Abstract
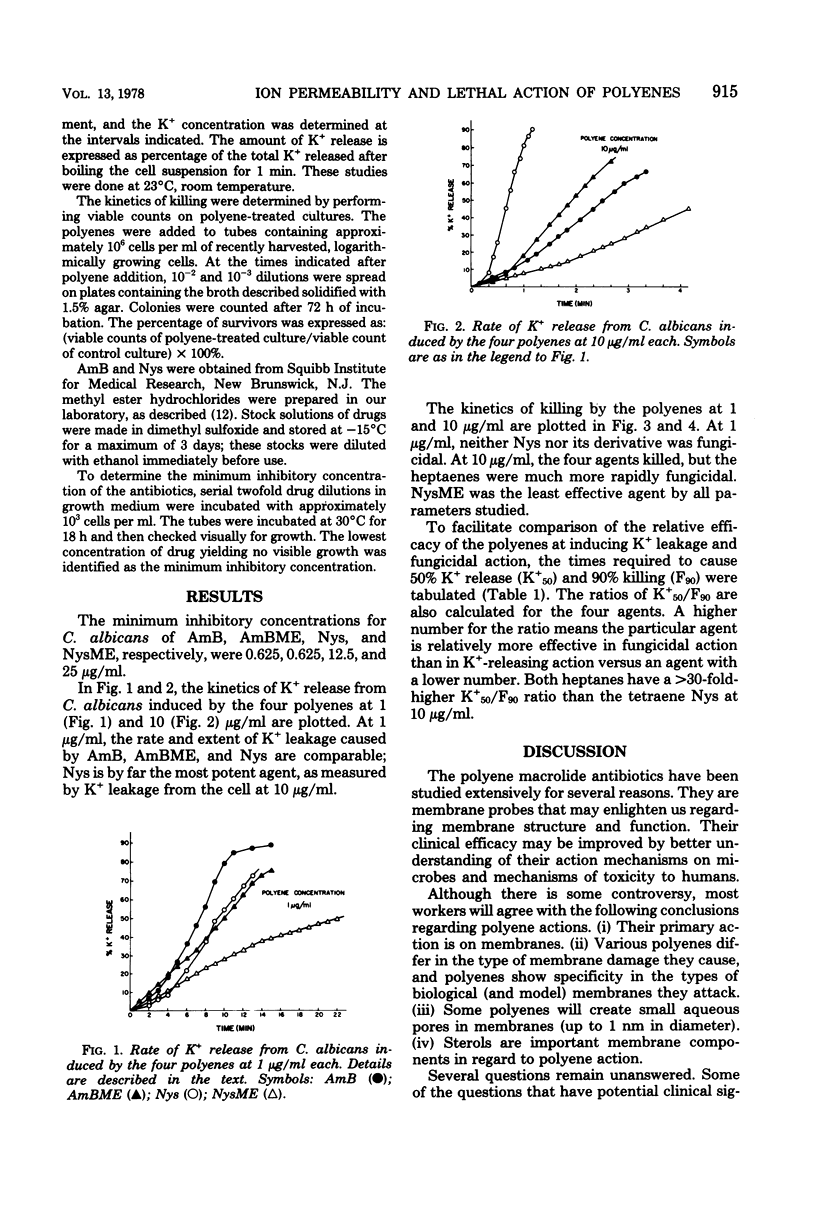
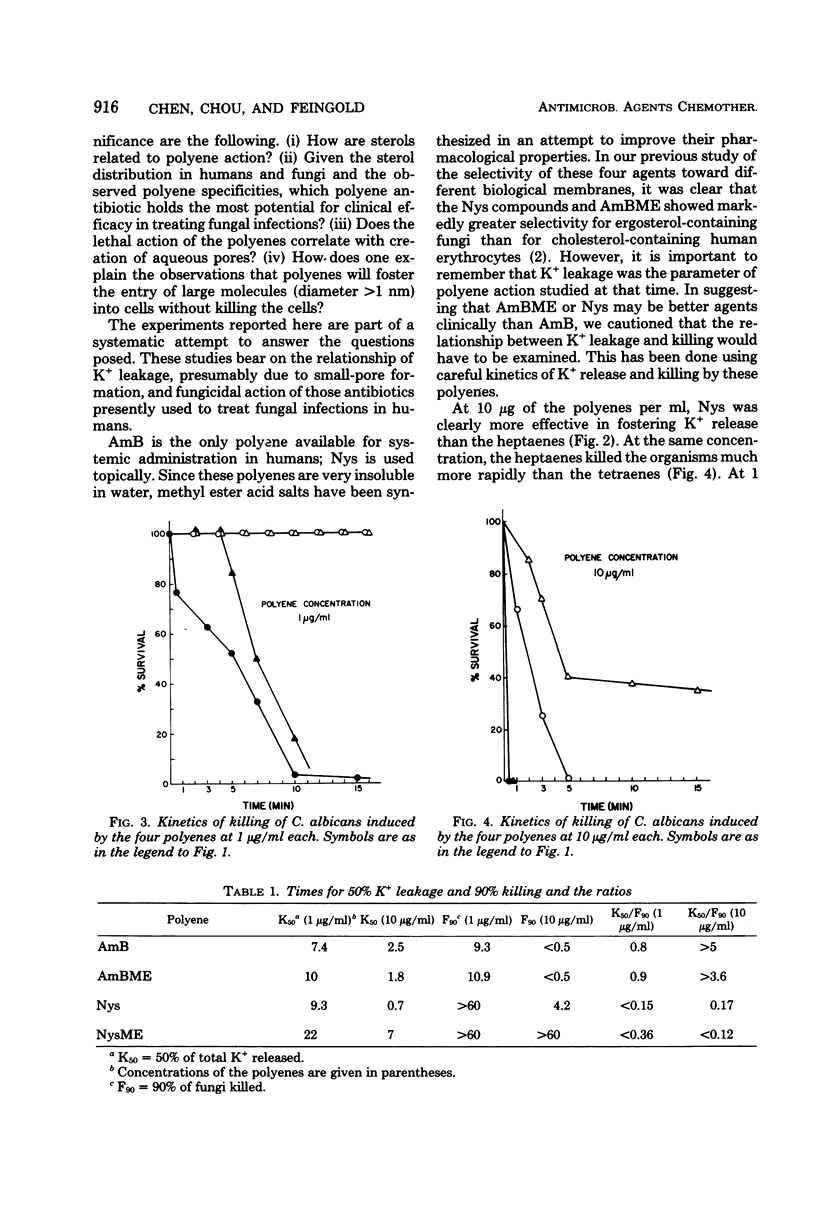
Kinetic data on potassium release from and killing of Candida albicans by the four polyene antibiotics amphotericin B, amphotericin B methyl ester hydrochloride, nystatin, and nystatin methyl ester hydrochloride are presented. The nystatins were relatively more effective than the amphotericins in causing potassium release rather than killing. These data suggest that the aqueous channels or pores formed by the polyene antibiotics are not central to the lethal action of the drugs.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cass A., Finkelstein A., Krespi V. The ion permeability induced in thin lipid membranes by the polyene antibiotics nystatin and amphotericin B. J Gen Physiol. 1970 Jul;56(1):100–124. doi: 10.1085/jgp.56.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. C., Sud I. J., Chou D. L., Feingold D. S. Selective toxicity of the polyene antibiotics and their methyl ester derivatives. Biochem Biophys Res Commun. 1977 Jan 24;74(2):480–487. doi: 10.1016/0006-291x(77)90329-1. [DOI] [PubMed] [Google Scholar]
- Dennis V. W., Stead N. W., Andreoli T. E. Molecular aspects of polyene- and sterol-dependent pore formation in thin lipid membranes. J Gen Physiol. 1970 Mar;55(3):375–400. doi: 10.1085/jgp.55.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein A., Holz R. Aqueous pores created in thin lipid membranes by the polyene antibiotics nystatin and amphotericin B. Membranes. 1973;2:377–408. [PubMed] [Google Scholar]
