alleles had a further decrease in T50 values compared with simple heterozygous flies (**Bonferroni’s multiple comparison test, p<0.05). (B) T50 values were decreased in flies heterozygous for mysXG or mysts2 compared to w[CS] controls (*Bonferroni’s multiple comparison test, p<0.05) and were further decreased in flies transheterozygous for both alleles (**Bonferroni’s multiple comparison test, p<0.05). (C) The development of rapid ethanol tolerance was significantly greater in mysts2 homozygous females compared to w[CS] controls (*t-test, p<0.0001). (D) Rapid ethanol tolerance was enhanced in flies heterozygous for mysXG or mysts2 as well as in transheterozygotes for these two alleles compared to control w[CS] flies (one-way ANOVA, p<0.0001, *Bonferroni’s multiple comparison test, p<0.05). Data were compiled from three independent experiments for a total of 10–20 vials of 25 flies per group. All data are mean ± S.E.M.
We also examined the influence of the mys locus on rapid ethanol tolerance in eRING assays. Homozygous and heterozygous mysts2 flies exhibited enhanced development of rapid ethanol tolerance (Figure 6C and 6D) as did flies heterozygous for mysXG (Figure 6D). Flies with mysts2 in trans to mysXG also had an enhanced development of rapid ethanol tolerance compared to control flies, although the phenotype of these transheterozygous mys mutants was not significantly different from mysts2 or mysXG simple heterozygotes (Figure 6D). These data implicate the mys locus in the development of rapid ethanol tolerance in Drosophila.
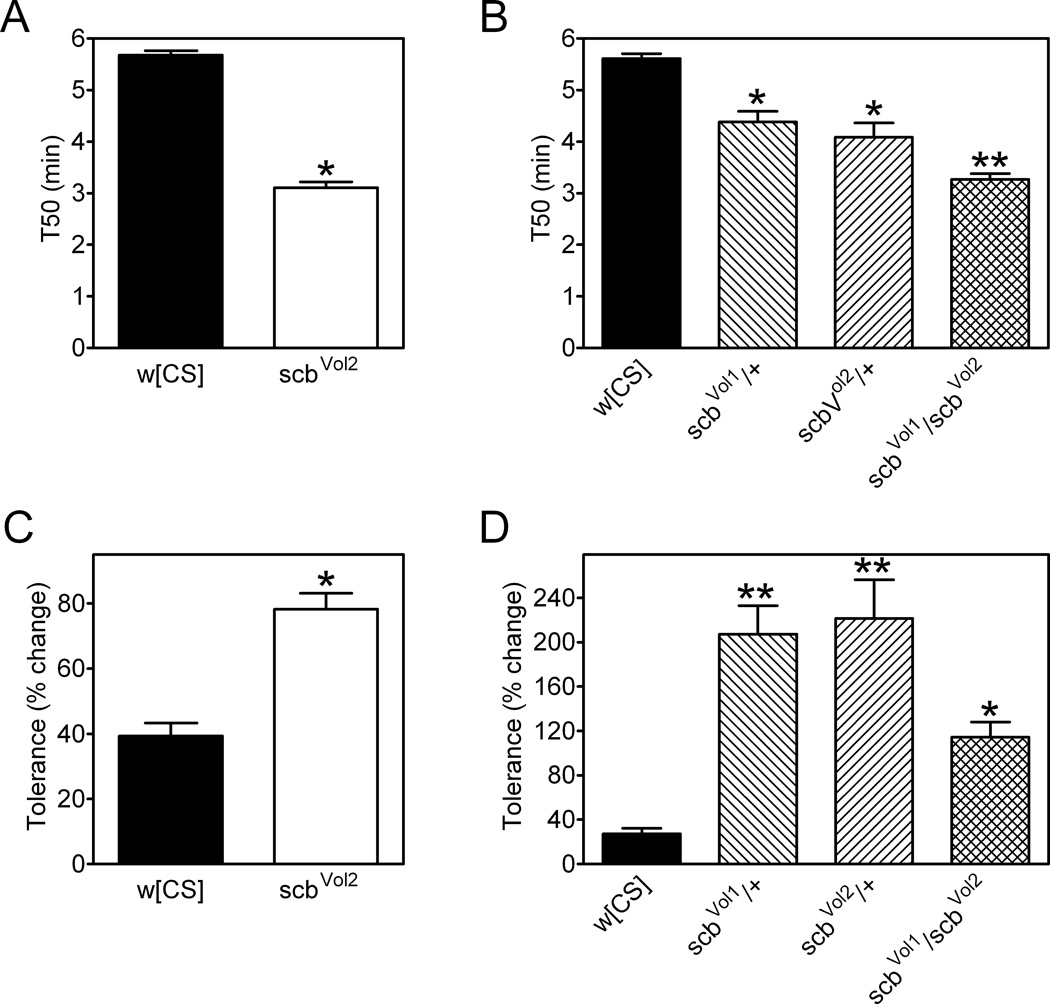
Integrins function as heterodimers of α and β subunits (Hynes, 1992). We therefore further explored the role of integrins in ethanol-related behaviors by assessing ethanol sensitivity and rapid ethanol tolerance in flies with mutations in scab (scb, a.k.a. Volado), the gene that encodes the α integrin αPS3 (Grotewiel et al., 1998; Stark et al., 1997). Flies homozygous for the partial loss of function allele scbVol2 (Grotewiel et al., 1998) had enhanced ethanol sensitivity compared to w[CS] controls (Figure 7A). Additionally, flies heterozygous for scbVol2 or scbVol1 (another partial loss of function allele (Grotewiel et al., 1998)) exhibited increased ethanol sensitivity (Figure 7B). Flies harboring these two scb alleles in trans had a further increase in ethanol sensitivity (Figure 7B). Compared to w[CS] controls, none of the scb mutant genotypes had significant changes in baseline negative geotaxis (Figure S2B), indicating that the increased sensitivity to ethanol in scb mutant flies is not related to altered locomotor behavior per se.
