Abstract
A number of herbal preparations have been shown to interact with prescription medications secondary to modulation of cytochrome P450 (CYP) and/or P-glycoprotein (P-gp). The purpose of this study was to determine the influence of Panax ginseng on CYP3A and P-gp function using the probe substrates midazolam and fexofenadine, respectively. Twelve healthy subjects (8 males) completed this open label, single sequence pharmacokinetic study. Healthy volunteers received single oral doses of midazolam 8 mg and fexofenadine 120 mg, before and after 28 days of P. ginseng 500 mg twice daily. Midazolam and fexofenadine pharmacokinetic parameter values were calculated and compared pre-and post P. ginseng administration. Geometric mean ratios (post-ginseng/pre-ginseng) for midazolam area under the concentration vs. time curve from zero to infinity (AUC0-∞), half life (T1/2), and maximum concentration (Cmax) were significantly reduced at 0.66 (0.55 – 0.78), 0.71 (0.53 – 0.90), and 0.74 (0.56 – 0.93), respectively. Conversely, fexofenadine pharmacokinetics were unaltered by P. ginseng administration. Based on these results, Panax ginseng appeared to induce CYP3A activity in the liver and possibly the gastrointestinal tract. Patients taking Panax ginseng in combination with CYP3A substrates with narrow therapeutic ranges should be monitored closely for adequate therapeutic response to the substrate medication.
Keywords: HIV, Panax ginseng, cytochrome P450, drug interaction, midazolam, herb
Introduction
Complimentary and alternative medicine (CAM) use has dramatically increased over the last 20 years. In a survey conducted by Eisenberg et al., approximately 34% of adults in the USA reported using at least one form of CAM.1 Typical uses for CAM include disease treatment and prevention and management of medication related side effects.1–2 Due to the widespread use of CAM in combination with proprietary medications, there is a strong possibility of drug-drug interactions between these groups of compounds. As such, there is continuing need to characterize the influence of herbal preparations on common metabolic and transport pathways.1–2
Over the past decade, a variety of pharmacokinetic interactions between herbal supplements and prescription medications have been described.3–7 Many of these interactions occurred secondary to CAM–related modulation of CYP3A4/5, which is responsible for metabolizing over half of all drugs that undergo Phase 1 metabolism.8,9 Indeed, St. John’s wort, has been shown to significantly decrease the exposure of a number of CYP3A substrates including indinavir, cyclosporine, alprazolam, nifedipine, and oral contraceptives.5 In addition, herbal supplements may also alter the disposition of coadministered medications secondary to modulation of efflux proteins such as the MDR–1 gene product, P–glycoprotein (P–gp).6
Among herbs that may interact with medications via CYP3A modulation is Panax ginseng (also referred to as ginseng or Chinese ginseng). P. ginseng is purported to improve vitality, immune function, cognitive function, and enhance overall well–being.10–12 A number of in vitro and in vivo studies have been conducted to assess the influence of P. ginseng on a variety of CYP isoforms.7,13–19 However, results from these investigations failed to yield consistent results. Therefore this study was conducted to characterize the influence of P. ginseng on CYP3A and P–gp activity, using the probe substrates, midazolam and fexofenadine, respectively, in healthy human volunteers.
Subjects and methods
Subjects
Healthy volunteers between the ages of 18 and 50 years were eligible to participate in this study. Evaluation of potential subjects included a medical history, physical examination and laboratory analyses to rule out any medical conditions that could increase subject risk or affect study results. Participants were also required to have a negative HIV ELISA test. Subjects were not allowed to have taken any medications (including non–prescription drugs, herbal supplements and oral contraceptives) within 30 days of study participation. Additional exclusion criteria included tobacco use within 6 weeks, active drug or alcohol abuse, history of intolerance to any of the study medications, and persistent diarrhea. Volunteers were instructed to abstain from grapefruit or grapefruit juice during the entire study period; subjects were prohibited from ingesting any fruit juices (ie. apple juice, orange juice) during fexofenadine administration and sampling periods, because fruit juices have been shown to impair fexofenadine absorption.20 Lastly, females of child–bearing potential were required to use a non–hormonal method of contraception throughout the study.
Informed consent was obtained from all study participants and clinical research was conducted in accordance with guidelines for human experimentation as specified by the U.S. Department of Health and Human Services. The study was approved by the National Institute of Allergy and Infectious Diseases Institutional Review Board.