- Gent M. P., Prestegard J. H. Interaction of the polyene antibiotics with lipid bilayer vesicles containing cholesterol. Biochim Biophys Acta. 1976 Feb 19;426(1):17–30. doi: 10.1016/0005-2736(76)90425-9. [DOI] [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Fungal sterols and the mode of action of the polyene antibiotics. Adv Appl Microbiol. 1974;17(0):109–134. doi: 10.1016/s0065-2164(08)70556-2. [DOI] [PubMed] [Google Scholar]
- HsuChen C. C., Feingold D. S. Two types of resistance to polyene antibiotics in Candida albicans. Nature. 1974 Oct 18;251(5476):656–659. doi: 10.1038/251656a0. [DOI] [PubMed] [Google Scholar]
- Kerridge D., Koh T. Y., Johnson A. M. The interaction of amphotericin B methyl ester with protoplasts of Candida albicans. J Gen Microbiol. 1976 Sep;96(1):117–123. doi: 10.1099/00221287-96-1-117. [DOI] [PubMed] [Google Scholar]
- Marty A., Finkelstein A. Pores formed in lipid bilayer membranes by nystatin, Differences in its one-sided and two-sided action. J Gen Physiol. 1975 Apr;65(4):515–526. doi: 10.1085/jgp.65.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechlinski W., Schaffner C. P. Polyene macrolide derivatives. I. N-acylation and esterification reactions with amphotericin B. J Antibiot (Tokyo) 1972 Apr;25(4):256–258. [PubMed] [Google Scholar]
- Medoff G., Kwan C. N., Schlessinger D., Kobayashi G. S. Permeability control in animal cells by polyenes: a possibility. Antimicrob Agents Chemother. 1973 Mar;3(3):441–443. doi: 10.1128/aac.3.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff G., Valeriote F., Lynch R. G., Schlessinger D., Kobayashi G. S. Synergistic effect of amphotericin B and 1,3-bis(2-chloroethyl)-1-nitrosourea against a transplantable AKR leukemia. Cancer Res. 1974 May;34(5):974–978. [PubMed] [Google Scholar]