Figure 7. Ethanol sensitivity and rapid ethanol tolerance in scab α integrin mutants.
eRING assays were performed with vapor from a 50% ethanol solution. (A and B) T50 was decreased in scb mutants (A, *t test, p<0.0001; B, one-way ANOVA, p<0.001). (B) scbvol1 and scbvol2 heterozygotes had lower T50 values than w[CS] (*Bonferroni’s multiple comparison test, p<0.05) while flies transheterozygous for these two alleles exhibited a further reduction in T50 (**Bonferroni’s multiple comparison test, p< 0.05). (C and D) The development of rapid ethanol tolerance is altered by mutations in scb (C, *t test, p<0.0001; D, one-way ANOVA, p<0.0001). (D) Rapid ethanol tolerance is elevated in scbVol1 and scbVol2 heterozygotes (**Bonferroni’s multiple comparison test, p<0.05), but less so in scbvol1/scbvol2 transheterozygotes (*Bonferroni’s multiple comparison test, p<0.05). Data in (A) and (B) were compiled from three independent experiments for a total of 10–30 vials of 25 flies per group. All data are mean ± S.E.M.
The development of rapid ethanol tolerance was also enhanced in scbVol2 homozygotes compared to w[CS] control flies (Figure 7C). Flies heterozygous for scbVol2 or scbVol1 had an enhanced development of rapid ethanol tolerance as did flies with these two alleles in trans (Figure 7D). While rapid ethanol tolerance in scbVol1/scbVol2 transheterozygotes was enhanced relative to w[CS] controls, the phenotype in these flies was not as great as in scbVol1 and scbVol2 simple heterozygotes (Figure 7D). These studies indicate that the integrin αPS3, encoded by the scb locus, influences ethanol sensitivity and the development of rapid ethanol tolerance in flies.
Toward addressing the possibility that βPS and αPS3 influence ethanol-related behavior by functioning within the same pathway, we evaluated mys mutants, scb mutants, and mys;scb double mutants in eRING assays. If βPS and αPS3 are in the same pathway, the combination of heterozygous mutations in mys and scb should have non-additive effects defined as a synergistic interaction (indicated by a phenotype double mutants that is more severe than the sum of the phenotypes from either mutation alone) or an alleviating interaction (indicated by the effect of one mutation masking the effect of the other mutation). Conversely, if the two integrins function within distinct pathways, the effect of mutations in mys and scb on the phenotype should be additive (Mani et al., 2008).