Study design
This study was a single–center, crossover, single–sequence, open–label investigation to evaluate the effect of orally administered P. ginseng on CYP3A and P–gp activity in humans using oral midazolam and fexofenadine, respectively, as probe substrates. The study was conducted at the Clinical Research Center at the National Institutes of Health (Bethesda, MD, USA).
Treatment and blood sampling
Subjects were administered a single 8 mg oral dose of midazolam syrup (Roche Laboratories, Nutley, NJ, USA) and 120 mg (two 60 mg tablets) of fexofenadine tablets (Sanofi–Aventis, Bridgewater, NJ, USA) together on an empty stomach. Blood samples were collected for determination of both midazolam and fexofenadine plasma concentrations at the following time points: 0 (pre–dose), 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 8 and 24 hours post-dose. After collection, samples were immediately centrifuged and plasma harvested and frozen at −80°C until chromatographic analysis. Subjects then began taking P. ginseng 500 mg twice daily (Vitamer Laboratories) for 28 days. The P. ginseng dose (500 mg) was selected based on the recommended dose listed on the product label; the duration of administration was chosen to allow for adequate CYP3A and/or P-gp induction to occur in the event that P. ginseng turned out to be an inducer of one or both of these proteins.21 P. ginseng was self–administered with morning and evening meals by study subjects. Adherence was assessed by self-report, and examination of diary cards and pill counts at scheduled study visits. On Day 28 of P. ginseng administration, subjects returned to clinic for repeat midazolam and fexofenadine administration and blood sampling as previously described.
Analytical methods
Fexofenadine and midazolam were separated using Ultra Performance Liquid Chromatography (UPLC) with detection by tandem mass spectrometry (MS) using multiple reaction monitoring (MRM) as previously described.3 Calibration curves for midazolam and fexofenadine were linear from 1.0 to 100 ng/mL (R2 ≥ 0.998). Percent errors, as a measure of accuracy, were < 15%, and inter–assay and intra–assay coefficients of variation for fexofenadine were 6.16–9.22% and 5.88–6.87%, respectively, and coefficients of variation for midazolam were 5.00–12.59% and 5.32–10.85%, respectively at three different concentrations. The limit of quantization for fexofenadine and midazolam was 1.0 ng/mL and the limit of detection was 0.50 ng/mL.
Panax ginseng formulation
Chinese Panax ginseng 500 mg capsules (Vitamer Laboratories, Irvine, CA; lot # 115007) were used in this investigation. The formulation was standardized to 5% ginsenosides and certified by the Natural Products Association, a non–profit USA organization dedicated to the quality of manufacturing of natural supplements, in accordance with Good Manufacturing Practices (GMP). In addition, the product conformed to the dosage and mode of administration as established by the German Commission E monograph, containing P. ginseng powder from the whole root. After purchase from a commercial source, we did not perform further content analysis on the P. ginseng formulation nor did we assess serum concentrations of ginsenosides in our study population. However, the identical Vitamer product used in this study (Chinese Panax ginseng 500 mg capsules) was used previously in a study conducted by Gurley et al. In their study, the Vitamer Panax ginseng product underwent independent assessment for ginsenosides Rb1, Rb2, Rc, Rd, Re, Rf, and Rg at the National Center for Natural Products Research (University of Mississippi, University, MS, USA) using a proprietary gradient HPLC method.13 All ginsenosides were detected in that analysis, with Rb1 and Rb2 comprising the highest amounts (7.39 and 6.8 mg/capsule, respectively). In total, each capsule contained 22.4 mg ginsenosides. Previously conducted studies suggest that a number of ginsenosides and their deglycosylated metabolites are involved in CYP3A modulation, although a direct relationship between ginsenosides and CYP modulation has not been identified.22,23 It is also possible that additional phytochemicals present in P. ginseng may contribute to CYP modulation
Pharmacokinetic Analysis
Within subject changes for midazolam concentrations (before and after 28 days of P. ginseng administration period) were assessed by calculating geometric means (GM) and geometric mean ratios (GMR) with 90% confidence intervals (CI), determined as linear values. The coefficient of variation (CV) for the GMs was also calculated to measure dispersion within the data set. Plasma concentrations of midazolam and fexofenadine were analyzed by non–compartmental methods using WinNonlin pharmacokinetic software, version 5.0 (Pharsight Corporation, Mountain View, CA, USA). The maximum plasma concentration (Cmax), and time to reach Cmax (Tmax) were obtained by direct inspection of the plasma concentration–time profiles. The elimination rate constant (λZ) was determined by calculating the absolute value of the slope of the log–linear regression using at least three points on the plasma concentration–time plot. The AUC from zero to the last quantifiable concentration (AUC0–last) was determined for midazolam and fexofenadine by the log–linear trapezoidal rule; AUC from zero to infinity (AUC0–∞) was calculated by dividing the last measured concentration by λZ and adding this value to AUClast. Apparent oral clearance (CL/F) was estimated for midazolam and fexofenadine as dose/AUC0–∞.