- Norman A. W., Spielvogel A. M., Wong R. G. Polyene antibiotic - sterol interaction. Adv Lipid Res. 1976;14:127–170. [PubMed] [Google Scholar]
- Zygmunt W. A. Intracellular Loss of Potassium in Candida albicans After Exposure to Polyene Antifungal Antibiotics. Appl Microbiol. 1966 Nov;14(6):953–956. doi: 10.1128/am.14.6.953-956.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kruijff B., Demel R. A. Polyene antibiotic-sterol interactions in membranes of Acholeplasma laidlawii cells and lecithin liposomes. 3. Molecular structure of the polyene antibiotic-cholesterol complexes. Biochim Biophys Acta. 1974 Feb 26;339(1):57–70. doi: 10.1016/0005-2736(74)90332-0. [DOI] [PubMed] [Google Scholar]
- de Kruijff B., Gerritsen W. J., Oerlemans A., van Dijck P. W., Demel R. A., van Deenen L. L. Polyene antibiotic-sterol interactions in membranes of Acholesplasma laidlawii cells and lecithin liposomes. II. Temperature dependence of the polyene antibiotic-sterol complex formation. Biochim Biophys Acta. 1974 Feb 26;339(1):44–56. doi: 10.1016/0005-2736(74)90331-9. [DOI] [PubMed] [Google Scholar]
- van Zutphen H., Demel R. A., Norman A. W., van Deenen L. L. The action of polyene antibiotics on lipid bilayer membranes in the presence of several cations and anions. Biochim Biophys Acta. 1971 Aug 13;241(2):310–330. doi: 10.1016/0005-2736(71)90031-9. [DOI] [PubMed] [Google Scholar]