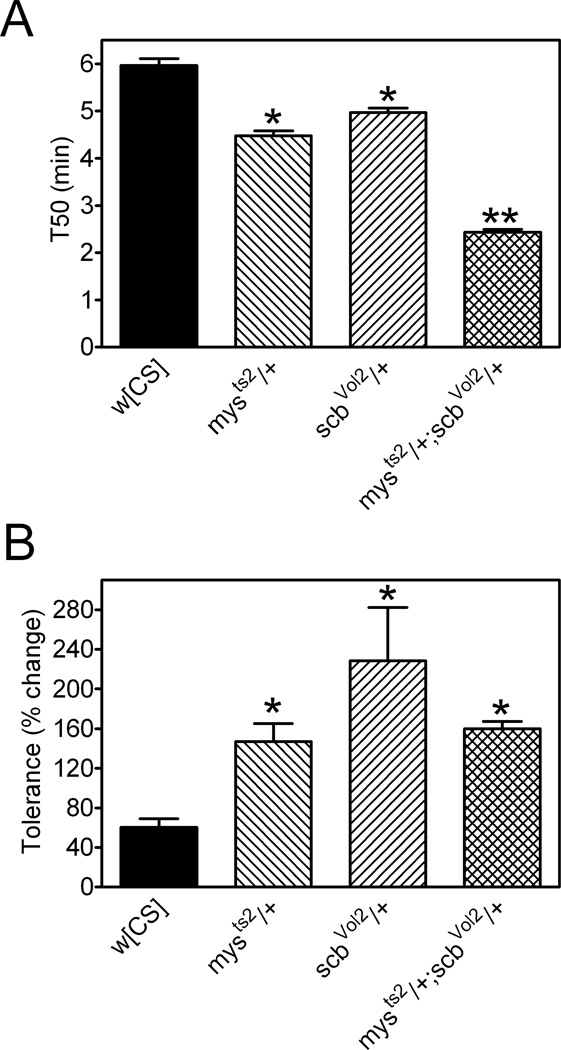
Consistent with our studies described above (Figures 6 and 7), mysts2 and scbVol2 heterozygotes exhibited increased ethanol sensitivity (Figure 8A). Double heterozygous mys;scb mutants had an increase in ethanol sensitivity that was greater than the sum of the phenotypes in flies with mutations in mys or scb alone (Figure 8A). The increased ethanol sensitivity in mys, scb and mys;scb mutants is not related to altered baseline negative geotaxis (Figure S2C). The synergistic genetic interaction between mys and scb mutations is consistent with a model in which βPS and αPS3 function within the same pathway to influence ethanol sensitivity.
Figure 8. Ethanol sensitivity and rapid ethanol tolerance in myospheroid/scab double mutants.
Female flies were assessed for ethanol sensitivity (A), rapid ethanol tolerance (B), and internal ethanol concentrations (C). (A) T50 values were lower in flies heterozygous for mysts2 or scbvol2 (one-way ANOVA, p<0.001, *Bonferroni’s multiple comparison test, p< 0.05) and further reduced in mysts2;scbvol2 double heterozygous flies compared to control (**p<0.05). (B) The development of rapid ethanol tolerance was enhanced relative to w[CS] flies in mysts2 and scbvol2 heterozygotes and in mysts2/+; scbvol2/+ double heterozygotes (non-Gaussian data, Kruskal-Wallis one-way ANOVA, p<0.0001, *Dunn’s multiple comparison test, p<0.05). Data (mean ± SEM) were compiled for n=10 vials (25 flies/vial) from three independent experiments. All data are mean ± S.E.M.
The development of rapid ethanol tolerance was enhanced in mys and scb heterozygotes (Figure 8B) as described above (Figures 6 and 7). Rapid ethanol tolerance was also enhanced in double heterozygous mys;scb mutants compared to w[CS], but this phenotype was not significantly different from that of mys or scb simple heterozygotes (Figure 8B). The alleviating genetic interaction between mys and scb mutants suggests that the two genes are in the same genetic pathway leading to rapid ethanol tolerance.
To determine whether the changes in ethanol sensitivity or rapid tolerance in integrin mutants could be due to altered ethanol absorption or metabolism, we measured internal ethanol concentrations. As a prelude to these measurements we estimated the volume of distribution for ethanol in flies by determining their water content. The water content in all integrin mutant genotypes except mysts2/+ was decreased compared to w[CS] controls (Figure S1B). We therefore used genotype-specific water content values to calculate internal ethanol concentrations.
Although genotype had a statistically significant overall effect on internal ethanol concentration during a first drug challenge in a study with mys mutants (Figure S3A, provided as supplementary information), internal ethanol concentrations in all flies at all time-points analyzed were statistically indistinguishable from w[CS] controls except for mysts2 mutants after 30 minutes of exposure (Figure S3A). Furthermore, internal ethanol concentrations during a first exposure were not altered in scb mutants (Figure S3B) or in a subsequent study with both mys and scb mutants (Figure S3C). The enhanced sensitivity to ethanol in integrin mutants is therefore not associated with a consistent change in internal ethanol concentration.