Statistical Analysis
Changes in pharmacokinetic parameters were considered significant when the confidence interval (CI) for the GMR did not cross the value of 1.24 SYSTAT Software, version 11 (Richmond, CA, USA) was used for sample size calculation and inferential statistics; Microsoft Excel 2003 (Microsoft Corp., Redmond, WA, USA) was used to generate descriptive statistical data.
Sample Size
A difference in midazolam AUC of at least 33% was considered to be clinically relevant for the purpose of estimating sample size. A standard deviation of 53 ng·hr/mL with an average AUC0–∞ of 143 ng·hr/mL was assumed for midazolam based on previous data.4 With α set at 0.05, a sample of 12 subjects was deemed necessary to provide 84% power to detect a 33% difference in midazolam AUC0–∞ before and after P. ginseng administration (SYSTAT software, version 11).
Results
Subjects
Fifteen subjects screened, and twelve (8 males) completed study participation. Demographic information for the study subjects is presented in Table 1. Two subjects were not enrolled due to elevated cholesterol, and participation in another research study, respectively. A third subject was removed from the study for non–compliance with protocol procedures. All subjects reported excellent adherence to P. ginseng with no subject missing more than 2 doses throughout the study.
Table 1.
Study Subject Demographics
| Subject | Age (years) | Sex | Race/Ethnicity | Weight (kg) | BMI (kg/m2) |
|---|---|---|---|---|---|
| 1 | 32 | Male | Hispanic | 97 | 35.0 |
| 2 | 28 | Male | White | 83 | 23.8 |
| 3 | 40 | Female | Black | 114 | 43.7 |
| 4 | 33 | Male | White | 70 | 24.0 |
| 5 | 41 | Male | White | 92 | 29.1 |
| 6 | 31 | Female | Unknown | 51 | 22.4 |
| 7 | 29 | Male | Unknown | 67 | 24.5 |
| 8 | 23 | Female | Black | 59 | 22.4 |
| 9 | 27 | Male | Asian | 63 | 20.6 |
| 10 | 38 | Male | Unknown | 75 | 27.9 |
| 11 | 23 | Female | Unknown | 94 | 38.4 |
| 12 | 42 | Male | White | 70 | 23.0 |
|
| |||||
| Median: | 32 | 73 | 24.3 | ||
Midazolam and fexofenadine pharmacokinetics
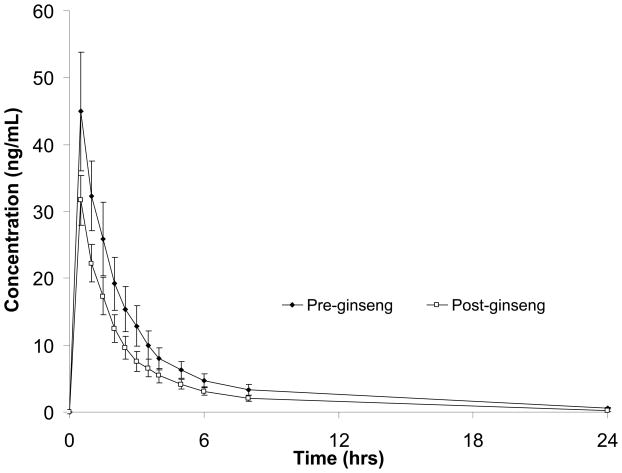
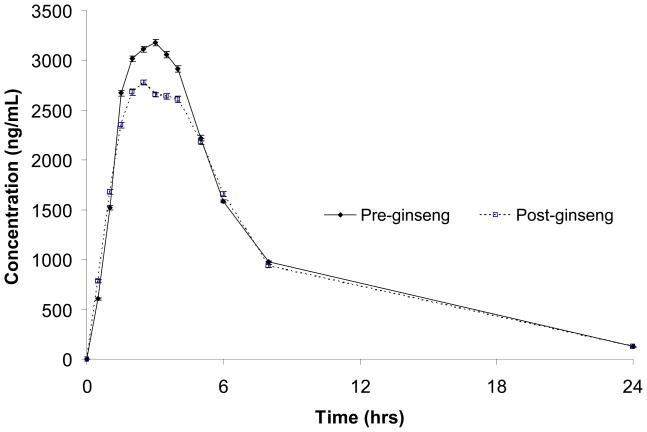
Pharmacokinetic parameter values and GMRs are presented for both midazolam and fexofenadine in Table 2. Concentration–versus–time profiles for midazolam and fexofenadine, before and after P. ginseng administration, are shown in Figures 1 and 2, respectively. Both midazolam AUC and Cl/F were significantly altered after 28 days of P. ginseng administration. The GMR for midazolam AUC (post– ginseng/pre–ginseng) was 0.66 (0.55–0.78); moreover, midazolam AUC decreased in 11 of the 12 study participants (up to 71%) after P. ginseng administration. Consistent with the observed changes in midazolam AUC, the apparent oral clearance of midazolam was significantly increased by 51% following P. ginseng administration (Table 2). Midazolam Cmax and T1/2 were also reduced by lesser, albeit statistically significant differences of 26 and 29%, respectively. In contrast to midazolam, fexofenadine pharmacokinetics were unchanged after 28 days of P. ginseng administration (Table 2).