A similar pattern emerged during a second challenge with ethanol. Internal ethanol concentrations during a second drug exposure were affected by genotype in mys mutants, but only mysts2 and mysXG/+ flies after 30 minutes were significantly different from w[CS] controls (Figure S3A). Internal ethanol concentrations were also not affected in scb mutants (Figure S3B) or in a second study with mys and scb alleles (Figure S3C). Furthermore, internal ethanol concentrations were statistically indistinguishable during the first and second challenges with the drug (Figure S3). The enhanced rapid ethanol tolerance in integrin mutants is not associated with a consistent change in ethanol uptake or metabolism.
All of the integrin mutants we analyzed had increased sensitivity to ethanol during a first drug exposure, but subsequently manifested an enhanced development of rapid ethanol tolerance during a second drug exposure. To determine if the enhanced development of rapid ethanol tolerance could compensate for the increased ethanol sensitivity in naïve integrin mutants, we compared the absolute ethanol sensitivity in w[CS] controls and integrin mutants during the second drug challenge. Relative to w[CS] flies, the absolute sensitivity to ethanol during the second drug exposure was similar in three mys genotypes and marginally higher in one mys genotype (Figure S4A, supplied as supporting information), similar in two scb mutants and decreased in two other scb mutants (Figure S4B), and similar in mys;scb double heterozygous mutants (Figure S4C). In seven out of nine integrin mutant genotypes, therefore, the enhanced development of rapid ethanol tolerance compensated for the increased ethanol sensitivity observed in naïve flies.
Discussion
A number of behavioral paradigms have been used to study the effects of ethanol in flies. Exposure to ethanol causes flies to lose postural control, a sedative effect than can be assessed in an inebriometer (a series of baffles enclosed within a vertical cylinder) (Weber, 1988) or in simpler containers oriented vertically or horizontally (Urizar et al., 2007; Wen et al., 2005). Additionally, both the stimulating and sedating properties of ethanol can be assessed in assays that measure spontaneous locomotor behavior (Parr et al., 2001; Wolf et al., 2002). Furthermore, recovery from the sedating effects of ethanol can be assessed in assays that measure postural control (Cowmeadow et al., 2006) or negative geotaxis (startle-induced climbing) (Berger et al., 2004; Ramazani et al., 2007). These behavioral paradigms can also be used to assess various forms of tolerance to ethanol in Drosophila (Berger et al., 2004; Cowmeadow et al., 2005; Cowmeadow et al., 2006; Ghezzi et al., 2004; Godenschwege et al., 2004; Scholz et al., 2005; Scholz et al., 2000).
We became interested in developing a negative geotaxis assay for assessing the onset of ethanol’s sedative effects on fly locomotor behavior. An important consideration was that an assay based on negative geotaxis would allow us to directly assess baseline behavioral performance in the absence of ethanol in mutants and controls. Additional considerations were that negative geotaxis is a robust, reproducible locomotor behavior and that it is relatively simple to assess in RING assays using methods we previously developed (Gargano et al., 2005). We therefore evaluated negative geotaxis in RING assays with flies exposed to ethanol (eRING).
In naïve flies, negative geotaxis measured in eRING assays becomes progressively impaired as the dose of ethanol or the time of exposure to ethanol (and consequently the internal ethanol concentration) increases. Additionally, flies are less sensitive to the sedating effects of ethanol during a second exposure than during a first exposure to the drug in eRING assays. The sensitivity to an initial exposure to ethanol and relative resistance to a second exposure are influenced by genetic background and single gene mutations (amn and hang) known to disrupt these processes in inebriometer studies (Moore et al., 1998; Scholz et al., 2005).
The effects of genetic background on sensitivity to a first exposure to ethanol in eRING assays are not related to differences in the internal ethanol concentrations in flies. This indicates that the differences in initial ethanol sensitivity in the strains tested are due to genetic background effects on the pharmacodynamic responses to ethanol as opposed to altered uptake or metabolism of the drug. Additionally, since the ethanol concentrations are indistinguishable during a first and second exposure to the drug in the four genetic background strains tested, the decreased sensitivity of flies to the sedative effects of ethanol during the second exposure reflects functional or pharmacodynamic tolerance (Fadda and Rossetti, 1998; Tabakoff et al., 1986). We consider the decreased sensitivity to be rapid ethanol tolerance based on the schedule of drug delivery (Berger et al., 2004). Together, these data indicate that the eRING assay is suitable for assessing genes that influence initial sensitivity and rapid ethanol tolerance in flies.
As noted above, other laboratories have used recovery of negative geotaxis behavior after ethanol sedation to assess sensitivity and tolerance (Berger et al., 2004; Ramazani et al., 2007). The eRING data described here extend beyond these published studies in several ways. The previous reports used negative geotaxis to evaluate recovery from sedation, whereas we used negative geotaxis to assess the onset of ethanol intoxication and the time-dependent behavioral effects of ethanol sedation. eRING assays, therefore, can be used to assess resistance and sensitivity to the acute intoxicating effects of ethanol during an initial exposure to the drug. This is important because resistance to the acute intoxicating effects of ethanol is associated with a higher risk for eventually developing alcohol dependence in humans (Schuckit and Smith, 1996). Additionally, since hang mutants are actually more sensitive to a second exposure to ethanol in eRING assays in contrast to retaining some rapid ethanol tolerance in inebriometer studies (Scholz et al., 2005), it is possible that subtly altered or more severe phenotypes associated with other genes implicated in ethanol-related behavior could be revealed by evaluating their performance in eRING studies.
In a screen of candidate mutants using eRING, we found that integrins influence ethanol-related behavior. Integrins are cell adhesion molecules that function as heterodimers of α and β subunits (Hynes, 1992). Our studies with eRING show that flies with partial loss of function mutations in mys and scb, Drosophila genes that encode the integrins βPS and αPS3, respectively, exhibit increased sensitivity to ethanol. Mutations in both genes fail to complement in genetic tests for increased ethanol sensitivity and flies with two mutant alleles have stronger phenotypes than flies with a single mutant allele. Furthermore, there is a synergistic genetic interaction between partial loss of function alleles in mys and scb for ethanol sensitivity. Together, these data strongly suggest that βPS and αPS3 influence sensitivity to the sedating effects of ethanol. These data are also consistent with a model in which these two integrin subunits function within the same pathway in this context.
Flies with mutations in mys and scb also exhibit an enhanced development of rapid ethanol tolerance. Mutations in these genes do not complement for rapid ethanol tolerance and mys;scb double mutants also exhibit an enhanced rapid ethanol tolerance phenotype. Interestingly, mutations in mys and scb exhibit an alleviating genetic interaction for rapid ethanol tolerance such that the presence of a mutation in one gene masks the phenotype of a mutation in the other gene. These genetic analyses implicate βPS and αPS3 in the development of rapid ethanol tolerance in flies and suggest that the two proteins function in the same pathway. Importantly, mutations in mys and scb do not consistently alter internal ethanol concentrations in flies tested in eRING assays, indicating that the changes in ethanol sensitivity and rapid ethanol tolerance in these animals reflects the role of integrins in the pharmacodynamic responses to ethanol.
While our data implicate mys and scb in both ethanol sensitivity and rapid ethanol tolerance, the results of genetic tests for these two traits were somewhat different. The mys and scb alleles we tested are semi-dominant for increased ethanol sensitivity whereas they are dominant for enhanced development of rapid ethanol tolerance. Additionally, mys and scb mutations exhibit a synergistic genetic interaction for increased ethanol sensitivity, whereas they have an alleviating or masking genetic interaction for the development of rapid ethanol tolerance. Our data support the idea that these two ethanol-related traits are governed by at least partially distinct mechanisms (Berger et al., 2008)
In principle, animals exposed to ethanol could appear sensitive to the drug in behavioral studies simply because they are uncoordinated or otherwise have defects in the behavior that is being measured. We exploited the ability of eRING assays to explicitly assess baseline negative geotaxis in the absence of ethanol to determine whether the enhanced ethanol sensitivity in integrin mutants might be secondary to defects in locomotor abilities. Mutations in mys and scb do not alter negative geotaxis in the absence of ethanol. Thus, the enhanced ethanol sensitivity of integrin mutants is not related to a mutation-induced impairment in the ability to perform negative geotaxis, the behavior that lies at the core of our studies. eRING and other assays that measure baseline behavior should be well suited for addressing whether ethanol sensitivity in other mutants is related to defects that impair behavior in the absence of the drug.
Integrin mutants exhibit an increased initial sensitivity to ethanol and an enhanced development of rapid ethanol tolerance. Interestingly, integrin mutants have essentially normal sensitivity to ethanol during a second exposure to the drug. This indicates that the enhanced development of rapid ethanol tolerance in integrin mutants can largely compensate for their heightened initial sensitivity to ethanol. These data also raise the possibility that a wide range of behavioral responses to ethanol might be enhanced in integrin mutants. Additional studies will be required to investigate whether integrins play a role in other behavioral responses to ethanol such as recovery from sedation and chronic tolerance.