Table 2.
Midazolam and fexofenadine pharmacokinetic parameter values before and after 28 days of Panax ginseng administration in 12 healthy volunteers
| Midazolam | Pre-ginseng | Post-ginseng | Post-ginseng/Pre-ginseng | ||
|---|---|---|---|---|---|
|
| |||||
| Geometric Mean | % CV | Geometric Mean | % CV | Geometric Mean Ratios | |
| AUC0-∞ (ng · hr/mL) | 120 | 69 | 79 | 51 | 0.66 (0.55–0.78) |
| Cmax (ng/mL) | 39 | 67 | 29 | 41 | 0.74 (0.56 – 0.93) |
| Tmax (hr)a | 0.5 | 0.5–1.0b | 0.5 | 0.5–1.5b | – |
| T ½(hr) | 5.6 | 32 | 4.0 | 84 | 0.71 (0.53–0.90) |
| Cl/F (mL/hr) | 67 | 43 | 101 | 58 | 1.51 (1.17–1.86) |
|
| |||||
|
Fexofenadine
| |||||
| AUC0-∞ (ng · hr/mL) | 2036 | 39 | 1860 | 59 | 0.91 (0.69–1.14) |
| Cmax (ng/mL) | 305 | 33 | 258 | 40 | 0.84 (0.67–1.02) |
| Tmax (hr)a | 2.3 | 1.5–4.0b | 2.8 | 1.0–5.0b | – |
| T ½ (hr) | 5.2 | 19 | 4.8 | 15 | 0.93 (0.85–1.01) |
| Cl/F (mL/hr) | 59 | 41 | 65 | 36 | 1.09 (0.84–1.35) |
Median.
Ranges.
Figure 1.
Midazolam concentrations (±SEM) before- and after 28 days of Panax ginseng
Figure 2.
Fexofenadine concentrations (±SEM) before- and after 28 days of Panax ginseng administration
Safety
Three of the twelve subjects experienced mild adverse events while taking P. ginseng; these included Grade 1 diarrhea, abdominal pain, and insomnia. No serious adverse events were reported. Abnormal laboratory findings included Grade 1 anemia (n=1) and a Grade 1 decrease in absolute neutrophil count (n=2) in two separate subjects; none of these abnormalities were felt to be related to the study medications.
Discussion
The use of herbal supplements has grown considerably during the past twenty years. As a result, clinicians must be constantly aware of potential herb–drug interactions.1,2,5 Indeed, a number of herb–drug interactions have been reported for St. John’s wort, ginkgo biloba, and garlic.3,5,25,26 The proposed mechanism(s) for these interactions frequently involves modulation of CYP3A and/or P–gp by the herbal supplement. Studies examining the influence of P. ginseng on CYP3A activity in humans are limited, and none have used midazolam AUC (the accepted standard for CYP3A phenotyping) to characterize CYP3A activity.27 Additionally, no studies have assessed the influence of P. ginseng on P–gp activity in humans. As such, we chose to characterize the influence of 28 days of P. ginseng administration on CYP3A4/5 and P–gp activity, using midazolam and fexofenadine probes, respectively.
In the current investigation, we observed significant reductions in midazolam AUC (−44%), T1/2 (−29%), and Cmax (−26%), and a significant increase in CL/F (51%). The observed increase in midazolam elimination and reduction in absorption by P. ginseng is consistent with increased CYP3A activity in the liver and perhaps the gastrointestinal tract as well. However, because we did not administer intravenous midazolam in this study, it is not possible to definitively characterize the differential effects of P. ginseng on hepatic versus intestinal CYP3A activity.