Integrins play important roles in developmental (Brown, 1993), learning and memory (Chan et al., 2003; Grotewiel et al., 1998), olfactory behavior (Bhandari et al., 2006), and aging (Goddeeris et al., 2003). The studies reported here indicate that integrins are also important for ethanol-related behaviors in flies. Given the conservation in the genetics of ethanol-related behavior in invertebrates and vertebrates (Cowmeadow et al., 2005; Davies et al., 2004; Davies et al., 2003; Kapfhamer et al., 2008; Wen et al., 2005), it would be interesting to investigate whether ethanol sensitivity and tolerance are influenced by altered expression of integrins in mice.
Intriguingly, a number of Drosophila mutants with altered learning and memory also have altered responses to ethanol (Berger et al., 2008; Cheng et al., 2001; LaFerriere et al., 2008; Moore et al., 1998). Although the overlap between the genetics of learning and the genetics of ethanol-related behavior is not absolute, our data on ethanol sensitivity and rapid ethanol tolerance in scb mutants do strengthen this connection since this gene is also involved in associative memory (Grotewiel et al., 1998). Additional studies in genetically-altered mice will be important for determining whether common genetic pathways serve both ethanol-related behavior and learning in vertebrates.
Supplementary Material
Acknowledgements
The authors appreciate the expert technical assistance of Devin Rhodenizer. The authors thank Ron Davis, Nick Brown, Danny Brower, Ulrike Heberlein, Henrike Scholz and the Drosophila Stock Center for providing fly stocks.
References
- Abramov U, Raud S, Innos J, Koks S, Matsui T, Vasar E. Gender specific effects of ethanol in mice, lacking CCK2 receptors. Behav Brain Res. 2006;175(1):149–156. doi: 10.1016/j.bbr.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Berger KH, Heberlein U, Moore MS. Rapid and chronic: two distinct forms of ethanol tolerance in Drosophila. Alcohol Clin Exp Res. 2004;28(10):1469–1480. doi: 10.1097/01.alc.0000141817.15993.98. [DOI] [PubMed] [Google Scholar]
- Berger KH, Kong EC, Dubnau J, Tully T, Moore MS, Heberlein U. Ethanol sensitivity and tolerance in long-term memory mutants of Drosophila melanogaster. Alcohol Clin Exp Res. 2008;32(5):895–908. doi: 10.1111/j.1530-0277.2008.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari P, Gargano JW, Goddeeris MM, Grotewiel MS. Behavioral responses to odorants in Drosophila require nervous system expression of the beta integrin gene myospheroid. Chem Senses. 2006;31(7):627–639. doi: 10.1093/chemse/bjl002. [DOI] [PubMed] [Google Scholar]
- Brown NH. Integrins hold Drosophila together. Bioessays. 1993;15(6):383–390. doi: 10.1002/bies.950150604. [DOI] [PubMed] [Google Scholar]
- Bunch TA, Salatino R, Engelsgjerd MC, Mukai L, West RF, Brower DL. Characterization of mutant alleles of myospheroid, the gene encoding the beta subunit of the Drosophila PS integrins. Genetics. 1992;132(2):519–528. doi: 10.1093/genetics/132.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CS, Weeber EJ, Kurup S, Sweatt JD, Davis RL. Integrin requirement for hippocampal synaptic plasticity and spatial memory. J Neurosci. 2003;23(18):7107–7116. doi: 10.1523/JNEUROSCI.23-18-07107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Endo K, Wu K, Rodan AR, Heberlein U, Davis RL. Drosophila fasciclinII is required for the formation of odor memories and for normal sensitivity to alcohol. Cell. 2001;105(6):757–768. doi: 10.1016/s0092-8674(01)00386-5. [DOI] [PubMed] [Google Scholar]
- Connolly JB, Tully T. Behaviour, learning, and memory. In: Roberts DB, editor. Drosophila, A Practical Approach. 2 ed. Oxford: IRL Press at Oxford University Press; 1998. pp. 265–317. [Google Scholar]
- Cowmeadow RB, Krishnan HR, Atkinson NS. The slowpoke gene is necessary for rapid ethanol tolerance in Drosophila. Alcohol Clin Exp Res. 2005;29(10):1777–1786. doi: 10.1097/01.alc.0000183232.56788.62. [DOI] [PubMed] [Google Scholar]
- Cowmeadow RB, Krishnan HR, Ghezzi A, Al'Hasan YM, Wang YZ, Atkinson NS. Ethanol tolerance caused by slowpoke induction in Drosophila. Alcohol Clin Exp Res. 2006;30(5):745–753. doi: 10.1111/j.1530-0277.2006.00087.x. [DOI] [PubMed] [Google Scholar]
- Davies AG, Bettinger JC, Thiele TR, Judy ME, McIntire SL. Natural variation in the npr-1 gene modifies ethanol responses of wild strains of C. elegans. Neuron. 2004;42(5):731–743. doi: 10.1016/j.neuron.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Davies AG, Pierce-Shimomura JT, Kim H, VanHoven MK, Thiele TR, Bonci A, Bargmann CI, McIntire SL. A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans. Cell. 2003;115(6):655–666. doi: 10.1016/s0092-8674(03)00979-6. [DOI] [PubMed] [Google Scholar]
- DHHS. 10th Special Report to the U.S. Congress on Alcohol and Health: Highlights from Current Research. 2000 [Google Scholar]
- Eckhardt CI, Crane C. Effects of alcohol intoxication and aggressivity on aggressive verbalizations during anger arousal. Aggress Behav. 2008;34(4):428–436. doi: 10.1002/ab.20249. [DOI] [PubMed] [Google Scholar]
- Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog Neurobiol. 1998;56(4):385–431. doi: 10.1016/s0301-0082(98)00032-x. [DOI] [PubMed] [Google Scholar]
- Feany MB, Quinn WG. A neuropeptide gene defined by the Drosophila memory mutant amnesiac. Science. 1995;268(5212):869–873. doi: 10.1126/science.7754370. [DOI] [PubMed] [Google Scholar]
- Gargano JW, Martin I, Bhandari P, Grotewiel MS. Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila. Exp Gerontol. 2005;40(5):386–395. doi: 10.1016/j.exger.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Ghezzi A, Al-Hasan YM, Larios LE, Bohm RA, Atkinson NS. slo K(+) channel gene regulation mediates rapid drug tolerance. Proc Natl Acad Sci U S A. 2004;101(49):17276–17281. doi: 10.1073/pnas.0405584101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancola PR, Zeichner A. An investigation of gender differences in alcohol-related aggression. J Stud Alcohol. 1995;56(5):573–579. doi: 10.15288/jsa.1995.56.573. [DOI] [PubMed] [Google Scholar]
- Goddeeris MM, Cook-Wiens E, Horton WJ, Wolf H, Stoltzfus JR, Borrusch M, Grotewiel MS. Delayed behavioural aging and altered mortality in Drosophila beta integrin mutants. Aging Cell. 2003;2(5):257–264. doi: 10.1046/j.1474-9728.2003.00060.x. [DOI] [PubMed] [Google Scholar]
- Godenschwege TA, Reisch D, Diegelmann S, Eberle K, Funk N, Heisenberg M, Hoppe V, Hoppe J, Klagges BR, Martin JR, Nikitina EA, Putz G, Reifegerste R, Reisch N, Rister J, Schaupp M, Scholz H, Schwarzel M, Werner U, Zars TD, Buchner S, Buchner E. Flies lacking all synapsins are unexpectedly healthy but are impaired in complex behaviour. Eur J Neurosci. 2004;20(3):611–622. doi: 10.1111/j.1460-9568.2004.03527.x. [DOI] [PubMed] [Google Scholar]
- Grotewiel MS, Beck CD, Wu KH, Zhu XR, Davis RL. Integrin-mediated short-term memory in Drosophila. Nature. 1998;391(6666):455–460. doi: 10.1038/35079. [DOI] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychol Med. 1997;27(6):1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Kapfhamer D, Bettinger JC, Davies AG, Eastman CL, Smail EA, Heberlein U, McIntire SL. Loss of RAB-3/A in Caenorhabditis elegans and the mouse affects behavioral response to ethanol. Genes Brain Behav. 2008;7(6):669–676. doi: 10.1111/j.1601-183X.2008.00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFerriere H, Guarnieri DJ, Sitaraman D, Diegelmann S, Heberlein U, Zars T. Genetic dissociation of ethanol sensitivity and memory formation in Drosophila melanogaster. Genetics. 2008;178(4):1895–1902. doi: 10.1534/genetics.107.084582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little HJ, O'Callaghan MJ, Butterworth AR, Wilson J, Cole J, Watson WP. Low alcohol preference among the "high alcohol preference" C57 strain of mice; preference increased by saline injections. Psychopharmacology (Berl) 1999;147(2):182–189. doi: 10.1007/s002130051159. [DOI] [PubMed] [Google Scholar]