While our data are suggestive of CYP3A induction by P. ginseng, other studies in healthy volunteers have reported alternate findings.7,14,19 Three other studies have assessed the influence of P. ginseng on CYP3A activity. Gurley et al. found that 28 days of P. ginseng administration (500 mg, three times daily) had no apparent effect on midazolam metabolism in 12 healthy subjects.7 One potential reason for the disparity between our results and those of Gurley et al. lies in the details of the approach of midazolam phenotyping used. Gurley et al. employed a 1–hour post–dose plasma concentration ratio of 1-hydroxymidazolam:midazolam to assess CYP3A activity, while we used midazolam AUC0-∞. These two approaches have not yielded consistent results in previous studies; thus we chose to use midazolam AUC0-∞ to assess CYP3A modulation with P. ginseng.27,28–31
Anderson et al. also investigated the potential of P. ginseng to modulate CYP3A by measuring the urinary metabolic ratio of 6–β–OH-cortisol:cortisol in healthy volunteers given 24 days of ginseng extract (4% ginsenosides, 100 mg twice daily).14 Results from this study showed no change in 6-βOH-coritsol:cortisol ratio, leading the authors to conclude that P. ginseng did not alter CYP3A activity. Limitations to this study included a low dose of P. ginseng (100 mg), which may not have been sufficient to produce changes in CYP3A activity, and the use of 6-β-OH-coritsol:cortisol ratios for CYP3A phenotyping, which has been shown to be suboptimal.32–34.
A third study, published in abstract form, reported a 29% increase (p value not reported) in the maximum concentration of the CYP3A substrate, nifedipine, after an 18 day course of ginseng 200 mg daily. The impact of ginseng on the systemic exposure (AUC) of nifedipine was not reported. As a result, it is not possible to accurately assess the impact of ginseng on nifedipine from these preliminary data.19
In contrast to its effect on CYP3A activity, ginseng did not alter P–gp function, using fexofenadine as a probe substrate. fexofenadine has been used as a probe to assess P–gp transport in previously published studies.3,4 However, use of fexofenadine as a P–gp specific probe is not without limitations. Though multiple in vitro and in vivo studies have confirmed that fexofenadine is indeed a substrate for P–gp,35–42 recent in vitro data suggest that fexofenadine transport may be more complex, involving other transporters, such as organic anion transporting polypeptides (OATPs), in addition to P–gp.43–45 The relative contribution and importance of other transport proteins on the absorption, distribution, and elimination of fexofenadine is not yet known. Therefore, results from studies, such as this one, using fexofenadine to assess P–gp–mediated drug transport need to take into account the potential role of additional transport proteins that may contribute to fexofenadine disposition.35 Nonetheless it appears likely that ginseng did not significantly alter the function of any of the proteins involved in fexofenadine transport. However, it is also possible that multiple fexofenadine transporters were equally altered by P. ginseng in opposite directions; resulting in no net change in fexofenadine pharmacokinetics. As a result, P. ginseng appears unlikely to interact with medications transported by P–gp and/or OATPs, but the possibility cannot be ruled out entirely..
Another potential limitation to this study is that we did not perform an independent phytochemical analysis to confirm percent ginsenosides in the ginseng product used in our study. Thus, it cannot be ruled out that the product we used differed in ginsenoside content – and the subsequent ability to modulate CYP3A – compared to other commercial preparations. However, this potential limitation appears minimal, considering that the P. ginseng formulation used in this study was tested for ginsenoside content by an independent organization (National Products Association) and found to contain percent ginsenosides consistent with the manufacturer’s label. The product also underwent independent analysis in a previous study by Gurley et al. (described herein) where it was found to contain appropriate amounts of ginsenosides.13
Results from this study indicate that ginseng has the potential to reduce the systemic exposure of medications that are largely metabolized by CYP3A. The magnitude of the interaction between ginseng and the CYP3A substrate midazolam (−34%) may be clinically relevant for a number of medications with narrow therapeutic ranges. Such medications may include, but are not limited to cyclosporine, tacrolimus, irinotecan, sildenafil and sirolimus. Conversely, ginseng is unlikely to interact with medications that are transported by P–gp and/or OATPs but are not metabolized by CYP3A (ie. digoxin, talinolol, and fexofenadine).
Conclusion
In conclusion, patients taking P. ginseng along with CYP3A4/5 substrates with narrow therapeutic ranges should be monitored closely for adequate therapeutic response to the substrate medication. Dosage increase in the substrate medication or discontinuation of P. ginseng may be warranted in some instances; therefore, patients should be assessed on an individual basis.
Acknowledgments
Sources of Support: The Intramural Research Programs of the National Institutes of Health (NIH) Clinical Center Pharmacy Department and the National Institute of Allergy and Infectious Diseases (NIAID).
Footnotes
Declaration of interest: The authors have no financial conflicts of interest or other commercial relationships to declare. No outside statistical or editorial assistance was provided to the authors. No official support or endorsement of this article by the US Food and Drug Administration is intended or should be inferred.
References
- 1.Eisenberg DM, Daivs RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990–1997: results of a follow-up national survey. JAMA. 1998;280:1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 2.Tindle HA, Davis RB, Phillips RS, Eisenberg DM. Trends in use of complementary and alternative medicine use by US adults: 1997–2002. Altern Ther Health Med. 2005;11:42–49. [PubMed] [Google Scholar]
- 3.Robertson SM, Davey RT, Voell J, et al. Effect of Ginkgo biloba extract on lopinavir, midazolam and fexofenadine pharmacokinetics in healthy subjects. Curr Med Res Opin. 2008;24:591–599. doi: 10.1185/030079908x260871. [DOI] [PubMed] [Google Scholar]
- 4.Penzak SR, Robertson SM, Hunt JD, et al. Echinacea purpurea significantly induces cytochrome P450 3A activity but does not alter lopinavir-ritonavir exposure in healthy subjects. Pharmacotherapy. 2010;30:797–805. doi: 10.1592/phco.30.8.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrelli F, Izzo AA. Herb-drug interactions with St John’s wort (Hypericum perforatum): an update on clinical observations. AAPS J. 2009;11:710–727. doi: 10.1208/s12248-009-9146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: an updated systematic review. Drugs. 2009;69:1777–1798. doi: 10.2165/11317010-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Gurley BJ, Gardner SF, Hubbard MA, et al. Cytochrome P450 phenotypic ratios for predicting herb-drug interactions in humans. Clin Pharmacol Ther. 2002;72:276–287. doi: 10.1067/mcp.2002.126913. [DOI] [PubMed] [Google Scholar]
- 8.Guengerich FP. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol. 1999;39:1–17. doi: 10.1146/annurev.pharmtox.39.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Wrighton SA, Stevens JC. The human hepatic cytochromes P450 involved in drug metabolism. Crit Rev Toxicol. 1992;22:1–21. doi: 10.3109/10408449209145319. [DOI] [PubMed] [Google Scholar]
- 10.Coon JT, Ernst E. Panax ginseng: a systematic review of adverse effects and drug interactions. Drug Saf. 2002;25:323–344. doi: 10.2165/00002018-200225050-00003. [DOI] [PubMed] [Google Scholar]
- 11.Christensen LP. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 12.Chen CF, Chiou WF, Zhang JT, et al. Comparison of the pharmacological effects of Panax ginseng and Panax quinquefolium. Acta Pharmacol Sin. 2008;29:1103–1108. doi: 10.1111/j.1745-7254.2008.00868.x. [DOI] [PubMed] [Google Scholar]
- 13.Gurley Bill J, Gardner SF, Hubbard MA, et al. Clinical Assessment of effects of botanical supplementation on cytochrome P450 phenotypes in the elderly. Drugs Aging. 2005;22:525–539. doi: 10.2165/00002512-200522060-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson GD, Rosito G, Mohustsy MA, Elmer GW. Drug interaction potential of soy extract and Panax ginseng. J Clin Pharmacol. 2003;43:643–648. [PubMed] [Google Scholar]
- 15.Gorski CJ, Huang SM, Pinto A, et al. The effect of Echinacea (Echinacea purpurea root) on cytochrome P450 activity in vivo. Clin Pharmacol Ther. 2004;75:89–100. doi: 10.1016/j.clpt.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Budzinski JW, Foster BC, Vandenhoek S, Arnason JT. An in vitro evaluation of human cytochrome P450 3A4 inhibition by selected commercial herbal extracts and tinctures. Phytomedicine. 2000;7:273–282. doi: 10.1016/S0944-7113(00)80044-6. [DOI] [PubMed] [Google Scholar]