- MacKrell AJ, Blumberg B, Haynes SR, Fessler JH. The lethal myospheroid gene of Drosophila encodes a membrane protein homologous to vertebrate integrin beta subunits. Proc Natl Acad Sci U S A. 1988;85(8):2633–2637. doi: 10.1073/pnas.85.8.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani R, St Onge RP, Hartman JLt, Giaever G, Roth FP. Defining genetic interaction. Proc Natl Acad Sci U S A. 2008;105(9):3461–3466. doi: 10.1073/pnas.0712255105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meliska CJ, Bartke A, Vandergriff JL, Jensen RA. Ethanol and nicotine consumption and preference in transgenic mice overexpressing the bovine growth hormone gene. Pharmacol Biochem Behav. 1995;50(4):563–570. doi: 10.1016/0091-3057(94)00345-9. [DOI] [PubMed] [Google Scholar]
- Moore MS, DeZazzo J, Luk AY, Tully T, Singh CM, Heberlein U. Ethanol intoxication in Drosophila: Genetic and pharmacological evidence for regulation by the cAMP signaling pathway. Cell. 1998;93(6):997–1007. doi: 10.1016/s0092-8674(00)81205-2. [DOI] [PubMed] [Google Scholar]
- Parr J, Large A, Wang X, Fowler SC, Ratzlaff KL, Ruden DM. The inebri-actometer: a device for measuring the locomotor activity of Drosophila exposed to ethanol vapor. J Neurosci Methods. 2001;107(1–2):93–99. doi: 10.1016/s0165-0270(01)00357-0. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Aggen SH, Kendler KS. Sex differences in the sources of genetic liability to alcohol abuse and dependence in a population-based sample of U.S. twins. Alcohol Clin Exp Res. 1999;23(7):1136–1144. doi: 10.1111/j.1530-0277.1999.tb04270.x. [DOI] [PubMed] [Google Scholar]
- Ramazani RB, Krishnan HR, Bergeson SE, Atkinson NS. Computer automated movement detection for the analysis of behavior. J Neurosci Methods. 2007;162(1–2):171–179. doi: 10.1016/j.jneumeth.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodenizer D, Martin I, Bhandari P, Pletcher SD, Grotewiel M. Genetic and environmental factors impact age-related impairment of negative geotaxis in Drosophila by altering age-dependent climbing speed. Exp Gerontol. 2008;43(8):739–748. doi: 10.1016/j.exger.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz H, Franz M, Heberlein U. The hangover gene defines a stress pathway required for ethanol tolerance development. Nature. 2005;436(7052):845–847. doi: 10.1038/nature03864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz H, Ramond J, Singh CM, Heberlein U. Functional ethanol tolerance in Drosophila. Neuron. 2000;28(1):261–271. doi: 10.1016/s0896-6273(00)00101-x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry. 1996;53(3):202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Stark KA, Yee GH, Roote CE, Williams EL, Zusman S, Hynes RO. A novel alpha integrin subunit associates with betaPS and functions in tissue morphogenesis and movement during Drosophila development. Development. 1997;124(22):4583–4594. doi: 10.1242/dev.124.22.4583. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Cornell N, Hoffman PL. Alcohol tolerance. Ann Emerg Med. 1986;15(9):1005–1012. doi: 10.1016/s0196-0644(86)80119-6. [DOI] [PubMed] [Google Scholar]
- Urizar NL, Yang Z, Edenberg HJ, Davis RL. Drosophila homer is required in a small set of neurons including the ellipsoid body for normal ethanol sensitivity and tolerance. J Neurosci. 2007;27(17):4541–4551. doi: 10.1523/JNEUROSCI.0305-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber KE. An apparatus for measurement of resistance to gas-phase reagents. Dros Info Serv. 1988;67:91–93. [Google Scholar]
- Wen T, Parrish CA, Xu D, Wu Q, Shen P. Drosophila neuropeptide F and its receptor, NPFR1, define a signaling pathway that acutely modulates alcohol sensitivity. Proc Natl Acad Sci U S A. 2005;102(6):2141–2146. doi: 10.1073/pnas.0406814102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf FW, Rodan AR, Tsai LT, Heberlein U. High-resolution analysis of ethanol-induced locomotor stimulation in Drosophila. J Neurosci. 2002;22(24):11035–11044. doi: 10.1523/JNEUROSCI.22-24-11035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.