- 17.He N, Edeki TI. Effects of ginseng and Ginkgo biloba components on CYP3A4 mediated testosterone 6beta-hydroxylation in human liver microsomes [abstract] Clin Pharmacol Ther. 2003;73:50, Abstract PII-81. [Google Scholar]
- 18.Henderson GL, Harkey MR, Gershwin ME, Hackman RM, Stern JS, Stresser DM. Effects of ginseng components on c-DNA-expressed cytochrome P450 enzyme catalytic activity. Life Sci. 1999;65:209–214. doi: 10.1016/s0024-3205(99)00407-5. [DOI] [PubMed] [Google Scholar]
- 19.Smith M, Lin KM, Zheng YP. An open trial of nifedipine-herb interactions: nifedipine with St. John’s wort, ginseng, or ginkgo biloba [abstract] Clin Pharmacol Ther. 2001;69:86, Abstract PIII-89. [Google Scholar]
- 20.Dresser GK, Bailey DG, Leake BF, et al. Fruit juices inhibit organic anion transporting polypeptide-mediated drug uptake to decrease the oral availability of fexofenadine. Clin Pharmacol Ther. 2002;71:11–20. doi: 10.1067/mcp.2002.121152. [DOI] [PubMed] [Google Scholar]
- 21.Kashuba ADM, Bertino JS., Jr . Mechanisms of Drug interactions I: Absorption, Metabolism and Excretion. In: Piscitelli SC, Rodvold KA, editors. Drug Interactions in Infectious Diseases. 2. Totowa, NJ: Human Press; 2005. pp. 13–39. [Google Scholar]
- 22.Liu Y, Zhang JW, Li W, et al. Ginsenoside metabolites, rather than naturally ocurribg ginsenosides, lead to inhibition of human cytochrome P450 enzymes. Toxicol Sci. 2006;91:356–64. doi: 10.1093/toxsci/kfj164. [DOI] [PubMed] [Google Scholar]
- 23.Hao H, Ba Q, Yin J, et al. Deglycosylated ginsenosides are more potent inducers of CYP1A1, CYP1A2, and CYP3A4 expression in HepG2 cells than glycosylated ginsenosides. Drug Metab Pharmacokinet. 2010 Dec 17; doi: 10.2133/dmpk.dmpk-10-nt-056. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.US Department of Health and Human Services. Food and Drug Administration. Guidance for Industry: Drug interaction studies – study design, data analysis, and implications for dosing and labeling. 2006 Sep; Available from: http://www.fda.gov/cder/guidance/index.htm.
- 25.Piscitelli SC, Burstein AH, Welden N, Gallicano KD, Falloon J. The effect of garlic supplements on the pharmacokinetics of saquinavir. Clin Infect Dis. 2002;34:234–8. doi: 10.1086/324351. [DOI] [PubMed] [Google Scholar]
- 26.Piscitelli SC, Burstein AH, Chaitt D, Alfaro RM, Falloon J. Indinavir concentrations and St. John’s wort Lancet. 2000;355:547–48. doi: 10.1016/S0140-6736(99)05712-8. [DOI] [PubMed] [Google Scholar]
- 27.Streetman DS, Bertino JS, Nafziger AN. Phenotyping of drug-metabolizing enzymes in adults: a review of in-vivo cyctochrome P450 phenotyping probes. Pharmacogenetics. 2000;10:187–216. doi: 10.1097/00008571-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Rogers JF, Nafziger AN, Kashuba AD, et al. Single plasma concentrations of 1′-hydroxymidazolam or the ratio of 1′-hydroxymidazolam: midazolam do not predict midazolam clearance in healthy subjects. J Clin Pharmacol. 2002;42:1079–1082. doi: 10.1177/009127002401382614. [DOI] [PubMed] [Google Scholar]
- 29.Eap CB, Buclin T, Cucchia G, et al. Oral administration of a low dose of midazolam (75 microg) as an in vivo probe for CYP3A activity. Eur J Clin Pharmacol. 2004;60:237–246. doi: 10.1007/s00228-004-0762-z. [DOI] [PubMed] [Google Scholar]
- 30.Lin YS, Lockwood GF, Graham MA, et al. In-vivo phenotyping for CYP3A by a single-point determination of midazolam plasma concentration. Pharmacogenetics. 2001;11:781–791. doi: 10.1097/00008571-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Nafziger AN, Bertino JS., Jr Low hepatic cytochrome P450 3A activity is a risk for corticosteroid-induced osteonecrosis. Clin Pharmacol Ther. 2007;82:379. doi: 10.1038/sj.clpt.6100105. [DOI] [PubMed] [Google Scholar]
- 32.Hunt CM, Watkins PB, Saenger P, Stave GM, Barlascini N, Watlington CO, et al. Heterogeneity of CYP3A isoforms metabolizing erythromycin and cortisol. Clin Pharmacol Ther. 1992;51:18–23. doi: 10.1038/clpt.1992.3. [DOI] [PubMed] [Google Scholar]
- 33.Kinirons MT, O’Shea D, Downing TE, Fitzwilliam AT, Joellenbeck L, Groopman JD, et al. Absence of correlations among three putative in-vivo probes of human cytochrome P-4503A4 activity in young healthy men. Clin Pharmacol Ther. 1993;54:621–629. doi: 10.1038/clpt.1993.199. [DOI] [PubMed] [Google Scholar]
- 34.Watkins PB, Turgeon DK, Saenger P, Lown KS, Kolars JC, Hamilton T, et al. Comparisons of urinary 6-beta-cortisol and the erythromycin breath test as measures of hepatic P450IIIA (CYP3A) activity. Clin Pharmacol Ther. 1992;52:265–273. doi: 10.1038/clpt.1992.140. [DOI] [PubMed] [Google Scholar]
- 35.Ma JD, Tsunoda SM, Bertino JS, Trivedi M, Beale KK, Nafziger AN. Evaluation of in vivo p-glycoprotein phenotyping probes: A need for validation. Clin Pharmacokinet. 2010;49:223–237. doi: 10.2165/11318000-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 36.Cvetkovic M, Leake B, Fromm MF, Wilkinson GR, Kim RB. OATP and P-glycoprotein transporters mediate the cellular uptake and excretion of fexofenadine. Drug Metab Dispos. 1999;27:866–871. [PubMed] [Google Scholar]
- 37.Putnam WS, Ramanathan S, Pan L, Takahashi LH, Benet LZ. Functional characterization of monocarboxylic acid, large neutral amino acid, bile acid and peptide transporters, and P-glycoprotein in MDCK and Caco-2 cells. J Pharm Sci. 2002;91:2622–2635. doi: 10.1002/jps.10264. [DOI] [PubMed] [Google Scholar]
- 38.Perloff MD, von Moltke LL, Greenblatt DJ. Fexofenadine transport in Caco-2 cells: inhibition with verapamil and ritonavir. J Clin Pharmacol. 2002;42:1269–1274. doi: 10.1177/009127002762491370. [DOI] [PubMed] [Google Scholar]
- 39.Gupta S, Banfield C, Kantesaria B, Marino M, Clement R, Affrime M, Batra V. Pharmacokinetic and safety profile of desloratadine and fexofenadine when coadministered with azithromycin: a randomized, placebo-controlled, parallel-group study. Clin Ther. 2001;23:451–466. doi: 10.1016/s0149-2918(01)80049-7. [DOI] [PubMed] [Google Scholar]
- 40.Hamman MA, Bruce MA, Haehner-Daniels BD, Hall SD. The effect of rifampin administration on the disposition of fexofenadine. Clin Pharmacol Ther. 2001;69:114–121. doi: 10.1067/mcp.2001.113697. [DOI] [PubMed] [Google Scholar]
- 41.Yasui-Furukori N, Uno T, Sugawara K, Tateishi T. Different effects of three transporting inhibitors, verapamil, cimetidine, and probenecid, on fexofenadine pharmacokinetics. Clin Pharmacol Ther. 2005;77:17–23. doi: 10.1016/j.clpt.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 42.Shimizu M, Uno T, Sufawara K, Tateishi T. Effects of itraconazole and diltiazem on the pharmacokinetics of fexofenadine, a substrate of P-glycoprotein. Brit J Clin Pharmacol. 2006;61:538–544. doi: 10.1111/j.1365-2125.2006.02613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nozawa T, Imai K, Nezu J, et al. Functional characterization of pH-sensitive organic anion transporting polypeptide OATP-B in human. J Pharmacol Exp Ther. 2004;308:438–445. doi: 10.1124/jpet.103.060194. [DOI] [PubMed] [Google Scholar]
- 44.Matsushima S, Maeda K, Ishiguro N, et al. Investigation of the inhibitory effects of various drugs on the hepatic uptake of fexofenadine in humans. Drug Metab Dispos. 2008;36:663–669. doi: 10.1124/dmd.107.017814. [DOI] [PubMed] [Google Scholar]
- 45.Shimizu M, Fuse K, Okudaira K, et al. Contribution of OATP (organic anion-transporting polypeptide) family transporters to the hepatic uptake of fexofenadine in humans. Drug Metab Dispos. 2005;33:1477–1481. doi: 10.1124/dmd.105.004622. [DOI] [PubMed] [Google Scholar]